Abstract
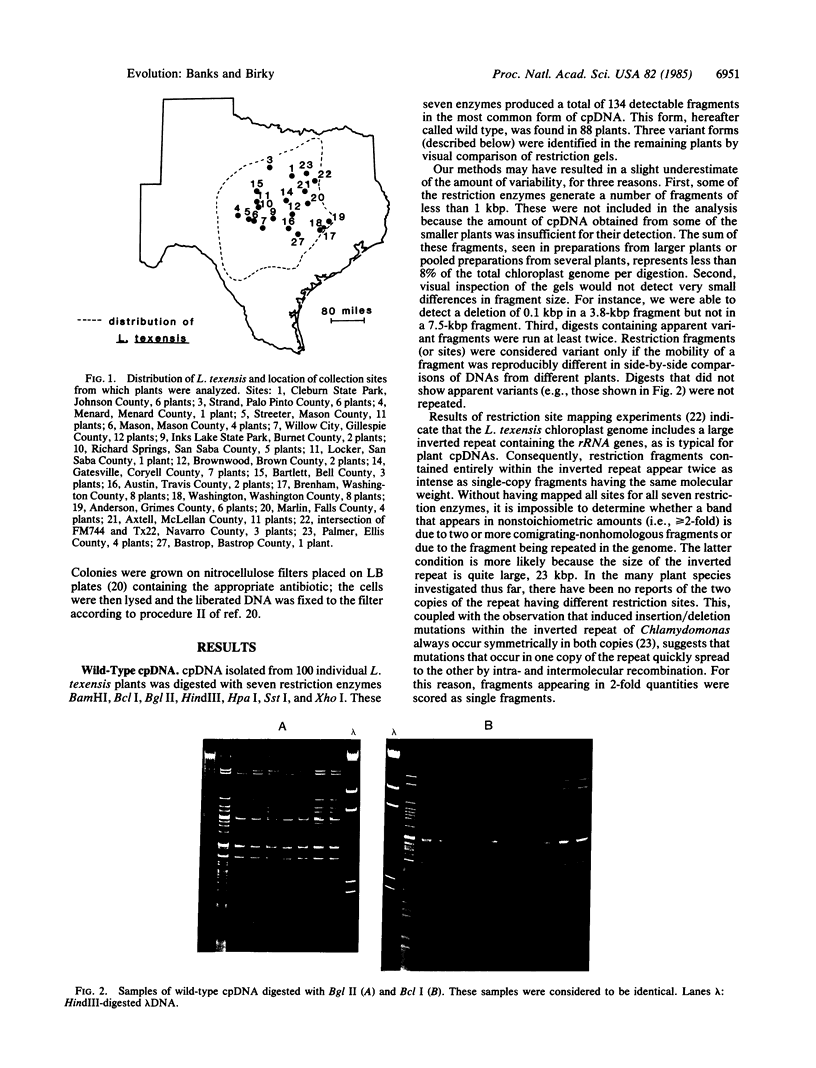
Chloroplast DNA diversity was measured in an annual flowering plant, Lupinus texensis. Individual plants were collected from 21 local populations throughout the range of the species in Texas. Chloroplast DNA was isolated separately from each plant and digested with seven restriction enzymes. The most common form of the 150-kilobase-pair genome was cut at 134 sites, so that about 0.5% of the base pairs in the genome were sampled. Of the 100 plants examined, 88 had identical restriction fragment patterns. Three variant forms were found in different local populations. Two, represented in single plants, differed from wild type in the presence or absence of single restriction sites. The third variant was fixed in one of the local populations; it had lost a restriction site and also had a deletion of approximately equal to 100 base pairs. The data suggest that chloroplast DNA in this plant is much less polymorphic than mitochondrial DNA from animals and is probably less polymorphic than nuclear genes in the same plant or in animals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birky C. W., Jr, Maruyama T., Fuerst P. An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts, and some results. Genetics. 1983 Mar;103(3):513–527. doi: 10.1093/genetics/103.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky C. W., Jr Relaxed cellular controls and organelle heredity. Science. 1983 Nov 4;222(4623):468–475. doi: 10.1126/science.6353578. [DOI] [PubMed] [Google Scholar]
- Birky C. W., Jr Transmission genetics of mitochondria and chloroplasts. Annu Rev Genet. 1978;12:471–512. doi: 10.1146/annurev.ge.12.120178.002351. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. W., Stephens J. C., Lansman R. A., Avise J. C. Models of mitochondrial DNA transmission genetics and evolution in higher eucaryotes. Genet Res. 1982 Aug;40(1):41–57. doi: 10.1017/s0016672300018899. [DOI] [PubMed] [Google Scholar]
- Clegg M. T., Rawson J. R., Thomas K. Chloroplast DNA variation in pearl millet and related species. Genetics. 1984 Mar;106(3):449–461. doi: 10.1093/genetics/106.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. E., Clegg M. T. Molecular evolution of chloroplast DNA sequences. Mol Biol Evol. 1984 Jul;1(4):291–301. doi: 10.1093/oxfordjournals.molbev.a040319. [DOI] [PubMed] [Google Scholar]
