Abstract
Gymnema sylvestre (Asclepiadaceae), popularly known as “gurmar” for its distinct property as sugar destroyer, is a reputed herb in the Ayurvedic system of medicine. The phytoconstituents responsible for sweet suppression activity includes triterpene saponins known as gymnemic acids, gymnemasaponins, and a polypeptide, gurmarin. The herb exhibits a broad range of therapeutic effects as an effective natural remedy for diabetes, besides being used for arthritis, diuretic, anemia, osteoporosis, hypercholesterolemia, cardiopathy, asthma, constipation, microbial infections, indigestion, and anti-inflammatory. G. sylvestre has good prospects in the treatment of diabetes as it shows positive effects on blood sugar homeostasis, controls sugar cravings, and promotes regeneration of pancreas. The herbal extract is used in dietary supplements since it reduces body weight, blood cholesterol, and triglyceride levels and holds great prospects in dietary as well as pharmacological applications. This review explores the transition of a traditional therapeutic to a modern contemporary medication with an overview of phytochemistry and pharmacological activities of the herb and its phytoconstituents.
1. Introduction
The naturopathic treatment for diseases has been explored extensively since ancient times and gaining momentum in the present scenario. Indian flora accounts for about 45,000 plant species out of which several thousands have pharmacological significance [1]. Diabetes mellitus is a major endocrine disorder affecting nearly 10% of the population worldwide [2] and a key issue of concern. The disease in its severe state affects major systems of the body, leading to multiorgan complications. Oral hypoglycemic agents like sulphonylureas and biguanides are the conventional drugs used for the treatment, but the adverse side effect associated with these drugs is a major limitation. The herbal medicines are becoming popular due to better results and safe use as compared to marketed drugs and more effective treatment of health problems [3]. Plants possessing antidiabetic activities are of significant interest for ethnobotanical community as they are recognized to contain valuable medicinal properties in different parts and a number of them have shown varying degree of hypoglycemic and antihyperglycemic activity [1]. The bioactive constituents found in many plant species are isolated for direct use as drugs, lead compounds, or pharmacological agents. These traditional approaches might offer a natural key to unlock diabetic complications [4]. The chemical structures of a phytomolecule play a critical role in its antidiabetic activity. Several plant species being a major source of terpenoids, flavonoids, phenolics, coumarins, and other bioactive constituents have shown reduction in blood glucose levels [5, 6]. Various antidiabetic plant extracts like aloe (Aloe vera L), bitter Melon (Momordica charantia), fenugreek (Trigonella foenum-graecum), Asian ginseng (Panax ginseng C.A.Meyer) and American ginseng (Panax quinquefolius L), gymnema (Gymnema sylvestre), milk thistle (Silybum marianum), nopal (Opuntia streptacantha), salacia (Salacia oblonga; Salacia Reticulate), and formulations like those of chromium have been used and clinically tested for their activity as well as potential side effect [7].
The present review is a research update on Gymnema sylvestre, a rare herb with significant medicinal attributes with an overview of its ethnobotanical uses, phytochemistry dealing with an in-depth study of its phytochemicals, and their bioactivities. It also explores the facts and prospects of its development into a modern and efficient therapeutic, contemporary with the present trends of pharmacology and drug development. Furthermore, it holds significant prospects in major health problems like cardiovascular disorders, obesity, osteoporosis, and asthma besides being a popular medication for number of other health ailments. The herb finds significant application in various food preparations for control of obesity and blood cholesterol levels besides regulation of sugar homeostasis. The herbal preparations of G. sylvestre are presently used in tea bags, health tablets and supplements, beverages, and confectioneries.
2. Traditional Perspective
G. sylvestre is an indigenous herb, belonging to the class dicotyledonous of the family Asclepiadaceae. The plant is a good source of a large number of bioactive substances [8]. It has deep roots in history, being one of the major botanicals used in Ayurvedic system of medicine to treat conditions ranging from diabetes, malaria, to snakebites [9]. The herb is cultivated worldwide and also known as Chigengteng or Australian Cowplant, Waldschlinge in German, periploca of the woods in English and gurmar in Hindi [10].
3. Taxonomy
G. sylvestre R.Br. is a perennial, woody climber belonging to family Asclepiadaceae or the “milk weed” family [11]. The genus is classified into 40 species, some of which like G. sylvestre, G. montanum, G. yunnanense, and G. inodorum have medicinal properties [12–14]. The plant is found in tropical and subtropical regions, well distributed in parts of central and southern India and in the southern part of China, tropical Africa, Malaysia, and Sri Lanka [9]. G. sylvestre is slow growing herb, found ideally in tropical and subtropical humid climate and common in hills of evergreen forests. It is a climber and generally requires support for growth. The seeds are sown in the months of November-December and harvested from September to February. The propagation through seed germination is difficult due to low viability of the seeds; therefore, the alternative has been root cuttings which are generally planted in the months of June and July [15]. Terminal cuttings with three of four nodes have also been used as for vegetative propagation and usually planted in the month of February-March [16]. The leaves are opposite, usually elliptic or ovate (1.25–2.0 inch × 0.5–1.25 inch), inflorescence is lateral umbel in cymes; follicles are terete and lanceolate, up to 3 inches in height. Corolla is pale yellow in colour, valvate, campanulate with single corona with 5 fleshy scales. The calyx-lobes are long, ovate, obtuse, and pubescent. Carpels-2, unilocular, ovules locules may be present, anther connective produced into a membranous tip [17, 18].
4. Phytochemical Profiling
The leaves of G. sylvestre contain triterpene saponins belonging to oleanane and dammarane classes. The major constituents like gymnemic acids and gymnemasaponins are members of oleanane type of saponins while gymnemasides are dammarane saponins [19, 20]. Other phytoconstituents include anthraquinones, flavones, hentriacontane, pentatriacontane, phytin, resins, tartaric acid, formic acid, butyric acid, lupeol, β-amyrin related glycosides, stigmasterol, and calcium oxalate [21]. The presence of alkaloids had been detected in plant extracts. Leaves of G. sylvestre have acidic glycosides and anthraquinones and their derivatives [22]. The major secondary metabolites in Gymnema includes a group of nine closely related acidic glycosides, the main are gymnemic acid A–D and found in all parts of the plant (see Supplementary Table 1 in supplementary materials available online at http://dx.doi.org/10.1155/2014/830285). The maximum content of gymnemic acid is found in shoot tips (54.29 mg-g−1 DW) and least in seeds (1.31 mg-g−1 DW). Antisaccharin property of gymnemic acid A1 was greatly reduced on conversion into A2, while no activity was observed in case of A3 suggesting that the ester group in the genin portion of gymnemic acid imparts the antisweet property to the triterpene saponins, the gymnemic acids. Gymnemic acids A2 and A3 possessed both glucuronic acid and galactose in their molecular structures while glucuronic acid was found to be the only moiety in gymnemic acid A1 [23]. Further, a series of gymnemic acids (gymnemic acid I, II, III, IV, V, VI, and VII) were isolated and characterized from the hot water extract of dry leaves of G. sylvestre [24, 25]. The Gymnemic acids comprise of several members designated as gymnemic acids I–VII, gymnemosides A–F, and gymnemasaponins Table 1. The derivatives of gymnemic acids are several acylated tigloyl, methylbutyryl group substituted members, derived from deacylgymnemic acid (DAGA) which is a 3-O-β-glucuronide of gymnemagenin (3β, 16β, 21β, 22α, 23, 28-hexahydroxy-olean-12-ene). Gymnemic acid A comprises of gymnemic acids A1, A2, A3, and A4 and named gymnemagenin. This constituent is a D-glucuronide of hexahydroxy-triterpene that esterifies with acids [26]. Other five gymnemic acids, namely, VIII, IX, X, XI, and XII, were isolated and characterized later [27]. Gymnemasaponins III, another antisweet compound, isolated from G. sylvestre was found to consist of 23 hydroxylongispinogenin as the aglycone moiety glycosylated with either one or two glucose molecules at both the 23 or 28 hydroxyl groups [28]. These compounds exhibited lesser antisweet effect than those of gymnemic acids [29].
Table 1.
Phytoconstituents in Gymnema sylvestre.
