Abstract
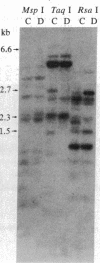
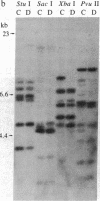
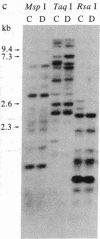
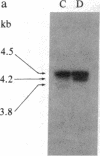
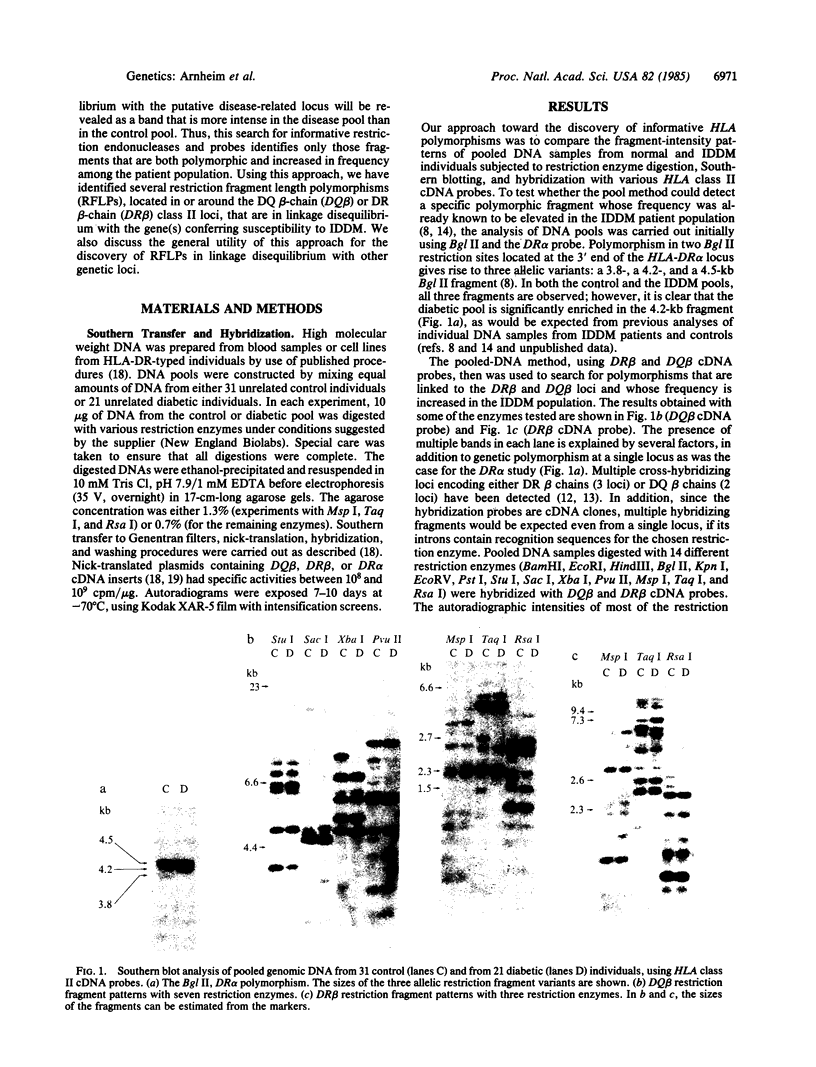
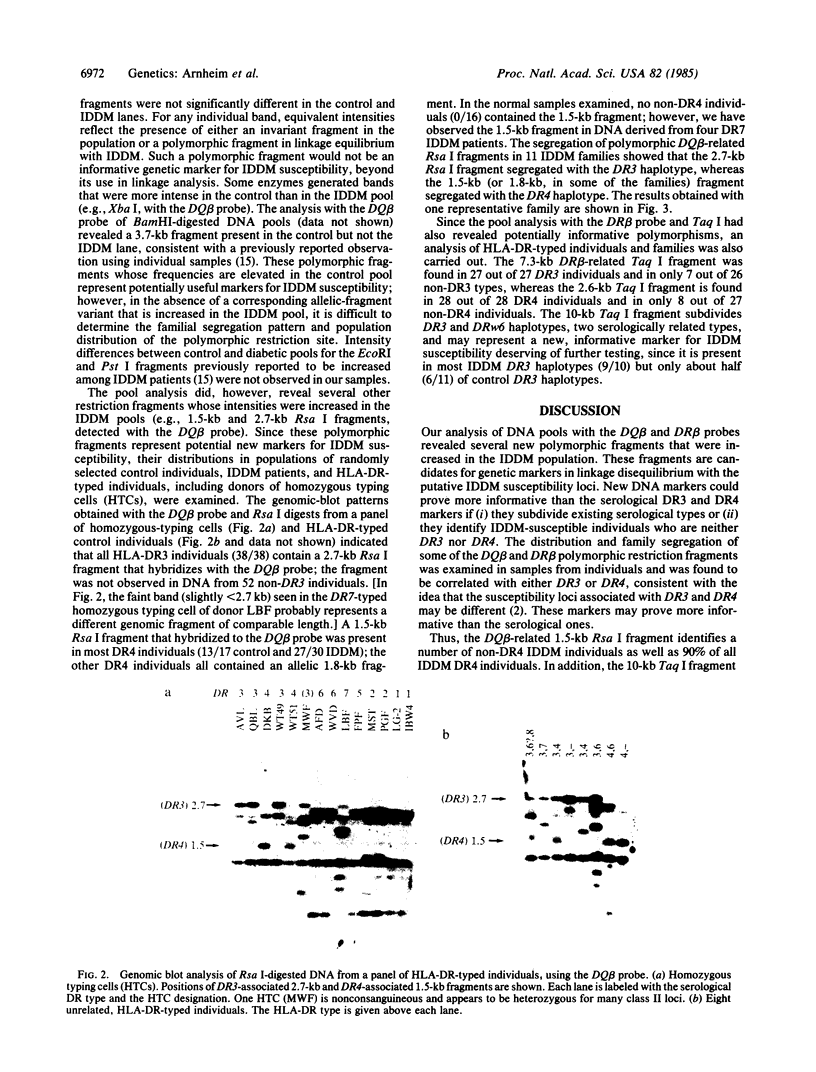
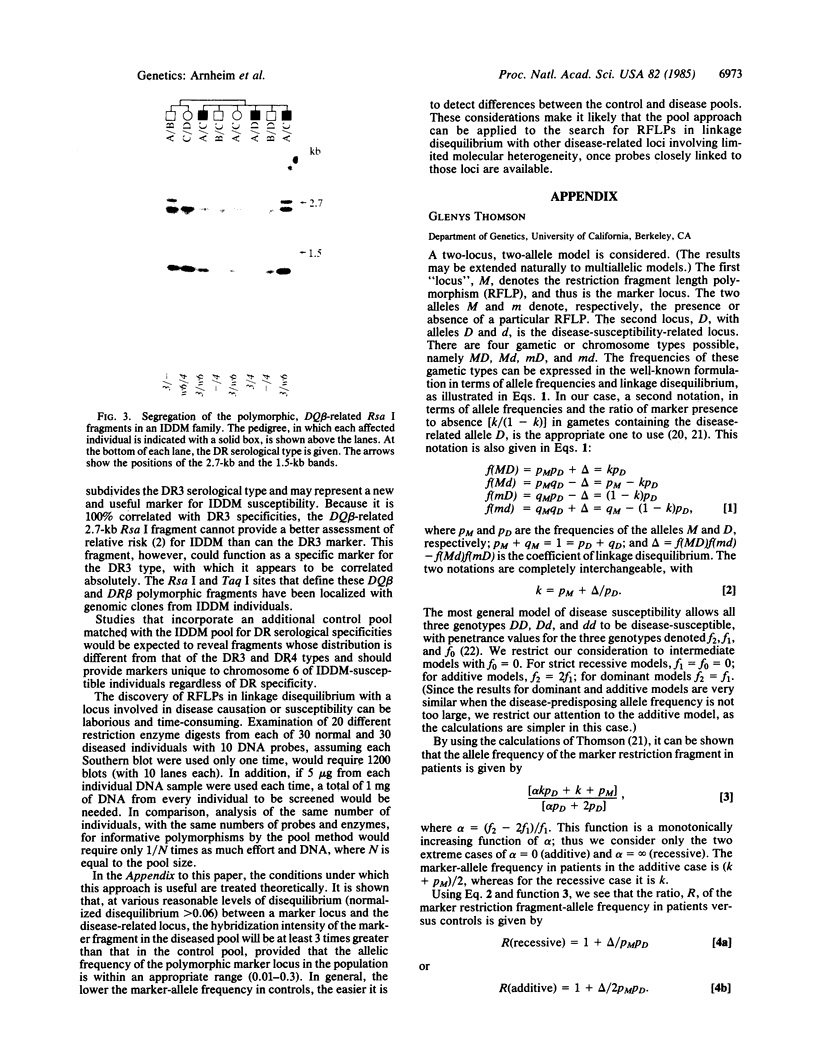
A rapid method has been developed and used to search for restriction fragment length polymorphisms (RFLPs) that are in linkage disequilibrium with disease-associated loci. By using genomic blot-hybridization analysis with DQ beta-chain and DR beta-chain cDNA probes, we examined DNA polymorphisms within the HLA class II loci associated with susceptibility to insulin-dependent mellitus (IDDM). To facilitate the search for informative RFLPs, we compared pooled DNA samples from IDDM patients with pooled DNA samples from randomly selected control individuals, instead of using the conventional approach of examining DNA samples from individuals in two groups. (The conditions under which this approach is useful are treated theoretically in the Appendix.) Several specific polymorphic restriction fragments associated with IDDM were revealed by using this economical and rapid approach. The restriction enzymes and probes identified as informative in this screening were then used to analyze HLA-DR-typed IDDM families, homozygous typing cells, and unrelated individuals to determine the association of the specific restriction fragments with HLA-DR serological type and the frequency in control and IDDM populations. Some individual polymorphic fragments for which the IDDM population was enriched correlated strongly with HLA-DR3, whereas others correlated strongly with HLA-DR4. Some fragments (e.g., a 10-kilobase Taq I fragment detected with the DR beta probe) that were more prevalent in the IDDM population subdivided the serologically defined HLA-DR type and may be informative markers for IDDM susceptibility.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhme J., Owerbach D., Denaro M., Lernmark A., Peterson P. A., Rask L. Human class II major histocompatibility antigen beta-chains are derived from at least three loci. Nature. 1983 Jan 6;301(5895):82–84. doi: 10.1038/301082a0. [DOI] [PubMed] [Google Scholar]
