Abstract
Background
Considerable controversy has transpired regarding the core features of myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS). Current case definitions differ in the number and types of symptoms required. This ambiguity impedes the search for biological markers and effective treatments.
Purpose
This study sought to empirically operationalize symptom criteria and identify which symptoms best characterize the illness.
Methods
Patients (n=236) and controls (n=86) completed the DePaul Symptom Questionnaire, rating the frequency and severity of 54 symptoms. Responses were compared to determine the threshold of frequency/severity ratings that best distinguished patients from controls. A Classification and Regression Tree (CART) algorithm was used to identify the combination of symptoms that most accurately classified patients and controls.
Results
A third of controls met the symptom criteria of a common CFS case definition when just symptom presence was required; however, when frequency/severity requirements were raised, only 5% met criteria. Employing these higher frequency/severity requirements, the CART algorithm identified three symptoms that accurately classified 95.4% of participants as patient or control: fatigue/extreme tiredness, inability to focus on multiple things simultaneously, and experiencing a dead/heavy feeling after starting to exercise.
Conclusions
Minimum frequency/severity thresholds should be specified in symptom criteria to reduce the likelihood of misclassification. Future research should continue to seek empirical support of the core symptoms of ME and CFS to further progress the search for biological markers and treatments.
Keywords: chronic fatigue syndrome, myalgic encephalomyelitis, case definition, criteria, symptoms
Introduction
Holmes et al. [1] constructed the first US working case definition of CFS. To meet criteria, participants were required to report at least eight of eleven minor symptoms (fever or chills, sore throat, lymph node pain, muscle weakness, muscle pain, post-exertional malaise, headaches of a new or different type, migratory arthralgia, neuropsychiatric complaints, sleep disturbance, and a sudden onset of symptoms). However, when these criteria were utilized in research and practice, it became evident that there were numerous inconsistencies in interpretation and classification of cases.[2–4] A major concern was that the requirement of eight or more minor symptoms could inadvertently select for individuals with psychiatric problems.[4] For example, Katon and Russo [5] noted that chronic fatigue patients with the highest numbers of unexplained physical symptoms had high rates of psychiatric disorders, while patients with the lowest numbers of unexplained symptoms displayed rates of psychiatric disorders that were similar to other clinical populations with chronic medical illnesses.
These difficulties were influential in the development of a revised US case definition for CFS by Fukuda and associates [6] (Fukuda CFS). This case definition requires the concurrence of at least four of eight symptoms (sore throat, lymph node pain, muscle pain, joint pain, post-exertional malaise, headaches of a new or different type, memory and concentration difficulties, and unrefreshing sleep), a reduced set of symptoms from the Holmes et al. criteria.[1] Several investigations have contrasted these two CFS case definitions. For example, Jason et al. [7] found that the Holmes et al. criteria,[1] compared to the Fukuda CFS criteria, selected a group of participants with more symptomatology and functional impairment. Because the Fukuda CFS criteria require only four symptoms of a possible eight, participants could meet criteria without having prominent CFS symptoms, such as post-exertional malaise and memory and concentration problems. The Fukuda CFS case definition has been criticized for not requiring core CFS symptoms [8] and lacking clear operational definitions and guidelines to assist researchers in its application.[9]
By 2003, the Canadian Clinical case definition had been developed, utilizing the term ME/CFS, as opposed to CFS, to refer to the illness (Canadian ME/CFS).[10] This case definition requires the occurrence of seven specific symptoms. Unlike the polythetic approach used in the Fukuda CFS criteria,[6] the Canadian ME/CFS criteria specify exactly which symptom domains must be present in a case of ME/CFS, such as post-exertional malaise. Jason et al. [11] compared persons meeting the Canadian ME/CFS criteria, the Fukuda CFS criteria, and people experiencing chronic fatigue explained by psychiatric reasons. The Canadian ME/CFS criteria, in contrast to the Fukuda CFS criteria, selected cases with less psychiatric comorbidity, more physical, functional impairment, greater fatigue or weakness, and more neuropsychiatric and neurological symptoms, such as confusion, disorientation, and difficulty retaining information.
In an effort to better operationalize the Canadian ME/CFS criteria, Jason et al. [12] specified explicit rules for determining ME/CFS status with this case definition. Using this method, Jason et al. [13] compared those meeting the Canadian ME/CFS case definition to those who did not meet the Canadian ME/CFS criteria but met the Fukuda CFS criteria [6] only. Findings indicated that the Canadian ME/CFS case definition identified individuals with more severe symptoms and greater functional disability than those who met the Fukuda CFS criteria only.
In another study, Jason et al. [14] compared the Canadian ME/CFS criteria [10] to a different set of empiric CFS criteria developed by Reeves et al [15] through the use of data mining with decision trees. Participants belonged to one of four groups: CFS (diagnosed by a physician), Idiopathic Chronic Fatigue (ICF; six months of fatigue, but insufficient symptoms to meet CFS criteria), Exclusionary (chronic fatigue explained by medical or psychiatric conditions), and Control (individuals with fewer than 6 months of fatigue). Two decision tree analyses were conducted: the first used items from the Reeves et al. CFS criteria to attempt to accurately classify participants as CFS, ICF, Exclusionary, or Control, and the second analysis used items from the Canadian ME/CFS criteria. When items from the Reeves et al. CFS criteria were used, the resulting classification correctly identified 79% of cases. When items from the Canadian ME/CFS criteria were used, 87% of the cases were classified correctly. In addition, the items that were identified as having the most discriminatory ability in the Canadian ME/CFS analysis represent core features of the illness, such as the inability to concentrate, post-exertional malaise, and unrefreshing sleep, whereas the analysis that used the Reeves et al. CFS criteria did not identify these items.
Prior studies [11,13,14] used a symptom questionnaire that was originally developed to measure the Fukuda CFS criteria [6], rather than the Canadian ME/CFS criteria[10] to identify participants for research studies. Jason et al. [16] corrected this limitation by analyzing three distinct samples, each collected through a different case ascertainment method, using the DePaul Symptom Questionnaire as the assessment instrument. The questionnaire was developed to measure the criteria of each case definition. Findings indicated that fewer individuals met the Canadian ME/CFS criteria than the Fukuda CFS criteria, and that those who met the Canadian ME/CFS criteria evidenced more severe symptoms and more physical impairment.