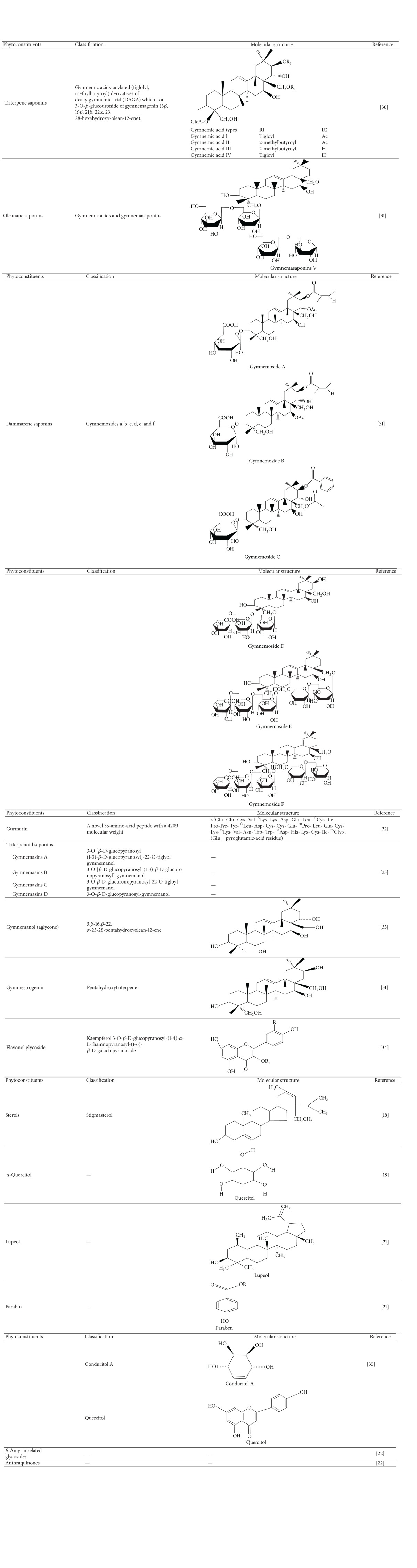
|
Gurmarin, an important 35 amino-acid peptide having a molecular weight of 4209, was isolated from G. sylvestre [32]. The sugar suppression activity of this compound was determined electrophysiologically on the taste responses of rat [36]. The antisweet effect of this polypeptide is very specific to sweet taste on tongue, affected by the pH change. It has been reported that the polypeptide exhibited maximum antisweetner property near its isoelectric point [37]. The hydrophobic, rather than the ionic, interaction plays a significant role in proper binding of gurmarin to the target molecules [32, 38]. The other important constituents isolated from leaves are gymnemasins A, B, C, and D and alkaloids [39]. A number of saponins such as gymnemic acid, deacyl gymnemic acid, gymnemagenin [40], 23-hydroxylnogispinogenin, and gymnestrogenin have been purified [33, 41, 42] from G. sylvestre. The phytochemicals in leaf extract were also analyzed through gas chromatography coupled to mass spectrometry and identified for the presence of terpenoids, glycosides, saturated and unsaturated fatty acids, and alkaloids in three different leaves extract, namely, petroleum ether, chloroform, and methanol as solvents used for extraction [43]. The bioactive constituents present in the plant were found to be mixture of diverse phytomolecules such as gymnemic acids, gymnemosides, gymnemasaponins, gurmarin, gymnemanol, stigmasterol, d-quercitol, β-amyrin related glycosides, anthraquinones, lupeol, hydroxycinnamic acids, and coumarols group.
5. Biosynthesis and Genomics
Saponins, natural products widespread in plant kingdom, are glycosides composed of triterpenoids or steroidal aglycones moieties [44] and the aglycones are known as sapogenins. Many plant-derived saponins, namely, ginsenosides, soyasaponins, and saikosaponins have been found to exhibit significant anticancer activity. Besides, some saponins display pharmacological properties, namely, anticholesterolemic, adjuvant hemolytic, and anticancer [45–47]. It was also found that the foods originating from plants having an increased level of triterpenes are thought to have a cholesterol lowering effect. Transgenics with altered levels of triterpenes may be resistant to pests and increased saponin content will confer enhanced nutritional value to the plant.
Triterpenoid saponins are a class of plant secondary metabolites originated via the isoprenoid pathway by cyclization of 2,3-oxidosqualene precursor in which one or more sugar residues are added [48] and leading to the formation of the triterpenoid skeleton of b-amyrin and related glycosides. The presence of polar nucleus, linked to one or more sugar residues, is responsible for the characteristic activities of these compounds [44]. Majority of the significant steps at molecular level in triterpene saponin biosynthesis remain uncharacterized. The steps involving the biosynthesis of b-amyrin by b-amyrin synthase, an oxidocyclase, have been well characterized in several plant species including Arabidopsis thaliana [49], oat [50], but steps involving the modification of the triterpenoid backbone by the cytochrome P450-dependent monooxygenases and uridine diphosphate glycosyltransferases remain less understood.
Extensive research has gone into the metabolic profiling of G. sylvestre, but there are very few reports pertaining to metabolomics and genomics. The structural elucidation of gymnemic acid revealed the presence of triterpene aglycone moiety known as sapogenin attached to a sugar chain. The occurrence of significant percentage of triterpene glycosides in plant indicates that glycosylation is a critical process in the modification/generation of triterpene saponins. Studies including the metabolomics and functional genomics with emphasis on the gene identification, cloning, and their functional characterization will be an important tool in deciphering the functional role of these genes in the biochemical pathway leading to medicinal properties of the phytoconstituents in the plant.
Further, in an attempt to understand the molecular mechanism of genes responsible for medicinal properties of G. sylvestre, two partial cds (accession nos. GU191124; GU181368) were submitted to NCBI database [51, 52]. Further studies into the identification and characterization of genes involved in the biosynthesis of triterpene glycosides, gymnemic acids will provide valuable information in deciphering the biosynthetic pathway of gymnemic acids and the mechanism of their pharmacological activities in the plant. Since, the transcriptome data of Gymnema sylvestre is unavailable and various proteins and enzymes at the biochemical level remain uncharacterized, so the exact mechanism of Gymnemic acid biosynthesis is not reported in the literature. However, extensive research is ongoing in our lab to decode the functional role of glycosyltransferases in biosynthesis of Gymnemic acids owing to its significant pharmacological importance (unpublished data). The biosynthesis pathway of gymnemic acid remains unknown; however, putatively pathway for triterpene glycosides is derived from the isoprenoid pathway with glycosylation of the triterpene aglycone at the terminal transformation of gymnemagenin. A general diagrammatic sketch has been drawn to represent a putative pathway with a focus on terminal pathway steps in biosynthesis of saponins from Gymnema sylvestre (Figure 1).
Figure 1.
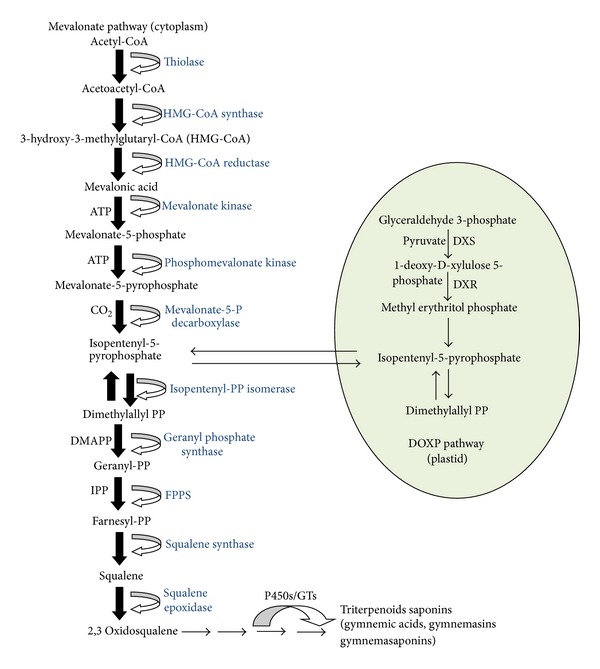
Hypothetical pathway of Gymnemic acid biosynthesis. The general sketch represents the formation of triterpenoids through Mevalonate pathway. Further, it was assumed that gymnemagenin (sapogenin) gave rise to gymnemic acids and derivatives through glycosylation mechanism by glycosyltransferases.
6. Mechanism of Action of Gymnemic Acids
The mode of action of the drug is through stimulation in insulin secretion from pancreas [53]. It also exerts a similar effect by delaying the glucose absorption in the blood. The atomic arrangements of gymnemic acids to the taste buds are similar to sugar molecules which fill the receptors in the taste buds preventing its activation by the sugar molecule in the food. Similarly, in the intestine it attaches to the receptor present in external layer of intestine, thereby preventing the absorption of sugar molecules by intestine, leading to reduction in blood sugar levels [33]. Gurmarin acts in a similar manner by interfering with the ability of taste buds on the tongue to differentiate between sweet and bitter. Hypoglycemic effect of gymnemic acids includes a cascade of events starting from modulation of incretin activity which triggers insulin secretion and release. It also increases regeneration of pancreatic islet cells to enhanced enzyme mediated uptake of glucose. This process decreased glucose and fatty acid assimilation in the small intestine and interferes in the ability of receptors in mouth and intestine to sensation of sweetness. It has been previously reported in the literature that the action of gymnemic acid is similar to that of incretin-mimetic mechanism of action [54]. Gymnemic acid has been found to interact with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a key enzyme in glycolysis pathway [55]. The findings also indicated that the acyl moieties present in gymnemic acids play important role for the GA-induced smearing of GAPDH and G3PDH and play an integral role in the antihyperglycemic activity of GA derivatives [56].
