Abstract
Menopause is the transition from reproductive to non-reproductive life well before natural death. Rather than involving a smooth, rapid change, it is normally preceded by a long period of erratic hormonal fluctuation that is accompanied by a plethora of unpleasant symptoms. Here, we (1) suggest that this turbulent period owes to conflict, between a woman's maternally inherited (MI) and paternally inherited (PI) genes, over the trade-off between reproduction and communal care; (2) perform a theoretical analysis to show that this conflict is resolved either through silencing or fluctuating expression of one of the genes; (3) highlight which of the symptoms preceding menopause may result from antagonistic co-evolution of MI and PI genes; (4) argue that ecological differences between ancestral human populations may explain the variability in menopause among different ethnic groups; (5) discuss how these insights may be used to inform family planning and cancer risk assessment based on a woman's ancestral background.
Keywords: Cancer, cooperation, demography, fertility, game theory, genomic imprinting, humans, hunter gatherers, kin selection, migration
Introduction
Menopause is the end of female reproductive capability long before natural death (Prior 1998). A woman's fertility ends with her last menstruation at age ∼51, but her life extends to 65–70 years in societies where there is little access to modern medicine (Prior 1998; Jones et al. 2002). Menopause is extremely rare among mammals and has been confirmed beyond doubt only in humans and killer whales (Johnstone & Cant 2010). This phenomenon has attracted much attention from researchers in different fields who have suggested a number of explanatory hypotheses. Non-adaptationist hypotheses regard menopause as a side effect of ovarian senescence and extended human lifespan caused by modern medicine (Austad 1997), whereas adaptationist hypotheses suggest that it owes to natural selection (see Table 1). Some adaptationist hypotheses suggest that natural selection has favoured post-reproductive life because care provided by post-reproductive women increases the survival of kin and reproductive cessation reduces kin competition (Williams 1957; Alexander 1974; Hawkes et al. 1998; Cant & Johnstone 2008). In particular, the ‘Grandmother Hypothesis’ (Hawkes et al. 1998; Cant & Johnstone 2008; Johnstone & Cant 2010) posits that a young woman at the beginning of her reproductive career, being relatively unrelated to her neighbours, would maximise her inclusive fitness by focusing upon her own reproductive effort, whereas an older woman, being surrounded by her descendants, would maximise her inclusive fitness by giving up her own reproduction and providing care for others. This hypothesis has gained empirical support in recent years (Lahdenpera et al. 2004, 2012).
Table 1.
Theories of menopause
| Explanation | Summary | References |
|---|---|---|
| Extended Lifespan | Recent extension of human lifespan results in women living beyond reproductive age | (Austad 1997) |
| Good Mother | Trade-off between giving up own reproduction (when there is a growing age-related mortality) in exchange of greater survival of depending children | (Williams 1957; Alexander 1974) |
| Grandmother Hypothesis | Menopause is the result of a trade-off between giving up own reproduction in exchange of: | |
| (1) Greater survival of kin | (Hawkes et al. 1998) | |
| (2) Reduced mate competition with relatives | (Cant & Johnstone 2008; Johnstone & Cant 2010) | |
| Better Longer | Post-reproductive lifespan results from unidirectional selection against death before completion of reproductive life | (Tully & Lambert 2011) |
Summary of evolutionary explanations for menopause.
However, it is not clear why an adaptive menopause should be such a long and difficult process, rather than a rapid and smooth transition from the reproductive to the non-reproductive phases of a woman's life. Specifically, menopause is preceded by a peri-menopausal period of 10–15 years, during which fertility decline is not driven by the demise of hormone production due to ovarian senescence but rather by dramatic fluctuations in oestrogen levels, resulting in irregular menstrual cycles and skipped ovulations (Prior 1998, 2006; Douma et al. 2005) (Fig. 1). Indeed, oestrogen reaches its highest levels in a woman's lifetime during peri-menopause; as high as 30% above the average level during the regular reproductive period (Santoro et al. 1996; Prior 1998, 2006; Douma et al. 2005) (Fig. 1). This hormonal deregulation during peri-menopause results in a plethora of unpleasant symptoms, including psychological (e.g. mood-swings, depression), vaso-motor (e.g. hot flashes, night sweats) and reproductive (e.g. painful intercourse, vaginal dryness) disorders (Gold et al. 2000; Avis et al. 2001). Interestingly, these symptoms often appear to be working at cross-purposes (Prior 1998, 2006). Duration, hormonal chaos and contradictory symptoms, taken together, suggest that peri-menopause is a detrimental process, from a woman's perspective.
Figure 1.
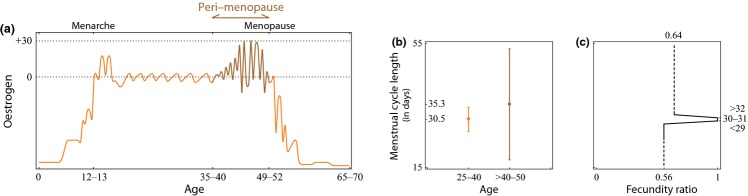
Menopause and peri-menopause endocrinology. (a) Schematic representation of oestrogen levels during a woman's lifetime [adapted from Prior (2006) and Douma et al. (2005)]. Menarche occurs between ages 12–13 (Butts & Seifer 2010), peri-menopause begins between ages 35 and 40 (Douma et al. 2005; Voorhuis et al. 2010) and terminates with menopause at age 51 (Butts & Seifer 2010), the life expectancy is 65–70 among modern hunter gatherers with no access to modern medicine (Jones et al. 2002). (b) Length of menstrual cycles at different ages. The average age and standard errors are provided. (Ferrell et al. 2005). (c) Fertility as a function of cycle length (Small et al. 2006).
Moreover, the turbulent physiology of peri-menopause and the timing of menopause do not appear to owe to constraint, as both phenomena are highly variable among different ethnic groups (Avis et al. 2001; Gold et al. 2001; Butts & Seifer 2010). For example, even after controlling for confounding variables: African Americans experience a longer peri-menopause, with intensified vaso-motor symptoms, that terminates in earlier menopause, whereas Japanese Americans experience a shorter peri-menopause, with milder vaso-motor symptoms, that terminates in later menopause (Gold et al. 2000, 2001; Avis et al. 2001; Henderson et al. 2008; Butts & Seifer 2010). In particular, vaso-motor symptoms are 2.5 times more prevalent among African Americans (1.56%) than among Japanese Americans (0.63%) (Gold et al. 2001). Furthermore, premature ovarian failure (i.e. menopause before the age of 40) is 10 times more prevalent among African Americans (1.4%) than among Japanese Americans (0.14%) (Luborsky et al. 2003).
Here, we suggest that the duration and turbulent nature of peri-menopause owes to intragenomic conflict between a woman's maternally inherited (MI) and paternally inherited (PI) genes. Intragenomic conflict refers to instances in which natural selection favours genes with opposing phenotypic effects expressed in the same individual, and has been associated with several medical disorders (Wilkins & Úbeda 2011). We show that the ecological factors which, according to the Grandmother Hypothesis (Hawkes et al. 1998; Cant & Johnstone 2008), have driven the evolution of a menopause in humans, may also drive intragenomic conflicts over reproductive schedules. In particular, as a result of female-biased dispersal and/or male-biased adult mortality or variance in reproductive success, a woman's PI genes are favoured to promote an earlier menopause, to the benefit of her social group, while her MI genes are favoured to promote a later menopause, to the detriment of her social group. Under this view, the period of active intragenomic conflict defines the turbulent peri-menopausal stage of a woman's life.
