Abstract
Cytochrome P450 CYP17A1 catalyzes a series of reactions that lie at the intersection of corticoid and androgen biosynthesis and thus occupies an essential role in steroid hormone metabolism. This multifunctional enzyme catalyzes the 17α-hydroxylation of Δ4- and Δ5-steroids progesterone and pregnenolone to form the corresponding 17α-hydroxy products through its hydroxylase activity, and a subsequent 17,20 carbon-carbon scission of pregnene-side chain produce the androgens androstenedione (AD) and dehydroepiandrosterone (DHEA). While the former hydroxylation reaction is believed to proceed through a conventional “Compound I” rebound mechanism, it has been suggested that the latter carbon cleavage is initiated by an iron-peroxy intermediate. We report on the role of Thr306 in CYP17 catalysis. Thr306 is a member of the conserved acid/alcohol pair thought to be essential for the efficient delivery of protons required for hydroperoxoanion heterolysis and formation of Compound I in the cytochromes P450. Wild type and T306A CYP17A1 self-assembled in Nanodiscs were used to quantitate turnover and coupling efficiencies of CYP17's physiological Δ4- and Δ5-substrates. We observed that T306A co-incorporated in Nanodiscs with its redox partner cytochrome P450 oxidoreductase, coupled NADPH only by 0.9% and 0.7% compared to the wild type (97% and 22%) during the conversion of pregnenolone and progesterone, respectively, to the corresponding 17∝-OH products. Despite increased oxidation of pyridine nucleotide, hydroxylase activity was drastically diminished in the T306A mutant, suggesting a high degree of uncoupling in which reducing equivalents and protons are funneled into non-productive pathways. This is similar to previous work with other P450 catalyzed hydroxylation. However, catalysis of carbon-carbon bond scission by the T306A mutant was largely unimpeded by disruption of the CYP17A1 acid-alcohol pair. The unique response of CYP17A1 lyase activity to mutation of Thr306 is consistent with a reactive intermediate formed independently of proton delivery in the active site, and supports involvement of a nucleophilic peroxo-anion rather than the traditional Compound I in catalysis.
Keywords: CYP17A1, T306A, proton delivery, Compound I, nucelophilic attack
Introduction
The human microsomal cytochrome P450, CYP17A1, is a key enzyme in steroid hormone biosynthesis, which is capable of both hydroxylase and carbon-carbon lyase activity in a series of chemical transfomations of pregnenolone that give rise to corticoid precursors and androgens. In human adrenal steroidogenesis, the hydroxylation process predominates in the adrenal zona fasciculata, where Δ5 (pregnenolone, PREG) and Δ4 (progesterone, PROG) steroid are converted into the 17α-hydroxy products OH-PREG and OH-PROG, respectively, by insertion of an oxygen into a C-H bond. In the adrenal zona reticularis, each of these hydroxylated products are converted to the estrogen and testosterone precursors dehydroepiandrosterone (DHEA) and androstenedione (AD) via 17,20-carbon-carbon bond scission in which the 21-carbon 17α-hydroxysteroids are cleaved to 19-carbon, 17-ketosteroids, and acetic acid [1]. This compartmentalization of CYP17A1 behavior in the adrenal gland is thought to be controlled by co-localization with its putative allosteric effector cytochrome b5 (cyt-b5) in the adrenal zona reticularis [2,3,4]. Interaction between CYP17A1 and cyt-b5 is known to substantially stimulate C-C lyase activity of this enzyme such that its presence can be considered essential for lyase chemistry. Furthermore, the absence of CYP17A1 in the adrenal zona glomerulosa helps to direct steroidogenesis in this region toward mineralocorticoid aldosterone production. As a result, varying expression levels of CYP17A1 and cyt-b5 throughout the adrenal gland controls an important branch point in human steroidogenesis between glucocorticoid and sex hormone biosynthesis. It has been shown that both PREG and PROG are good substrates for human CYP17A1 for the 17α-hydroxylase reaction, however, 17-OH-PREG is preferred over 17-OH-PROG for the 17,20-lyase reaction [5,6,7].
The C-C lyase reaction catalyzed by CYP17A1 is notable not only for the profound rate enhancement exerted by association with cyt-b5, but also in the chemistry performed. While the hydroxylase activity of CYP17A1 is expected to proceed through the “Compound I” initiated hydrogen abstraction observed in other members of the P450 superfamily, significant debate exists regarding the nature of the intermediate responsible for catalysis of carbon-carbon bond scission. The nucleophilic peroxoanion attacking the C-20 carbonyl of OH-PREG and OH-PROG with subsequent decomposition to form the androgen product was suggested [8,9,10]. On the other hand, the traditional mechanism catalyzed by Compound I (Cpd I) has also been proposed [11].