7. Pharmacological Activities of Extracts and Pure Compounds Isolated from Gymnema sylvestre
Although the herb is widely used as a naturopathic treatment for diabetes [57, 58], it also demonstrates promising effects in the treatment of obesity, arthritis, hyperlipidemia, Parkinsonism, and hypercholesterolemia [59–61]. Furthermore, the bioactive compounds of plant have antimicrobial, anti-inflammatory, and anticancer properties. The leaves of the plant are used for the treatment of obesity [62], dental caries [63], antibiotic, in stomachache, blood purifier, and in rheumatism [64]. Some of the significant pharmacological properties of the herb had been discussed in detail. Various plant parts, namely, leaves, roots possess medicinal properties and used for the treatment of various diseases in Ayurvedic system of medicine (supplementary Table 2). Numerous bioactive compounds isolated from the plant either as pure compounds or as crude extracts possess medicinal properties and clinically tested in animal model systems for scientific validation (supplementary Table 3).
7.1. Antidiabetic Property
The herb accounts for its sweet inactivation property to the presence of triterpene saponins known as gymnemic acids, gymnemasaponins, and gurmarin. Experimental trials confirmed the hypoglycemic effect of G. sylvestre on beryllium nitrate and streptozotocin treated rats. There was a slight increase in body weight and protein and a significant decrease in fasting blood glucose in diabetic rats treated with G. sylvestre, C. auriculata, E. jambolanum, and S. reticulata and the effects were quite similar to insulin and glibenclamide treated mice.
An investigation to determine the antioxidant activity of Gymnema leaf extract and the role of antioxidants in diabetic rats was performed by Kang et al. [65] using ethanolic extracts. Several antioxidant assays, namely, thiobarbituric acid (TBA) assay with slight modifications, using egg yolk lecithin or 2-deoxyribose (associated with lipid peroxidation), superoxide dismutase- (SOD-) like activity assay, and 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay (involved in electron or radical scavenging), depicted significant antioxidant activity of the ethanolic extract. Further LC/MS analysis revealed the presence of antihyperglycemic compounds like gymnemagenin and gymnemic acids in G. sylvestre extract and the level of lipid peroxidation reduced by 31.7% in serum, 9.9% in liver, and 9.1% in kidney in diabetic rats fed with the ethanolic extract. The activity of transaminases in gluconeogenesis and ketogenesis in diabetes like glutamate pyruvate transaminase (GPT) in serum and glutathione peroxidase in cytosolic liver returned to normal levels after the administration of ethanolic leaf extract in diabetic rats [66]. Antihyperglycemic effect of crude saponin fraction and five triterpene glycosides (gymnemic acids I–IV and gymnemasaponin V), isolated from the methanolic extract of the leaves, was reported [56]. It was found that gymnemic acid IV (3.4/13.4 mg/kg) decreased blood glucose levels by 14.0–60.0% within 6 hours of administration as compared to glibenclamide. It has been reported that gymnemic acid IV increased plasma insulin levels in STZ-diabetic mice at a concentration of 13.4 mg/kg while it did not cause inhibitory effect on α-glucosidase activity in the brush border membrane vesicles of small intestine in normal rat.
Similarly, in an experimental study, the antidiabetic and hypolipidemic potential of dried powdered leaves of G. sylvestre was investigated. The effect of G. sylvestre leaf extract was administered to nondiabetic and alloxan-diabetic rats. It was found that the Gymnema leaf extract had no effect on the alleviated glycemia caused by balanced meal or due to the administration of glucose or amylose but increased serum lipid level after SOC treatment. However, in nondiabetic and alloxan diabetic rats the subacute and chronic treatment with Gymnema extract had no effect on the ingestion of food and water, gain of body weight, and the level of glucose and lipid in blood. But the herbal formulation requires clinical approval and scientific validation before being used for the treatment of diabetes and hyperlipidemia [67]. Finally, it was concluded from the studies that the herb possesses antidiabetic effect and sugar inactivation properties.
7.2. Antiarthritic Activity
The leaf extract of G. sylvestre was examined for antiarthritic activity on albino rats. The water soluble and petroleum ether (40–60°C) extract was found to be significantly effective in controlling arthritis. It was also assumed that the most potent antiarthritic activity of the leaves may be due to the nature of triterpenoids, steroids, and saponin glycosides [59]. Different extracts were suspended with 1% Tween 80, and the drug Diclofenac sodium was administered once daily through oral route and the effect was monitored for 21 days. It was observed that the rats developed swelling in multiple joints on induction with an adjuvant and exhibited inflammation in cells, bone destruction, and reshaping. The petroleum ether extract treated group showed significant reduction in paw swelling possibly due to inhibiting the response of inflammatory cells or blocking the release of mediators like cytokines (IL-Ib and TNF-a), GM-CSF, interferons, and PGDF which are responsible for pain and disabilities arising due to destruction of bone and cartilage [68]. The other possible mechanism of action suggested protection of the release of joint cartilage and bone destruction in chronic arthritic model [59]. The multiple studies employing use of polar solvents in extract preparations by investigators demonstrated the antiarthritic potential of the leaf extract.
7.3. Treatment of Dental Caries
Dental caries can be defined as infection of tooth, occurring due to various kinds of gram-positive cariogenic bacteria [69] like S. aureus, S. mitis, and S. mutans, and fungus-like Candida albicans which attaches to the tooth surface through release of extracellular polysaccharides from sucrose and metabolize sugar to organic acid mainly lactic acid resulting in demineralization of the tooth enamel [70]. The chloroform, petroleum ether, and methanolic leaf extracts of G. sylvestre at various concentrations of 25, 50, and 100 mg/mL were tested against microbial dental infections and found to be significantly effective against these cariogenic bacteria particularly the methanolic extract which showed highest activity at minimum concentration. The good potential of the hydroalcoholic extract of the plant leads to the development and manufacture of gurmar tooth powdered marketed as “Gurmar Herbal tooth paste” and “Gurmar Herbal Tooth powder.” These herbal formulations offer new prospects in the treatment of dental caries once clinically approved by the scientific community [63].
7.4. Antibiotic and Antimicrobial Activity
The antibiotic and antimicrobial activity of different extracts of G. sylvestre was determined [71] against a number of pathogens, namely, S. aureus, E. coli, and B. subtilis while no activity was observed against gram-negative bacteria. G. sylvestre leaf extracts showed good prospects as an antibiotic herbal remedy was effective as herbal formulation for the treatment of microbe's related infections [71]. The antibacterial activity of G. sylvestre and gymnemic acid was also studied against E. coli and B. cereus and the antimicrobial effect was significant against the microbes [72]. Bhuvaneswari et al. [73] demonstrated that the methanolic extracts of G. sylvestre were assessed for antimicrobial activity of aerial and root parts separately. The result exhibited that the methanol extracts in acidic range have good activity towards all the pathogens showing its broad spectrum nature. In a similar study, the antimicrobial effect of ethanolic extract of G. sylvestre against Bacillus pumilus, B. subtilis, P. aeruginosa, and S. aureus showed promising antimicrobial effect [74]. It can be inferred from the studies that the methanolic and ethanolic leaf extract of Gymnema sylvestre possesses considerable antibiotic and antimicrobial activity.
7.5. Anti-Inflammatory Activity
In the Ayurvedic system of medicine, the leaf of G. sylvestre has been widely used and is considered as bitter, acrid, thermogenic, digestive, liver tonic, anodyne, and anti-inflammatory [75]. The bioactive constituents in G. sylvestre known as tannins and saponins are responsible for the anti-inflammatory activity of the plant [76]. In the study, carrageenin induced paw oedema and cotton pellet induced granuloma rats were taken, and the aqueous extract of G. sylvestre leaf was investigated for its anti-inflammatory activity at the doses of 200, 300, and 500 mg/kg with drug, phenylbutazone as standard. It was found that the gymnema aqueous extract at a concentration of 300 mg/kg significantly decreased the paw oedema volume by 48.5% within 4 hours of administration while the drug phenylbutazone decreased the paw oedema volume by 57.6%. Also, the aqueous extract at a concentration of 200 and 300 mg/kg exhibited reduction in granuloma when compared with the control group [77].