We develop a ‘battleground model’ to assess how this conflict between a woman's MI and PI genes unfolds as she ages. We also develop two ‘resolution models’, founded upon the biology of fertility, to assess possible outcomes of this conflict. We use these models to make explicit predictions about the imprinting status of genes that underpin menopause in general and primary ovarian insufficiency in particular. We also show that the erratic hormonal fluctuations (and related symptoms) experienced by peri-menopausal women are a suggestive of such conflict. These models allow us to link, for the first time, the symptoms of menopause to ancestral human ecology. We discuss the implications for personalised family planning and medicine.
Materials and Methods
We employ an inclusive-fitness model to explore selection acting on genes with different parental origin coding for fertility in females. Models that explore the existence of conflict between evolutionary agents are termed battleground models. Having established the conditions under which genes with different parental origin might be in conflict, we employ two game theory models to explore the evolutionary outcomes of this conflict. These models differ in the mechanisms for controlling fertility. Models that explore the outcome of conflict between evolutionary agents are termed resolution models.
Battleground model
We build upon the model of Úbeda & Gardner (2012). This considers an infinite population of diploid individuals subdivided into social groups. Upon reaching maturity, women and men disperse to new groups with probabilities df and dm respectively, and otherwise remain in their natal groups. This allows consideration of any type of residential system, including matrilocality (df < dm), bilocality (df = dm) and patrilocality (df > dm). Mating follows dispersal, and occurs at random between women and men within each group. The probabilities that two random juveniles born in the same group share the same mother and same father are α and β, respectively. This allows consideration of any type of reproductive system, including monogamy (α ≈ β), polygyny (α < β) and polyandry (α > β) (Úbeda & Gardner 2012).
Whereas Úbeda & Gardner (2012) considered generic social traits, here we focus on reproductive traits. We consider that a woman can allocate her limited resources either into raising juveniles in her social group or else into producing her own offspring. By increasing her investment into raising neighbouring juveniles, a woman improves their survival to adulthood by an amount b while reducing the number of her own children she would otherwise have raised to adulthood by an amount c. We are interested in finding out how a woman's valuation of neighbouring juveniles relative to the production of her own offspring changes as she ages. This is a combination of the coefficient of relatedness of a woman of age t to her neighbouring juveniles rAx|t , and the level of competition a between related individuals. First, we determine the condition for selection to favour a reduction in fertility of a woman of age t, and write this as c/b < St , where St defines the ‘potential for sterility at age t ’. That is, menopause is selectively favoured when St is above the threshold 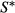 = c/b, which we term the ‘menopause threshold’.
= c/b, which we term the ‘menopause threshold’.
More specifically, we separate the potentials for sterility at age t when the gene expressed is MI 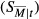 vs. PI
vs. PI 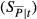 :
:
| (1a) |
| (1b) |
These expressions correspond to eqns (A.01–A.14) of Úbeda & Gardner (2012). Substituting 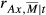 ,
, 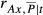 and a into
and a into 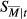 and
and 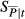 — our eqns (1a) and (1b) respectively — yields recursions for the potentials for sterility via the MI and PI genes in terms of the ecological parameters: dispersal (dm, df ), mating system (α, β) and mortality (μm, μf ). Iterating these recursions, we calculate the potentials for sterility via the MI and PI genes as functions of the age of the focal female, given a set of numerical values for the ecological parameters (method used to generate Fig. 2).
— our eqns (1a) and (1b) respectively — yields recursions for the potentials for sterility via the MI and PI genes in terms of the ecological parameters: dispersal (dm, df ), mating system (α, β) and mortality (μm, μf ). Iterating these recursions, we calculate the potentials for sterility via the MI and PI genes as functions of the age of the focal female, given a set of numerical values for the ecological parameters (method used to generate Fig. 2).
Figure 2.
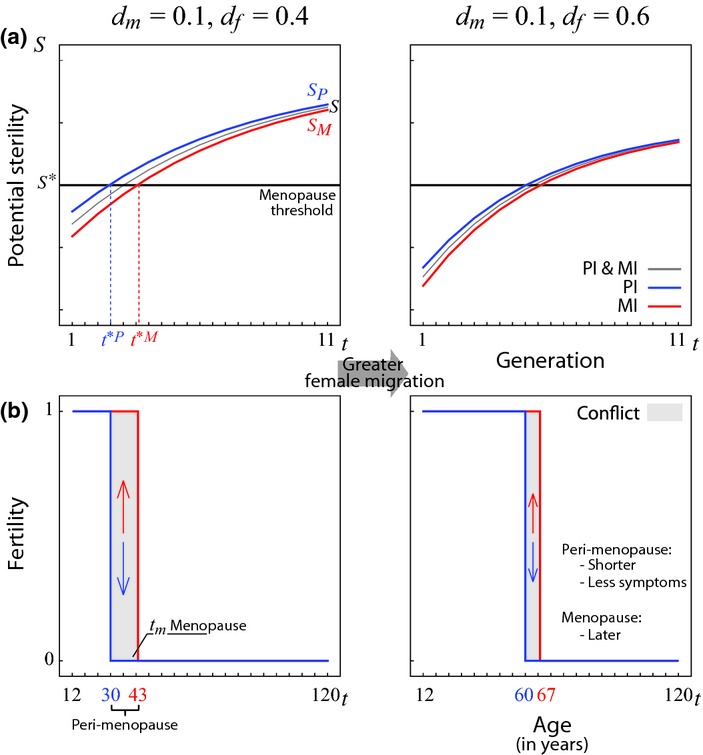
Conflict over menopause. (a) Potential for sterility of the MI gene (red), PI gene (blue), and MI and PI combined (black) as a function of age. (b) Intragenomic conflict as a function of age. Before the age t*P natural selection favours genes that promote fertility both when MI and PI (no conflict). Between the ages t*P and t*M natural selection favours genes that promote fertility when MI but sterility when PI (conflict). From t*M on, natural selection favours genes that promote sterility both when MI and PI (no conflict). To elaborate (a and b) we assume that α = β = 0.1 and μm = μf = 0.1 and we consider two cases of female-biased dispersal: less female bias when dm = 0.1 and df = 0.4, and more female bias when dm = 0.1 and df = 0.6.
Resolution models
The battleground model establishes the ecological conditions under which there is a conflict of interest between a woman's MI and PI genes. Here, we develop two game theory models to investigate the antagonistic interaction between MI and PI genes. The difference between these two models lies in the different modes by which a woman's genes control her fertility.
Directional control
For the purpose of biological concreteness and illustration, we consider the role played by the number of primordial ovarian follicles on a woman's fertility. Ovaries carry a stock of ∼300 000 ovarian follicles by 5 months of gestational age, which then undergoes a decline throughout a woman's life (Wallace & Kelsey 2010). Menopause is caused by the depletion of the ovarian follicle stock, and occurs at age ∼51 when ∼1000 follicles remain (Wallace & Kelsey 2010). However, this decline is not uniform: at age ∼38, when ∼16 000 ovarian follicles remain, there is a marked increase in the rate of ovarian follicle attrition (Richardson et al. 1987; Faddy et al. 1992; Wallace & Kelsey 2010), a phenomenon that is unknown in other mammals (Jones & Krohn 1961; Gosden & Telfer 1987; Nichols et al. 2005; Jones et al. 2007).