A new case definition, the International Consensus Criteria for myalgic encephalomyelitis (ME-ICC), was recently published.[17] To meet the ME-ICC, a person must have symptoms from the following four domains: (1) Post-Exertional Neuroimmune Exhaustion; (2) Neurological Impairments; (3) Immune, Gastro-intestinal, and Genitourinary Impairments; and (4) Energy Production/Transportation Impairments. Brown et al. [18] contrasted the ME-ICC [17] with the Fukuda CFS criteria.[6] Findings indicated that the ME-ICC identified a subset of patients with more functional impairments and physical, mental, and cognitive problems than the larger group of patients who met the Fukuda CFS criteria. However, the patients who met the ME-ICC also had significantly greater rates of psychiatric comorbidity. Jason et al. [19] also compared the ME-ICC to the Fukuda CFS criteria. In general, participants who met the ME-ICC were more functionally impaired than those with Fukuda-defined CFS.
As evidenced by prior studies, patients who met different case definitions displayed differences in symptomatology and impairment. Studying dissimilar samples hinders the search for causal factors, biological markers, and effective treatments. In the present study, we examined individuals who were diagnosed by a physician using the Fukuda CFS [6] or Canadian ME/CFS [10] criteria and compared this patient group to a control group. We examined the prevalence of the symptom domains defined by the Fukuda CFS, Canadian ME/CFS, and ME-ICC [17] case definitions in the patient and control groups. Specifically, we determined the frequency and severity thresholds necessary to distinguish patients from controls and applied these thresholds to identify the most salient, core symptoms of this illness. For a summary of the participant and case definition terminology used throughout this article, please refer to Table 1.
Table 1.
Article Terminology
| Term | Definition |
|---|---|
|
| |
| Patient | Individuals in the SolveCFS BioBank sample who were diagnosed with CFS or ME/CFS by a licensed physician |
| Control | Individuals in the SolveCFS BioBank sample without a diagnosis of CFS or ME/CFS |
| Fukuda CFS | The Fukuda et al. (1994) case definition for CFS |
| Canadian ME/CFS | The Canadian Clinical ME/CFS case definition (Carruthers et al., 2003) |
| ME-ICC | The Myalgic Encephalomyelitis International Consensus Criteria (Carruthers et al., 2011) |
When referencing previous studies, the authors use the illness name associated with the case definition employed in the referenced study.
Method
Research participants
SolveCFS BioBank Sample
Data from the SolveCFS BioBank were de-identified and shared with the DePaul Research Team by the CFIDS Association of America. The SolveCFS BioBank has clinical information and blood samples on a group of individuals who were diagnosed by a licensed physician using the Fukuda CFS [6] or Canadian ME/CFS [10] criteria. All individuals included in the present study were over 18 years of age. Participants were recruited by the CFIDS Association of America from expert physician clinics. All participants who met eligibility criteria completed a written informed consent process. Control participants were recruited who were in generally good physical and mental health and did not have a substance use disorder or any disorder that causes immunosuppression. Participants completed the study measures electronically or by hard copy.
Case definitions
Fukuda et al. CFS Case Definition (Fukuda CFS)
Fukuda et al. [6] defined chronic fatigue syndrome by the presence of the following criteria: (1) clinically evaluated, unexplained, persistent or relapsing chronic fatigue that is of new or definite onset (has not been lifelong); is not the result of ongoing exertion; is not substantially alleviated by rest and results in substantial reduction in previous levels of occupational, educational, social, or personal activities, and (2) the concurrent occurrence of four or more of the following symptoms, all of which must have persisted or recurred during six or more consecutive months of illness and must not have predated the fatigue: memory or concentration problems, sore throat, tender lymph nodes, muscle pain, joint paint, headaches, unrefreshing sleep, and post-exertional malaise.[2,p.956]
Canadian Clinical ME/CFS Case Definition (Canadian ME/CFS)
The Canadian ME/CFS case definition [10] requires that the following symptoms be present: unexplained, chronic physical or mental fatigue, post-exertional malaise (i.e., a worsening of symptoms after physical or mental effort) from which at least 24 hours are required to recover, significant pain (e.g., myalgias, arthralgias), sleep dysfunction (e.g., unrefreshing sleep, sleep rhythm disturbance), and two neurological or cognitive symptoms (e.g., confusion, memory impairment, loss of concentration). Additionally, individuals must report symptoms from two of the following categories: autonomic manifestations (e.g., orthostatic intolerance, nausea, irritable bowel problems), neuroendocrine manifestations (e.g., intolerance of temperature extremes, loss of appetite), and immune manifestations (e.g., fever, recurrent sore throats).
International Consensus Criteria for Myalgic Encephalomyelitis (ME-ICC)
The ME-ICC [17] state that the impact of symptom severity must lead to a 50% or greater reduction of a patient’s premorbid activity level. Additionally, the criteria require symptoms from four major symptom groupings. (1) Patients must report Post-Exertional Neuroimmune Exhaustion (i.e., worsening of symptoms after physical or mental activity). (2) Additionally, patients must have at least one symptom from three of the following four neurological impairment domains: neurocognitive impairments (e.g., difficulty processing information, short-term memory loss), pain; sleep disturbance; and neurosensory, perceptual and motor disturbances (e.g. inability to focus vision, sensitivity to light, muscle weakness, feeling unsteady on feet). (3) Patients also must report at least one symptom from three of the following five immune, gastro-intestinal and genitourinary impairments: flu-like symptoms; susceptibility to viral infections with prolonged recovery periods; gastro-intestinal tract symptoms (e.g., nausea, abdominal pain); genitourinary symptoms (e.g., urinary urgency); and sensitivities to foods, medications, odors, or chemicals. (4) The final category is Energy Production/Transportation Impairments. Patients must have at least one symptom from one of the following four categories: cardiovascular (e.g. orthostatic intolerance), respiratory (e.g. labored breathing), loss of thermostatic stability (e.g. feeling feverish), and intolerance of extremes of temperature.