Decades of interrogation of the cytochromes P450, including the mechanistic exemplar for this family of enzymes, P450cam, has yielded a wealth of information regarding the key catalytic intermediates and active site environment necessary for the generation of the Cpd I high-valent porphyrin cation radical utilized in hydrogen abstraction and substrate hydroxylation, thought to operate in all P450s (Figure 1) [12,13,14]. Of a particular relevance to this study is the structural motif of the I-helix and its conserved residue Threonine (Thr), located in the distal side of the heme-plane [15]. Thr252 in P450cam plays an important role in proton transfer essential for O-O bond scission required for Cpd I formation [16,17,18], and dioxygen activation during catalytic turnover [19,20]. It has been shown that mutation of this Thr residue to Ala results in a dramatic inhibition of substrate hydroxylation catalyzed by several P450s such as CYP102A1 (P450BM3) [21], CYP2E1 [22,23], CYP2D6 [24] and CYP1A2 [25]. The critical role of this threonine in successful formation of Cpd I and productive catalysis in cytochromes P450 is now well established [18,26,27].
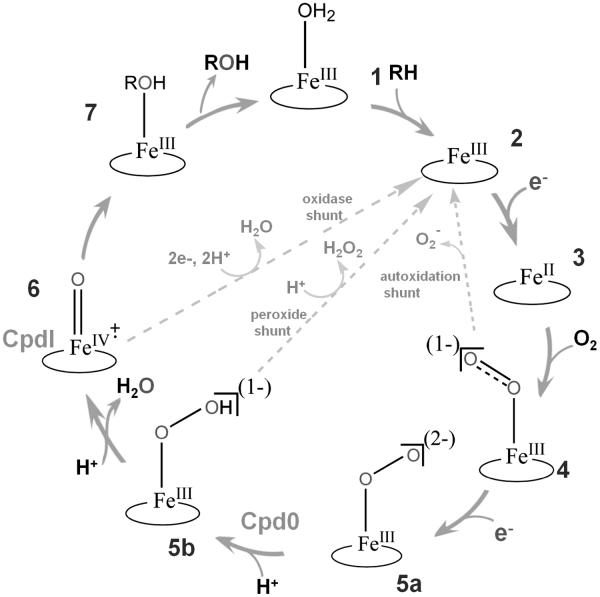
Figure 1. A reaction cycle of cytochrome P450 catalysis.
The seven steps indicating the binding of substrate (1), formation of a high spin ferric state (2), transfer of an electron from the redox partner to form the ferrous protein (3), generation of the ferrous oxygenated (oxy-ferrous) state (4), reduction to form the peroxo states after receiving second electron (5a), transfer of proton to the distal oxygen atom and production of the “hydroperoxo” state (5b), a second protonation and formation of a higher valent metal-oxo species “Compound I” (Cpd I) (6), and the hydroxylation and release of product (7) of P450 catalysis are illustrated. The three unproductive pathways are shown in grey.
In this work, we evaluate the mechanistic differences in the two steps reaction comprised with hydroxylation and lyase activity performed by CYP17A1 using the analogous mutation, T306A, within steroid metabolizing P450. Previously, Akthar's group studied the T306A mutant of CYP17A1, using Δ5-steroids (PREG and 17-OH-PREG) as substrates [9]. Other reports of CYP17A1 activity used various subsets of Δ4- and Δ-5-steroids as substrates and detergent solubilized CYP17A1 reconstituted with CPR and cyt-b5 at different stoichiometries [8,9,28,29,30], which makes a direct comparison of the hydroxylase and lyase activity for specific Δ4- or Δ5-steroids difficult. To overcome these shortfalls, we incorporated CYP17A1 and its variant T306A in Nanodiscs and reconstituted it with redox partners in a well controlled stoichiometry [31,32]. We also employed the consistent substrate sets, the Δ5 (PREG and 17-OH-PREG) and Δ4 (PROG and 17-OH-PROG) steroids to study the hydroxylase and lyase activity using the same preparation of cytochrome P450 reductase (CPR) and cyt-b5 throughout the in vitro reconstitution experiments. Importantly, we also documented the preference of substrate and coupling efficiency during the hydroxylation and lyase reaction by the wild type CYP17A1 and its mutant T306A.
Material and Methods
Construction of T306A
In order to obtain the conserved I-helix variant of human CYP17A1 T306A, the pCWori_CYP17A1 construct [7] was mutated using primers 5'-ggt gcc ggt gtg gaa gcc acc acc agc gtc gtc-3' (forward) and 5'- gac gac gct ggt ggt ggc ttc cac acc ggc acc-3' (Reverse). The sequence of the mutant was verified by automated DNA sequencing (ACGT, Inc.).
