Chronic myeloid leukemia (CML) was a fatal disease for almost all patients until the introduction of allogeneic stem cell transplantation (SCT) and of interferon-alfa (IFNα). However, these were of benefit only for a minority of patients.1 The targeted therapies, first imatinib, then the other tyrosine kinase inhibitors (TKIs), have dramatically changed the scenario. The scientific community enjoyed the expected normal life span for most TKI-treated CML patients, considering the extrapolation of the survival curves.2,3 More recently, the scientific community is focusing on the importance of achieving a deeper and deeper response that can only be measured through molecular methods.4–7 It is expected and predicted that the deeper the response the better the outcomes, where, today, outcome is considered in terms of overall survival, while tomorrow it is likely to be treatment-free survival.8,9 Accordingly, the choice of treatment has traditionally been based on efficacy criteria including rate, time and depth of response.8,9 This policy has sound clinical bases because CML is a cancer, and the ultimate objective is to provide a cure. Consequently, outcome assessment in CML has, till now, been heavily disease oriented. While this policy must be implemented, we should also bear in mind the fact that the disease course and treatment approaches have radically changed over the last decade. Currently, based on at least ten years of experience with imatinib and on the availability of other TKIs, less than 20% of patients are still at risk of dying of leukemia, less than 20% can achieve a treatment-free remission, and more than 60% are facing a situation of chronic, life-long treatment.9
For many years, we have dedicated our efforts and resources to the evaluation of the response, achieving remarkable success in the standardization of the methods used to assess minimal residual disease (MRD), and widespread agreement on the evaluation of treatment response and on treatment recommendations.7–9 While biochemical or laboratory abnormalities can be recorded objectively, clinical side-effects, for example, are typically recorded and collected by health care professionals (HCPs) who interpret and evaluate reports from the patients themselves. Probably only a few side-effects, like skin rash, alopecia, edema and fluid retention, can really be evaluated directly and, to a certain extent, objectively by the investigators. All available TKIs for first-line therapy, that is imatinib (i.e. 1st-generation TKIs) and dasatinib or nilotinib (i.e. 2nd-generation TKIs), have side-effects that one should consider when deciding which therapy is best for the individual patient. However, some side-effects can be more frequent with a given TKI. As an example, while fatigue has been reported to be similar amongst the three TKIs,10 others, such as rash, have been reported to be worse with both 2nd-generation TKIs.11,12 In any case, in most studies, recording and assessing the type, the intensity and the duration of the side-effects are not planned, apart from formal reference to some internationally recognized scoring systems (e.g. NCI, SWOG). The data obtained with this methodology can be very different, even in company-sponsored, registrative studies.
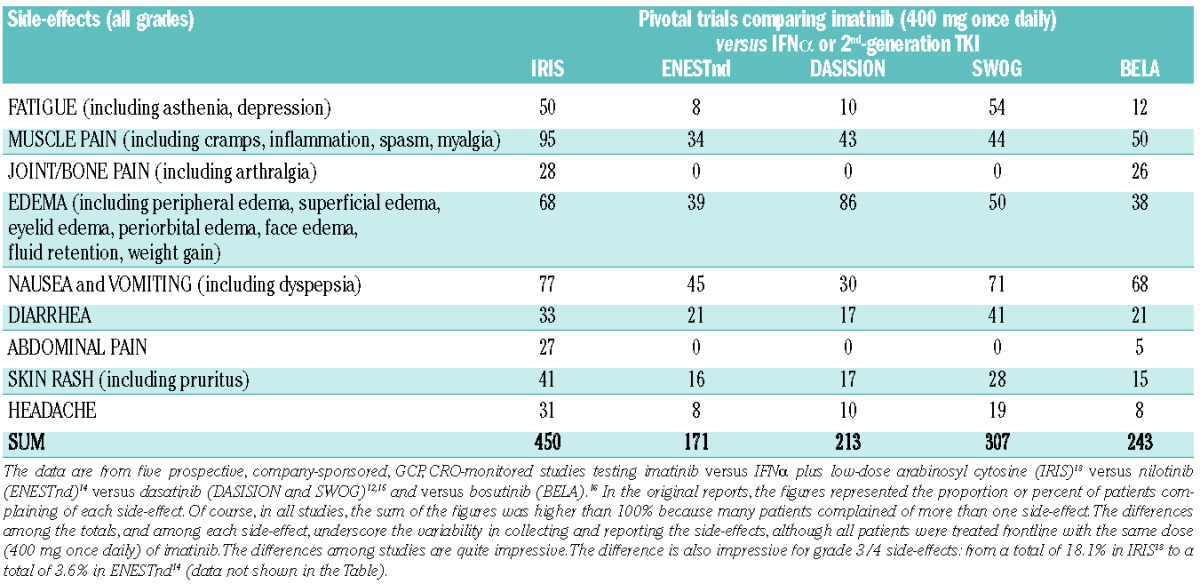