Measures
The DePaul Symptom Questionnaire
All participants completed the DePaul Symptom Questionnaire (DSQ)[12], a self-report measure of symptomatology, demographics, and medical, occupational, and social history. The DSQ has items that measure the dimensions of the Fukuda CFS,[6] Canadian ME/CFS,[10] and ME-ICC [17] case definitions.[20] Participants were asked to rate each of 54 symptom’s frequency and severity over the past six months on a 5-point Likert scale. Symptom frequency was rated: 0=none of the time, 1=a little of the time, 2=about half the time, 3=most of the time, and 4=all of the time. Likewise, severity was rated: 0=symptom not present, 1=mild, 2=moderate, 3=severe, and 4=very severe. The DSQ has evidenced good test-retest reliability among both patient and control groups.[21] The development of the DSQ was based on the CFS Questionnaire, which was able to sensitively distinguish among individuals with CFS, individuals with Major Depressive Disorder, and healthy controls.[22] The CFS Questionnaire also assesses for frequency and severity of symptoms over the past six months, but the severity rating is on a scale from 0–100 whereas the DSQ utilizes Likert scales. Additionally, the CFS Questionnaire was not developed to examine the Canadian ME/CFS criteria and the ME-ICC, whereas the DSQ was specifically developed to assess these criteria. The DSQ is available at REDCap’s [23] shared library (<Insert Link; Will Be Available Soon>).
Demographic analysis
The Fukuda CFS [6], Canadian ME/CFS [10], and ME-ICC [17] case definitions were applied to the patient group based on responses to items in the DSQ. Data were unavailable in the SolveCFS BioBank database for two symptoms defined in the ME-ICC: susceptibility to viral infections with prolonged recovery periods (within the Immune, Gastro-Intestinal, and Genitourinary Impairments category) and intolerance of extremes of temperature (within the Energy Production/Transportation Impairments category). Thus, these criteria were adjusted slightly, but 49 of the 51 symptoms used to operationalize these criteria were still available to classify participants. Newer versions of the DSQ do have questions that measure these symptoms.
The demographic information of individuals who met each case definition was compared to the demographic information of the control group. As those individuals meeting the Fukuda CFS [6] definition also met the Canadian ME/CFS [10] and adjusted ME-ICC [17] case definitions, we did not examine statistical differences between these groups. However, we did examine differences between each case definition group and the control group. T-tests were calculated to statistically compare mean ages, and Fisher’s exact tests were used to compare gender, race, marital status, work status, and education level.
Threshold analysis
The frequency and severity scores of all patients and controls were analyzed for each of the 54 symptoms in the DSQ (described in Jason et al. [12]). To determine what percentage of patients and controls had each symptom, two different frequency/severity requirements were examined: (1) participants needed to report a frequency and severity score of at least 1 (severity of mild and frequency of at little of the time) to be counted as having the symptom; and (2) participants had to report a frequency and severity score of at least 2 (severity of moderate and frequency of half of the time) for the symptom to count. The percentages of patients and controls who met these requirements were compared for each symptom. Charts were created that displayed the results of this analysis for the symptoms used in the Fukuda CFS,[6] Canadian ME/CFS,[10] and adjusted ME-ICC [17] case definitions. This analysis identified symptoms of high prevalence within the patient group, but low prevalence within the control group.
Data mining analysis
Data mining with decision trees was used to further analyze symptom data. Data mining techniques, such as classification using decision trees, provide statistical analyses that identify which questionnaire items best predict class membership and are useful for indicating which symptoms should be required in the diagnostic process to ensure the most accurate classifications. In the current study, decision trees were used to determine which symptoms were most effective at accurately classifying participants as patient or control.
The 54 symptoms listed in the DSQ were converted into binary variables for use in this analysis. Each symptom variable specified whether or not the participant reported frequency and severity levels for that symptom that met a minimum threshold. To meet this threshold, a symptom’s frequency and severity scores needed to be greater than or equal to 2 (symptoms of at least moderate severity that occur at least half of the time). The resulting 54 binary variables were used as inputs in the decision tree analysis, one associated with each symptom in the DSQ.
Decision trees consist of a series of successive binary choices (branch points) that will ideally result in an accurate classification of participants. At each branch point of the tree, all of the symptom variables are examined to determine which symptom has the most effect on the entropy of the classifications. Here, entropy indicates the certainty of the diagnosis. The symptom selected at each branch point is the one that best predicts classifications at that point in the tree, and is used to split all of the cases into two groups. This process is repeated, and more symptoms are chosen, until the resulting series of branch points produces groupings of correctly classified participants.
SPSS Statistics software was used to build our decision tree models. To build the models, a Classification and Regression Tree (CART) algorithm was applied to a training set consisting of 66% of the cases, stratified to reflect the distribution of patient and control groups. The value of the model was measured by evaluating its classification performance when applied to cases reserved for testing (34% of the data), allowing this technique the ability to be generalized to new data.
Results
Demographics
Table 2 presents demographic data for patients who met the Fukuda CFS,[6] Canadian ME/CFS,[10] and adjusted ME-ICC [17] case definitions. Significant differences existed in work status between the control group and the Fukuda CFS [p < 0.000, two-tailed Fisher’s exact test], Canadian ME/CFS [p < 0.000, two-tailed Fisher’s exact test], and ME-ICC groups [p < 0.000, two-tailed Fisher’s exact test]. Most of the individuals in the control group were working, while the majority of the Fukuda CFS, Canadian ME/CFS, and ME-ICC groups were on disability. Additionally, a significant difference was found when comparing the marital status of the Fukuda CFS and control groups [p = 0.03, two-tailed Fisher’s exact test], as a larger proportion of the Fukuda CFS group was single.
Table 2.