- Engels W. R. Estimating genetic divergence and genetic variability with restriction endonucleases. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6329–6333. doi: 10.1073/pnas.78.10.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Brown W. M., Davidson W. S., Wilson A. C. Extensive polymorphism in the mitochondrial DNA of apes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6319–6323. doi: 10.1073/pnas.78.10.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. C., Mills K. A., Demopulos C. M., Hornung S., Motulsky A. G. Linkage disequilibrium and evolutionary relationships of DNA variants (restriction enzyme fragment length polymorphisms) at the serum albumin locus. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3486–3490. doi: 10.1073/pnas.81.11.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. M., Grant D. M., Rabert D. K., Harris E. H., Boynton J. E., Gillham N. W. Mutants of Chlamydomonas reinhardtii with physical alterations in their chloroplast DNA. Plasmid. 1982 Mar;7(2):133–151. doi: 10.1016/0147-619x(82)90073-7. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Tajima F. DNA polymorphism detectable by restriction endonucleases. Genetics. 1981 Jan;97(1):145–163. doi: 10.1093/genetics/97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Jorgensen R. A., Thompson W. F. Chloroplast DNA variation and evolution in pisum: patterns of change and phylogenetic analysis. Genetics. 1985 Jan;109(1):195–213. doi: 10.1093/genetics/109.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D. Physical and gene mapping of chloroplast DNA from Atriplex triangularis and Cucumis sativa. Nucleic Acids Res. 1982 Mar 11;10(5):1593–1605. doi: 10.1093/nar/10.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Zamir D. Chloroplast DNA evolution and phylogenetic relationships in Lycopersicon. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5006–5010. doi: 10.1073/pnas.79.16.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Langley C. H. Inter- and intraspecific variation in restriction maps of Drosophila mitochondrial DNAs. Nature. 1979 Oct 25;281(5733):696–699. doi: 10.1038/281696a0. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Takahata N., Palumbi S. R. Extranuclear differentiation and gene flow in the finite island model. Genetics. 1985 Feb;109(2):441–457. doi: 10.1093/genetics/109.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson G. The effect of a selected locus on linked neutral loci. Genetics. 1977 Apr;85(4):753–788. doi: 10.1093/genetics/85.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timothy D. H., Levings C. S., Pring D. R., Conde M. F., Kermicle J. L. Organelle DNA variation and systematic relationships in the genus Zea: Teosinte. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4220–4224. doi: 10.1073/pnas.76.9.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T., Brown A. H. The Nature of Nucleotide Sequence Divergence between Barley and Maize Chloroplast DNA. Genetics. 1984 Apr;106(4):735–749. doi: 10.1093/genetics/106.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]