Abstract
Although preservation of the spleen following abdominal trauma and spleen-preserving surgical procedures have become gold standards, about 22,000 splenectomies are still conducted annually in the USA. Infections, mostly by encapsulated organisms, are the most well-known complications following splenectomy. Recently, thrombosis and cancer have become recognized as potential adverse outcomes post-splenectomy. Among more than 4 million hospitalized USA veterans, we assessed incidence and mortality due to infections, thromboembolism, and cancer including 8,149 cancer-free veterans who underwent splenectomy with a follow-up of up to 27 years. Relative risk estimates and 95% confidence intervals were calculated using time-dependent Poisson regression methods for cohort data. Splenectomized patients had an increased risk of being hospitalized for pneumonia, meningitis, and septicemia (rate ratios=1.9–3.4); deep venous thrombosis and pulmonary embolism (rate ratios=2.2); certain solid tumors: buccal, esophagus, liver, colon, pancreas, lung, and prostate (rate ratios =1.3–1.9); and hematologic malignancies: non-Hodgkin lymphoma, Hodgkin lymphoma, multiple myeloma, acute myeloid leukemia, chronic lymphocytic leukemia, chronic myeloid leukemia, and any leukemia (rate ratios =1.8–6.0). They also had an increased risk of death due to pneumonia and septicemia (rate ratios =1.6–3.0); pulmonary embolism and coronary artery disease (rate ratios =1.4–4.5); any cancer: liver, pancreas, and lung cancer, non-Hodgkin lymphoma, Hodgkin lymphoma, and any leukemia (rate ratios =1.3–4.7). Many of the observed risks were increased more than 10 years after splenectomy. Our results underscore the importance of vaccination, surveillance, and thromboprophylaxis after splenectomy.
Introduction
In the past, the spleen was considered unnecessary for life. Today, we know that the spleen is a reticuloendothelial organ with important hematologic and immunological functions, including clearance of bacteria from the blood and generation of immune responses to certain pathogens.1
Although preservation of the spleen following abdominal trauma and spleen-preserving surgical procedures have gained significant attention in recent years,2 about 22,000 splenectomies are still conducted annually (for all causes) in the USA.3 In most hospitals, trauma and incidental splenectomy remain the primary indications; however, spleen removal in trauma patients is becoming less common as a result of more conservative non-operative management of splenic injury.3 The most frequent medical indication is a hematologic disorder, such as autoimmune hemolytic anemia.
Bacterial infections, mostly by encapsulated organisms, are the best known complications of splenectomy4–14 but other types of infections also occur, including those caused by Gram-negative bacteria.15–17 Immunological and hematologic abnormalities have been described as well, including depressed phagocytic activity, diminished immunoglobulin M (IgM) production, depressed T-cell function, and leukocytosis and thrombocytosis, all of which may contribute to late complications.4,18–21 Post-splenectomy infections may be fatal, particularly in younger patients, those with an underlying malignant disease, and during the initial years following splenectomy.5,6,8,9
More recently, venous thromboembolism has become appreciated as another potential complication of splenectomy,9,22,23 although some studies have reported no excess risk.24 Portal vein thrombosis has been reported most often,25–27 while the risk of other types of thromboembolism is poorly defined.
It is unclear whether splenectomy increases the risk of developing cancer. In both rat and mouse models splenectomy has been associated with a significant increase of malignant tumor induction,28,29 along with a decrease in the peripheral blood lymphocyte count after tumor inoculation in a mouse model.30 In some,9,31 but not all,10,32 epidemiological studies, splenectomy has been associated with an excess risk of developing cancer. An important limitation of these studies is their lack of exclusion of patients with a malignancy prior to splenectomy.
To expand our insights regarding long-term risks of splenectomy, we have conducted the largest follow-up study to date of cancer-free subjects who have undergone this procedure. In over four million male military veterans admitted to Veterans Affairs (VA) hospitals we identified 8,149 cancer-free veterans who underwent splenectomy with a follow-up of up to 27 years. In this cohort, we assessed patterns of hospitalization for infections, thromboembolism, and malignancies following splenectomy.
