Abstract
Prior to Janus kinase inhibitors, available therapies for myelofibrosis were generally supportive and did not improve survival. This analysis compares efficacy outcomes of patients with myelofibrosis in the control arms (placebo [n=154] and best available therapy [n=73]) from the two phase 3 COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment (COMFORT) studies. Spleen volume was assessed by magnetic resonance imaging/computed tomography at baseline and every 12 weeks through week 72; spleen length was assessed by palpation at each study visit. Health-related quality of life and symptoms were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 Items at baseline and in weeks 4, 8, 12, 16 and 24 in COMFORT-I and in weeks 8, 16, 24 and 48 in COMFORT-II. The demographic and baseline characteristics were similar between the control arms of the two studies. One patient who received placebo and no patients who received best available therapy had a ≥35% reduction in spleen volume from baseline at week 24. At 24 weeks, neither placebo nor best available therapy had produced clinically meaningful changes in global quality of life or symptom scales. Non-hematologic adverse events were mostly grade 1/2; the most frequently reported adverse events in each group were abdominal pain, fatigue, peripheral edema and diarrhea. These data suggest that non–Janus kinase inhibitor therapies provide little improvement in splenomegaly, symptoms or quality of life as compared with placebo. Both COMFORT-I (NCT00952289) and COMFORT-II (NCT00934544) studies have been appropriately registered with clinicaltrials.gov.
Introduction
Myelofibrosis has the worst median overall survival of the BCR-ABL–negative myeloproliferative neoplasms and can present as primary disease or evolve from polycythemia vera or essential thrombocythemia.1 Regardless of its origin, myelofibrosis is characterized by bone marrow fibrosis, progressive splenomegaly, cytopenias and burdensome debilitating constitutional symptoms,2 leading to a severely diminished quality of life (QoL).3 The median survival of patients with myelofibrosis ranges from 2 to 11 years, depending on defined prognostic factors.4
Treatment options for myelofibrosis have generally been palliative without impact on the natural history of the disease. Although stem cell transplant is a potentially curative treatment for patients with myelofibrosis, it is an option for only a minority of patients, and the rates of mortality and treatment-related toxicities and complications remain high.5 However, the recent identification of aberrant activation of the Janus kinase (JAK) signaling pathway – along with an improved understanding that cytokines that signal through JAK1 and JAK2 play a role in the development of myeloproliferative neoplasms – has led to the development of new therapeutic approaches.6–9
Ruxolitinib (also known as INC424 and INCB018424) is a potent and selective inhibitor of JAK1 and JAK2. It was approved in November 2011 by the US Food and Drug Administration for the treatment of intermediate- or high-risk myelofibrosis10 and more recently by Health Canada11 and the European Commission for the treatment of myelofibrosis-related splenomegaly or symptoms.12 Ruxolitinib was approved based on data from two pivotal phase 3 COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment (COMFORT) trials:13,14 COMFORT-I was double-blind and placebo-controlled and COMFORT-II was an open-label study that compared ruxolitinib with best available therapy (BAT). In both studies, patients who received ruxolitinib had rapid and durable reductions in splenomegaly and improvements in disease-related symptoms, role functioning and QoL measures. In contrast, patients in the control arms of both of these studies generally had increases in splenomegaly and worsening of symptoms, demonstrating that non–JAK inhibitor treatments were not efficacious. Here, we present a post hoc analysis specifically evaluating the efficacy outcomes and safety of the placebo arm of COMFORT-I compared with those of the BAT arm of COMFORT-II.