Demographics
| Control N = 86 |
Fukuda CFS N = 224 |
Canadian ME/CFS N = 176 |
ME-ICC (adjusted) N = 149 |
||||
|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | Sig. | M (SD) | Sig. | M (SD) | Sig. | |
|
| |||||||
| Age | 49.7 (13.6) | 49.6 (12.7) | 48.9 (12.7) | 48.5 (13.0) | |||
|
| |||||||
| Other Demographic Information: | N (%) | N (%) | Sig. | N (%) | Sig. | N (%) | Sig. |
|
| |||||||
| Gender | |||||||
| Male | 19 (22) | 60 (27) | 45 (26) | 41 (28) | |||
| Female | 67 (78) | 164 (73) | 131 (74) | 108 (73) | |||
| Race | |||||||
| Caucasian | 83 (98) | 221 (99) | 175 (99) | 147 (99) | |||
| Black or African American | 1 (1) | 0 (0) | 0 (0) | 0 (0) | |||
| Chinese | 0 (0) | 1 (0) | 0 (0) | 0 (0) | |||
| Japanese | 0 (0) | 1 (0) | 0 (0) | 0 (0) | |||
| Other | 1 (1) | 1 (0) | 1 (1) | 2 (1) | |||
| Marital status | |||||||
| Single | 13 (15) | 60 (27) | * | 47 (27) | 40 (27) | ||
| Married | 49 (57) | 121 (54) | 93 (53) | 77 (52) | |||
| Widowed | 5 (6) | 4 (2) | 4 (2) | 4 (3) | |||
| Divorced | 11 (13) | 31 (14) | 25 (14) | 23 (15) | |||
| Separated | 1 (1) | 1 (0) | 0 (0) | 0 (0) | |||
| Live-in partner | 7 (8) | 7 (3) | 7 (4) | 5 (3) | |||
| Work status | |||||||
| On disability | 0 (0) | 148 (66) | *** | 125 (71) | *** | 108 (73) | *** |
| Student | 5 (6) | 10 (4) | 9 (5) | 9 (6) | |||
| Homemaker | 7 (8) | 12 (5) | 4 (2) | 3 (2) | |||
| Retired | 11 (13) | 22 (10) | 13 (7) | 9 (6) | |||
| Unemployed | 4 (5) | 2 (1) | 2 (1) | 1 (1) | |||
| Temporarily not working | 1 (1) | 1 (0) | 1 (1) | 1 (1) | |||
| Working | 56 (65) | 24 (11) | 19 (11) | 15 (10) | |||
| Other | 2 (2) | 5 (2) | 3 (2) | 3 (2) | |||
| Educational level | |||||||
| GED | 2 (2) | 15 (7) | 12 (7) | 12 (8) | |||
| High school graduate | 9 (11) | 11 (5) | 8 (5) | 7 (5) | |||
| Some college | 21 (25) | 46 (21) | 40 (23) | 34 (23) | |||
| College graduate | 32 (38) | 98 (44) | 75 (43) | 59 (40) | |||
| Graduate degree | 15 (18) | 32 (14) | 23 (13) | 19 (13) | |||
| Professional degree | 5 (6) | 21 (9) | 17 (10) | 17 (11) | |||
| Other | 1 (1) | 1 (0) | 1 (1) | 1 (1) | |||
p < 0.05;
p < 0.01;
p < 0.001
Threshold symptoms
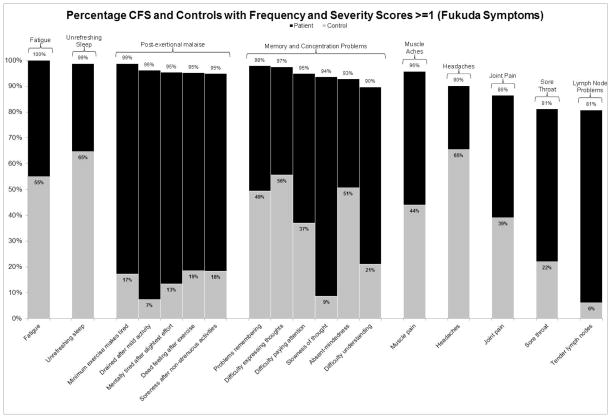
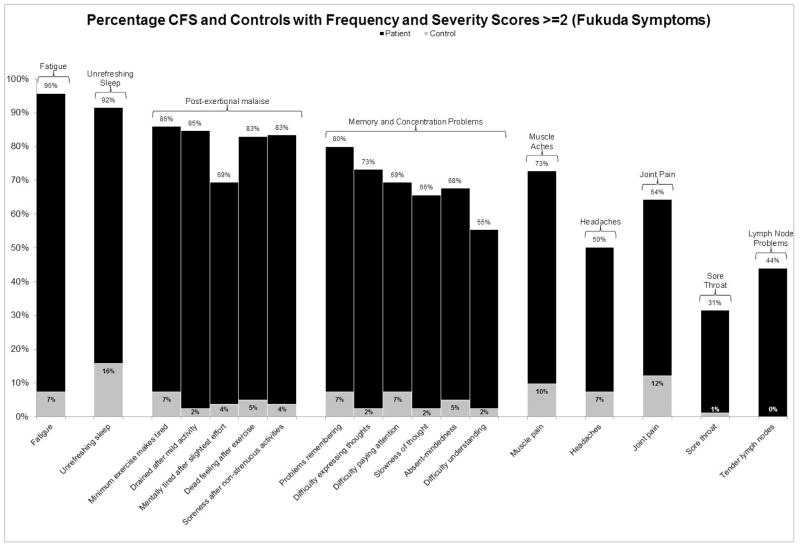
Figure 1 displays the percentage of patient and control participants who met frequency and severity threshold scores of 1 (symptoms of at least minor severity that occur at least a little of the time) for symptoms specified by the Fukuda CFS criteria.[6] Figure 2 shows the percentage of participants who met frequency and severity thresholds of 2 (symptoms of at least moderate severity that occur at least half of the time) for these symptoms. The bars denoting patient frequencies are displayed in black, whereas the control frequencies are grey. It is clear from these figures that a high proportion of controls have these symptoms when using lower minimum frequency and severity thresholds of 1. In fact, using this threshold, 33.7% of controls would meet the Fukuda CFS symptom requirement of having four of the eight specified symptoms, while only 4.7% of controls would meet this requirement if the frequency and severity threshold were raised to scores of 2 or higher. Similarly, 20.7% of controls would meet the seven symptom requirements of the Canadian ME/CFS criteria [10] when using a minimum frequency and severity threshold of 1, while just 3.7% of controls would meet these symptom requirements if the minimum threshold were raised to 2. The adjusted ME-ICC [17] result in the same trend: 14.6% of controls would meet the eight symptom requirements using a minimum threshold of 1, while 3.7% of controls would meet the requirements when employing the more stringent threshold of 2.
Figure 1.
The percentage of patients and controls who reported frequency and severity scores of at least 1 (symptoms of at least mild severity that occur at least a little of the time) for symptoms specified by the Fukuda et al. criteria [2]
Figure 2.