Methods
Study population
Based on USA census data, an estimated 30 million veterans were entitled to admission to VA hospitals during the study period.33 The VA database has been previously described.34,35
In the present study, splenectomized patients were identified from the hospital discharge summary records [coded in the 8th and 9th revisions of the USA version of the International Classification of Diseases (ICD): ICD8 45.1, ICD9 41.2, 41.43, 41.5] and were included in the study (n=8,149). To minimize the influence of reverse causality (i.e., undetected cancer requiring the splenectomy), all analyses were restricted to individuals whose first VA discharge with a splenectomy occurred at least 1 year prior to the first hospitalization listing a diagnosis of cancer. Thus, patients were followed from 1 year after their initial hospital discharge until the first discharge diagnosis of infection, thromboembolism, malignancy, death, or end of study, whichever occurred first. The time to develop infection, thromboembolism, or malignancy (i.e., latency) was estimated by subtracting the date of discharge from the first hospitalization listing a splenectomy from the date of the first hospitalization listing a diagnosis of infection, thromboembolism, or malignancy.
Dates of death were ascertained from record linkage to Social Security Administration mortality files. With such linkage, death reporting is believed to be 96% complete.36 Among the 8,149 splenectomized men who were selected for the study, 6,731 were eligible for matching to the National Death Index (alive as of January 1, 1979). In addition, a random sample (n=6,731) of the eligible VA cohort was selected to match these patients on the basis of race and year of birth (1:1 sample). The National Death Index provided death certificate matching for the men who had undergone splenectomy and for the matched controls (n=13,462).
Statistical analysis
Relative risk (RR) estimates and 95% confidence intervals (CI) were calculated using time-dependent Poisson regression methods for cohort data.37 Calculations were performed using the AMFIT program (Epicure Version 2.0; HiroSoft International Corporation, Seattle, Washington, USA). All risk estimates were adjusted for attained age (<40, 40–49, 50–59, 60–69, 70–79, 80 or more years) and calendar year (1969–1974, 1975–1979, 1980–1984, 1985–1989, 1990–1996), race (African-American or white), number of hospital visits (1–2, 3–4, 5 or more), and time between study entry and exit (2–3, 4–5, 6–9, 10–14, 15 or more years). Risk estimates for buccal, esophageal, and liver cancers were also adjusted for a hospital discharge diagnosis of alcohol-related disorders because inclusion of this variable in the regression models resulted in a >10% change in the risk estimates. Adjustment for a hospital discharge diagnosis of hypertension was necessary for the kidney cancer analysis. No other diagnoses were found to materially (>10%) change the risk estimates for the other outcomes. All P values and confidence intervals were two-sided, and P values <0.05 were considered statistically significant. We conducted sensitivity analyses when numbers allowed (>5 exposed cases). Analyses were stratified by race, age at splenectomy/entry (<50, 50+), calendar year at splenectomy/entry (1969–1979, 1980–1996), latency (2–5 years, 5+), and subsets of patients with only trauma or no autoimmune conditions (as in previous splenectomy studies).
An exemption from Institutional Review Board review was obtained from the NIH Office of Human Subjects Research because we analyzed existing data without personal identifiers. Informed consent was waived because there was no contact with study subjects.
Results
Among over 4.5 million military USA veterans admitted to a VA hospital, a total of 8,149 cancer-free men were splenectomized during the study period. The characteristics of the study population are shown in Table 1. The majority were white (84%) and their median age at splenectomy was 53 years.
Table 1.
Patients’ characteristics.

Risk and mortality due to infection
Risk of hospitalization for infections and associated mortality due to infections is shown in Table 2. Splenectomized patients had a significantly increased risk of pneumococcal pneumonia (RR=2.06; 95% CI 1.85–2.30), pneumonia not otherwise specified (RR=1.94; 95% CI 1.84–2.04), meningitis (RR=2.44; 95% CI 1.77–3.38), and septicemia (RR=3.38; 95% CI 3.12–3.67). Splenectomized patients had a 1.58-fold and 3.02-fold increased risk of death from pneumonia (95% CI 1.20–2.08) and septicemia (95% CI 1.80–5.06), respectively. When analyzing risk and mortality by race, calendar year at splenectomy/entry (1969–1979 versus 1980–1996), age at splenectomy/entry (below versus above 50 years), in trauma patients only (n=1,831), and in patients with a previous autoimmune disease (n=1,843), the risk estimates were essentially the same in all subgroups (Online Supplementary Tables S1 and S2). In analyses based on latency (the time between splenectomy and subsequent infection), the risk of infectious diseases was still significantly elevated more than 10 years after splenectomy (Table 3). Risk of death from infections was also significantly increased more than 10 years after splenectomy (data not shown).
Table 2.