Methods
Patients, study design and treatment
COMFORT-I was a randomized (1:1), double-blind, multicenter study that compared the safety and efficacy of ruxolitinib 15 or 20 mg twice daily (n=155) with placebo (n=154) in patients with primary myelofibrosis, post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis.13 COMFORT-II was a randomized (2:1), open-label, multicenter study that compared ruxolitinib 15 or 20 mg twice daily (n=146) with BAT (n=73) in patients with patients with primary myelofibrosis, post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis.14 The choice of dose of ruxolitinib (15 or 20 mg twice daily) was based on platelet count at baseline (100–200 or >200×109/L, respectively). Eligibility criteria were previously reported elsewhere.13,14 BAT included any commercially available agents (as monotherapy or in combination) or no therapy at all and could be changed during the treatment phase (Table 1).14 Crossover was permitted in each study according to the protocol-specified criteria previously described.13,14 The COMFORT-I and COMFORT-II studies were approved by the institutional review boards of the respective institutions and conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
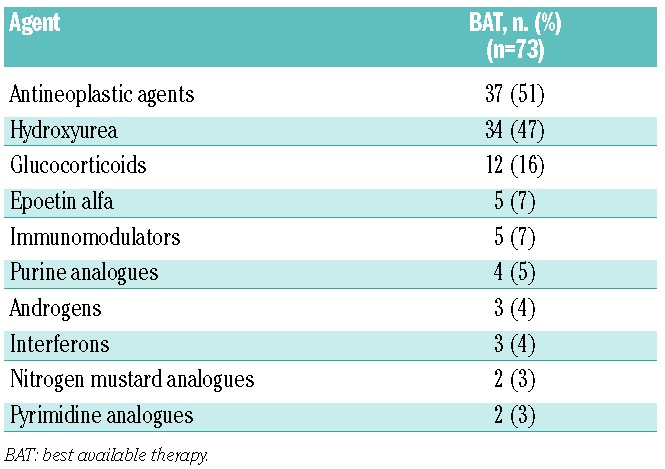
Table 1.
Patients’ treatments on the BAT-treated arm.14
Assessments
As previously described, the analysis for this study was conducted when all enrolled patients completed week 24 for COMFORT-I (data cut-off date, November 2, 2010)13 or week 48 for COMFORT-II (data cut-off date, January 4, 2011)14 or were withdrawn from the study. The primary end-point was a reduction of ≥35% in spleen volume from baseline at week 24 and week 48 for COMFORT-I and COMFORT-II, respectively. COMFORT-II had a key secondary end-point (type I error controlled) of reduction of ≥35% in spleen volume from baseline at week 24. Spleen volume was assessed by magnetic resonance imaging (MRI) or computed tomography (CT; in those patients who were not candidates for MRI) every 12 weeks up to week 72. In COMFORT-I, any patient who crossed over before week 24 or discontinued from the study was counted as a non-responder for response measures of reduction in spleen volume and symptom improvement. In COMFORT-II, any patient who discontinued from the study or had a protocol-defined event of disease progression14 before week 48 was considered to be a non-responder. Spleen length was assessed by manual palpation at every study visit.
Health-related QoL and symptoms were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 Items (EORTC QLQ-C30), which was completed by patients at baseline in both studies, in weeks 4, 8, 12, 16 and 24 in COMFORT-I and in weeks 8, 16, 24 and 48 in COMFORT-II. The EORTC QLQ-C30 includes five functional scales (physical, role, emotional, cognitive and social functioning), a global health status/QoL scale and nine symptom scales (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties). All scores range from 0 to 100. For functional and global health status/QoL scales, higher scores indicate better QoL; for symptom scales, higher scores indicate more severe symptoms. For the EORTC QLQ-C30 global health status/QoL scale, patients with a 10% (or 10-point) improvement in score were considered to have clinically meaningful improvements.15
Results
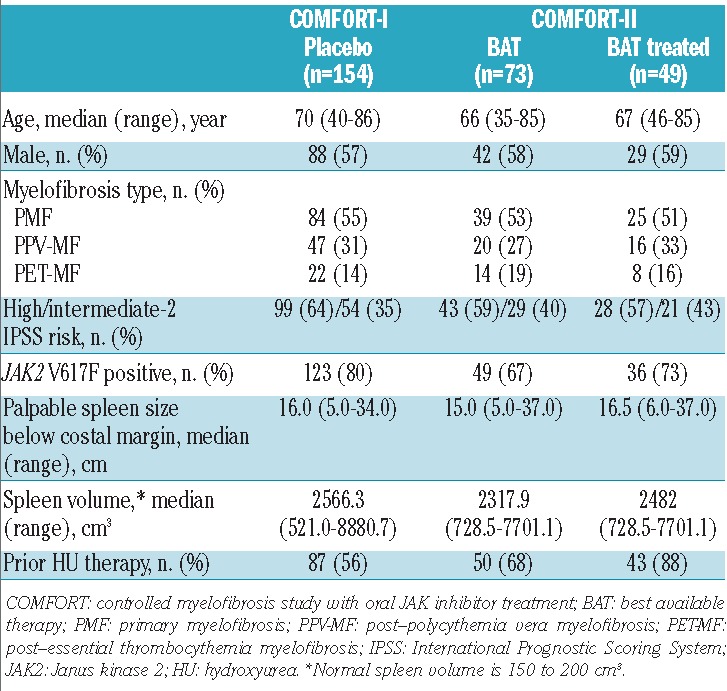
In the COMFORT-I and COMFORT-II studies, 154 patients received placebo and 73 patients received BAT, respectively, and all were included in the primary efficacy analyses except for one patient in each group with a missing baseline spleen volume assessment. Among those who were randomized to BAT, 49 patients (67%) received any BAT medication – referred to hereafter as BAT-treated –and 24 patients (33%) received no therapy as BAT during the randomized treatment phase. As previously described by Harrison et al.,14 of the 49 BAT-treated patients, 37 patients (76%) were given antineoplastic agents, most commonly hydroxyurea (n=32; 65%), and 12 patients (24%) were given glucocorticoids. The demographic and baseline characteristics were generally similar between the control arms of the two studies (Table 2), including spleen length below the costal margin: median, 16.0 cm (range, 5.0–34.0) and 15.0 cm (range, 5.0–37.0) in the placebo and BAT arms, respectively. Compared with patients who received BAT, those who received placebo were older and higher proportions had high-risk disease and were JAK2 V617F-positive and hydroxyurea-naïve.