Expression and purification of recombinant proteins
The expression and purification of T306A was performed as described for wild type CYP17A1 [7,33]. The expression and purification of membrane scaffold protein (MSP), rat P450 reductase (CPR) and cyt-b5 was performed as described [34,35,36].
Nanodisc assembly of T306A
The detailed protocol for the incorporation of CYP17A1 into Nanodiscs has been previously reported [7,33] and same procedure was employed for the T306A mutant.
Spectroscopic characterization
UV-visible spectra for CYP17A1 and T306A were recorded at room temperature using a Cary 300 UV–visible spectrophotometer. The P450 concentration was estimated by CO-difference spectra assuming Δε (450–490) = 91 mM−1cm−1 as described [37].
NADPH oxidation
Incorporation of CPR into preformed and purified CYP17A1 (or T306A) Nanodiscs was made by direct addition of oligomeric CPR at 1:4 CYP17A1 (or T306A)/CPR molar ratio, as described [38]. Briefly, 1 ml of CYP17A1 (or T306A) and CPR solution, in presence or absence of cytochrome b5 (1:4:4 molar ratio), in 100 mM potassium phosphate buffer, pH 7.4, containing 50 mM NaCl and 50 μM substrate (PROG, 17-OH-PROG, PREG or 17-OH-PREG) was brought 37 °C in a stirred quartz cuvette. The sample was incubated for 5 min and the reaction was initiated by addition of 100 μM of NADPH. The consumption of NADPH was monitored by recording the absorbance at 340 nm for 5 min. The reaction was stopped by adding 50 μl of 9 M sulfuric acid to bring the pH below 4.0. The sample was removed from the cuvette, flash frozen in liquid nitrogen, and stored at −80 °C until product analysis. The optical measurements were performed on Hitachi U-3300 spectrophotometer supplied with temperature controller and built-in magnetic stirrer. For 17-OH-PROG and 17-OH-PREG assay a three-fold higher concentration of NADPH was used with a path length of 0.4 cm rather than 1 cm. The rate of NADPH oxidation was determined from the slope of absorption at 340 nm during the first two minutes using an extinction coefficient of 6.22 cm−1mM−1.
Catalytic turnover
Analysis of the hydroxylated and lyase products of Δ4- and Δ5-steroids by CYP17A1 and T306A was performed as follows.
The conversion of PREG to 17-OH-PREG was analyzed by TLC (EMD TLC Silica gel 60 F254, 20 × 20 cm), using radiolabeled [7-3H] pregnenolone (8.5 nmol, 0.8 μCi) as described [39]. The conversion of PROG to 17-OH-PROG was analyzed by HPLC (Waters). Briefly, 1 μl of 27 mM cortexolone solution in methanol were added to 500 μl each of the reaction sample and vortexed for 10 sec. 2 ml of chloroform was added to each aliquots and vortexed for 30 sec. The organic phage was removed and dried under the stream of nitrogen. The dried sample was dissolved in 100 μl of methanol and 40 μl was injected onto C18-HPLC column, using a 150 × 2.1 mm, 3 μm (ACE-111-1502) with the mobile phase of 45% each of methanol and acetonitrile in water and a flow rate of 0.2 ml/min. The 17-hydroxylated product of PROG was separated in the linear gradient of methanol and acetonitrile from 20% to 80% in 28 min. Peak integration was performed with GRAM/32 software (Thermo Fischer Scientific). The analysis of 17-hydroxylated product of both Δ4- and Δ5-steroids was performed on an FID equipped gas chromatograph (Agilent 6890) as described [39].
Results
Expression, purification, spectrophotometric characterization and assembly of T306A Nanodiscs
The mutant protein T306A was successfully overexpressed and purified from the bacterial membrane. The protein was pure by SDS-PAGE with a mass of ~58 kDa. The yield of the protein was 4–6 mg/L E. coli cell culture. The oxidized, reduced and CO-complexed spectra of the progesterone bound T306A displayed the Soret band at 391 nm, a diminished absorption maximum at 408 nm and 447 nm, respectively. The mutant protein was incorporated into Nanodiscs and the purity was confirmed by SDS-PAGE and HPLC size exclusion chromatography. The oxidized substrate free T306A showed the characteristic absorption maxima: Q bands at 568 nm (α band) and 535 nm (β band), and the Soret band at 417 nm. The pronounced Soret peak at 417 nm in the absence of substrate was shifted to 391 nm by the addition of progesterone. The reduced protein displayed a peak maximum at 408 nm and the reduced CO-complex showed a typical “P450” peak at 447 nm.