Risk of being hospitalized (SIR) and risk of dying (SMR) due to selected infectious and thromboembolic conditions following splenectomy.
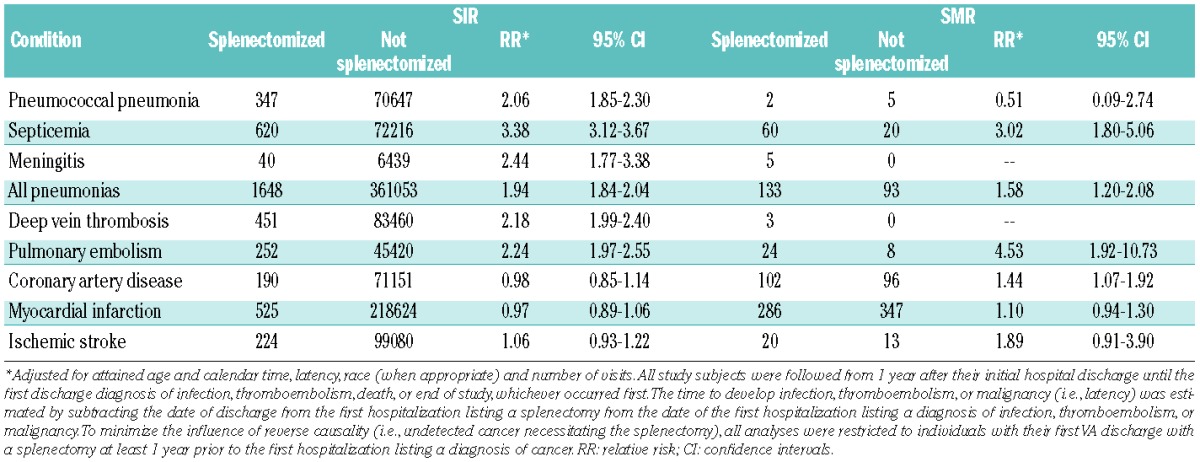
Table 3.
Risk of being hospitalized (SIR) due to selected infectious and thromboembolic conditions following splenectomy, by latency.

Risk and mortality due to thromboembolism
Table 2 shows risks of hospitalization for thromboembolic disease and associated mortality. Splenectomized patients had an increased risk of developing deep vein thrombosis (RR=2.18; 95% CI 1.99–2.40) and pulmonary embolism (RR=2.24; 95% CI 1.97–2.55), but not acute myocardial infarction, coronary artery disease, or ischemic stroke. Splenectomized patients also had an increased risk of death from pulmonary embolism (RR=4.53; 95% CI 1.92–10.73) and coronary artery disease (RR=1.44; 95% CI 1.07–1.92; Table 4). Risk estimates were not significantly different when analyzing risk and mortality by latency (Table 3), race, calendar year and age at splenectomy/entry (data not shown), in trauma patients only, and in patients with a previous autoimmune disease (Online Supplementary Tables S1 and S2).
Table 4.
Risk of being hospitalized (SIR) and risk of dying (SMR) due to selected hematologic and solid tumors following splenectomy.

Risk and mortality due to malignancy
A total of 1,094 (13%) splenectomized patients were diagnosed with cancer during the follow-up. As shown in Table 4, there was an increased risk of any cancer (RR=1.51; 95% CI 1.42–1.60). In particular, the risk was significantly elevated for buccal (RR=1.26; 95% CI 1.00–1.58), esophageal (RR=1.60; 95% CI 1.12–2.27), liver (RR=1.88; 95% CI 1.22–2.89), colon (RR=1.33; 95% CI 1.01–1.76), pancreatic (RR=1.87; 95% CI 1.27–2.75), lung (RR=1.24; 95% CI 1.09–1.40), and prostate cancer (RR=1.26; 95% CI 1.06–1.48), and also non-Hodgkin lymphoma (RR=3.21; 95% CI 2.47–4.17), Hodgkin lymphoma (RR=3.74; 95% CI 2.01–6.97), multiple myeloma (RR=1.82; 95% CI 1.08–3.07), acute myeloid leukemia (RR=6.04; 95% CI 3.92–9.29), chronic lymphocytic leukemia (RR=2.86; 95% CI 1.80–4.55), chronic myeloid leukemia (RR=5.81; 95% CI 3.54–9.51), and any leukemia (RR=5.20; 95% CI 4.23–6.39).