Just to illustrate how challenging it is to draw conclusions with regards to toxicity data, we report (for descriptive purposes only) five studies,12–16 all in newly diagnosed, chronic phase, CML patients treated with imatinib 400 mg once daily (Table 1). Interestingly, the reported proportion of patients with any grade fatigue and muscle pain ranged from 8% and 34% (ENESTnd)14 to 50% and 95% (IRIS),13 respectively. Similarly, marked differences were also reported for all the other major groups of side-effects (Table 1). The purpose of these studies, that tested imatinib versus other drugs, was to compare the type and severity of the side-effects, but not the duration, between two different drugs. However, the reported data in the imatinib arm differed so greatly that it was difficult to assess the imatinib-related burden of symptoms, and can raise doubts as to the value of the comparison. Limitations of standard physician-reported toxicity with regards to the documentation of drug safety have been acknowledged17 and have prompted the National Cancer Institute (NCI) to create a version of the NCI’s Common Terminology Criteria for Adverse Events (PRO-CTCAE) that can be completed by patients themselves, providing direct patient feedback on their experience of their symptoms during treatment.18
Table 1.
Percentage of newly diagnosed, chronic phase, CML patients who were reported to complain of the listed side-effects with imatinib.
In the context of CML treated with long-term TKI therapy, it has been shown that even low-grade side-effects can substantially impact quality of life (QoL).19 Also, a recent CML study has shown that physicians tend to under-estimate symptom severity and over-estimate the overall health status of their patients.20 This evidence underscores the need for directly asking patients themselves about their disease and treatment burden, and also confirms, with empirical data, the previously raised concerns about the current practice of assessing intolerance to TKIs (i.e. not patient-reported).21
While major stakeholders are pointing out the importance of patient-centered outcome research (PCOR) in medicine,22 evidence-based data in CML are lacking.23 This is striking considering the great potential that patient-reported data could have to facilitate clinical decision-making in the current CML arena, where treatment decisions are often highly challenging. We believe that the lack of CML-specific instruments for patient-reported outcome (PRO) is the major cause of the lack of PCOR in CML. The development of methodologically sound PRO instruments requires major financial investment and research efforts as it has to comply with several and rigorous methodological criteria. The patient’s unique viewpoint on the burden of disease and the effect of treatment on his/her life can only be known through the use of such instruments and cannot be otherwise inferred from other indirect measures (e.g. physician-reported toxicity).24
Use and area of applications of PRO instruments in CML
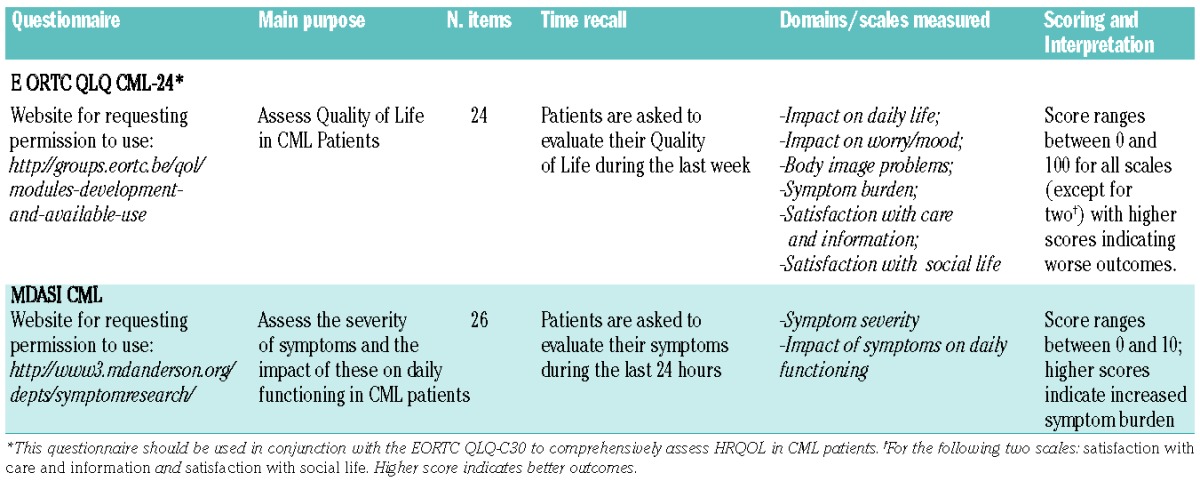
The good news for the CML community is that two CML-specific instruments, the EORTC QLQ CML-24 and the MDASI-CML, have recently been developed and published in full. Both questionnaires have followed high-quality methodological criteria recommended for the development of PRO measures. The EORTC QLQ CML-24 was developed by the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Group.25 This questionnaire has been devised to supplement the EORTC QLQ-C30 to comprehensively assess QoL in CML patients. The development of the EORTC QLQ CML-24 involved overall 655 CML patients on treatment with various TKIs from 10 different countries (in Europe, the USA and Asia).25 A major strength of this tool was its international development, which ensured satisfactory validity and applicability across multiple languages and cultures. Questionnaire items were tested in a pilot study, and debriefing cognitive interviews were held simultaneously in different cultural contexts. This has important implications for using this tool in CML international studies.