The percentage of patients and controls who reported frequency and severity scores of at least 2 (symptoms of at least moderate severity that occur at least half of the time) for symptoms specified by the Fukuda et al. criteria [2]
Core symptoms
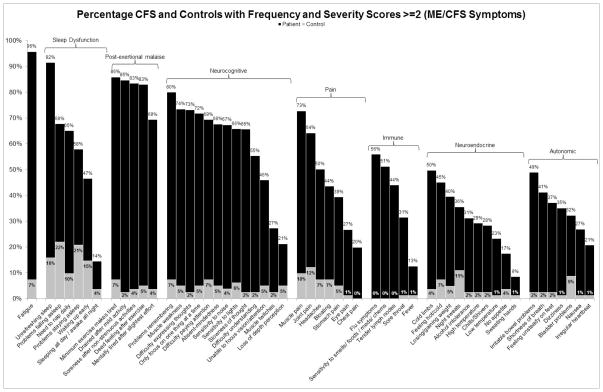
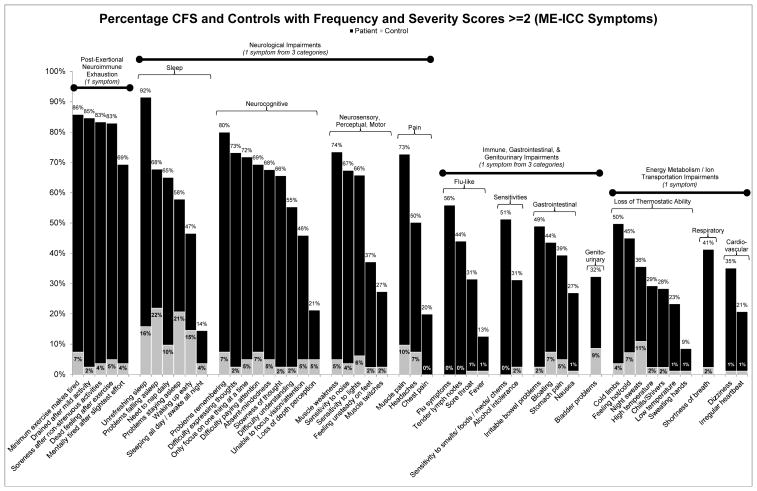
Figures 3 and 4 present the percentage of patients and controls that experienced core symptoms of the Canadian ME/CFS [10] and ME-ICC [17] case definitions respectively, employing a threshold of 2 for frequency and severity scores. As data were unavailable for the two symptoms of the ME-ICC, described above, these symptoms are not displayed in Figure 4. These graphs demonstrate that three of the symptom domains (post-exertional malaise, memory and concentrations problems, and unrefreshing sleep) are more prevalent among patients in comparison to the other specified domains. This trend is also present in Figures 1 and 2.
Figure 3.
The percentage of patients and controls who reported frequency and severity scores of at least 2 (symptoms of at least moderate severity that occur at least a half of the time) for symptoms specified by the Canadian ME/CFS criteria [3]
Figure 4.
The percentage of patients and controls who reported frequency and severity scores of at least 2 (symptoms of at least moderate severity that occur at least a half of the time) for symptoms specified by the Myalgic Encephalomyelitis International Consensus Criteria [4]. Data was unavailable for the following two symptoms: susceptibility to viral infections and intolerance of extremes of temperature; therefore, these symptoms are not displayed.
The data mining analysis identified three symptoms from the initial 54 analyzed that accurately classified 95.4% of participants as patient or control when employing minimum frequency and severity scores of 2 (moderate; half of the time): fatigue or extreme tiredness, inability to focus on more than one thing at a time, and experiencing a dead or heavy feeling after starting to exercise. Using these three symptoms, the resulting classification had a sensitivity of 96.3% and specificity of 92.9%.
Discussion
The results of this study indicate that identifying minimum frequency and severity thresholds for symptom criteria is necessary in order to accurately distinguish patients from controls. While a large proportion of controls met the symptom criteria of the various case definitions when low frequency and severity thresholds were employed, raising these thresholds differentiated controls from patients. Among researchers using the different case definitions, there is considerable variability in how thresholds for symptom criteria are operationalized. For example, Baraniuk et al. [24] operationalized the Fukuda CFS criteria [6] by collecting data on fatigue and the eight specified symptoms over the previous six months, using the following severity ratings: 0 for no symptom, 1 for trivial, 2 for mild, 3 for moderate and 4 for severe. To be diagnosed with CFS, a participant needed to report a fatigue score of at least 3 (moderate), but scores had to be just 2 (mild) or higher for four of the eight Fukuda CFS symptoms. Simply rating severity rather than both frequency and severity could lead to measurement and classification problems, and allowing only mild symptoms to meet criteria might allow some individuals without CFS to be classified as having CFS. Neither the Canadian ME/CFS criteria [10] nor the ME-ICC [17] provided guidance for rating the severity or frequency of symptoms. Moreover, the ME-ICC [25] did not specify a time frame over which to rate the symptoms.
In another effort to operationalize the Fukuda CFS criteria [6], Wagner et al. [26] developed the CDC Symptom Inventory (SI), which assesses information about the presence, frequency, and intensity of fatigue-related symptoms during the past one month. The frequency and severity scores are multiplied for each of the eight critical Fukuda CFS symptoms and are then summed. To meet the Reeves et al. [15] symptom criteria, a person needs to have four or more symptoms and a total SI score greater than or equal to 25. A few issues have been noted for the SI.[27] For example, the threshold score of 25 may be low for patients with classic CFS symptoms. A score of 25 could be met if a patient rated just two symptoms as occurring all the time, with one of moderate severity and the other severe. In addition, the SI specifies a time period of the past one month for rating the eight symptoms, while the Fukuda CFS criteria specifies a time period of the last six months. The use of varied threshold specifications in ME and CFS studies inhibits researchers’ ability to replicate results. Furthermore, the use of lower symptom thresholds may inadvertently bring individuals without ME or CFS into patient samples. Thus, standardized, empirically-based thresholds should be identified in ME and CFS case definitions. The results of the current study indicate that defining symptom presence as symptoms of at least moderate severity that occur at least half of the time accurately distinguishes patients from controls.
Symptom prevalence
Moving from issues of thresholds to prevalence of symptoms, the findings of this study indicate that patients’ most common symptoms are fatigue, post-exertional malaise, neurocognitive problems, and unrefreshing sleep. Other symptoms (such as pain, autonomic, immune, and neuroendocrine symptoms) are not as prevalent. Current case definitions vary in the symptoms they require. Whereas the original US case definition of CFS required patients to have eight of eleven specified symptoms,[1] the Fukuda CFS criteria [6] reduced the number required to four of eight symptoms. However, the polythetic nature of the Fukuda CFS criteria makes it possible to meet criteria without experiencing core symptoms of the illness. The Canadian ME/CFS criteria later required seven specific symptoms.[10] The most recent case definition, the ME-ICC, increased the number of required symptoms to eight.[17] Requiring larger numbers of symptoms can inadvertently increase the rate of psychiatric comorbidity of the group that meets criteria.[5]
Future refinement of case definitions may wish to focus on requiring a small set of core symptoms, such as post-exertional malaise, neurocognitive symptoms, and possibly unrefreshing sleep. These symptoms were among the most prevalent that patients in this study’s sample experienced. Furthermore, the data mining analysis identified one symptom from each of the post-exertional malaise and neurocognitive domains in order to accurately distinguish patients from controls. The use of a split cohort and the validating of findings were methodological strengths of this study.