We consider a locus that modifies a woman's fertility by altering the stock or rate of decline of ovarian follicles (atresia). We denote the expression levels of the MI and PI genes by 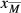 and
and 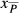 respectively. We assume that these combine additively to determine the total level of expression of that locus, i.e.
respectively. We assume that these combine additively to determine the total level of expression of that locus, i.e. 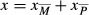 .
.
We consider that fertility is a function of the total level of expression of this gene, i.e. f = F(x). We use the term ‘fertility inhibitor’ to refer to any locus whose greater expression reduces the stock (or accelerates atresia) of ovarian follicles, inhibiting fertility (Fig. A2). In contrast, we use the term ‘fertility enhancer’ to refer to any locus whose greater expression augments the stock (or slows atresia) of ovarian follicle, enhancing fertility (Monget et al. 2012) (Fig. A2). For simplicity, we model a fertility enhancer but symmetrical results can be derived for a fertility inhibitor.
We assume that the payoffs 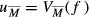 and
and 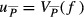 — gained by the MI and PI genes respectively — are unimodal functions, with
— gained by the MI and PI genes respectively — are unimodal functions, with 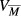 and
and 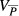 reaching their maxima at
reaching their maxima at  and
and 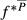 respectively (Fig. A2-i). For simplicity, we assume that the MI gene is favoured for greater fertility than the PI gene, i.e.
respectively (Fig. A2-i). For simplicity, we assume that the MI gene is favoured for greater fertility than the PI gene, i.e. 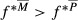 (Fig. A2; symmetrical results can be derived when the direction of the conflict is reversed, i.e.
(Fig. A2; symmetrical results can be derived when the direction of the conflict is reversed, i.e. 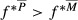 .
.
Consequently, the payoffs for the MI and PI genes are functions of the total level of expression, i.e. 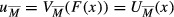 and
and 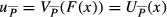 . Let
. Let 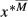 and
and 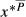 be the total levels of expression that maximise fertility for the MI and PI copies, respectively, i.e.
be the total levels of expression that maximise fertility for the MI and PI copies, respectively, i.e. 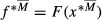 and
and 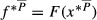 (Fig. A2). From our assumption that
(Fig. A2). From our assumption that 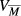 and
and 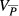 are unimodal it follows that
are unimodal it follows that 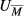 and
and 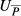 are also unimodal, with
are also unimodal, with 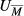 and
and 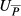 reaching their maxima at
reaching their maxima at 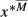 and
and 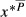 respectively. From our assumptions that the locus is a fertility inhibitor and the sense of conflict being
respectively. From our assumptions that the locus is a fertility inhibitor and the sense of conflict being 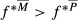 it follows that
it follows that  (Fig. A2).
(Fig. A2).
In particular, we use Gaussian payoff functions, which satisfy the above assumptions:
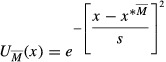 |
(2a) |
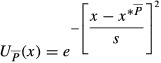 |
(2b) |
where s determines the variance of both functions.
Stabilising/destabilising control
For the purpose of biological concreteness and illustration, we consider the role played by hormonal levels and menstrual cycle length on a woman's fertility. Four hormones are mainly responsible for a woman's menstrual cycle, namely oestrogen and progesterone, produced by the ovaries; and luteinising hormone (LH) and follicle-stimulating hormone (FSH), produced by the pituitary gland (Prior 1998). The levels of these hormones alternate regularly during a woman's reproductive life (Prior 1998), and their relative levels determine the duration of the menstrual cycle (Landgren et al. 1980). In particular, higher levels of oestrogen result in relatively short cycles, whereas lower levels of oestrogen result in relatively long cycles (Landgren et al. 1980).
The regularity in the alternation of these hormones results in minimal cycle length variability during a woman's reproductive life (ages 15–40) with an average duration of ∼30 days per menstrual cycle (Vitzthum 2009) (Fig. 1). Upon entering peri-menopause, levels of oestrogen increase and begin to fluctuate erratically (Prior 1998). Oestrogen disrupts the regular alternation of hormones, and menstrual cycles shorter or longer than 30 days become increasingly frequent (Prior 1998). Fertility is maximised when menstrual cycles are regular (i.e. every 30 days) and declines if menstrual cycles become irregular [i.e. before or after 30 days (Small et al. 2006); Fig. 1].
We consider a locus that modifies a woman's fertility by altering the rate of production of hormones during the menstrual cycle and thus altering its length. We denote the levels of expression of the MI and PI genes by 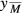 and
and 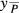 respectively. We assume that these combine additively to determine the total level of expression of that locus, i.e.
respectively. We assume that these combine additively to determine the total level of expression of that locus, i.e. 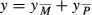 .
.
We assume that fertility is a function of the total level of expression of this gene, i.e. f = F(y). We use the term ‘fertility maximiser’ to refer to any locus whose default expression maximises fertility. That is, its enhanced expression reduces the length of the menstrual cycle, reducing fertility; and its inhibited expression increases the length of the menstrual cycle, also reducing fertility (Fig. A2). The fertility function F is thus unimodal, reaching a single maximum at 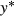 . Notice that this, in practice, corresponds to any locus that mediates the length of a woman's menstrual cycle (Fig. 1). For completeness, we use the term ‘fertility minimiser’ refer to any locus whose regular expression minimises fertility. That is, both its enhanced and its inhibited expression reduce fertility (Fig. A2). For simplicity, we model a fertility maximiser but symmetrical results can be derived for a fertility minimiser.
. Notice that this, in practice, corresponds to any locus that mediates the length of a woman's menstrual cycle (Fig. 1). For completeness, we use the term ‘fertility minimiser’ refer to any locus whose regular expression minimises fertility. That is, both its enhanced and its inhibited expression reduce fertility (Fig. A2). For simplicity, we model a fertility maximiser but symmetrical results can be derived for a fertility minimiser.
We assume that the payoffs 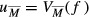 and
and 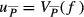 — gained by the MI and PI genes respectively — are unimodal with
— gained by the MI and PI genes respectively — are unimodal with 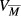 and
and 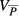 reaching their maxima at
reaching their maxima at 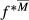 and
and 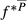 respectively (Fig. A2-ii). For simplicity, we assume that the MI gene is favoured for greater fertility than the PI gene, i.e.
respectively (Fig. A2-ii). For simplicity, we assume that the MI gene is favoured for greater fertility than the PI gene, i.e. 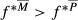 (Fig. A2-ii; symmetrical results can be derived when the direction of the conflict is reversed, i.e.
(Fig. A2-ii; symmetrical results can be derived when the direction of the conflict is reversed, i.e. 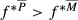 .
.
Consequently, the payoffs functions for the MI and PI genes are functions of the total level of expression, i.e. 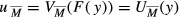 and
and 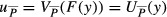 respectively. Let
respectively. Let 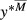 and
and 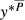 be the total levels of expression that maximise fertility for the MI and PI copies, respectively, i.e.
be the total levels of expression that maximise fertility for the MI and PI copies, respectively, i.e. 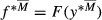 and
and 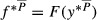 . We assume that the total level of expression that maximises fertility for the PI copy corresponds to the fertility maximum, i.e.