The MDASI-CML was developed at the MD Anderson Cancer Center (Houston, TX, USA) and involved 187 patients on treatment with different TKIs.26 Unlike the EORTC QLQ CML-24, this questionnaire has been devised to evaluate symptom burden (rather than QoL). The strength of this tool was the longitudinal analysis performed in the development process, which further supported validity data. Basic information on scoring and interpretation of both questionnaires are summarized in Table 2.
Table 2.
Summary of basic characteristics for the EORTC QLQ CML-24 and the MDASI CML questionnaires.
Two broad areas can be identified for the implementation of these measures: clinical research and routine clinical practice. With regard to clinical research, the introduction of such measures would be of particular value in observational studies and randomized clinical trials (RCTs). Just to illustrate this, in this latter case, it would be used as a study end point to weigh the potential clinical benefit of new therapeutic approach against risk and toxicities from the patient’s perspective. If PRO is to be used in an RCT setting, this assessment should be carefully planned a priori in the research protocol and a number of issues should be examined in detail. Typically, these issues would be addressed in dedicated PRO chapter in the protocol. The selection of the most appropriate PRO questionnaire/s to be used is an important issue and should always be guided by specific rationale. However, this is just one of several other issues that should be considered in the protocol. Other topics include: i) stating the specific PRO hypothesis being tested; ii) the methods for data collection; iii) management of missing data; and iv) a statistical analysis plan. A naïve approach to PRO implementation in RCTs is unlikely to generate solid data that could be used to facilitate clinical decision making therefore methodological rigor is essential.27 To date, several guidelines are available to assist investigators in the planning, conducting and reporting of PRO in RCTs, including the recently issued PRO-specific CONSORT standards.27
Another important area of application of PRO instruments would be their implementation in routine practice. Previous research has shown that the use of PRO instruments in clinical practice is feasible and can facilitate discussion of health problems between patients and physicians.28 Systematic use of these questionnaires in follow-up visits might help physicians in the early identification of those CML patients for whom the given therapy is particularly burdensome, and this would enable the timely consideration of alternative treatments. Guidelines and suggestions for implementing PRO instruments in clinical practice are available.29
To conclude, while continued efforts towards the definitive cure of CML are necessary, integration of PRO data with clinical and laboratory information (e.g. MRD) is also now needed to capture the real patient burden of treatment and to facilitate a transition to a more patient-centered decision-making approach.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370(9584):342–50 [DOI] [PubMed] [Google Scholar]
- 2.Bjorkholm M, Ohm L, Eloranta S, Derolf A, Hultcrantz M, Sjoberg J, et al. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29(18):2514–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian H, O’Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30(3):232–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branford S, Kim DW, Soverini S, Haque A, Shou Y, Woodman RC, et al. Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or -intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib. J Clin Oncol. 2012;30(35):4323–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanfstein B, Muller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. 2012;26(9):2096–102 [DOI] [PubMed] [Google Scholar]