- Cohen D., Cohen O., Marcadet A., Massart C., Lathrop M., Deschamps I., Hors J., Schuller E., Dausset J. Class II HLA-DC beta-chain DNA restriction fragments differentiate among HLA-DR2 individuals in insulin-dependent diabetes and multiple sclerosis. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1774–1778. doi: 10.1073/pnas.81.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniach D., Bottazzo G. F., Cudworth A. G. Etiology of Type I diabetes mellitus: heterogeneity and immunological events leading to clinical onset. Annu Rev Med. 1983;34:13–20. doi: 10.1146/annurev.me.34.020183.000305. [DOI] [PubMed] [Google Scholar]
- Erlich H., Stetler D., Sheng-Dong R., Saiki R. Analysis by molecular cloning of the human class II genes. Fed Proc. 1984 Dec;43(15):3025–3030. [PubMed] [Google Scholar]
- Gorski J., Rollini P., Long E., Mach B. Molecular organization of the HLA-SB region of the human major histocompatibility complex and evidence for two SB beta-chain genes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3934–3938. doi: 10.1073/pnas.81.13.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner J. P., Watson A. J., Bach F. H. Dw/LD-related molecular polymorphism of DR4 beta-chains. J Exp Med. 1983 May 1;157(5):1687–1691. doi: 10.1084/jem.157.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Bodmer W. F. cDNA clones coding for the heavy chain of human HLA-DR antigen. Proc Natl Acad Sci U S A. 1982 Jan;79(2):545–549. doi: 10.1073/pnas.79.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin R C. The Interaction of Selection and Linkage. I. General Considerations; Heterotic Models. Genetics. 1964 Jan;49(1):49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Gorski J., Mach B. Complete sequence of an HLA-dR beta chain deduced from a cDNA clone and identification of multiple non-allelic DR beta chain genes. EMBO J. 1983;2(3):389–394. doi: 10.1002/j.1460-2075.1983.tb01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom B. S., Nepom G. T., Mickelson E., Antonelli P., Hansen J. A. Electrophoretic analysis of human HLA-DR antigens from HLA-DR4 homozygous cell lines: correlation between beta-chain diversity and HLA-D. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6962–6966. doi: 10.1073/pnas.80.22.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owerbach D., Lernmark A., Platz P., Ryder L. P., Rask L., Peterson P. A., Ludvigsson J. HLA-D region beta-chain DNA endonuclease fragments differ between HLA-DR identical healthy and insulin-dependent diabetic individuals. Nature. 1983 Jun 30;303(5920):815–817. doi: 10.1038/303815a0. [DOI] [PubMed] [Google Scholar]
- Platz P., Jakobsen B. K., Morling N., Ryder L. P., Svejgaard A., Thomsen M., Christy M., Kromann H., Benn J., Nerup J. HLA-D and -DR antigens in genetic analysis of insulin dependent diabetes mellitus. Diabetologia. 1981 Aug;21(2):108–115. doi: 10.1007/BF00251276. [DOI] [PubMed] [Google Scholar]
- Rotter J. I., Landaw E. M. Measuring the genetic contribution of a single locus to a multilocus disease. Clin Genet. 1984 Dec;26(6):529–542. doi: 10.1111/j.1399-0004.1984.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Ryder L. P., Svejgaard A., Dausset J. Genetics of HLA disease association. Annu Rev Genet. 1981;15:169–187. doi: 10.1146/annurev.ge.15.120181.001125. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spielman R. S., Lee J., Bodmer W. F., Bodmer J. G., Trowsdale J. Six HLA-D region alpha-chain genes on human chromosome 6: polymorphisms and associations of DC alpha-related sequences with DR types. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3461–3465. doi: 10.1073/pnas.81.11.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez B. K. The affected sib pair IBD distribution for HLA-linked disease susceptibility genes. Tissue Antigens. 1978 Aug;12(2):87–93. doi: 10.1111/j.1399-0039.1978.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Thomson G. A review of theoretical aspects of HLA and disease associations. Theor Popul Biol. 1981 Oct;20(2):168–208. doi: 10.1016/0040-5809(81)90009-5. [DOI] [PubMed] [Google Scholar]
- Thomson G. Investigation of the mode of inheritance of the HLA associated diseases by the method of antigen genotype frequencies among diseased individuals. Tissue Antigens. 1983 Feb;21(2):81–104. doi: 10.1111/j.1399-0039.1983.tb00377.x. [DOI] [PubMed] [Google Scholar]