It is clear from Figures 3 and 4 that, at a threshold of 2 for both frequency and severity, the majority of patients do not experience immune, neuroendocrine, autonomic, and pain symptoms. Although a moderate percentage of the patient group reported muscle pain, this symptom is not as prominent as other core symptoms and a relatively large percentage of controls reported muscle pain as well. Future research could search for commonly co-occurring symptoms within the less prevalent symptom domains to develop subtypes of the illness. Using this approach, the number of symptoms required could remain at four, with one symptom being from the subtype domain (e.g., immune, neuroendocrine, autonomic, pain) and with the remaining three requirements being the symptoms of highest prevalence (post-exertional malaise, neurocognitive symptoms, and unrefreshing sleep). A factor analysis by Brown and Jason [28] resulted in a three-factor solution that supports such a case definition structure. The analysis resulted in one factor comprised of post-exertional malaise items, one factor of neurocognitive items, and one larger factor that encompassed pain, immune, neuroendocrine, and autonomic items.
Subtypes and symptom factors
Recent pathophysiologic research also supports the existence of subtypes among patients with CFS.[29] For example, Light et al. [30] found that after moderate exercise, two subtypes of changes occurred within the study’s CFS group. In 71% of the CFS group, large gene expression occurred for multiple systems, including sensory receptors (2PX4, 2PX5, TPRIV1), adrenergic (sympathetic nervous system) receptors (Alpha 2a, Beta-1, Beta-2, COMT), and cytokine receptors (IL-10). However, for 29% of the CFS group, decreases after exercise were only found in the Alpha 2a mRNA, indicating that for this group, there was only dysregulation in the adrenergic sympathetic nervous system (71% of these patients had orthostatic intolerance, but only 18% had this symptom in the larger CFS subgroup).
Several additional studies have used statistical techniques to determine the factor structure of symptoms experienced by patients with this illness. For example, Friedberg et al. [31] found the following three-factor solution: cognitive problems, flu-like symptoms, and neurologic symptoms. Jason et al. [32] found a six-factor solution, consisting of: neurocognitive, vascular, inflammation, muscle/joint, infectious, and sleep/post-exertional malaise symptoms. Arroll and Senior [33] reported a five-factor solution: fibromyalgia syndrome-like, depression/anxiety, fatigue/post-exertional malaise, cognitive/neurological, and irritable bowel syndrome-like symptoms. Finally, Hickie et al. [34] found a five-factor model involving musculoskeletal pain/fatigue, neurocognitive difficulties, inflammation, sleep disturbance/fatigue, and mood disturbance. It is clear from these studies that the domains of post-exertional malaise, unrefreshing sleep, and neurocognitive impairments are most common, whereas fewer studies report autonomic, immune and neuroendocrine factors.
Different types of fatigue
The symptom of fatigue did emerge in the data mining analysis; however, the disabling fatigue experienced by individuals with ME and CFS differs from that associated with other illnesses or everyday activity. Of importance, fatigue at low thresholds was common within the control group. However, fatigue was infrequent among controls at moderate severity levels and frequency levels of at least half of the time. Thus, steps must be taken to clarify and differentiate the unique type of fatigue associated with ME and CFS. Jason et al. [35] illustrated this difference by using the ME/CFS Fatigue Types Questionnaire (MFTQ) to capture the various aspects of fatigue frequently described by patients with ME and CFS. Items were written for the following five hypothesized dimensions of fatigue: Post-Exertional, Wired, Brain Fog, Energy, and Flu-Like. Jason et al. [35] found a five-factor structure that was confirmed in the patient sample. In contrast, among the control group, only one factor emerged. These findings suggest that the symptom of fatigue in this illness is a multi-dimensional entity that is distinct from the fatigue experienced by the general population. Case definitions may better capture this distinct type of fatigue through requiring fatigue of higher frequency and severity.
Case definitions and their limitations
The Fukuda CFS case definition [6] has been extensively used by researchers for the past two decades. Unfortunately, it is possible that some individuals who meet these criteria do not have core symptoms of the illness, such as post-exertional malaise, unrefreshing sleep, or memory/concentration problems. The Canadian ME/CFS criteria [10] do identify a smaller subset of patients with more severe functional impairment and post-exertional malaise symptoms. For example, using the DSQ, Jason et al. [16] found that fewer individuals met the Canadian ME/CFS criteria (from 72.7% to 77.2% of three distinct samples) than the Fukuda CFS criteria (from 86.5% to 96.3% of the same three samples), and those who met the Canadian ME/CFS criteria evidenced more severe symptoms and physical functioning impairment. These findings were replicated across three samples and provide evidence supporting the ME construct as originally proposed by earlier researchers.[36–38]
An additional limitation of current case definitions is that only one symptom within a symptom domain must be present for a participant to meet criteria for that domain. For example, while there are many possible symptoms within the neurocognitive domain, some symptoms are highly prevalent among patients, while others are less prevalent; however, the occurrence of either a high or low prevalence item will allow a participant to fulfill that symptom domain requirement. Empirical approaches might help specify which symptoms within a given domain have both the needed sensitivity and specificity to create a more valid case definition. The results of this study support the need for well-defined symptom criteria thresholds and an empirical approach in identifying core symptom domains of the illness; however, this study had a few limitations. For example, symptoms were not asked about in relation to activity; as some patients may only experience certain symptoms in response to activity, the prevalence of these symptoms may be underrepresented in this study’s results. While this study focused on comparing patients to healthy controls, future research should also identify thresholds and symptom domains that best distinguish CFS and ME from other illnesses.