. We assume that the total level of expression that maximises fertility for the PI copy corresponds to the fertility maximum, i.e. 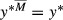 (Fig. A2-ii-a). From the assumptions that the locus is a fertility maximiser and
(Fig. A2-ii-a). From the assumptions that the locus is a fertility maximiser and 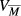 and
and 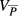 are unimodal, it follows that
are unimodal, it follows that 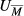 is unimodal with its maximum at
is unimodal with its maximum at 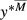 but
but 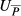 is bimodal, with two maxima at
is bimodal, with two maxima at 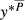 and
and 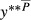 . From the assumptions that the locus is a fertility maximiser and
. From the assumptions that the locus is a fertility maximiser and 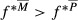 , it follows that
, it follows that 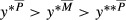 (Fig. A2).
(Fig. A2).
In particular, we use the following Gaussian functions that satisfy the above assumptions:
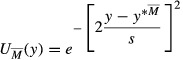 |
(3a) |
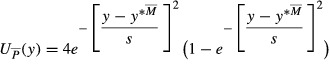 |
(3b) |
Adaptive dynamics
We assume that level of gene expression at any time is a random variable with a uniform probability distribution over a range of values defined by their lower and upper bounds. In particular, the window of expression is 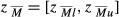 for the MI gene and
for the MI gene and 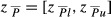 for the PI gene, where z ∈ [x,y]. When lower and upper bounds coincide (zl ≈ zu), the genes exhibit the same level of expression in each menstrual cycle. When lower and upper bounds differ (zl ≠ zu), the gene may exhibit a different level of expression from one menstrual cycle to the next.
for the PI gene, where z ∈ [x,y]. When lower and upper bounds coincide (zl ≈ zu), the genes exhibit the same level of expression in each menstrual cycle. When lower and upper bounds differ (zl ≠ zu), the gene may exhibit a different level of expression from one menstrual cycle to the next.
Because the levels of expression of the MI and PI genes may fluctuate stochastically, the payoff of the MI and PI genes are given by the expectation taken over all possible pairs of levels of expression, that is,
| (4a) |
| (4b) |
The adaptive dynamics of the window of expression of a gene is given by the dynamic system:
| (5) |
We assume that there is a lower bound at 0 in our evolutionary dynamics, since gene-expression levels cannot be negative. In practice, this is done by fixing negative time-derivatives to 0 as required. Given a set of initial conditions, we use equation (5) to generate Fig. 3.
Figure 3.
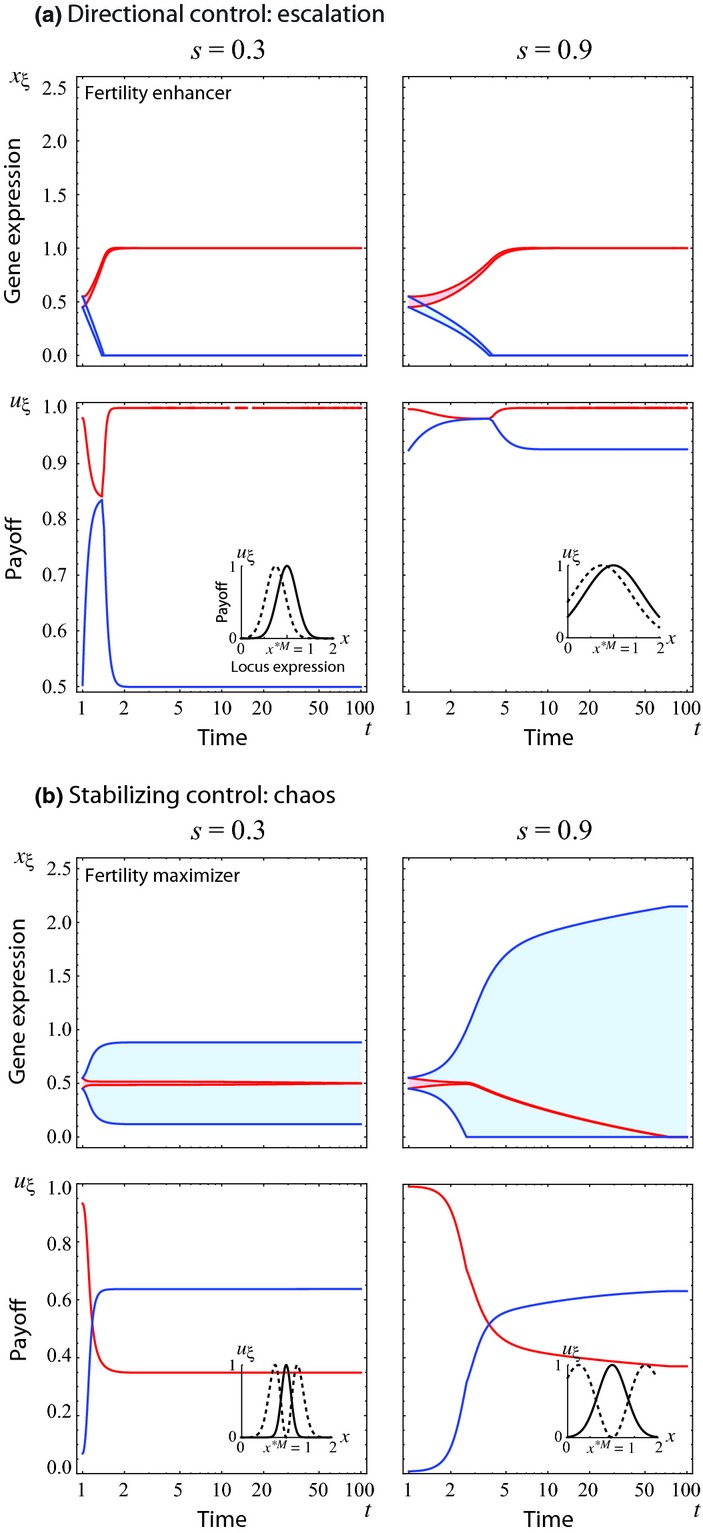
Adaptive dynamics of gene expression. We consider the cases when gene expression exerts: (a) directional control of fertility (in particular, a fertility inhibitor), with payoff functions characterised by x*M = 1.00, x*P = 0.75 and s = 0.3 or s = 0.9; and (b) stabilising control of fertility (fertility maximiser), with payoff functions characterised by x*M = 1.00 and s = 0.3 or s = 0.9. In each case, the first row represents the evolution of parent-of-origin-conditional gene expression over time. We consider that gene expression can take any value within a range (shadowed area) determined by its lower and upper bounds (continuous lines) with equal probability (uniform probability density). We assume that there are initially no differences between the MI and PI genes, and both are expressed within the range [0.45, 0.55] whose combined expression [0.90, 1.10] is approximately optimal for the MI gene, x*M = 1. The second row represents the evolution of parent-of-origin expected payoff over time. Insets illustrate the payoff functions.
Qualitatively similar results are obtained using an alternative modelling approach, namely formulating payoff matrices for each gene and finding the evolutionarily stable strategy (ESS) for each game (see Appendix).