7.6. Anticancer and Cytotoxic Activity
Many plant-derived saponins, namely, ginsenosides, soyasaponins, and saikosaponins have been found to exhibit significant anticancer activity. Anticancer potential of gymnemagenol on HeLa cancer cell lines in in vitro conditions, was determined [78]. The cytotoxic activity of the saponins was tested by MTT cell proliferation assay. Different concentrations of gymnemagenol (5, 15, 25, and 50 μg/mL) were taken and plates were incubated for 48 hours. The IC50 value was found to be 37 μg/mL for gymnemagenol and after 96 hours, the extract at a concentration of 50 μg/mL showed good cytotoxic activity on 73% on HeLa cells. The isolated bioactive constituent, gymnemagenol, was found to show a high degree of inhibition to the proliferation of HeLa cancer cell line. Further, these saponins were not toxic to the growth of normal cells under in vitro conditions [79]. With the rising percentage of cancer in people, the herbal formulation is a prospective medication in cancer therapy.
7.7. Antihyperlipidemic Activity
The prevalence of coronary artery disease is the cause of higher incidence of mortality than other causes combined [80]. The major factor contributing to atherosclerosis and related disorders like coronary artery diseases is hyperlipidemia [81]. Reduction in serum cholesterol levels may significantly reduce the chances of coronary heart disease [80]. Due to the limitations of synthetic drugs in having adverse effects, plant-based formulations offer a good prospect for the treatment of heart disease. Gymnemic acids preparations have been found to be effective against obesity [42]. The triterpene saponins constitute several acylated (tigloyl, methylbutyryl, etc.) derivatives of deacylgymnemic acid. Gymnemic acids consist of gymnemic acids I–VII, gymnemosides A–F, gymnemasaponins, and so forth [17]. In the study, high cholesterol diet, standard atorvastatin, and high cholesterol diet with hydroalcoholic extract of gymnemic acid were fed to female rats for seven days. It was observed that the rats fed with high cholesterol diet showed increase in serum cholesterol, serum triglycerides, low-density lipoprotein cholesterol, and very low-density lipoprotein and significant decrease in high-density lipoprotein cholesterol in comparison to normal animals. The group administered with hydroalcoholic extract of Gymnema leaves at a dose of 200 mg/kg showed significant reduction in the levels of all lipids with increase in HDL-C as compared to high cholesterol diet control [60]. A study demonstrated that the hexane extract of the leaves of G. sylvestre possesses antiobesity activity. It was found that, after 45 days of administration of hexane extract of G. sylvestre, a significant reduction in increased body weight and high temperature due to obesity was observed. Also, the hexane extract improved the cholesterol, triglyceride, LDL, and HDL levels. The hexane extract of the leaves of G. sylvestre have the potential to treat obesity comparable with that of standard drug, atorvastatin [82]. The studies showed that the leaf extract has good prospects in the reduction of cholesterol levels and as a herbal medication for obesity.
7.8. Immunostimulatory Activity
Immunomodulation is referred to as the regulation or control of the immunity which involves the enhancement or reduction in the immune responses. The body response to a particular condition might be regulated by agent that enhances or suppresses its action [83]. G. sylvestre is reported to be an immunostimulatory plant and the leaves possess immunostimulatory effect [84]. The aqueous leaf extract was tested for immunostimulatory activities by detecting the movement of neutrophils, chemotaxis tests, phagocytosis of killed C. albicans, and nitroblue tetrazolium assays. Aqueous leaf extract of G. sylvestre showed remarkable immunostimulatory activity at 10, 25, 50, 100, and 1000 μg/mL on human neutrophils under in vitro conditions [85].
7.9. Hepatoprotective Activity
The hepatoprotective effect of hydro-alcoholic extract of G. sylvestre was evaluated by Srividya et al. [86]. The rat hepatocytes (freshly prepared) were subject to treatment with different concentration of hydroalcoholic extract prepared by the hot maceration process. The extract at a concentrations of 200, 400, and 600 μg/mL showed significant antihepatotoxicity against the D-galactosamine-induced hepatotoxicity, and the concentration of 800 μg/mL was found to be cytotoxic. The cells exhibited a significant restoration of the altered biochemical parameters towards the normal (P < 0.001) when compared to D-galactosamine treated groups in a dose-dependent manner, when treated with the hydroalcoholic extract different extracts of G. sylvestre.
7.10. Wound Healing Activity
The alcoholic extract of leaves of G. sylvestre was found to exhibit significant wound healing activity in rats [85]. According to Kiranmai et al. [87], hydroalcoholic extract of G. sylvestre has good wound healing property as compared with control group. TLC analysis, wound contraction, and qualitative tests supported the synergistic wound healing effect of the plant. The increased wound healing activity of hydroalcoholic extracts may be attributed to the free radical scavenging action and the presence of phytoconstituents (flavonoids) which may act individually or have additive effect. The flavonoids in alcoholic extract were detected by TLC and phytochemical analysis [88].
7.11. Ethnobotanical Uses
Traditionally, the leaves of G. sylvestre were used for the treatment of diabetes and other disorders, while the flowers and bark are given in diseases related to phlegm [89]. The ancient literature on Indian medicine, Sushruta, describes gurmar as a destroyer of madhumeha (glycosuria) and other urinary disorders. The extract of G. sylvestre is reported to be a bitter acrid, anti-inflammatory, anodyne, digestive, liver tonic, emetic, diuretic, thermogenic, stomachic, stimulant, anthelmintics, laxative, cardiotonic, expectorant, antipyretic, and uterine tonic. The plant also exhibits medicinal importance in the treatment of jaundice, constipation, cardiopathy, asthma, bronchitis, amenorrhoea, conjunctivitis, renal and vesical calculi, dyspepsia, leucoderma, and Parkinsonism [90]. Reports in the ancient literature suggested that the plant has multiple medicinal applications, namely, antihelminthic, antipyretic, astringent, an alexipharmic, anodyne, cardiotonic, digestive, diuretic, cough dyspepsia, hemorrhoids, hepatosplenomegaly, laxative, stimulant, stomachic, uterine tonic, intermittent fever, jaundice, and leucoderma. The root bark is useful as an emetic, expectorant, and analgesic for bodyache and root juice in the treatment of snakebite [91]. The plant extract is also useful in the treatment of piles, colic pain, dropsy, phlegm, eye troubles, cardiac, and respiratory diseases.
8. Bioavailability and Toxicity
Bioavailability is a key issue in terms of effectiveness of any herbal medicine as a drug and will determine its effective delivery into the circulatory system in the body. Bioavailability of gymnemic acid is an important parameter for its in vivo pharmacological applications. Gymnemic acid has poor lipid solubility and complex structure and difficult to pass through the biomembranes for its absorption in circulatory system. Pathan and coworkers have developed a herbal formulation (gymnemic acid: phospholipid complex) with an aim to improve its bioabsorption and pharmacokinetics. A phytosome exhibits better absorption and utilization in body due to its increased capacity to cross lipid biomembranes and reach the systemic circulation. The complex exhibits antiapoptotic potential in doxorubicin-induced cardiotoxicity in rats and shows cardioprotective effect [92]. Toxicity studies of Gymnema sylvestre extract have shown its safety when taken in recommended doses. High doses may lead to side effects including hypoglycemia, weakness, shakiness, excessive sweating, and muscular dystropy. Administration of 1.00% basal powder (GSE) in the diet in Wistar rats for 52 weeks has shown no toxic effects and no animal died during the experiment [93]. Treatment of diabetic patients with Gymnema sylvestre has been shown to cause toxic hepatitis or drug-induced liver injury (DILI) [94].
9. In Vitro Cultivation of Gymnema sylvestre
Cultured plant cells and tissues are widely recognized as promising alternatives for the production of valuable secondary metabolites [95, 96]. Plant tissue culture techniques have been employed on an industrial scale for the production of bioactive compounds [97]. Various techniques were employed for propagation of the herb in plant tissue culture through in vitro multiplication for shoot regeneration from mature nodal explants of G. sylvestre [98] and large-scale production of gymnemic acids in plant cell suspension cultures [99]. Somatic embryogenesis was optimized and whole plant regeneration was achieved in callus cultures derived from hypocotyl, cotyledon, and leaf explants excised from seedlings of G. sylvestre. Globular/heart shaped embryos developed and produced torpedo and cotyledon stage embryos upon subculturing on embryo maturation medium EM8 (medium containing MS salts, B5 vitamins, 0.5 μM BA, and 2% sucrose). The mature embryos were subcultured on fresh EM8 medium for embryo germination and plantlet formation. These plantlets were grown in glasshouse, respectively [100].