Table 2.
Patients’ demographic and baseline characteristics.
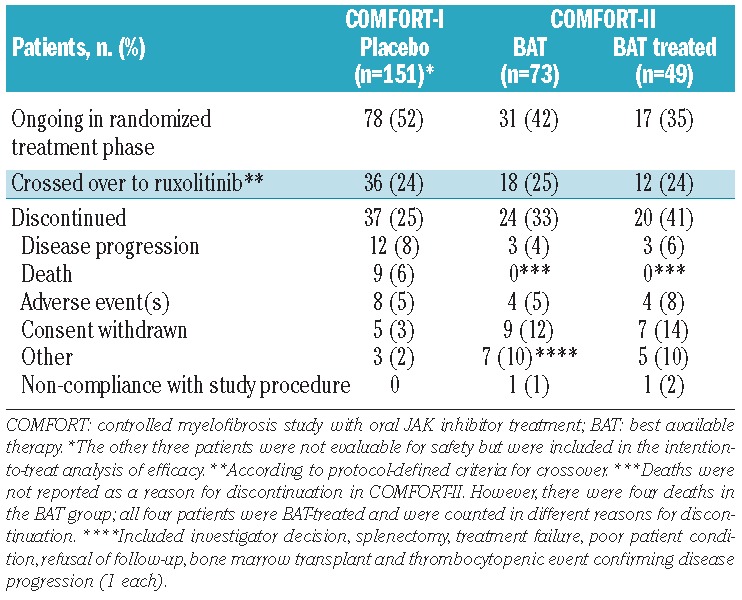
Overall, 52% of patients receiving placebo and 42% of patients receiving BAT (35% BAT-treated) were ongoing in the randomized treatment phase at the data cut-off date (Table 3). Of those who discontinued the study, a similar proportion of patients in each group crossed over to receive ruxolitinib [placebo, 36 of 151 (24%); BAT, 18 of 73 (25%; of whom 12 of 18 (67%) were BAT-treated)].
Table 3.
Patients’ disposition.
Efficacy
Spleen size
As shown in Figure 1, only one patient (0.7%) who received placebo and no patients who received BAT achieved a ≥35% reduction in spleen volume from baseline at week 24 (the primary end-point of COMFORT-I and the key secondary end-point of COMFORT-II). The patient in the placebo arm who achieved this reduction in spleen volume died from disease progression 4 days later, possibly from splenic infarction.16 During the 24-week period, the majority of patients treated with placebo (75%) or BAT (69%) had measurable increases in spleen volume. Of those patients in the BAT group, a similar proportion of BAT-treated patients [18 of 26 (69%)] and of those who received no BAT medications (13 of 19 [68%]) had spleen volume increases.
Figure 1.