Results
Battleground model
For consistency with the Grandmother Hypothesis, and for the purpose of concreteness and illustration, we will focus on female-biased dispersal as the driver of intragenomic conflict over menopause. However, the same results will be found for any ecology resulting in females being more related to her neighbours through their PI genes (namely, male-biased adult mortality and/or variance in reproductive success), and symmetrical results for any ecology resulting in females being more related to their neighbours through their MI genes (namely, female-biased adult mortality and/or variance in reproductive success).
Consistent with the Grandmother Hypothesis (Cant & Johnstone 2008), we find that female-biased dispersal (df > dm) with monogamy (α ≈ β) leads to women having a lower potential for sterility early in reproductive life, which then monotonically increases with age (i.e. St+1 > St for all t ; Fig. 2). Moreover, this qualitative pattern also holds both for MI and PI genes (i.e.  and
and  for all t; Fig. 2). However, the potential for sterility is always higher for a woman's PI genes and lower for her MI genes (i.e.
for all t; Fig. 2). However, the potential for sterility is always higher for a woman's PI genes and lower for her MI genes (i.e.  for all t; Fig. 2). Consequently, the menopause threshold will be crossed at different ages for these two sets of genes (i.e.
for all t; Fig. 2). Consequently, the menopause threshold will be crossed at different ages for these two sets of genes (i.e. 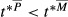 such that
such that 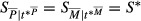 , resulting in intragenomic conflict over fertility during the intervening ages (Fig. 2). That is, for
, resulting in intragenomic conflict over fertility during the intervening ages (Fig. 2). That is, for 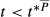 , both sets of genes are favoured to maintain fertility (no conflict); for
, both sets of genes are favoured to maintain fertility (no conflict); for 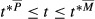 , the PI genes are favoured to reduce fertility while MI genes are favoured to increase fertility (conflict); and for
, the PI genes are favoured to reduce fertility while MI genes are favoured to increase fertility (conflict); and for 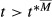 , both sets of genes are favoured to suppress fertility (no conflict; Fig. 2).
, both sets of genes are favoured to suppress fertility (no conflict; Fig. 2).
Our model predicts that there is conflict between PI and MI genes during the approach to menopause (peri-menopause) (Fig. 2). We suggest that peri-menopause initiates when the PI genes cross their menopause threshold (i.e. at age 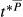 ) and terminates in actual menopause by the time that the MI genes cross their menopause threshold (i.e. at age
) and terminates in actual menopause by the time that the MI genes cross their menopause threshold (i.e. at age 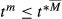 ). Hence, intragenomic conflict could play an important role in explaining the unpleasant symptoms observed during peri-menopause.
). Hence, intragenomic conflict could play an important role in explaining the unpleasant symptoms observed during peri-menopause.
Finally, our model predicts that greater female-biased dispersal (df′ > df > dm) leads to a shorter window of conflict preceding menopause 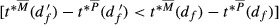 , and a smaller degree of conflict
, and a smaller degree of conflict 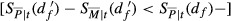
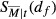 for
for 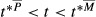 ; Fig. 2]. We therefore predict that, in populations with greater female-biased dispersal, women will experience shorter and less symptomatic peri-menopause. Our model also predicts that in populations with greater female-biased dispersal women experience menopause later in life (Fig. 2).
; Fig. 2]. We therefore predict that, in populations with greater female-biased dispersal, women will experience shorter and less symptomatic peri-menopause. Our model also predicts that in populations with greater female-biased dispersal women experience menopause later in life (Fig. 2).
Resolution models
Directional control and escalation
When the locus is a fertility enhancer, our battleground model predicts that natural selection will favour a greater level of expression when the gene is MI (e.g. increasing fertility by preserving ovarian follicles) and a lower level of expression when the gene is PI (e.g. decreasing fertility by destroying ovarian follicles). Our resolution model shows that every increase in the level of expression of the MI gene is followed by a decrease in the level of expression of the PI gene (Fig. 3a). The end-point of this escalation is the self-imposed silencing of the PI gene and the expression of the MI gene at its optimal level (Fig. 4a). In contrast, when the locus is a fertility inhibitor, our battleground model predicts that natural selection will favour a greater level of expression when the gene is PI but a lower level of expression when the gene is MI. The outcome of this conflict is self-imposed silencing of the MI gene and expression of the PI gene at its optimal level. These results recover the ‘loudest voice prevails’ principle (Haig 1996).
Figure 4.
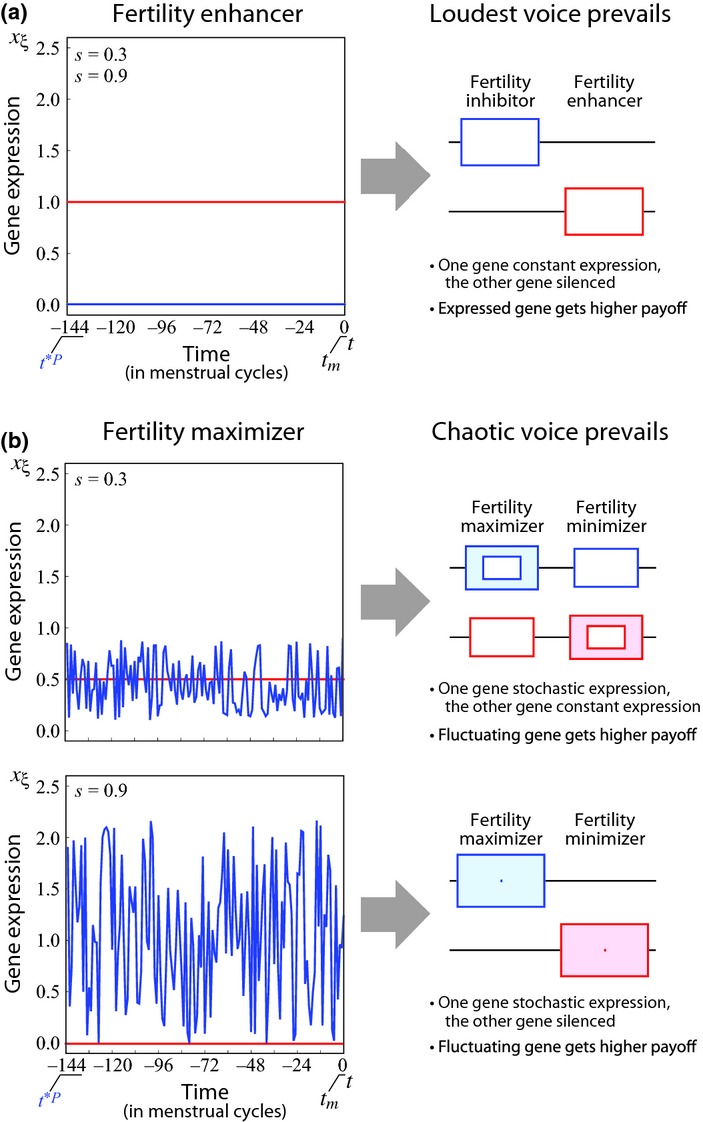
Resolution of conflict over menopause. We consider the cases when: (a) Gene expression exerts directional control of fertility (in particular a fertility inhibitor); (b) Gene expression exerts stabilising control of fertility (fertility maximiser). We illustrate the cases when s = 0.3 and s = 0.9 respectively. In each case, the first column presents the ESS level of expression for the MI (red) and PI (blue) genes during peri-menopause. The second column presents the schematic representation of the ESS gene expression for each copy and each type of gene.