- 7.Muller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23(11):1957–63 [DOI] [PubMed] [Google Scholar]
- 8.O’Brien S, Abboud CN, Akhtari M, Altman J, Berman E, DeAngelo DJ, et al. Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia, Version 1.2013, National Comprehensive Cancer Network (NCCN) Available from: http://www.nccn.org (Accessed November, 2013). [Google Scholar]
- 9.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013; 122(6):872–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin D. Initial choice of therapy among plenty for newly diagnosed chronic myeloid leukemia. American Society of Hematology Educational Program Book Hematology. 2012:115–121, 10.1182/asheducation-2012.1.115 [DOI] [PubMed] [Google Scholar]
- 11.Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012; 26(10):2197–203 [DOI] [PubMed] [Google Scholar]
- 12.Radich JP, Kopecky KJ, Appelbaum FR, Kamel-Reid S, Stock W, Malnassy G, et al. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic-phase chronic myeloid leukemia. Blood. 2012;120(19):3898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004 [DOI] [PubMed] [Google Scholar]
- 14.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9 [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70 [DOI] [PubMed] [Google Scholar]
- 16.Cortes JE, Kim DW, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicius L, et al. Bosutinib Versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia: Results From the BELA Trial. J Clin Oncol. 2012;30(28):3486–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485–90 [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. US Department of Health and Human Services Division of Cancer Control and Population Sciences, Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Available from: http://outcomes.cancer.gov/tools/pro-ctcae_fact_sheet.pdf (Accessed November, 2013).
- 19.Efficace F, Baccarani M, Breccia M, Cottone F, Alimena G, Deliliers GL, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27(7):1511–9 [DOI] [PubMed] [Google Scholar]
- 20.Efficace F, Rosti G, Aaronson N, Cottone F, Angelucci E, Molica S, et al. Patient versus Physician Symptom and Health Status Reporting in Chronic Myeloid Leukemia. Haematologica 2013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: Definitions and clinical implications. Cancer. 2011;117(4):688–97 [DOI] [PubMed] [Google Scholar]
- 22.Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspective. JAMA. 2012; 307(15):1636–40 [DOI] [PubMed] [Google Scholar]
- 23.Efficace F, Cardoni A, Cottone F, Vignetti M, Mandelli F. Tyrosine-kinase inhibitors and patient-reported outcomes in chronic myeloid leukemia: A systematic review. Leuk Res. 2013;37(2):206–13 [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration: Guidance for Industry Patient-reported outcome measures: Use in medical product development to support labeling claims. US Department of Health and Human Services Food and Drug Administration; December, 2009. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf (Accessed November, 2013). [Google Scholar]
- 25.Efficace F, Baccarani M, Breccia M, Saussele S, Abel G, Caocci G, et al. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res. 2013. September 13 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Williams LA, Gonzalez AG, Ault P, Mendoza TR, Sailors ML, Williams JL, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122(5):641–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–22 [DOI] [PubMed] [Google Scholar]
- 28.Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–24 [DOI] [PubMed] [Google Scholar]
- 29.Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012;21(8):1305–14 [DOI] [PubMed] [Google Scholar]