NADPH oxidation and turnover of Δ4- and Δ5- steroids by CYP17WT and T306A
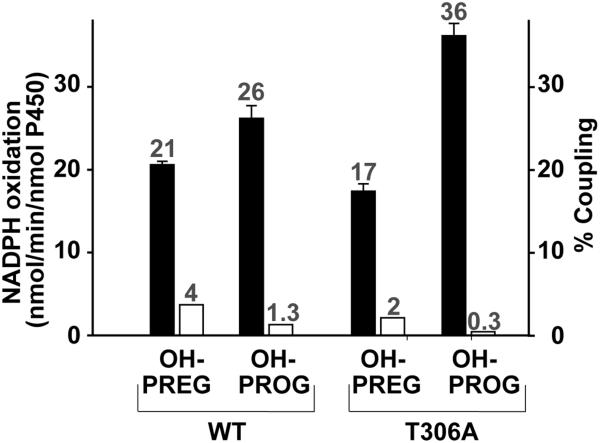
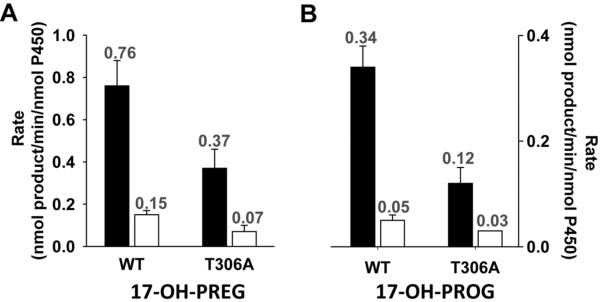
The rates of pyridine nucleotide oxidation and product formation were measured for T306A and compared with that of the wild type enzyme. We evaluated both the hydroxylase and lyase activities in the presence of Δ4- and Δ5- steroids. Using PREG and PROG as substrates, the “normal” hydroxylation reaction is followed by the production of the 17α-OH product. Using 17-OH-PREG and 17-OH-PROG allowed us to specifically examine the 17,20-carbon-carbon lyase reaction of the corresponding substrates by monitoring the production of dehydroepiandrosterone (DHEA) and androstenedione (AD), respectively. Since Thr306 is a member of the conserved acid/alcohol pair and thought to be essential for the efficient delivery of protons required for hydroperoxoanion heterolysis and formation of Cpd I in P450cam [16,28,18], the change of T306A at the analogous position in CYP17A1 would be expected to disrupt the Cpd I mediated hydroxylation chemistry. We observed that T306A consumed NADPH at about 6.6 times and 2.3 times faster than WT during the conversion of PREG and PROG, respectively, to their corresponding 17α-OH product (Figure 2.A, Table 1). However, the product formation rate was dramatically reduced by ~94% and 92% (Figure 2.B, Table 1). As a result, the coupling efficiency of T306A was less than 1% during the hydroxylation of PREG and PROG as compared to 97 and 22%, for WT (Figure 2. C). These two reactions were highly uncoupled in T306A mutant where reducing equivalents and protons are funneled into the formation of superoxide and hydrogen peroxide without O-O bond cleavage rather than productive hydroxylation of the substrates. This suggests the disruption on proton delivery to the distal oxygen atom of the peroxoanion which is required to initiate O-O bond scission and formation of the Cpd I reactive intermediate. These results are consistent with previous investigations of P450cam [16,40] and other systems, in which mutation of this critical residue severely impairs formation of Cpd I with a concomitant decoupling of product formation from NADPH oxidation. On the other hand, catalysis of carbon-carbon bond scission by the T306A mutant was largely unimpeded by disruption of the CYP17A1 acid-alcohol pair (Figure 3, Table 1). During carbon-carbon bond scission reaction monitored by the conversion of 17-OH-PREG to DHEA, the rate of NADPH oxidation was almost similar 21 vs 17 nmol/min/nmolP450 for WT and T306A, respectively, and did not show the dramatic difference in coupling (4% vs 2% for WT and mutant) (Figure 3, Table 1). Likewise, the lyase reaction of 17α-OH-PROG to AD also showed the similar rates, having moderately increasing rate of NADPH consumption in T306A mutant, of NADPH oxidation of 26 vs 36 nmol/min/nmolP450 with the coupling of 1.3 vs 0.3 percentage for WT and T306A. However, in both the lyase reactions, we did not observe dramatic changes like the one that we have observed during hydroxylase activity. These data suggested that C-C lyase chemistry conducted by CYP17A1 proceeds independently of the proton delivery pathway and O-O bond scission and Cpd I formation and are consistent with a reactive peroxo anion that initiates the 17, 20-lyase activity through nucleophilic attack on the 20-keto group of the substrate [39].
Figure 2. Comparative study on 17-α-hydroxylase activity of pregnenolone and progesterone by wild type CYP17A1 and T306A.