Thus, our models predict that genes mediating fertility in a directional mode (i.e. fertility enhancers and fertility inhibitors) are likely to exhibit parent-of-origin differential expression with silencing of one of the genes (classic genomic imprinting). Moreover, our model predicts the direction of this asymmetry in expression: paternally silenced and maternally expressed if the gene product increases fertility during peri-menopause and delays menopause (e.g. via decelerated follicle destruction), and the reverse if the gene product reduces fertility during peri-menopause and hastens menopause (e.g. via accelerated follicle destruction) (Fig. 4a). While our model provides a resolution for the intralocus conflict, interlocus conflict between maternally expressed fertility enhancers and paternally expressed fertility inhibitors persists.
Stabilising/destabilising control and chaos
When the locus is a fertility maximiser (in the case of genes controlling the length of menstrual cycles via oestrogen production; Fig. 1), our battleground model predicts that natural selection will favour changing the level of expression when the gene is PI (e.g. decreasing fertility by changing the length of the menstrual cycle) but maintaining the level of expression when the gene is MI (for example, maintaining fertility by maintaining the length of the menstrual cycle). Our resolution model shows that every increase in the stochasticity of expression of the PI gene is countered by a decrease in the stochasticity of expression of the MI gene (Fig. 3b). The end-point of this co-evolutionary process is stochastic expression of the PI gene within the range of values that attains the PI gene's desired fertility, and constant expression of the MI gene at a level equal to or lower than its original (Fig. 4b). Whether MI genes are ultimately silenced depends on whether the lower bound of the range of values that maximise the PI gene's fertility is sufficiently low (Fig. 4b s = 0.3) or not (Fig. 4b s = 0.9).
In contrast, when the locus is a fertility minimiser, natural selection will favour changing the level of expression when the gene is MI but maintaining the level of expression when the gene is PI. The outcome of this conflict is a constant expression of the PI gene at equal or lower level than its original one, and the stochastic expression of the MI gene within the range of values that attains the MI gene's desired fertility. Whether PI genes are ultimately silenced depends on whether the lower bound of the range of values that maximise the MI gene's fertility is positive. As an analogy to the loudest-voice-prevails principle, we term this the ‘chaotic-voice-prevails’ principle.
Thus, our models predict that genes mediating fertility in a stabilising/destabilising mode (i.e. fertility maximisers and fertility minimisers) are likely to exhibit parent-of-origin differential expression with stochastic expression of one copy (a novel form of genomic imprinting). In particular, our model predicts the direction of this asymmetry: constant expression when MI but stochastic expression when PI, if changes in the gene product decrease fertility during peri-menopause (e.g. via hormonal fluctuations that shorten or lengthen the interval between menstrual cycles); and constant expression when PI but stochastic expression when MI, if changes in the gene product promote fertility during peri-menopause (Fig. 4b).
Discussion
We have shown that the ancestral ecology responsible for driving the evolution of human menopause, according to the Grandmother Hypothesis, may also drive intragenomic conflict between a woman's MI and PI genes over her fertility during peri-menopause and the timing of her menopause. We suggest that a woman's menopause is characterised by the struggle between her PI genes, which are favoured to end her reproductive capacity, and her MI genes, which are favoured to maintain her reproductive capacity. Under this view, several of the unpleasant symptoms associated with peri-menopause may be explained by intragenomic conflict arising as a consequence of human ecology.
As a result of this intragenomic conflict, we predict that genes underpinning peri-menopause will show different patterns of expression when MI and PI. Specifically, we predict that genes mediating fertility during the menopausal transition will exhibit two patterns of expression: (1) silencing of one copy and expression of the other, when determining fertility in a directional manner (resulting from the loudest-voice-prevails principle); and (2) stable expression of one copy and random expression of the other, when determining fertility in a stabilising manner (resulting from the chaotic-voice-prevails principle).
If ancestral human populations exhibited female-biased dispersal, then we predict that those genes whose greater expression promotes fertility (fertility enhancers) during peri-menopause will be paternally silenced and maternally expressed, whereas those genes whose greater expression reduces fertility (fertility inhibitors) will be maternally silenced and paternally expressed. This might be the case for genes mediating the stock of ovarian follicles and the rate of atresia at the onset of peri-menopause, when follicle destruction accelerates (Cant & Johnstone 2008). These predictions provide a first evolutionary explanation for why autosomal genes GNAS1, DLX5 and WT1-AS controlling ovarian follicle stock as fertility enhancers appear to be maternally expressed and paternally silenced in agreement with our model predictions (see Table 2 and references therein). Recently, the maternally expressed gene KCNQ1 (Wilkins & Úbeda 2011) has been associated with age of menopause (Spencer et al. 2013), although its specific role remains unknown. In addition, conflict between fertility-enhancing and fertility-inhibiting loci can explain the overexpression of antagonistic genes that mediate fertility. This could provide an explanation for those menopausal symptoms that appear to work at cross-purposes.
Table 2.
Imprinted genes linked to fertility and menopause
| Gene | Imprint status-Fertility-Imprint prediction | References |
|---|---|---|
| GNAS1 | Direct evidence shows that gene GNAS1 is imprinted in some human tissues, in particular maternally expressed and paternally silenced in the pituitary gland and ovaries. | (Hayward et al. 1998; Mantovani et al. 2002; Wilkins & Úbeda 2011) |
| A loss-of-function mutation of GNAS1 results in follicle-stimulating hormone (FSH) resistance (among other symptoms) when the gene is MI but not when it is PI. FSH resistance results in reduced ovarian follicle stock and premature ovarian failure. Thus a loss-of-function mutation of GNAS1 results in premature ovarian failure when MI but not when PI. Greater expression of GNAS1 contributes to greater fertility thus acting as a fertility enhancer. | (Nakamoto et al. 1998; Weinstein et al. 2001; Persani et al. 2010) | |
| Our model predicts that GNAS1 will be paternally silenced in tissues affecting fertility, prediction supported by the evidence of imprinting in GNAS1. | ||
| DLX5 | Direct evidence shows that DLX5 is imprinted in humans; maternally expressed and paternally silenced. | (Horike et al. 2005; Miyano et al. 2008) |
| Mutant mice that are monoallelic for DLX5 experience reduced fertility and early follicular exhaustion that results in premature ovarian failure. Given that expression of DLX5 contributes to greater fertility, this locus can be classified as a fertility enhancer. | (Bouhali et al. 2011) | |
| Our model predicts that DLX5 will be paternally silenced, prediction supported by the evidence of imprinting in DLX5. | ||
| WT1-AS | The antisense transcript of gene WT1 (WT1-AS) is imprinted in humans, in particular paternally expressed and maternally silenced. The antisense transcript antagonises the sense transcript of the same parental origin. | (Dallosso et al. 2004) |
| WT1 knockout mice develop without the establishment of an ovarian reserve and are non-viable. Given that expression of WT1 contributes to establishing an ovarian reserve, this locus can be classified as a fertility enhancer and its antisense as a fertility inhibitor. | (Wilhelm & Englert 2002; Monget et al. 2012) | |
| Our model predicts that WT1-AS will be maternally silenced, prediction supported by the evidence of imprinting in WT1-AS. |
Summary of the evidence linking imprinted genes to menopause.