Data mining techniques
In addition, the current study’s analyses used self-report data, so future research may benefit from using data mining techniques with results of medical testing. For example, using Neural-Network Classifiers to differentiate CFS and non-CFS control groups, Hanson, Gause, and Natelson [39] found that only one cytokine, interleukin-4 (IL-4), remained in their final model, suggesting a shift in the CFS group to a type 2 cytokine pattern. Another study evaluated various computational tools in predicting CFS through single nucleotide polymorphisms.[40] They found a significant association of NR3C1 in the CFS group compared to non-fatigued controls. The NR3C1 gene is involved in a number of physiological functions, including energy metabolism and immune response. Others have used machine learning algorithms to show that an inflammatory adipokin leptin could distinguish, with 78.3% accuracy, high from low fatigue days among a sample of ten women with ME.[41]
Need for empirical approaches
Over time, there have been a number of terms and criteria used to define this illness, including CFS (operationalized by using the Fukuda CFS [6] or Reeves et al. CFS [15] criteria), ME/CFS (operationalized by using the Canadian ME/CFS criteria [10]), or ME [38] (operationalized by using the ME-ICC [17] or guidelines specified by Jason et al. [20]). As more researchers begin to use varying case definitions and criteria to select samples, it is imperative for researchers to specify the criteria used so that findings can be better compared.[42] Ultimately, researchers should develop more sophisticated and empiric approaches, rather than relying on consensus, to identify the core domains of this illness, as well as the best items to represent those domains. Close inspection of the limitations of past case definitions should guide the efforts to reduce criterion variance and ultimately develop more valid diagnostic criteria.
Acknowledgments
The authors appreciate the CFIDS Association of America, which approved the use of de-identified SolveCFS BioBank registry data in this analysis.
Funding
Funding was provided by NIAID (Grant numbers AI 49720 and AI 055735).
Contributor Information
Leonard A. Jason, Email: ljason@depaul.edu, Center for Community Research, DePaul University, Chicago, IL USA.
Madison Sunnquist, Email: msunnqui@depaul.edu, Center for Community Research, DePaul University, Chicago, IL USA
Abigail Brown, Email: abrown57@depaul.edu, Center for Community Research, DePaul University, Chicago, IL USA
Meredyth Evans, Email: mevans24@depaul.edu, Center for Community Research, DePaul University, Chicago, IL USA
Suzanne D. Vernon, Email: sdvernon@cfids.org, The CFIDS Association of America
Jacob Furst, Email: jfurst@cdm.depaul.edu, College of Computing and Digital Media, DePaul University, Chicago, USA
Valerie Simonis, Email: valerie.simonis@gmail.com, College of Computing and Digital Media, DePaul University, Chicago, USA
References
- 1.Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Strauss SE, Jones JF, Dubois RE, Cunningham-Rundles C, Pahwa S, Tosato G, Zegans LS, Purtilo DT, Brown N, Schooley RT, Brus I. Chronic Fatigue Syndrome: A working case definition. Ann Intern Med. 1988;108:387–389. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- 2.Matthews DA, Lane TJ, Manu P. Definition of the Chronic Fatigue Syndrome. Ann Int Med. 1988;109(6):511–512. doi: 10.7326/0003-4819-109-6-511. [DOI] [PubMed] [Google Scholar]
- 3.Schluederberg A, Straus SE, Peterson P, Blumenthal S, Komaroff AL, Spring SB, Landay A, Buchwald D. Chronic Fatigue Syndrome Research: Definition and Medical Outcome Assessment. Ann Int Med. 1992;117(4):325–331. doi: 10.7326/0003-4819-117-4-325. [DOI] [PubMed] [Google Scholar]
- 4.Straus SE. Defining the chronic fatigue syndrome. Arch Int Med. 1992;152(8):1569. [PubMed] [Google Scholar]
- 5.Katon W, Russo J. Chronic fatigue syndrome criteria: a critique of the requirement for multiple physical complaints. Arch Int Med. 1992;152(8):1604. doi: 10.1001/archinte.152.8.1604. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Jason LA, Torres-Harding SR, Taylor RR, Carrico AW. A comparison of the 1988 and 1994 diagnostic criteria for chronic fatigue syndrome. J Clin Psychol Med Settings. 2001;8:337–343. [Google Scholar]
- 8.Jason LA, King CP, Richman JA, Taylor RR, Torres SR, Song S. US case definition of chronic fatigue syndrome: Diagnostic and theoretical issues. J Chronic Fatigue Syndr. 1999;5:3–33. [Google Scholar]
- 9.Reeves WC, Lloyd A, Vernon SD, Klimas N, Jason LA, Bleijenberg G, Evengard B, White PD, Nisenbaum R, Unger ER International Chronic Fatigue Syndrome Study Group. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res. 2003;3:25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, Bested AC, Flor-Henry P, Joshi P, Powles ACP, Sherkey JA, van de Sande MI. Myalgic Encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatments protocols. J Chronic Fatigue Syndr. 2003;11:7–115. [Google Scholar]
- 11.Jason LA, Torres-Harding SR, Jurgens A, Helgerson J. Comparing the Fukuda et al. criteria and the Canadian case definition for chronic fatigue syndrome. J Chronic Fatigue Syndr. 2004;12(1):37–52. [Google Scholar]
- 12.Jason LA, Evans M, Porter N, Brown M, Brown A, Hunnell J, Anderson V, Lerch A, De Meirleir K, Friedberg F. The Development of a Revised Canadian Myalgic Encephalomyelitis-Chronic Fatigue Syndrome Case Definition. Am J Biochem Biotechnol. 2010;6:120–135. [Google Scholar]