Determination of rate of NADPH oxidation (A) and the rate of conversion of pregnenolone (Δ5-steroid) and progesterone (Δ4-steroid) to the corresponding 17-OH-product (B) are shown. The black and open bars represent the WT and T306A, respectively. The coupling efficiency (C) of WT and T306A during the hydroxylation of Δ5- (stripped-bar) and Δ4-steroids (grey bar) are illustrated. The number on the bar represents the values of the activity.
Table 1.
Comparison of hydroxylation and lyase reactivities of Δ5- and Δ4-steroids by human CYP17A1.
| Δ5-steroid | Δ4-steroid | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| PREG → 17α-OH-PREG | 17α-OH-PREG → DHEA | PROG → 17α-OH-PROG | 17α-OH-PROG → AD | Ref | ||
|
| ||||||
| CYP17 | CYP17 | CYP17 | CYP17 | |||
|
| ||||||
| −b5 | −b5 | +b5 | −b5 | −b5 | +b5 | |
|
| ||||||
| 5.63±0.31a | 0.15±0.02b | 0.76±0.21b | 4.81 ±0.15c | 0.05±0.01b | 0.34 ± 0.04b | This study |
| - | 0.17±0.042d | - | 2.9±0.39d | - | - | 28 |
| 2.20d | 0.00d | 2.45d | 3.33d | 0.00d | 1.80d | 8 |
| 2.17±0.04d | 0.32±0.05d | 3.06±0.05d | - | - | 9 | |
| - | - | - | 3.5d,e | 0.12d,e | 0.64d,e | 29 |
| - | 0.12±0.001f,c | 0.28±0.03f,c | 6.89±0.24f,c | - | - | 30 |
| - | 0.04±0.009c,g | 0.21±0.04c,g | 2.10±0.17c,g | - | - | 30 |
Note: Conversion monitored by
TLC using [7-3H]PREG,
GC,
HPLC,
TLC using 3H-labeled substrates ([3H]PREG, [3H]PROG, [3H]17α-OH-PROG, [3H]17α-OH-PREG);
Partically purified protein;
Full length CYP17;
CYP17 variant with the deletion of N-terminal hydrophobic amino acid sequence; - Not studied.
Figure 3. Comparison of NADPH oxidation and coupling efficiency during 17,20-lyase activity by wild type CYP17A1 and T306A.
Determination of rate of NADPH oxidation (black bar) and percentage of coupling (white bar) for 17-OH-PREG and 17-OH-PROG for wild type CYPC17A1 and T306A are shown. The number on the bar represents the corresponding value related with the unit on the y-axis. The error bar represents the standard deviation of 6–8 independent measurements.
Effect of cytochrome b5 on the lyase reaction
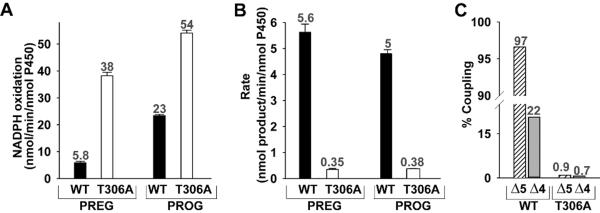
It is well documented that cyt-b5 augments the 17,20 carbon-carbon bond scission of mammalian CYP17 [2,3,41,42, 43], although detailed studies as a function of the P450:CPR:cyt-b5 ratio have not been reported. Therefore, we explored the effect of cyt-b5 during lyase activity on our well defined assay system consisting of CYP17A1 Nanodiscs and co-incorporated CPR and cyt-b5. We unambiguously observed the preference of 17-OH-PREG (>2 fold higher compared to 17-OH-PROG) during 17,20-lyase activity for both the WT and T306A (Figure 4, Table 1) in presence of cyt-b5. It has also been shown that the human, bovine, ovine and caprine CYP17 enzymes use 17OH-PREG as a preferred substrate for the 17,20-lyase reaction [3,41,42, 44,45,46]. In this study, we also observed that the presence of cyt-b5 enhanced the lyase activity by ~5- and 7-fold in the WT and ~ 5- and 4-fold in T306A for 17-OH-PREG and 17-OH-PROG, respectively. Though a detailed mechanism for cyt-b5 participation in catalysis is not clear, the most recent study on NMR chemical shift mapping of cyt-b5 titrations with CYP17A1 by Scott's group [47] showed evidence that cyt-b5 and CPR compete for a binding surface on CYP17A1, and the CYP17A1/cyt-b5 interaction is stronger in the presence of hydroxylase substrate pregnenolone in the CYP17A1 active site than when the presence of lyase substrate 17α-OH-progesterone.
Figure 4. Cytochrome b5 effect on CYP17A1 lyase activity.