Furthermore, splenectomized patients had an increased risk of death from any cancer (RR=1.53; 95% CI 1.36–1.73); especially liver (RR=1.8; 95% CI 1.20–3.13), pancreatic (RR=2.18; 95% CI 1.20–3.98), and lung cancer (RR=1.32; 95% CI 1.10–1.59), as well as non-Hodgkin lymphoma (RR=4.69; 95% CI 1.97–11.18) and any leukemia (RR=2.45; 95% CI 1.36–4.42). When analyzing risk estimates and mortality stratified by race, calendar year and age at splenectomy/entry, and in analyses limited to trauma patients, the risk estimates were essentially the same as the overall pattern. In sensitivity analyses limited to patients with previous autoimmune disease, the risks were also similar, except that the risks for hematologic malignancies were higher (Online Supplementary Tables S3 and S4). When excluding patients with a prior autoimmune disease, the risks of malignancies were still significantly elevated (Online Supplementary Tables S3 and S4).
In analyses stratified by latency, the risk of most malignancies tended to be highest during the first 2–5 years following splenectomy. However, after more than 10 years, there was still a significantly increased risk of esophageal, liver and lung cancers, as well as non-Hodgkin lymphoma, Hodgkin lymphoma, acute myeloid leukemia, chronic lymphocytic leukemia, chronic myeloid leukemia, and any leukemia (Table 5).
Table 5.
Risk of being hospitalized (SIR) due to selected hematologic and solid tumors following splenectomy, by latency.

Discussion
This large study involving over four million USA veterans enabled several important observations. Based on 8,149 cancer-free veterans who underwent splenectomy, compared to all other subjects in the database, we observed an increased risk of infections, thromboembolism, and malignancies. Furthermore, we found the overall risk of death from these disorders to be elevated. Increased risks persisted even more than 10 years after splenectomy.
Splenectomized patients had a 2- to 3-fold increased risk of pneumococcal pneumonia, other pneumonias, meningitis, and septicemia. It is well known that splenectomized patients have an increased risk of infections due to Gram-positive encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitides, and Haemophilus influenza type B.4–14 We did not have information on the underlying pathogen, but a large proportion of community-acquired pneumonias and meningitis are caused by encapsulated microbes.38,39 The excess risk of septicemia may be caused by Gram-positive or Gram-negative bacteria, as previously reported.15–17 The patterns of risk and mortality in our study and in others indicate the importance of pneumococcal vaccination in this population of patients. We previously showed that antibody levels are decreased in immunocompromised individuals and patients who undergo splenectomy because of chronic lymphocytic leukemia or Hodgkin lymphoma.40 These patients might require re-evaluation of antibody levels to determine if there is a need to re-vaccinate. Furthermore, patients with a poor response to vaccination might benefit from prophylactic penicillin41 or prompt treatment with antibiotics in the event of fever or other signs of infections.42
We found a 2-fold increased risk of deep vein thrombosis and pulmonary embolism following splenectomy, and 4.5-fold and 1.4-fold increased risks of death from pulmonary embolism and coronary artery disease, respectively. These complications appear due to a hypercoagulable state post-splenectomy along with a transient thrombocytosis. Underlying mechanisms may include platelet activation, disturbance and activation of endothelium, and altered lipid profile.3 In our study, the risk of thrombosis was increased not only in the immediate period following splenectomy, but also more than 10 years following the procedure, suggesting a life-long susceptibility state. In the Swedish mortality study, a significant 3- to 5-fold increase in death due to venous thromboembolism was observed in splenectomized patients, but no latency analyses were presented.9
An older epidemiological study based on 740 USA veterans who underwent splenectomy after trauma during the Second World War found no increase in the risk of cancer.10 Similarly, in a Danish study of 1,103 patients (average follow-up of 6.8 years), no increase in cancer was observed in those who underwent splenectomy after traumatic rupture of the spleen; however, an increased risk for some specific cancer sites was found among 5,212 patients who underwent splenectomy for non-traumatic reasons.31 A Swedish study of 1,295 patients (average follow-up 11.1 years) splenectomized for external trauma showed no significant excess of cancer, while 985 patients (average follow-up 9.0 years) whose splenectomy accompanied surgical treatment of non-malignant conditions of adjacent organs (mostly peptic ulcers) had a non-significant 40% elevated risk of total cancer, with significant increases of lung and ovarian cancers.32 Based on sparse data, rat and mouse models have suggested that splenectomy is associated with increased tumor induction.28,29 On the basis of 8,149 cancer-free splenectomized patients (average follow-up 12.6 years), excluding cancer diagnoses <1 year post-splenectomy, we observed a 50% increased risk of solid and hematologic malignancies (n=1,094); the risks were significantly elevated more than 10 years after splenectomy. To minimize the effect of underlying disease, we performed subanalyses restricted to patients with an ICD code of trauma at the time of splenectomy and found essentially the same results. Because autoimmunity is associated with an increased risk of cancer43 we conducted sensitivity analyses excluding patients with an autoimmune disease prior to splenectomy. In these analyses, the risks of malignancies remained significantly elevated. Furthermore, on the basis of 6,731 cancer-free splenectomized patients, we found a 50% increased risk of death from solid and hematologic malignancies (n=497). Future epidemiological and molecular investigations are needed to confirm and expand our findings.