Moreover, we predict that those genes whose lower or greater expression reduces fertility (fertility maximisers) will exhibit random fluctuations in expression when PI but constant expression when MI. This may be the case for genes that mediate the production of oestrogen during the menstrual cycle. Hormonal deregulation results in shorter or longer menstrual cycles at the onset of peri-menopasue, ensuing the increased volatility of the cycle length (Fig. 1) (Santoro et al. 1996; Prior 1998, 2006; Douma et al. 2005). This prediction provides a first evolutionary explanation for the erratic endocrinology observed during peri-menopause. This hormonal fluctuation is responsible for many of the unpleasant symptoms experienced during peri-menopause (Avis et al. 2001; Gold et al. 2001; Prior 2006). We suggest this novel form of gene expression may also illuminate other turbulent phases of human life history in which the allocation of resources from the individual to her relatives, and vice versa, undergoes a major shift.
We predict that women whose ancestors evolved in populations with lower female bias in dispersal will experience greater conflict over fertility during peri-menopause (manifesting as more severe symptoms) and earlier menopause than women whose ancestors evolved in populations with higher female bias in dispersal (Fig. 2). Our model suggests that the observed variability between ethnic groups in peri-menopausal symptoms and age of menopause may be explained by ecological differences in their ancestral populations.
In particular, vaso-motor symptoms — hot flushes and night sweats — are the most common symptoms experienced by peri-menopausal women (Avis et al. 2001). They are caused by large fluctuations in oestrogen levels (Prior 1998). The incidence of vaso-motor symptoms is 2.5 times lower among Japanese Americans than among African Americans (after controlling for confounding factors like body mass index, smoking habits, among others) (Gold et al. 2000; Avis et al. 2001), lending support to the idea that Japanese Americans experience less severe fluctuations in oestrogen levels than do African Americans.
Premature ovarian failure refers to menopause before the age of 40 and affects 5–10% of menopausal women (Toniolo 2006). It is caused mainly by early depletion of the ovarian follicle stock due to low initial stock or accelerated atresia (Toniolo 2006; Persani et al. 2010). The prevalence of premature ovarian failure among Japanese Americans is ten times lower than between Hispanic and African Americans (Luborsky et al. 2003), lending support to the idea that Japanese Americans experience later menopause than Hispanic and African Americans. Empirical evidence does show that Japanese Americans experience later menopause (median 51.8 years) than do other ethnic groups (Gold et al. 2001; Henderson et al. 2008). The median age of menopause for Caucasian Americans, after correcting for other factors, ranges between 51.2 and 51.4 years (Bromberger et al. 1997; Gold et al. 2001); for Hispanic Americans it is 51.0 years; and for African Americans ranges between 49.3 and 51.4 (Bromberger et al. 1997; Henderson et al. 2008).
If the female ancestors of modern Japanese Americans (i.e. located in East Asia) followed their sexual partners after mating more often than the female ancestors of modern African Americans, the predictions of our model would be consistent with the evidence for: (1) Japanese Americans experiencing less severe VMS (and the underlying hormonal fluctuations) than African Americans; and (2) Japanese Americans experiencing lower incidence of premature ovarian failure and later menopause than African Americans. However, information about the ancestral ecologies of these populations is lacking. We suggest that a proper comparative analysis of patrilocality among the ancestral populations of Japanese and African Americans is a useful avenue for future research, noting that this will be complicated by the extremely diverse ancestry of African Americans. A link between ancestral ecology and age of menopause would have direct implications for the development of personalised family planning based on a woman's ethnic background.
Finally, our model suggests that genomic imprinting may play an important role in mediating the symptoms of peri-menopause and the age of menopause, and this provides opportunities for testing the model. Under our hypothesis, peri-menopausal symptoms and age at menopause would be susceptible to epigenetic modifications. In particular, we predict that experimental silencing of paternally expressed genes will alleviate peri-menopausal symptoms and delay menopause, while silencing of maternally expressed genes will hasten menopause. This is important because the timing of menopause has implications not only for a woman's fertility but also for her health in general. A delay in the onset of menopause reduces the risk of suffering osteoporosis and cardiovascular diseases, but increases the risk of suffering breast, endometrial and ovarian cancers (Hartge 2009). Therefore, overexpression of paternally expressed genes will reduce the risk of cancer while overexpression of maternally expressed genes will increase the risk of cancer. Thus, confirmation of these predictions would have direct implications for fertility treatment and indirect implications for the management of cancers and cardiovascular diseases.
For the purpose of illustration and synthesis with the existing literature on the Grandmother Hypothesis, we have focused upon female-biased dispersal as the driver of intragenomic conflict over menopause. Evidence for female-biased dispersal in ancestral human populations comes from three sources: our closest non-human relatives, contemporary hunter gatherers and genetics [reviewed by Cant & Johnstone (Cant & Johnstone 2008)]. However, some researchers favour an unbiased-dispersal interpretation [reviewed by Cant & Johnstone (2008)]. In the event that dispersal was unbiased, other sex-specific demographies could have fuelled the intragenomic conflict, by making a female more related to her neighbours via one of her parents than the other. Specifically, our qualitative results are recovered under male-biased mortality and/or variance in reproductive success, both of which are ubiquitous in humans.
Acknowledgments
We thank Emma de Torres for an original source of intragenomic conflict, Chris Boake, Berni Crespi, David Haig, Manus Patten and two anonymous referees for comments, the Royal Society (AG) and JSPS KAKENHI Grant Numbers 23870009 and 25118006 (HO) for funding.
Authorship
FU and AG designed the study and wrote the manuscript, FU, HO and AG performed the research and provided new methods.
Supporting Information
Additional Supporting Information may be downloaded via the online version of this article at Wiley Online Library (http://www.ecologyletters.com).