The rate conversion of 17-OH-PREG to DHEA and 17-OH-PROG to AD was measured in the presence (black bar) and absence (white bar) of cytochrome b5. The number on the bar represents the rate of conversion of the substrate and the error bar the standard deviation of 6–8 independent measurements.
In conclusion, we have shown that the highly conserved acid-alcohol pair in human CYP17A1 supports a proton delivery pathway necessary only for oxygen-oxygen bond scission to form Cpd I which initiates normal hydroxylation process. This residue, and the associated proton delivery, is not operating the lyase reaction. The unique response of CYP17A1 lyase activity to mutation of Thr306 is consistent with a reactive intermediate formed independently of proton delivery in the active site and suggests the involvement of a nucelophilic peroxo-anion rather than the traditional Cpd I.
Highlights
The disruption of PREG/PROG hydroxylation activity by T306A showed the participation of Cpd I
T306A supports the involvement of a nucleophilic peroxo-anion during lyase activity
The presence of cytochrome b5 augments C-C lyase activity
Δ5-steroids are preferred substrates for CYP17 catalysis
Acknowledgements
This work was supported by the National Institutes of Health grants GM31756 and GM33775.
Abbreviations
- PREG
pregnenolone
- OH-PREG
17α-hydroxy-pregnenolone
- PROG
progesterone
- OH-PROG
17α-hydroxy-progesterone
- DHEA
dehydroepiandrosterone
- AD
Androstenedione
- cyt-b5
cytochrome b5
- Cpd I
Compound I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auchus RJ, Miller WL. Molecular Modeling of Human P450c17 (17α-Hydroxylase/17,20-Lyase): Insights into Reaction Mechanisms and Effects of Mutations. Mol. Endocrinol. 1999;13:1169–1182. doi: 10.1210/mend.13.7.0326. [DOI] [PubMed] [Google Scholar]
- 2.Kominami S, Ogawa N, Morimune R, Huang DY, Takemori S. The role of cytochrome b5 in adrenal microsomal steroidogenesis. J. Steroid Biochem. Mol. Biol. 1992;42:57–64. doi: 10.1016/0960-0760(92)90011-7. [DOI] [PubMed] [Google Scholar]
- 3.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch. Biochem. Biophys. 1995;317:343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 4.Pandey AV, Miller WL. Regulation of 17,20 Lyase Activity by Cytochrome b5 and by Serine Phosphorylation of P450c17. J. Biol. Chem. 1995;280:13265–13271. doi: 10.1074/jbc.M414673200. [DOI] [PubMed] [Google Scholar]
- 5.Cutler GB, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: A Survey of Rodents, Domestic Animals, and Primates, Endocrinology. 1978;103:2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- 6.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17, Endocrinol. Metab. Clin. North Am. 2001;30:101–119. doi: 10.1016/s0889-8529(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 7.Gregory M, Mak PJ, Sligar SG, Kincaid JR. Differential hydrogen bonding in human CYP17 dictates hydroxylation versus lyase chemistry, Angew. Chem. Int. Ed. Engl. 2013;52:5342–5. doi: 10.1002/anie.201300760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17 alpha-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem. J. 1995;308:901–908. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee-Robichaud P, Akhtar ME, Akhtar M. An analysis of the role of active site protic residues of cytochrome P-450s: mechanistic and mutational studies on 17alpha-hydroxylase-17,20-lyase (P-45017alpha also CYP17) Biochem. J. 1998;330:967–974. doi: 10.1042/bj3300967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhtar M, Wright JN, Lee-Robichaud P. A review of mechanistic studies on aromatase (CYP19) and 17α-hydroxylase-17,20-lyase (CYP17) J. Steroid. Biochem. Mol. Biol. 2011;125:2–12. doi: 10.1016/j.jsbmb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Akhtar M, Corina D, Miller S, Shyadehi AZ, Wright JN. Mechanism of the acyl-carbon cleavage and related reactions catalyzed by multifunctional P-450s: studies on cytochrome P-450(17)alpha. Biochemistry. 1994;33:4410–8. doi: 10.1021/bi00180a039. [DOI] [PubMed] [Google Scholar]
- 12.Makris TM, Davydov R, Denisov IG, Hoffman BM, Sligar SG. Mechanistic enzymology of oxygen activation by the cytochromes P450. Drug Metab. Rev. 2002;34:691–708. doi: 10.1081/dmr-120015691. [DOI] [PubMed] [Google Scholar]