Physicians and patients need to be aware of the long-term complications associated with splenectomy. Laparoscopic removal of the spleen is increasingly being used and is associated with fewer immediate complications than open surgery.44,45 The substantial risks of infections, thrombosis and possibly malignancy need to be weighed against the benefits of the splenectomy. Splenectomized patients should be vaccinated and given empirical antibiotics according to international guidelines.42 The possible increase in the risk of developing malignancy, suggests that asplenic patients should have life-long surveillance for cancer.
The strengths of the current study include its large size in a population of patients with relatively stable and standardized access to medical care that is provided to USA veterans independently of their socioeconomic status. Medical diagnoses were obtained from medical records and were not, therefore, subject to recall bias. Study subjects were followed for up to 27 years, so the numbers of incident cases and mortality outcomes were high. Furthermore, several sensitivity analyses were performed, including comparability over time, age, race, and for subsets of patients with diagnoses of autoimmune disease or trauma, without changing the overall results. Limitations of the study include the incompleteness of clinical and laboratory data for individual patients (including vaccination status), potential under-ascertainment of cancer due to passive rather than active ascertainment of cases, and lack of independent validation of cancer diagnoses. However, we previously found a very high validity for cancer diagnoses in VA discharge records.46,47
In summary, we found that cancer-free splenectomized patients have an increased risk of infections, thromboembolism, and possibly cancer. Risks were increased after a long latency period (>10 years) underscoring the importance of life-long follow-up including vaccination and thromboprophylaxis. Future studies are needed to clarify the risks of cancer, the mechanisms underlying susceptibility to infection and thromboembolism, and clinical strategies aimed at preventing and managing the complications.
Supplementary Material
Acknowledgments
We thank the Medical Administration Service of the USA Veterans Health Services and Research Administration for providing the data on which this study is based; Robert Bilgrad of the National Center for Health Statistics for his advice and help with cause of death matching; Jay Lubin of the Biostatistics Branch, DCEG, NCI for statistical advise; and Dave Campbell, Eric Boyd, and Heather Morris of Information Management Services, Inc for computer programming support.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was funded by the Intramural Research Program of the National Cancer Institute (NCI), National Institutes of Health (NIH), Bethesda, Maryland, USA.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bowdler AJ. The Complete Spleen: Structure, Function and Clinical Significance: Humana Pr Inc, 2001 [Google Scholar]
- 2.Sheikha AK, Salih ZT, Kasnazan KH, Khoshnaw MK, Al-Maliki T, Al-Azraqi TA, et al. Prevention of overwhelming post-splenectomy infection in thalassemia patients by partial rather than total splenectomy. Can J Surg. 2007;50(5):382–6 [PMC free article] [PubMed] [Google Scholar]
- 3.Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood. 2009;114(14):2861–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulkind ML, Ellis EF, Smith RT. Effect of antibody upon clearance of I–125-labelled pneumococci by the spleen and liver. Pediatr Res. 1967;1(3):178–84 [DOI] [PubMed] [Google Scholar]
- 5.Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43(3):182–6 [DOI] [PubMed] [Google Scholar]
- 6.Cullingford GL, Watkins DN, Watts AD, Mallon DF. Severe late postsplenectomy infection. Br J Surg. 1991;78(6):716–21 [DOI] [PubMed] [Google Scholar]