For in vitro regeneration of mature nodal explants of G. sylvestre, Murashige and Skoog (MS) media were used for the inoculation of single node explants having different combinations of 6-benzylaminopurine (BAP) or kinetin with naphthaleneacetic acid (NAA) and auxins like indoleacetic acid (IAA) alone or in combinations. The MS medium containing BAP (5 mg/L) and NAA (0.2 mg/L) exhibited maximum number of shoot (7 per explants). Further, the regenerated shoots were subjected to rooting on MS half strength medium in absence of any growth regulator (IAA, IBA, and NAA). In cultures where the shoot explants were inoculated on auxin-free half strength MS basal medium, root primordia emerged from the shoot base 15–20 days after implantation and subsequently developed into roots without basal callus as compared to MS media supplemented with different concentrations of auxins, which did not lead to root formation [15].
Plant cell suspension cultures were generated for large-scale production of gymnemic acids, the antisweet phytoconstituents. The methodology employed led to the development of a novel cell culture system for in vitro growth and cultivation of this species. The conditions for the production and HPLC quantification of gymnemic acids were optimized. The gymnemic acids were not accumulated in callus but were released into the medium. For the production of gymnemic acid commercially, this needs to be further optimized. In another study, the extraction of gymnemic acid through gymnemagenin from callus culture of G. sylvestre was reported. The aglycon component, known as gymnemagenin, was extracted, detected, and quantified in different callus cultures of G. sylvestre. HPTLC method was standardized for the rapid and accurate quantitative estimation of gymnemagenin in callus cultures of G. sylvestre [101].
Recently, Devi and Srinivasan [102] attempted the large-scale production of gymnemic acids under in vitro conditions, through the mediation of fungal elicitors. The use of bioelicitors, such as Aspergillus niger cell extract, enhanced the production of secondary metabolite, namely, gymnemic acids from G. sylvestre suspension culture. It is interesting to note that the elicitation of Gymnema suspension culture by A. niger significantly enhanced the production gymnemic acid as compared to nonelicited cultures. The technique is a potential means for the establishment of large-scale production of gymnemic acids through the employment of shaking flask and bioreactors. Due to the limited availability of G. sylvestre formulation, this technique holds good prospects for large-scale commercial production of bioactive phytoconstituents [102].
Gymnemic acid being an important bioactive compound, cell suspension cultures of G. sylvestre were generated and optimized for the production of gymnemic acids [103, 104].
10. Summary and Future Prospects
Medicinal plants served as a platform for ancient Ayurvedic system of medicine. In the present scenario, herbal therapeutics are gaining momentum in pharmacological applications and as molecular targets in the drug development. The emerging trend in rising incidence of diseases and associated complications with commercial medications poses a serious threat to mankind. Naturopathic treatments offer respite from the high cost of expensive drugs as well as in being comparatively safe with less side effects. It is estimated that nearly 80% of population depends on the natural remedies for health care. Plants are a valuable source of a number of bioactive compounds like alkaloids, quinine, paclitaxel, opium alkaloids, quinine, atropine, and cardiac glycosides (digitalis, ouabain) to name a few. The first antidiabetic drug, metformin, isolated from Galega officinalis, was a herbal formulation. Thus, it becomes very important to screen plants with pharmacological significance as a basis for the development of newer and more effective therapeutics. In spite of the good prospects of herbal medicines, these have gained little importance due to absence of scientific validation. The lack of availability of standards for herbal formulations is a major limitation. Although, a vast repertoire of plant resources is available but very few have experimentally validated and scientifically approved as medications for the treatment of diseases.
One major factor that comes into play is that many medicinal plants of commercial importance face threat of extinction due to increase in demand and destruction of their habitats due to urbanization and industrialization. The prime initiative should focus on the cultivation and conservation of medicinal plants with pharmacological importance. Although, the herb has immense prospects in drug development, but it faces threat of extinction due to continuous deforestation and absence of established lines or varieties. The in vitro propagation of plants, in plant tissue culture offers a promising alternative for the production of valuable secondary metabolite. G. sylvestre, being a valuable medicinal plant and source of bioactive substances, needs to be propagated and conserved. In vitro propagation of plants with high bioactive content and cell culture technologies for large-scale production of such secondary metabolites with medicinal significance will be highly prospective and will provide new dimensions to this area of research. Studies have been made in the past few years to understand the complex and incompletely understood nature of plant cells in vitro cultures [105]. Bioelicitors based strategies (from Xanthomonas spp. and A. niger cell extract) for enhanced production of gymnemic acids have been employed [102, 106], and the technique finds relevance for large-scale production of these bioactive compounds in bioreactors based industrial applications. These new technologies will be new beginning for further production and utilization of these sweet suppressing compounds invaluable as an antidiabetic herbal cure.
G. sylvestre holds a unique position among the sweetness modifying materials of natural origin. The herb accounts for multiple pharmacological significance as a naturopathic medication since ancient times and gaining popularity in the present scenario as well. Various polyherbal formulations like Dihar [53] and D-400 [105] containing G. sylvestre extract have been used for the treatment of diabetes mellitus. Several clinical trials and experimental studies indicated that the plant is an invaluable source of bioactive compounds and phytoconstituents like gymnemic acids have been used as molecular targets in drug development. Besides having pharmacological importance, the herbal extract exhibits good prospects in dietary applications. G. sylvestre dried leaf powder is orally consumed by Paliyan tribes of Sirumalai hills for treatment of diabetes. Several products such as GNC Herbal Plus Standardized G. sylvestre (herbal supplement), Vitamin Shoppe G. Sylvestre (sugar destroyer), Gymnema gold (Nutrigold) abolishe the taste of sugar and help support healthy glucose; Gurmar capsules (stimulates the heart and circulatory system and activate uterus) are some of the products composed of Gymnema extract and are marketed and sold as herbal preparations. Among the medicinal plants, G. sylvestre is a herb less exploited for its innumerable advantages. The aim of this review is to highlight the prospects of this rare herb as a potential medication for treatment of diseases from diabetes, obesity to cardiovascular disorders as well as a very good dietary and health supplements in food industry as an health tablets, beverages, tea bags, energy supplements, and in food items which regulates body weight. Gymnema sylvestre 75 is a herbal preparation which contains 75% Gymnemic acid from leaf extract and provides nutritional support to pancreas and maintain healthy blood sugar balance when used as part of diet.
The whole genome sequencing projects and functional elucidation of pathway genes have made significant contributions in deciphering the biological role and properties of biomolecules. With the functional characterization of genes, their relevance in the plant and functional role in the bioactivity of phytomolecules are being established. Information about such genes which code for economically viable traits or pharmacologically important bioactive molecules holds great prospects in crop engineering. The development of genetic transformation systems will provide an edge in the propagation and maintenance of such pharmacologically important plant having applications in drug discovery and development.
Supplementary Material
Supplementary Table 1 describes the occurrence of gymnemic acid in various plant parts of Gymnema sylvestre. The highest percentage of gymnemic acid is present in shoot tip (54.29 mg g−1 DW) and lowest is in seeds (1.31 mg g−1 DW) respectively. Supplementary Table 2 summarizes the therapeutic potential of various plant parts and their application in pharmacological studies. Supplementary Table 3: Various bioactive phytoconstituents present in Gymnema sylvestre, their isolation and application in treatment of various health ailments.