- 13.Sligar SG, Makris TM, Denisov IG. Thirty years of microbial P450 monooxygenase research: peroxo-heme intermediates--the central bus station in heme oxygenase catalysis. Biochem. Biophys. Res. Commun. 2005;338:346–54. doi: 10.1016/j.bbrc.2005.08.094. [DOI] [PubMed] [Google Scholar]
- 14.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem Rev. 2005;105:2253–77. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 15.Poulos TL, Finzel BC, Gunsalus IC, Wagner GC, Kraut J. The 2.6-A crystal structure of Pseudomonas putida cytochrome P-450. J. Biol. Chem. 1985;260:16122–30. [PubMed] [Google Scholar]
- 16.Raag R, Martinis SA, Sligar SG, Poulos TL. Crystal structure of the cytochrome P-450CAM active site mutant Thr252Ala. Biochemistry. 1991;30:11420–9. doi: 10.1021/bi00112a008. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Nakamura M. Point mutations at threonine-301 modify substrate specificity of rabbit liver microsomal cytochromes P-450 (laurate (omega-1)-hydroxylase and testosterone 16 alpha-hydroxylase) Biochem. Biophys. Res. Commun. 1989;158:717–22. doi: 10.1016/0006-291x(89)92780-0. [DOI] [PubMed] [Google Scholar]
- 18.Nagano S, Poulos TL. Crystallographic study on the dioxygen complex of wild-type and mutant cytochrome P450cam. Implications for the dioxygen activation mechanism. J. Biol. Chem. 2005;280:31659–63. doi: 10.1074/jbc.M505261200. [DOI] [PubMed] [Google Scholar]
- 19.Vidakovic M, Sligar SG, Li H, Poulos TL. Understanding the role of the essential Asp251 in cytochrome p450cam using site-directed mutagenesis, crystallography, and kinetic solvent isotope effect. Biochemistry. 1998;37:9211–9. doi: 10.1021/bi980189f. [DOI] [PubMed] [Google Scholar]
- 20.Schlichting I, Berendzen J, Chu K, Stock AM, Maves SA, Benson DE, Sweet RM, Ringe D, Petsko GA, Sligar SG. The catalytic pathway of cytochrome p450cam at atomic resolution. Science. 2000;287:1615–22. doi: 10.1126/science.287.5458.1615. [DOI] [PubMed] [Google Scholar]
- 21.Clark JP, Miles CS, Mowat CG, Walkinshaw MD, Reid GA, Daff SN, Chapman SK. The role of Thr268 and Phe393 in cytochrome P450 BM3. J. Inorg. Biochem. 2006;100:1075–90. doi: 10.1016/j.jinorgbio.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Vatsis KP, Coon MJ. Ipso-substitution by cytochrome P450 with conversion of p hydroxybenzene derivatives to hydroquinone: evidence for hydroperoxo-iron as the active oxygen species. Arch. Biochem. Biophys. 2002;397:119–29. doi: 10.1006/abbi.2001.2665. [DOI] [PubMed] [Google Scholar]
- 23.Blobaum AL, Kent UM, Alworth WL, Hollenberg PF. Novel reversible inactivation of cytochrome P450 2E1 T303A by tert-butyl acetylene: the role of threonine 303 in proton delivery to the active site of cytochrome P450 2E1. J. Pharmacol. Exp. Ther. 2004;310:281–90. doi: 10.1124/jpet.104.065508. [DOI] [PubMed] [Google Scholar]
- 24.Keizers PH, Schraven LH, de Graaf C, Hidestrand M, Ingelman-Sundberg M, van Dijk BR, Vermeulen NP, Commandeur JN. Role of the conserved threonine 309 in mechanism of oxidation by cytochrome P450 2D6. Biochem. Biophys. Res. Commun. 2005;338:1065–74. doi: 10.1016/j.bbrc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 25.Hiroya K, Ishigooka M, Shimizu T, Hatano M. Role of Glu318 and Thr319 in the catalytic function of cytochrome P450d (P4501A2): effects of mutations on the methanol hydroxylation. FASEB J. 1992;6:749–51. doi: 10.1096/fasebj.6.2.1347023. [DOI] [PubMed] [Google Scholar]
- 26.Vaz AD, Pernecky SJ, Raner GM, Coon MJ. Peroxo-iron and oxenoid-iron species as alternative oxygenating agents in cytochrome P450-catalyzed reactions: switching by threonine-302 to alanine mutagenesis of cytochrome P450 2B4. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4644–8. doi: 10.1073/pnas.93.10.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulos TL, Madrona Y. Oxygen activation and redox partner binding in cytochromes P450. Biotechnol. Appl. Biochem. 2013;60:128–33. doi: 10.1002/bab.1056. [DOI] [PubMed] [Google Scholar]
- 28.Imai T, Globerman H, Gertner JM, Kagawa N, Waterman MR. Expression and purification of functional human 17 alpha-hydroxylase/17,20-lyase (P450c17) in Escherichia coli. Use of this system for study of a novel form of combined 17 alpha-hydroxylase/17,20-lyase deficiency. J. Biol. Chem. 1993;268:19681–19689. [PubMed] [Google Scholar]