References
- Alexander RD. The evolution of social behaviour. Annu. Rev. Ecol. Syst. 1974;5:325. [Google Scholar]
- Austad SN. Comparative aging and life histories in mammals. Exp. Gerontol. 1997;32:23. doi: 10.1016/s0531-5565(96)00059-9. [DOI] [PubMed] [Google Scholar]
- Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc. Sci. Med. 2001;52:345. doi: 10.1016/s0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- Bouhali K, Dipietromaria A, Fontaine A, Caburet S, Barbieri O, Bellessort B, et al. Allelic reduction of Dlx5 and Dlx6 results in early follicular depletion: a new mouse model of primary ovarian insufficiency. Hum. Mol. Genet. 2011;20:2642. doi: 10.1093/hmg/ddr166. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P, et al. Prospective study of the determinants of age at menopause. Am. J. Epidemiol. 1997;145:124. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- Butts SF, Seifer DB. Racial and ethnic differences in reproductive potential across the life cycle. Fertil. Steril. 2010;93:681. doi: 10.1016/j.fertnstert.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Cant MA, Johnstone RA. Reproductive conflict and the separation of reproductive generations in humans. Proc. Natl Acad. Sci. USA. 2008;105:5332. doi: 10.1073/pnas.0711911105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallosso AR, Hancock AL, Brown KW, Williams AC, Jackson S, Malik K, et al. Genomic imprinting at the WT1 gene involves a novel coding transcript (AWT1) that shows deregulation in Wilms' tumours. Hum. Mol. Genet. 2004;13:405. doi: 10.1093/hmg/ddh038. [DOI] [PubMed] [Google Scholar]
- Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders - Reproductive life cycle factors. Adv. Nurs. Sci. 2005;28:364. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in midlife - implications for forecasting menopause. Hum. Reprod. 1992;7:1342. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Ferrell RJ, O'Connor KA, Rodriguez G, Gorrindo T, Holman DJ, Brindle E, et al. Monitoring reproductive aging in a 5-year prospective study: aggregate and individual changes in steroid hormones and menstrual cycle lengths with age. Menopause. 2005;12:567. doi: 10.1097/01.gme.0000172265.40196.86. [DOI] [PubMed] [Google Scholar]
- Gold EB, Sternfeld B, Kelsey J, L.Brown C, Mouton C, Reame N, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am. J. Epidemiol. 2000;152:463. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am. J. Epidemiol. 2001;153:865. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Telfer E. Numbers of follicles and oocytes in mammalian ovaries and their allometric relationships. J. Zool. 1987;211:169. [Google Scholar]
- Haig D. Placental hormones, genomic imprinting, and maternal-fetal communication. J. Evol. Biol. 1996;9:357. [Google Scholar]
- Hartge P. Genetics of reproductive lifespan. Nat. Genet. 2009;41:637. doi: 10.1038/ng0609-637. [DOI] [PubMed] [Google Scholar]
- Hawkes K, O'Connell JF, Jones NGB, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA. 1998;95:1336. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward BE, Kamiya M, Strain L, Moran V, Campbell R, Hayashizaki Y, et al. The human GNAS1 gene is imprinted and encodes distinct paternally and biallelically expressed G proteins. Proc. Natl Acad. Sci. USA. 1998;95:10038. doi: 10.1073/pnas.95.17.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the multiethnic cohort study. Am. J. Epidemiol. 2008;167:1287. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- Horike S, Cai ST, Miyano M, Cheng JF, Kohwi-Shigematsu T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat. Genet. 2005;37:31. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- Johnstone RA, Cant MA. The evolution of menopause in cetaceans and humans: the role of demography. Proc. Biol. Sci. 2010;277:3765. doi: 10.1098/rspb.2010.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EC, Krohn PL. Relationships between age, numbers of oocytes and fertility in virgin and multiparous mice. J. Endocrinol. 1961;21:469. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- Jones NGB, Hawkes K, O'Connell JF. Antiquity of postreproductive life: are there modern impacts on hunter-gatherer postreproductive life spans? Am. J. Hum. Biol. 2002;14:184. doi: 10.1002/ajhb.10038. [DOI] [PubMed] [Google Scholar]
- Jones KP, Walker LC, Anderson D, Lacreuse A, Robson SL, Hawkes K. Depletion of ovarian follicles with age in chimpanzees: similarities to humans. Biol. Reprod. 2007;77:247. doi: 10.1095/biolreprod.106.059634. [DOI] [PubMed] [Google Scholar]
- Lahdenpera M, Lummaa V, Helle S, Tremblay M, Russell AF. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428:178. doi: 10.1038/nature02367. [DOI] [PubMed] [Google Scholar]
- Lahdenpera M, Gillespie DO, Lummaa V, Russell AF. Severe intergenerational reproductive conflict and the evolution of menopause. Ecol. Lett. 2012;15:1283–1290. doi: 10.1111/j.1461-0248.2012.01851.x. [DOI] [PubMed] [Google Scholar]
- Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94:89. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum. Reprod. 2003;18:199. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A. The Gs alpha gene: predominant maternal origin of transcription in human thyroid gland and gonads. J. Clin. Endocrinol. Metab. 2002;87:4736. doi: 10.1210/jc.2002-020183. [DOI] [PubMed] [Google Scholar]
- Miyano M, Horike SI, Cai S, Oshimura M, Kohwi-Shigematsu T. DLX5 expression is monoallelic and Dlx5 is up-regulated in the Mecp2-null frontal cortex. J. Cell Mol. Med. 2008;12:1188. doi: 10.1111/j.1582-4934.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monget P, Bobe J, Gougeon A, Fabre S, Monniaux D, Dalbies-Tran R. The ovarian reserve in mammals: a functional and evolutionary perspective. Mol. Cell. Endocrinol. 2012;356:2. doi: 10.1016/j.mce.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Nakamoto JM, Sandstrom AT, Brickman AS, Christenson RA, Dop Van C. Pseudohypoparathyroidism type Ia from maternal but not paternal transmission of a G(s)alpha gene mutation. Am. J. Med. Genet. 1998;77:261. [PubMed] [Google Scholar]
- Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum. Reprod. 2005;20:79. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure (vol 45, pg 257, 2010) J. Mol. Endocrinol. 2010;45:405. doi: 10.1677/JME-10-0070. [DOI] [PubMed] [Google Scholar]
- Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr. Rev. 1998;19:397. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- Prior JC. Perimenopause lost - reframing the end of menstruation. J. Reprod. Infant Psychol. 2006;24:323. [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition - Evidence for accelerated loss and ultimate exhaustion. J. Clin. Endocrinol. Metab. 1987;65:1231. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J. Clin. Endocrinol. Metab. 1996;81:1495. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- Small CM, Manatunga AK, Klein M, Feigelson HS, Dominguez CE, McChesney R, et al. Menstrual cycle characteristics - Associations with fertility and spontaneous abortion. Epidemiology. 2006;17:52. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- Spencer KL, Malinowski J, Carty CL, Franceschini N, Fernandez-Rhodes L, Young A, et al. Genetic variation and reproductive timing: African American women from the Population Architecture Using Genomics and Epidemiology (PAGE) Study. PLoS ONE. 2013;8:e55258. doi: 10.1371/journal.pone.0055258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo D. X-linked premature ovarian failure: a complex disease. Curr. Opin. Genet. Dev. 2006;16:293. doi: 10.1016/j.gde.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Tully T, Lambert A. The evolution of postreproductive life span as an insurance against indeterminacy. Evolution. 2011;65:3013. doi: 10.1111/j.1558-5646.2011.01347.x. [DOI] [PubMed] [Google Scholar]
- Úbeda F, Gardner A. A model for genomic imprinting in the social brain: elders. Evolution. 2012;66:1567. doi: 10.1111/j.1558-5646.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. The ecology and evolutionary endocrinology of reproduction in the human female. In: Sussman RW, editor. Yearbook of Physical Anthropology. Vol. 52. New York: Wiley-Liss, Inc; 2009. pp. 95–136. [DOI] [PubMed] [Google Scholar]
- Voorhuis M, Onland-Moret NC, Schouw van der YT, Fauser B, Broekmans FJ. Human studies on genetics of the age at natural menopause: a systematic review. Hum. Reprod. Update. 2010;16:364. doi: 10.1093/humupd/dmp055. [DOI] [PubMed] [Google Scholar]
- Wallace WHB, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS ONE. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein LS, Yu SH, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr. Rev. 2001;22:675. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16:1839. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JF, Úbeda F. Diseases associated with genomic imprinting. In: Cheng XD, Blumenthal RM, editors. Modifications of Nuclear. Vol. 101. San Diego: Elsevier Academic Press Inc; 2011. pp. 401–445. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