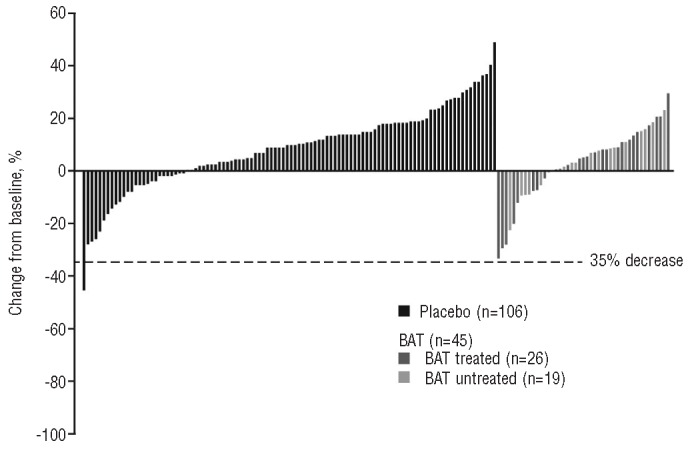
Percent change from baseline in spleen volume as assessed by magnetic resonance imaging/computed tomography at week 24 for patients with baseline and ≥1 post-baseline assessment. BAT: best available therapy.
The mean percentage change in spleen volume over time increased in the control groups of both studies and, in general, these increases were observed by the first assessment (12 weeks; Figure 2A). Changes in palpable spleen length in both groups mirrored the increases in spleen volume assessed by MRI/CT (Figure 2B). Although treatment in the BAT group was not blinded to the patients, all scans were read by a central blinded reader and, in general, similar increases in spleen volume assessed by MRI/CT were observed between the overall BAT and BAT-treated groups over time. With palpable spleen length, in which measurements were not blinded, some differences in the mean percentage change in spleen length over time between the BAT-treated and overall BAT populations were observed, with an apparent tendency toward smaller changes.
Figure 2.
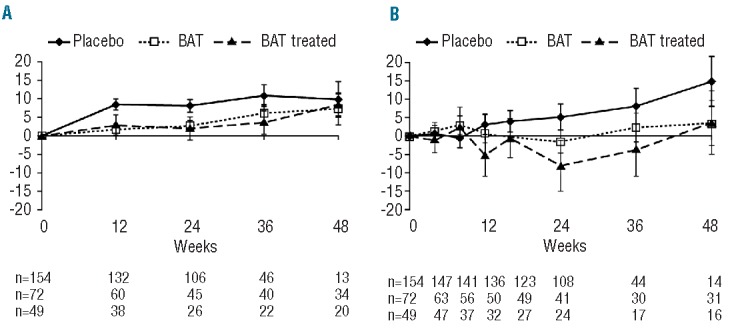
Mean percentage change from baseline in (A) spleen volume as assessed by magnetic resonance imaging/computed tomography and (B) palpable spleen length over time. BAT: best available therapy.
Symptoms and other patient-reported outcomes
At week 24, patients in the placebo arm and BAT arms did not have clinically meaningful improvements from baseline in any of the health-related QoL (Figure 3) or symptom scores (Figure 4), whereas the BAT-treated subgroup had a clinically meaningful improvement in global health status/QoL. Patients in each of the control groups had worsening from baseline in role functioning, which was clinically significant for the placebo group at week 24. Additionally, regardless of the control group, patients generally had worsening of myelofibrosis-associated symptoms, including appetite loss, dyspnea, insomnia and pain. In particular, worsening of appetite loss and dyspnea was observed in BAT-treated patients, and worsening of pain was noted in placebo patients. Although scores for insomnia showed some improvements with placebo and scores for pain and fatigue showed improvements in BAT-treated patients, no clinically meaningful improvements were observed in either group.
Figure 3.
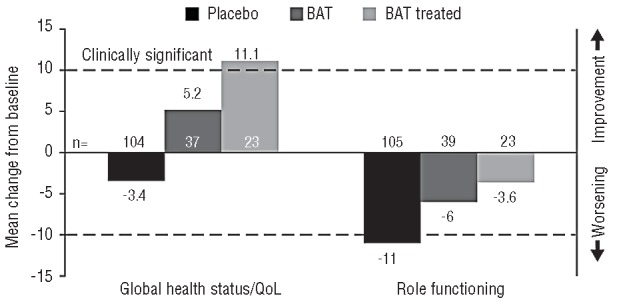
Mean change from baseline to week 24 in European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QoL) Questionnaire-Core 30 Items (QLQ-C30) scores. Only patients with measurements at both baseline and week 24 were included. EORTC QLQ-C30 global health status/QoL and functioning scales range from 0 to 100, with higher scores indicating better health-related QoL. A clinically meaningful treatment difference was defined as a 10-point improvement or 10-point worsening from baseline for global health status/QoL. BAT: best available therapy.
Figure 4.