Acknowledgments
The authors are thankful to CSIR Network Project NWP09 for the financial grant. Pragya Tiwari thanks CSIR, New Delhi, for the award of Senior Research Fellowship.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. Journal of Ethnopharmacology. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 2.Burke JP, Williams K, Narayan KMV, Leibson C, Hafener SM, Stern MP. A population perspective on diabetes prevention: whom should we target for preventing weight gain? Diabetes Care. 2003;26(7):1999–2004. doi: 10.2337/diacare.26.7.1999. [DOI] [PubMed] [Google Scholar]
- 3.Smith CM, Reynard AM. Essentials of Pharmacology. Philadelphia, Pa, USA: WB Saunders; 1995. [Google Scholar]
- 4.Babu PA, Suneetha G, Boddepalli R, et al. A database of 389 medicinal plants for diabetes. Bioinformation. 2006;1(4):130–131. doi: 10.6026/97320630001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji H-F, Li X-J, Zhang H-Y. Natural products and drug discovery: can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Reports. 2009;10(3):194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung M, Park M, Lee HC, Kang Y-H, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Current Medicinal Chemistry. 2006;13(10):1203–1218. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- 7.Shane-McWhorter L. Dietary supplements for diabetes: an evaluation of commonly used products. Diabetes Spectrum. 2009;22(4):206–213. [Google Scholar]
- 8.Manohar SH, Naik PM, Praveen N, Murthy HN. Distribution of gymnemic acid in various organs of Gymnema sylvestre . Journal of Forestry Research. 2009;20(3):268–270. [Google Scholar]
- 9.Singh VK, Umar S, Ansari SA, Iqbal M. Gymnema sylvestre for diabetics. Journal of Herbs, Spices and Medicinal Plants. 2008;14(1-2):88–106. [Google Scholar]
- 10.Kanetkar P, Singhal R, Kamat M. Gymnema sylvestre: a memoir. Journal of Clinical Biochemistry and Nutrition. 2007;41(2):77–81. doi: 10.3164/jcbn.2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saneja A, Sharma C, Aneja KR, Pahwa R. Gymnema Sylvestre (Gurmar): a review. Der Pharmacia Lettre. 2010;2(1):275–284. [Google Scholar]
- 12.Persaud SJ, Al-Majed H, Raman A, Jones PM. Gymnema sylvestre stimulates insulin release in vitro by increased membrane permeability. Journal of Endocrinology. 1999;163(2):207–212. doi: 10.1677/joe.0.1630207. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Ozeki M, Iino A, Nakajyo S, Urakawa N, Atsuchi M. Structure-activity relationships of triterpenoid derivatives extracted from Gymnema inodorum leaves on glucose absorption. Japanese Journal of Pharmacology. 2001;86(2):223–229. doi: 10.1254/jjp.86.223. [DOI] [PubMed] [Google Scholar]
- 14.Xie J-T, Wang A, Mehendale S, et al. Anti-diabetic effects of Gymnema yunnanense extract. Pharmacological Research. 2003;47(4):323–329. doi: 10.1016/s1043-6618(02)00322-5. [DOI] [PubMed] [Google Scholar]
- 15.Reddy PS, Gopal GR, Sita GL. In vitro multiplication of Gymnema sylvestre R.Br., an important medicinal plant. Current Science. 2004;10(1–4) [Google Scholar]
- 16. National Medicinal Plants Board, Department of Ayush, Ministry of Health and Family Welfare, Government of India, 2008.
- 17.Gurav S, Gulkari V, Duragkar N, Patil A. A. Systemic review: pharmacognosy, phytochemistry, pharmacology and clinical applications of Gymnema sylvestre R Br. Pharmacognosy Reviews. 2007;1:338–343. [Google Scholar]
- 18.Potawale SE, Shinde VM, Anandi L, Borade S, Dhalawat H, Deshmukh RS. Gymnema sylvestre: a comprehensive review. Pharmacologyonline. 2008;2:144–157. [Google Scholar]
- 19.Foster S. Alternative Medicine Reviews Monographs. Thorne Research Inc.; 2002. Gymnema sylvestre; pp. 205–207. [Google Scholar]
- 20.Khramov VA, Spasov AA, Samokhina MP. Chemical composition of dry extracts of Gymnema sylvestre leaves. Pharmaceutical Chemistry Journal. 2008;42(1):30–32. [Google Scholar]
- 21.Sinsheimer JE, Rao GS, McIlhenny HM. Constuents from Gymnema sylvestre leaves. V: isolation and preliminary characterization of the gymnemic acids. Journal of Pharmaceutical Sciences. 1970;59(5):622–628. doi: 10.1002/jps.2600590510. [DOI] [PubMed] [Google Scholar]
- 22.Dateo GP, Jr., Long L., Jr. Gymnemic acid, the antisaccharine principle of Gymnema sylvestre. Studies on the isolation and heterogeneity of gymnemic acid A1. Journal of Agricultural and Food Chemistry. 1973;21(5):899–903. doi: 10.1021/jf60189a030. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarti D, Debnath NB. Isolation of gymnemagenin the sapogenin from Gymnema sylvestre R.Br. (Asclepiadaceae) Journal of the Institution of Chemists. 1981;53:155–158. [Google Scholar]
- 24.Yoshikawa K, Amimoto K, Arihara S, Matsuura K. Structure studies of new antisweet constituents from Gymnema sylvestre . Tetrahedron Letters. 1989;30(9):1103–1106. [Google Scholar]
- 25.Yoshikawa K, Amimoto K, Arihara S, Matsuura K. Gymnemic acid V, VI and VII from gur-ma, the leaves of Gymnema sylvestre R.Br. Chemical and Pharmaceutical Bulletin. 1989;37(3):852–854. [Google Scholar]
- 26.Stocklin W, Weiss E, Resichstein T. Gymnemasaure das anti saccharine prinzip von Gymnema sylvestre R.Br. isolierngen und identifizierungen. Helvetica Chimica Acta. 1967;50(2):474–490. [Google Scholar]
- 27.Yoshikawa K, Nakagawa M, Yamamoto R, Arihara S, Matsuura K. Antisweet natural products. V. Structures of gymnemic acids VIII-XII from Gymnema sylvestre R.Br. Chemical and Pharmaceutical Bulletin. 1992;40(7):1779–1782. [Google Scholar]
- 28.Murakami N, Murakami T, Kadoya M, Matsuda H, Yamahara J, Yoshikawa M. New hypoglycemic constituents in “gymnemic acid“ from Gymnema sylvestre . Chemical and Pharmaceutical Bulletin. 1996;44(2):469–471. doi: 10.1248/cpb.44.469. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa K, Arihara S, Matsuura K. A new type of antisweet principles occurring in Gymnema sylvestre . Tetrahedron Letters. 1991;32(6):789–792. [Google Scholar]
- 30.Liu H-M, Kiuchi F, Tsuda Y. Isolation and structure elucidation of gymnemic acids, antisweet principles of Gymnema sylvestre R.Br. Chemical and Pharmaceutical Bulletin. 1992;40(6):1366–1375. doi: 10.1248/cpb.40.1366. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa M, Murakami T, Kadoya M, et al. Medicinal foodstuffs. IX. The inhibitors of glucose absorption from the leaves of Gymnema sylvestre R.Br. (Asclepiadaceae): structures of gymnemosides A and B. Chemical and Pharmaceutical Bulletin. 1997;45(10):1671–1676. doi: 10.1248/cpb.45.1671. [DOI] [PubMed] [Google Scholar]
- 32.Imoto T, Miyasaka A, Ishima R, Akasaka K. A novel peptide isolated from the leaves of Gymnema sylvestre — I. Characterization and its suppressive effect on the neural responses to sweet taste stimuli in the rat. Comparative Biochemistry and Physiology. 1991;100(2):309–314. doi: 10.1016/0300-9629(91)90475-r. [DOI] [PubMed] [Google Scholar]
- 33.Sahu NP, Mahato SB, Sarkar SK, Poddar G. Triterpenoid saponins from Gymnema sylvestre . Phytochemistry. 1996;41(4):1181–1185. doi: 10.1016/0031-9422(95)00782-2. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Ye W, Yu B, Zhao S, Wu H, Che C. Two new flavonol glycosides from Gymnema sylvestre and Euphorbia ebracteolata . Carbohydrate Research. 2004;339(4):891–895. doi: 10.1016/j.carres.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Zhen H-S, Zhu X-Y, Lu R-M, Liang J, Qiu Q, Meng Q-M. Research on chemical constituents from stem of Gymnema sylvestre . Journal of Chinese Medicinal Materials. 2008;31(8):1154–1156. [PubMed] [Google Scholar]
- 36.Gent JF, Hettinger TP, Frank ME, Marks LE. Taste confusions following gymnemic acid rinse. Chemical Senses. 1999;24(4):393–403. doi: 10.1093/chemse/24.4.393. [DOI] [PubMed] [Google Scholar]