- 13.Jason LA, Brown AA, Clyne E, Bartgis L, Evans M, Brown M. Contrasting case definitions for chronic fatigue syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, and myalgic encephalomyelitis. Eval Health Prof. 2012;35:280–304. doi: 10.1177/0163278711424281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jason LA, Skendrovic B, Furst J, Brown A, Weng A, Bronikowski C. Data mining: Comparing the Empiric CFS to the Canadian ME/CFS case definition. J Clin Psychol. 2012;68:41–49. doi: 10.1002/jclp.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves WC, Wagner D, Nisenbaum R, Jones JF, Gurbaxani B, Solomon L, Papanicolaou DA, Unger ER, Vernon SD, Heim C. Chronic fatigue syndrome – A clinical empirical approach to its definition and study. BMC Med. 2005;3(1):19. doi: 10.1186/1741-7015-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jason LA, Brown A, Evans M, Sunnquist M, Newton JL. Contrasting chronic fatigue syndrome versus Myalgic Encephalomyelitis/chronic fatigue syndrome. Fatigue: Biomed Health Behav. 2013;1(3):168–183. doi: 10.1080/21641846.2013.774556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles ACP, Speight N, Vallings R, Bateman L, Baumgarten-Austrheim B, Bell DS, Carlo-Stella N, Chia J, Darragh A, Jo D, Lewis D, Light AR, Marshall-Gradisbik S, Mena I, Mikovits JA, Miwa K, Murovska M, Pall ML, Stevens S. Myalgic Encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270(4):327–338. doi: 10.1111/j.1365-2796.2011.02428.x. doi: 10.1111/j.1365- 2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown AA, Jason LA, Evans MA, Brown M, Flores S. Contrasting case definitions: The ME International Consensus Criteria vs. the Fukuda et al. CFS Criteria. North Am J Psychol. 2013;15(1):103–120. [PMC free article] [PubMed] [Google Scholar]
- 19.Jason LA, Sunnquist M, Brown A, Evans M, Newton JL. Are Myalgic Encephalomyelitis and chronic fatigue syndrome different illnesses? 2013. p. 26. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jason LA, Damrongvachiraphan D, Hunnell J, Bartgis L, Brown A, Evans M, Brown M. Myalgic Encephalomyelitis: Case definitions. Auton Control Physiol State Funct. 2012;1:1–14. doi: 10.4303/acpsf/K11060. [DOI] [Google Scholar]
- 21.Jason LA, So S, Brown A, Sunnquist M, Evans M. Test-retest reliability of the DePaul Symptom Questionnaire. 2014. p. 29. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawk C, Jason LA, Torres-Harding S. Reliability of a chronic fatigue syndrome questionnaire. J Chronic Fatigue Syndr. 2006;13(4):41–66. [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraniuk JN, Adewuyi O, Merck SJ, Ali M, Ravindran MK, Timbol CR, Rayhan R, Zheng Y, Le U, Esteitie R, Petrie KN. A chronic fatigue syndrome (CFS) severity score based on case designation criteria. Am J Translational Res. 2013;5(1):53–68. [PMC free article] [PubMed] [Google Scholar]
- 25.Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles ACP, Speight N, Vallings R, Bateman L, Bell DS, Carlo-Stella N, Chia J, Darragh A, Gerken A, Jo D, Lewis D, Light AR, Light K, Marshall-Gradisbik S, McLaren-Howard J, Mena I, Miwa K, Murovska M, Steven S. Myalgic encephalomyelitis – Adult & Paediatric: International Consensus Primer for Medical Practitioners. Vancouver: 2012. [Google Scholar]
- 26.Wagner D, Nisenbaum R, Heim C, Jones JF, Unger ER, Reeves WC. Psychometric properties of the CDC symptom inventory for assessment of chronic fatigue syndrome. Popul Health Metrics. 2005;3:8. doi: 10.1186/1478-7954-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jason LA, Najar N, Porter N, Reh C. Evaluating the Centers for Disease Control’s empirical chronic fatigue syndrome case definition. J Disabil Policy Stud. 2009;20:93–100. [Google Scholar]
- 28.Brown AA, Jason LA. Validating a measure of Myalgic Encephalomyelitis/chronic fatigue syndrome symptomatology. 2013. Paper in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009;10(10):1099–1112. doi: 10.1016/j.pain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Light AR, Bateman L, Jo D, Hughen RW, VanHaitsma TA, White AT, Light KC. Gene expression alternations at baseline and following moderate exercise in patients with chronic fatigue syndrome and Fibromyalgia Syndrome. J Int Med. 2011;271:64–81. doi: 10.1111/j.1365-2796.2011.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedberg F, Dechene L, McKenzie MJ, Fontanetta R. Symptom patterns in long-duration chronic fatigue syndrome. J Psychosom Res. 2000;48:59–68. doi: 10.1016/s0022-3999(99)00077-x. [DOI] [PubMed] [Google Scholar]
- 32.Jason LA, Corradi K, Torres-Harding S. Toward an empirical case definition of CFS. J Soc Serv Res. 2007;34(2) doi: 10.1300/J079v34n02_0. [DOI] [Google Scholar]
- 33.Arroll MA, Senior V. Symptom typology and sub-grouping in chronic fatigue syndrome. Bulletin of the IACFS/ME. 2009;17(2):39–52. [Google Scholar]
- 34.Hickie I, Davenport T, Vernon SD, Nisenbaum R, Reeves WC, Hadzi-Pavlovic D, Lloyd A. Are chronic fatigue and chronic fatigue syndrome valid clinical entities across countries and health-care settings? Aust N Z J Psychiatry. 2009;43:25–35. doi: 10.1080/00048670802534432. [DOI] [PubMed] [Google Scholar]
- 35.Jason LA, Jessen T, Porter N, Boulton A, Njoku MG, Friedberg F. Examining types of fatigue among individuals with ME/CFS. Disabil Stud Q. 2009;29(3) Retrieved from: http://www.dsq-sds.org/article/view/938/1113. [Google Scholar]
- 36.Dowsett EG, Goudsmit EM, Macintyre A, Shepherd C. London criteria for Myalgic Encephalomyelitis. Report from the National Task Force on Chronic Fatigue Syndrome (CFS), Post Viral Fatigue Syndrome (PVFS), Myalgic Encephalomyelitis (ME) Westcare. 1994:96–98. [Google Scholar]
- 37.Goudsmit E, Shepherd C, Dancey CP, Howes S. ME: Chronic fatigue syndrome or a distinct clinical entity? Health Psychol Update. 2009;18:26–31. [Google Scholar]
- 38.Ramsay MA. Myalgic Encephalomyelitis and postviral fatigue states: the saga of Royal Free Disease. 2. London: Gower Publishing Co; 1988. [Google Scholar]
- 39.Hanson SJ, Gause W, Natelson B. Detection of immunologically significant factors for chronic fatigue syndrome using neural-network classifiers. Clin Diag Lab Immun. 2001;8(3):658–662. doi: 10.1128/CDLI.8.3.658-662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang LC, Hsu SY, Lin E. A comparison of classification methods for predicting chronic fatigue syndrome based on genetic data. J Trans Med. 2009;7(81) doi: 10.1186/1479-5876-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stringer EA, Baker KS, Carroll IR, Montoya JG, Chu L, Maecker HT, Younger JW. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J Trans Med. 2013;11:93. doi: 10.1186/1479-5876-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jason LA, Unger ER, Dimitrakoff JD, Fagin AP, Houghton M, Cook D, Marshall CD, Jr, Klimas N, Snell C. Minimum data elements for research reports on CFS. Brain Behav Immun. 2012;26:401–406. doi: 10.1016/j.bbi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]