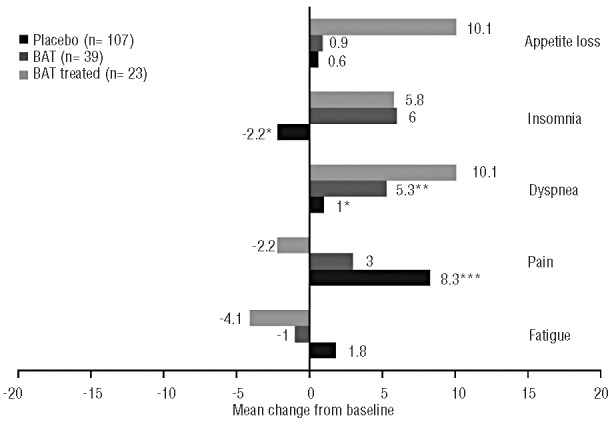
Mean change from baseline to week 24 in select European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QoL) Questionnaire Core 30 Items (QLQ-C30) scores. EORTC QLQ-C30 symptom scales range from 0 to 100, with higher scores indicating worse symptoms. BAT: best available therapy. *n=105. **n=38. ***n=104.
Safety
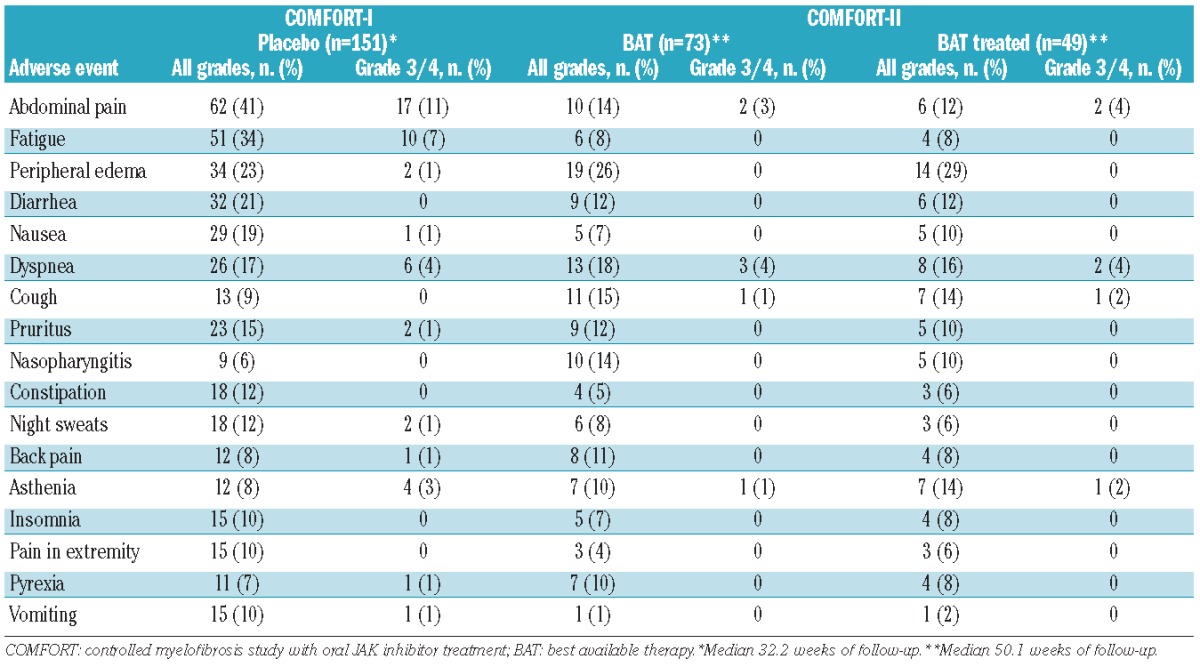
As shown in Table 4, patients who received placebo generally reported higher rates of the most frequent non-hematologic adverse events. Events that occurred with approximately ≥10% higher incidence in the placebo arm than in the BAT arm were abdominal pain (41% with placebo versus 14% with BAT), fatigue (34% versus 8%), diarrhea (21% versus 12%) and nausea (19% versus 7%). Peripheral edema was the most frequently reported adverse event in the BAT group (26%) and was also reported at a similar rate in the placebo group (23%). There were no events that occurred with a ≥10% higher incidence in the BAT arm than in the placebo arm; cough and nasopharyngitis were reported at a ≥5% higher incidence in patients who received BAT compared with those who received placebo. When BAT-treated patients were considered, peripheral edema, cough and asthenia were reported at a ≥5% higher rate than in patients who received placebo. In general, the rates of grade 3/4 adverse events were similar between the control arms, although rates of abdominal pain (11% with placebo versus 3% with BAT) and fatigue (7% versus 0%) were higher in the placebo arm than in the BAT arm. Of the most common grade 3/4 events reported in the BAT arm of COMFORT-II, all but one (a case of dyspnea) occurred in BAT-treated patients.