- 37.Chattopadhyay RR. A comparative evaluation of some blood sugar lowering agents of plant origin. Journal of Ethnopharmacology. 1999;67(3):367–372. doi: 10.1016/s0378-8741(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 38.Arai K, Ishima R, Morikawa S, et al. Three-dimensional structure of gurmarin, a sweet taste-suppressing polypeptide. Journal of Biomolecular NMR. 1995;5(3):297–305. doi: 10.1007/BF00211756. [DOI] [PubMed] [Google Scholar]
- 39.Suttisri R, Lee I-S, Douglas Kinghorn A. Plant-derived triterpenoid sweetness inhibitors. Journal of Ethnopharmacology. 1995;47(1):9–26. doi: 10.1016/0378-8741(95)01248-c. [DOI] [PubMed] [Google Scholar]
- 40.Rao GS, Sinsheimer JE. Constituents from Gymnema sylvestre leaves. 8. Isolation, chemistry, and derivatives of gymnemagenin and gymnestrogenin. Journal of Pharmaceutical Sciences. 1971;60(2):190–193. doi: 10.1002/jps.2600600205. [DOI] [PubMed] [Google Scholar]
- 41.Yoshikawa K, Arihara S, Matsuura K, Miyaset T. Dammarane saponins from Gymnema sylvestre . Phytochemistry. 1992;31(1):237–241. [Google Scholar]
- 42.Yoshikawa K, Murakami T, Matsuda H. Medicinal food stuffs. IX. The inhibitors of glucose absorption from the leaves of Gymnema sylvestre R.Br. (Asclepiadaceae): structures of gymnemosides A and B. Chemical and Pharmaceutical Bulletin. 1997;45(10):1671–1676. doi: 10.1248/cpb.45.1671. [DOI] [PubMed] [Google Scholar]
- 43.Sathya A, Ramasubramaniaraja R, Brindha P. Pharmacognostical, phytochemical and GC-MS investigation of successive extract of Gymnema sylvestre R.Br. Journal of Pharmacy Research. 2010;3:984–987. [Google Scholar]
- 44.Vincken J-P, Heng L, de Groot A, Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68(3):275–297. doi: 10.1016/j.phytochem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Haridas V, Higuchi M, Jayatilake GS, et al. Avicins: triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(10):5821–5826. doi: 10.1073/pnas.101619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marciani DJ, Press JB, Reynolds RC, et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18(27):3141–3151. doi: 10.1016/s0264-410x(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 47.Park H-J, Kwon S-H, Lee J-H, Lee K-H, Miyamoto K-I, Lee K-T. Kalopanaxsaponin A is a basic saponin structure for the anti-tumor activity of hederagenin monodesmosides. Planta Medica. 2001;67(2):118–121. doi: 10.1055/s-2001-11516. [DOI] [PubMed] [Google Scholar]
- 48.Yendo ACA, De Costa F, Gosmann G, Fett-Neto AG. Production of plant bioactive Triterpenoid saponins: elicitation strategies and target genes to improve yields. Molecular Biotechnology. 2010;46(1):94–104. doi: 10.1007/s12033-010-9257-6. [DOI] [PubMed] [Google Scholar]
- 49.Shibuya M, Katsube Y, Otsuka M, et al. Identification of a product specific β-amyrin synthase from Arabidopsis thaliana . Plant Physiology and Biochemistry. 2009;47(1):26–30. doi: 10.1016/j.plaphy.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Qi X, Bakht S, Leggett M, Maxwell C, Melton R, Osbourn A. A gene cluster for secondary metabolism in oat: implications for the evolution of metabolic diversity in plants. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8233–8238. doi: 10.1073/pnas.0401301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiwari P, Sabir F, Asha, Sangwan NS. Gymnema sylvestre glycosyltransferase mRNA, partial cds (accession no. GU181368) NCBI Database, 2009.
- 52.Sabir F, Tiwari P, Asha, Sangwan NS. Gymnema sylvestre UDP-glucosyltransferase mRNA, partial cds (accession no. GU191124) NCBI Database, 2009.
- 53.Patel SS, Shah RS, Goyal RK. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian Journal of Experimental Biology. 2009;47(7):564–570. [PubMed] [Google Scholar]
- 54.Bone K. Phytotherapy Review and Commentary. National Institute of Herbalists: National Herbalists Association of Australia; 2002. Gymnema: a key herb in the management of diabetes. [Google Scholar]
- 55.Ishijima S, Takashima T, Ikemura T, Izutani Y. Gymnemic acid interacts with mammalian glycerol-3-phosphate dehydrogenase. Molecular and Cellular Biochemistry. 2008;310(1-2):203–208. doi: 10.1007/s11010-007-9681-5. [DOI] [PubMed] [Google Scholar]
- 56.Sugihara Y, Nojima H, Matsuda H, Murakami T, Yoshikawa M, Kimura I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. Journal of Asian Natural Products Research. 2000;2(4):321–327. doi: 10.1080/10286020008041372. [DOI] [PubMed] [Google Scholar]
- 57.Shanmugasundaram ERB, Rajeswari G, Baskaran K, Kumar BRR, Shanmugasundaram KR, Ahmath BK. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. Journal of Ethnopharmacology. 1990;30(3):281–294. doi: 10.1016/0378-8741(90)90107-5. [DOI] [PubMed] [Google Scholar]
- 58.Baskaran K, Ahamath BK, Shanmugasundaram KR, Shanmugasundaram ERB. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. Journal of Ethnopharmacology. 1990;30(3):295–305. doi: 10.1016/0378-8741(90)90108-6. [DOI] [PubMed] [Google Scholar]
- 59.Malik JK, Manvi FV, Nanjware BR, Dwivedi DK, Purohit P, Chouhan S. Anti-arthritic activity of leaves of Gymnema sylvestre R.Br. leaves in rats. Der Pharmacia Lettre. 2010;2:336–341. [Google Scholar]
- 60.Rachh PR, Rachh MR, Ghadiya NR, et al. Antihyperlipidemic activity of Gymenma sylvestre R.Br. leaf extract on rats fed with high cholesterol diet. International Journal of Pharmacology. 2010;6(2):138–141. [Google Scholar]
- 61.Spasov AA, Samokhina MP, Bulanov AE. Antidiabetic properties of Gymnema sylvestre (a review) Pharmaceutical Chemistry Journal. 2008;42(11):22–26. [Google Scholar]
- 62.Government of India Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha & Homoeopathy (AYUSH) The Ayurvedic Pharmacopoeia of India, Part I. 1st edition. Vol. 5. New Delhi, India: The Controller of Publications; 2006. Mesasrngi; pp. 110–114. [Google Scholar]
- 63.Parimala Devi B, Ramasubramaniaraja R. Pharmacognostical and antimicrobial screening of Gymnema sylvestre R.Br, and evaluation of Gurmar herbal tooth paste and powder, composed of Gymnema sylvestre R.Br, extracts in dental caries. International Journal of Pharma and Bio Sciences. 2010;1(3):1–16. [Google Scholar]
- 64.Evans WC, Trease GE, Evans D. Trease and Evans, Pharmacognosy. 15th edition. Philadelphia, Pa, USA: WB Saunders; 2002. [Google Scholar]
- 65.Kang M-H, Lee MS, Choi M-K, Min K-S, Shibamoto T. Hypoglycemic activity of Gymnema sylvestre extracts on oxidative stress and antioxidant status in diabetic rats. Journal of Agricultural and Food Chemistry. 2012;60(10):2517–2524. doi: 10.1021/jf205086b. [DOI] [PubMed] [Google Scholar]
- 66.Patil PM, Chaudhari PD, Duragkar NJ, Katolkar PP. Formulation of anti-diabetic liquid preparation of Gymnema sylvestre and qualitative estimated by TLC. Asian Journal of Pharmaceutical and Clinical Research. 2012;5, supplemet 1:16–19. [Google Scholar]
- 67.Galletto R, Siqueira VLD, Ferreira EB, Oliveira A, Bazotte R. Absence of antidiabetic and hypolipidemic effect of Gymnema sylvestre in non-diabetic and alloxan-diabetic rats. Brazilian Archives of Biology and Technology. 2004;47(4):545–551. [Google Scholar]
- 68.Eric GB, Lawrence JL. Rheumatoid Arthritis and Its Therapy—The Text Book of Therapeutics: Drug and Disease Management. 16th edition. Vol. 16. Baltimore, Md, USA: Williams and Wilkins Company; 1996. [Google Scholar]
- 69.Marsh P, Martin M. Oral Microbiology. Vol. 3. London, UK: Chapman and Hall; 1992. [Google Scholar]
- 70.Akhtar MS, Bhakuni V. Streptococcus pneumoniae hyaluronate lyase: an overview. Current Science. 2004;86(2):285–295. [Google Scholar]
- 71.Saumendu DR, Sarkar K, Dipankar S, Singh T, Prabha B. In vitro antibiotic activity of various extracts of Gymnema sylvestre . International Journal of Pharmaceutical Research and Development. 2010;2:1–3. [Google Scholar]