- 29.Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38:1598–1606. doi: 10.1021/bi9821059. [DOI] [PubMed] [Google Scholar]
- 30.Pechurskaya TA, Lukashevich OP, Gilep AA, Usanov SA. Engineering, expression, and purification of “soluble” human cytochrome P45017alpha and its functional characterization. Biochemistry. 2008;73:806–811. doi: 10.1134/s0006297908070092. [DOI] [PubMed] [Google Scholar]
- 31.Denisov IG, Sligar SG. Cytochromes P450 in Nanodiscs. Biochim. Biophys. Acta. 2011;1814:223–9. doi: 10.1016/j.bbapap.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuler MA, Denisov IG, Sligar SG. Nanodiscs as a new tool to examine lipid-protein interactions. Methods Mol. Biol. 2013;74:415–33. doi: 10.1007/978-1-62703-275-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luthra A, Gregory M, Grinkova YV, Denisov IG, Sligar SG. Nanodiscs in the studies of membrane-bound cytochrome P450 enzymes. Methods Mol. Biol. 2013;987:115–27. doi: 10.1007/978-1-62703-321-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 2004;126:3477–87. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 35.Shen ES, Guengerich FP, Olson JR. Biphasic response for hepatic microsomal enzyme induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J and DBA/2J mice. Biochem. Pharmacol. 1989;38:4075–84. doi: 10.1016/0006-2952(89)90689-8. [DOI] [PubMed] [Google Scholar]
- 36.Mulrooney SB, Waskell L. High-level expression in Escherichia coli and purification of the membrane-bound form of cytochrome b5. Protein Expr. Purif. 2000;19:173–8. doi: 10.1006/prep.2000.1228. [DOI] [PubMed] [Google Scholar]
- 37.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes: Solubilization, purification, and properties. J. Biol. Chem. 1964;239:2379–85. [PubMed] [Google Scholar]
- 38.Grinkova YV, Denisov IG, Sligar SG. Functional reconstitution of monomeric CYP3A4 with multiple cytochrome P450 reductase molecules in Nanodiscs. Biochem. Biophys. Res. Commun. 2010;398:194–8. doi: 10.1016/j.bbrc.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregory M, Denisov IG, Grinkova YV, Khatri Y, Sligar SG. Kinetic Solvent Isotope Effect in Human P450 CYP17A1 Mediated Androgen Formation: Evidence for a Reactive Peroxoanion Intermediate. J. Am. Chem. Soc. 2013 doi: 10.1021/ja4086403. In press. DOI: 10.1021/ja4086403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada H, Sligar SG, Yeom H, Ishimura Y. Heme Monoxygenases - A chemical mechanism for cytochrome P450 oxygen activation. In: Funaniki T, editor. Oxygenases and Model systems. Kluwer Academic Publisher; London: pp. 195–221. [Google Scholar]
- 41.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J. Biol. Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 42.Flück CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome P450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J. Clin. Endocrinol. Metab. 2003;88:3762–3766. doi: 10.1210/jc.2003-030143. [DOI] [PubMed] [Google Scholar]
- 43.Nakajin S, Takahashi M, Shinoda M, Hall PF. Cytochrome b5 promotes the synthesis of Δ16-C19 steroids by homogeneous cytochrome P-450 C21 side-chain cleavage from pig testis. Biochem. Biophys. Res. Commun. 1985;132:708–713. doi: 10.1016/0006-291x(85)91190-8. [DOI] [PubMed] [Google Scholar]
- 44.Swart P, Lombard N, Swart AC, van der Merwe T, Murry BA, Nicol M, Ian Mason J. Ovine steroid 17α-hydroxylase cytochrome P450: characteristics of the hydroxylase and lyase activities of the adrenal cortex enzyme. Arch. Biochem. Biophys. 2003;409:145–152. doi: 10.1016/s0003-9861(02)00547-7. [DOI] [PubMed] [Google Scholar]
- 45.Zuber MX, Simpson ER, Waterman MR. Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science. 1986;234:1258–61. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 46.Storbeck KH, Swart AC, Slabbert JT, Swart P. The identification of two CYP17 alleles in the South African Angora goat. Drug Metab. Rev. 2007;39:467–480. doi: 10.1080/03602530701468649. [DOI] [PubMed] [Google Scholar]
- 47.Estrada DF, Laurence JS, Scott EE. Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR. J. Biol. Chem. 2013;288:17008–18. doi: 10.1074/jbc.M113.468926. [DOI] [PMC free article] [PubMed] [Google Scholar]