Table 4.
Non-hematologic adverse events regardless of study drug relationship (≥10% in any group).
Discussion
More than 130 years after the first description of myelofibrosis by Heuck in 1879,17 the treatment of patients with this condition has remained mostly supportive and the natural progression of myelofibrosis has not been significantly improved by drug therapy.18 In both of the COMFORT studies, it was demonstrated that significantly more patients who received ruxolitinib had rapid and durable reductions in splenomegaly as well as improvements in symptoms and QoL compared with patients in the control arms, and that these reductions were maintained throughout each study period.13,14 The lack of efficacy with non–JAK inhibitor therapeutics in COMFORT-II is of the utmost relevance because conclusions from previous studies of therapeutics in patients with myelofibrosis have been hampered by small numbers and the lack of objective response criteria and a control arm.19–22 For example, one retrospective study showed that the use of hydroxyurea, which was the most common treatment in the BAT arm, resulted in a 40% rate of clinical improvement by International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) criteria, with improvements in splenomegaly, symptoms and blood counts.20 However, this study only included 40 patients, and the subgroups evaluated were small, with 18 patients evaluable for spleen size and 22 patients evaluable for constitutional symptoms; in addition, the lack of a control arm makes the reported response rates difficult to interpret.
We acknowledge that a direct side-by-side comparison would be ideal to assess the efficacy of BAT compared with placebo and recognize that the data presented here are inevitably limited because they are derived from two different studies. However, although there are some differences in the patient populations and eligibility and inclusion criteria of the COMFORT-I and COMFORT-II studies, the general similarities in the trial end-points, period of accrual and responses achieved with ruxolitinib in each of the studies allow for some meaningful comparisons. In this post hoc analysis, patients who received BAT had numerically similar increases in spleen size as those who received placebo, and no clinically meaningful improvements in QoL or symptoms were seen in either the placebo or BAT arm. These data suggest that non–JAK inhibitor treatments for myelofibrosis provide little improvement in spleen size, symptoms or health-related QoL compared with placebo. Although it is conceivable that some patients in the BAT arm of COMFORT-II were not sufficiently aggressively treated, this is unlikely as evidenced by the observation that some patients had spleen regression and that anemia and transfusion rates were equivalent across the two arms of COMFORT-II.
Whereas COMFORT-I was a double-blind, placebo-controlled study, COMFORT-II was an open-label study in which treatment in the BAT arm was based on physicians’ choice and could, therefore, include any single treatment or combination of treatments, including any sequence of treatments or no treatment (i.e. “watchful waiting”). Based on these options, approximately one-third of the patients in the BAT arm received no treatment.14 To account for this possible confounding factor, the efficacy and safety data of the 49 patients (67%) who received any BAT medication were evaluated as a separate subgroup. A similarly low proportion of patients who received BAT medications had reductions in spleen volume as assessed by MRI/CT compared with those who did not receive any BAT medication. The increases in spleen volume in the BAT-treated and overall BAT groups also mirrored those observed with placebo, suggesting that non–JAK inhibitor therapies do not have any significant effect on spleen size.