- 72.Yogisha S, Raveesha KA. In vitro antibacterial effect of selected medicinal plant extracts. Journal of Natural Products. 2009;2:64–69. [Google Scholar]
- 73.Bhuvaneswari CH, Rao K, Giri A. Evaluation of Gymnema sylvestre antimicrobial activity in methanol. Recent Research in Science and Technology. 2011;3:73–75. [Google Scholar]
- 74.Satdive RK, Abhilash P, Fulzele DP. Antimicrobial activity of Gymnema sylvestre leaf extract. Fitoterapia. 2003;74(7-8):699–701. doi: 10.1016/s0367-326x(03)00154-0. [DOI] [PubMed] [Google Scholar]
- 75.Kokate CK. Pharmacognosy. Vol. 12. Nirali Prakashan; 1999. [Google Scholar]
- 76.Diwan PV, Margaret I, Ramakrishna S. Influence of Gymnema sylvestre on inflammation. Inflammopharmacology. 1995;3:271–277. [Google Scholar]
- 77.Malik JK, Manvi FV, Alagawadi KR, et al. Evaluation of anti-inflammatory activity of Gymnema sylvestre leaves extract in rats. International Journal of Green Pharmacy. 2007;2:114–115. [Google Scholar]
- 78.Jain KS, Kathiravan MK, Somani RS, Shishoo CJ. The biology and chemistry of hyperlipidemia. Bioorganic and Medicinal Chemistry. 2007;15(14):4674–4699. doi: 10.1016/j.bmc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 79.Khanna V, Kannabiran K. Anticancer-cytotoxic activity of saponins isolated from the leaves of Gymnema sylvestre and Eclipta prostrata on HeLa cells. International Journal of Green Pharmacy. 2009;3(3):227–229. [Google Scholar]
- 80.Hardman JG, Limbird LE, Goodman LS, Gilman AG. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 10th edition. New York, NY, USA: McGraw Hill; 2001. [Google Scholar]
- 81.Kaushik M, Kaushik A, Arya R, Singh G, Malik P. Anti-obesity property of hexane extract from the leaves of Gymnema sylvestre in high fed cafeteria diet induced obesity rats. International Research Journal of Pharmacy. 2011;2:112–116. [Google Scholar]
- 82.Shivaprasad HN, Kharya MD, Rana AC, Mohan S. Preliminary immunomodulatory activities of the aqueous extract of Terminalia chebula . Pharmaceutical Biology. 2006;44(1):32–34. [Google Scholar]
- 83.Trease GE, Evans WC. Pharmacognosy. Vol. 12. London, UK: Balliere Tindale; 1983. [Google Scholar]
- 84.Gupta SN, Pramanik S, Tiwari OP, Thacker N, Pande M, Upmanyu N. Immunomodulatory activity of Gymnema sylvestre leaves. Internet Journal of Pharmacology. 2010;8(2):1531–2976. [Google Scholar]
- 85.Malik JK, Manvi FV, Nanjware BR, et al. Wound healing properties of alcoholic extract of Gymnema sylvestre R.Br. leaves in rats. Journal of Pharmacy Research. 2009;2:1029–1030. [Google Scholar]
- 86.Srividya AR, Varma SK, Dhanapal SP, Vadivelan R, Vijayan P. In vitro and in vivo evaluation of hepatoprotective activity of Gymnema sylvestre . International Journal of Pharmaceutical Sciences and Nanotechnology. 2010;2:768–773. [Google Scholar]
- 87.Alam G, Singh MP, Singh A. Wound healing potential of some medicinal plants. International Journal of Pharmaceutical Sciences Review and Research. 2011;9:136–145. [Google Scholar]
- 88.Kiranmai M, Kazim SM, Ibrahim M. Combined wound healing activity of Gymnema sylvestre and Tagetes erecta linn . International Journal of Pharmaceutical Applications. 2011;2:135–140. [Google Scholar]
- 89.Kirtikar KR, Basu BD. Indian Medicinal Plants. Vol. 3. Delhi, India: Periodicals Experts; 1975. [Google Scholar]
- 90.Anis M, Sharma MP, Iqbal M. Herbal ethnomedicine of the Gwalior forest division in Madhya Pradesh, India. Pharmaceutical Biology. 2000;38(4):241–253. doi: 10.1076/1388-0209(200009)3841-AFT241. [DOI] [PubMed] [Google Scholar]
- 91.Sastry BS. Gymnema sylvestre. Varanasi, India: Bhav Prakash Nighantu, Chaukhambha; 1994. [Google Scholar]
- 92.Pathan RA, Bhandari U, Javed S, Nag TC. Anti-apoptotic potential of gymnemic acid phospholipid complex pretreatment in wistar rats with experimental cardiomyopathy. Indian Journal of Experimental Biology. 2012;50(2):117–127. [PubMed] [Google Scholar]
- 93.Ogawa Y, Sekita K, Umemura T, et al. Gymnema sylvestre leaf extract: a 52-week dietary toxicity study in wistar rats. Shokuhin Eiseigaku Zasshi. 2004;45(1):8–18. doi: 10.3358/shokueishi.45.8. [DOI] [PubMed] [Google Scholar]
- 94.Shiyovich A, Sztarkier I, Nesher L. Toxic hepatitis induced by Gymnema sylvestre, a natural remedy for type 2 diabetes mellitus. American Journal of the Medical Sciences. 2010;340(6):514–517. doi: 10.1097/MAJ.0b013e3181f41168. [DOI] [PubMed] [Google Scholar]
- 95.Sabir F, Sangwan RS, Singh J, Misra LN, Pathak N, Sangwan NS. Biotransformation of withanolides by cell suspension cultures of Withania somnifera (Dunal) Plant Biotechnology Reports. 2011;5(2):127–134. [Google Scholar]
- 96.Sabir F, Sangwan RS, Kumar R, Sangwan NS. Salt stress-induced responses in growth and metabolism in callus cultures and differentiating in vitro shoots of Indian ginseng (Withania somnifera Dunal) Journal of Plant Growth Regulation. 2012:1–12. [Google Scholar]
- 97.Ramachandra Rao S, Ravishankar GA. Plant cell cultures: chemical factories of secondary metabolites. Biotechnology Advances. 2002;20(2):101–153. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 98.Reddy PS, Gopal GR, Sita GL. In vitro multiplication of Gymnema sylvestre R.Br.—an important medicinal plant. Current Science. 1998;75(8):843–845. [Google Scholar]
- 99.Devi CS, Murugesh S, Srinivasan VM. Gymnemic acid production in suspension cell cultures of Gymnema sylvestre . Journal of Applied Sciences. 2006;6(10):2263–2268. [Google Scholar]
- 100.Ashok Kumar HG, Murthy HN, Paek KY. Somatic embryogenesis and plant regeneration in Gymnema sylvestre . Plant Cell, Tissue and Organ Culture. 2002;71(1):85–88. [Google Scholar]
- 101.Kanetkar PV, Singhal RS, Laddha KS, Kamat MY. Extraction and quantification of gymnemic acids through gymnemagenin from callus cultures of Gymnema sylverstre . Phytochemical Analysis. 2006;17(6):409–413. doi: 10.1002/pca.939. [DOI] [PubMed] [Google Scholar]
- 102.Devi CS, Srinivasan VM. In vitro studies on stimulation of gymnemic acid production using fungal elicitor in suspension and bioreactor based cell cultures of Gymnema sylvestre R.Br. Recent Research in Science and Technology. 2011;3:101–104. [Google Scholar]
- 103.Praveen N, Murthy HN, Chung IM. Improvement of growth and gymnemic acid production by altering the macro elements concentration and nitrogen source supply in cell suspension cultures of Gymnema sylvestre R.Br. Industrial Crops and Products. 2011;33(2):282–286. [Google Scholar]
- 104.Ch B, Rao K, Gandi S, Giri A. Abiotic elicitation of gymnemic acid in the suspension cultures of Gymnema sylvestre . World Journal of Microbiology and Biotechnology. 2012;28(2):741–747. doi: 10.1007/s11274-011-0870-8. [DOI] [PubMed] [Google Scholar]
- 105.Anturlikar SD, Gopumadhavan S, Chauhan BL, Mitra SK. Effect of D-400, a herbal formulation, on blood sugar of normal and alloxan-induced diabetic rats. Indian Journal of Physiology and Pharmacology. 1995;39(2):95–100. [PubMed] [Google Scholar]
- 106.Veerashree V, Anuradha CM, Kumar V. Elicitor-enhanced production of gymnemic acid in cell suspension cultures of Gymnema sylvestre R.Br. Plant Cell, Tissue and Organ Culture. 2012;108(1):27–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 describes the occurrence of gymnemic acid in various plant parts of Gymnema sylvestre. The highest percentage of gymnemic acid is present in shoot tip (54.29 mg g−1 DW) and lowest is in seeds (1.31 mg g−1 DW) respectively. Supplementary Table 2 summarizes the therapeutic potential of various plant parts and their application in pharmacological studies. Supplementary Table 3: Various bioactive phytoconstituents present in Gymnema sylvestre, their isolation and application in treatment of various health ailments.