The symptomatic burden of patients is an important clinical feature of myelofibrosis because these debilitating symptoms contribute to a diminished QoL and interfere with activities of daily living.3 At 24 weeks, neither patients in the placebo arm nor those in the BAT arm had clinically meaningful improvements from baseline in any of the QoL or symptom scores. Patients in the placebo group had a clinically significant worsening in role functioning from baseline at week 24. Although BAT-treated patients showed a clinically meaningful improvement in global health status/QoL, these patients did not have significant improvements in role functioning or in any myelofibrosis-associated symptom and, in fact, had significant worsening of appetite loss and dyspnea at week 24. These data confirm those reported by Mesa et al.3 in which patients reported having a significant burden of disease-related symptoms, even though the majority received one or more non–JAK inhibitor treatment. Thus, even with the flexibility of therapeutic options in the BAT arm, non–JAK inhibitor treatments were inadequate at controlling patients’ symptoms and QoL, just as was placebo. This inadequacy highlights the natural progression of myelofibrosis in these patients, even over the short 24-week study period. Although it is interesting to compare the control arms of the COMFORT trials to illustrate the unmet need of patients treated with BAT, it is important to note that the outcomes of patients in both the placebo and BAT arms were significantly inferior to those of the ruxolitinib-treated patients in both studies.13,14
In summary, patients who received BAT in COMFORT-II appeared to fare no better than patients who received placebo in COMFORT-I, and these findings illustrate that conventional therapeutic alternatives for patients with myelofibrosis do not alleviate the symptom burden of the disease in a meaningful way, underscoring the need for better treatments. The clinical success of JAK1/2 inhibitors, including the superiority of ruxolitinib in the COMFORT studies,13,14 indicates that JAK inhibitor therapy for myelofibrosis provides a valuable and effective new treatment option for patients.
Acknowledgments
The authors would like to thank Candice Willmon, PhD, for her medical editorial assistance with this manuscript. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): consensus on terminology by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT). Leuk Res. 2007;31(6):737–40 [DOI] [PubMed] [Google Scholar]
- 2.Cervantes F, Passamonti F, Barosi G. Life expectancy and prognostic factors in the classic BCR/ABL-negative myeloproliferative disorders. Leukemia. 2008;22(5):905–14 [DOI] [PubMed] [Google Scholar]
- 3.Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76 [DOI] [PubMed] [Google Scholar]
- 4.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901 [DOI] [PubMed] [Google Scholar]
- 5.Ballen K. How to manage the transplant question in myelofibrosis. Blood Cancer J. 2012;2(3):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8 [DOI] [PubMed] [Google Scholar]
- 7.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61 [DOI] [PubMed] [Google Scholar]
- 8.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352 (17):1779–90 [DOI] [PubMed] [Google Scholar]
- 9.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97 [DOI] [PubMed] [Google Scholar]
- 10.Jakafi (ruxolitinib) [package insert]. Wilmington, DE: Incyte Corporation; 2011 [Google Scholar]
- 11.Novartis drug Jakavi first medication to receive European Commission approval to treat patients with myelofibrosis [Internet]. Switzerland: Novartis Pharmaceuticals; [updated 28 August 2012; cited 15 May 2013]. Available from: http://www.novartis.com/newsroom/media-releases/en/2012/1636508.shtml [Google Scholar]
- 12.Jakavi (ruxolitinib) product monograph. Dorval, QC, Canada: Novartis Pharmaceuticals Canada Inc; 2012 [Google Scholar]
- 13.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9): 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98 [DOI] [PubMed] [Google Scholar]
- 15.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–44 [DOI] [PubMed] [Google Scholar]
- 16.Mesa RA, Shields A, Hare T, Erickson-Viitanen S, Sun W, Sarlis NJ, et al. Progressive burden of myelofibrosis in untreated patients: assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study. Leuk Res 2013;37(8):911–6 [DOI] [PubMed] [Google Scholar]
- 17.Heuck G. Zwei fälle von leukämie mit eigenthümlichem blut-resp knochenmarks-befund. Arch Pathol Antat Physiol Virchows. 1879;78:475–96 [Google Scholar]
- 18.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesa RA, Pardanani AD, Hussein K, Wu W, Schwager S, Litzow MR, et al. Phase1/-2 study of pomalidomide in myelofibrosis. Am J Hematol. 2009;85(2):129–30 [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Trillos A, Gaya A, Maffioli M, Arellano-Rodrigo E, Calvo X, Diaz-Beya M, et al. Efficacy and tolerability of hydroxy-urea in the treatment of the hyperproliferative manifestations of myelofibrosis: results in 40 patients. Ann Hematol. 2010;89(12): 1233–7 [DOI] [PubMed] [Google Scholar]
- 21.Besses C, Martínez-Sellés M. Anagrelide and cardiovascular events. Much ado about nothing? Leuk Res. 2011;35(12):1543–4 [DOI] [PubMed] [Google Scholar]
- 22.Mesa RA, Green A, Barosi G, Verstovsek S, Vardiman J, Gale RP. MPN-associated myelofibrosis (MPN-MF). Leuk Res. 2011; 35(1):12–3 [DOI] [PubMed] [Google Scholar]