- 7.Dickerman JD. Bacterial infection and the asplenic host: a review. J Trauma. 1976;16 (08):662–8 [DOI] [PubMed] [Google Scholar]
- 8.Ejstrud P, Kristensen B, Hansen JB, Madsen KM, Schonheyder HC, Sorensen HT. Risk and patterns of bacteraemia after splenectomy: a population-based study. Scand J Infect Dis. 2000;32(5):521–5 [DOI] [PubMed] [Google Scholar]
- 9.Linet MS, Nyren O, Gridley G, Adami HO, Buckland JD, McLaughlin JK, et al. Causes of death among patients surviving at least one year following splenectomy. Am J Surg. 1996;172(4):320–3 [DOI] [PubMed] [Google Scholar]
- 10.Robinette CD, Fraumeni JF., Jr Splenectomy and subsequent mortality in veterans of the 1939–45 war. Lancet. 1977;2(8029):127–9 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz PE, Sterioff S, Mucha P, Melton LJ, 3rd, Offord KP. Postsplenectomy sepsis and mortality in adults. JAMA. 1982;248(18): 2279–83 [PubMed] [Google Scholar]
- 12.Walker W. Splenectomy in childhood: a review in England and Wales, 1960–4. Br J Surg. 1976;63(1):36–43 [DOI] [PubMed] [Google Scholar]
- 13.Eraklis AJ, Kevy SV, Diamond LK, Gross RE. Hazard of overwhelming infection after splenectomy in childhood. N Engl J Med. 1967;276(22):1225–9 [DOI] [PubMed] [Google Scholar]
- 14.King H, Shumacker HB., Jr Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952;136 (2):239–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green JB, Shackford SR, Sise MJ, Fridlund P. Late septic complications in adults following splenectomy for trauma: a prospective analysis in 144 patients. J Trauma. 1986;26 (11):999–1004 [DOI] [PubMed] [Google Scholar]
- 16.Alebouyeh M, Moussavi F. Occurrence of overwhelming gram-negative infections in splenectomised patients with thalassaemia major. Eur J Pediatr. 2003;162(9):637–8 [DOI] [PubMed] [Google Scholar]
- 17.Altamura M, Caradonna L, Amati L, Pellegrino NM, Urgesi G, Miniello S. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol Immunotoxicol. 2001;23(2):153–61 [DOI] [PubMed] [Google Scholar]
- 18.Constantopoulos A, Najjar VA, Wish JB, Necheles TH, Stolbach LL. Defective phagocytosis due to tuftsin deficiency in splenectomized subjects. Am J Dis Child. 1973;125 (5):663–5 [DOI] [PubMed] [Google Scholar]
- 19.Rowley DA. The formation of circulating antibody in the splenectomized human being following intravenous injection of heterologous erythrocytes. J Immunol. 1950;65(5):515–21 [PubMed] [Google Scholar]
- 20.Hirsh J, Dacie JV. Persistent post-splenectomy thrombocytosis and thromboembolism: a consequence of continuing anaemia. Br J Haematol. 1966;12(1):44–53 [DOI] [PubMed] [Google Scholar]
- 21.Salter PP, Jr, Sherlock EC. Splenectomy, thrombocytosis, and venous thrombosis. Am Surg. 1957;23(6):549–54 [PubMed] [Google Scholar]
- 22.Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G, Mannucci AP. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111 (2):467–73 [DOI] [PubMed] [Google Scholar]
- 23.Pimpl W, Dapunt O, Kaindl H, Thalhamer J. Incidence of septic and thromboembolic-related deaths after splenectomy in adults. Br J Surg. 1989;76(5):517–21 [DOI] [PubMed] [Google Scholar]
- 24.Dawson AA, Bennett B, Jones PF, Munro A. Thrombotic risks of staging laparotomy with splenectomy in Hodgkin's disease. Br J Surg. 1981;68(12):842–5 [DOI] [PubMed] [Google Scholar]
- 25.Hassn AM, Al-Fallouji MA, Ouf TI, Saad R. Portal vein thrombosis following splenectomy. Br J Surg. 2000;87(3):362–73 [DOI] [PubMed] [Google Scholar]
- 26.Rossi P, Passariello R, Simonetti G. Portal thrombosis: high incidence following splenectomy for portal hypertension. Gastrointest Radiol. 1976;1(3):225–7 [DOI] [PubMed] [Google Scholar]
- 27.Stamou KM, Toutouzas KG, Kekis PB, Nakos S, Gafou A, Manouras A, et al. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg. 2006;141(7):663–9 [DOI] [PubMed] [Google Scholar]
- 28.Hull CC, Galloway P, Gordon N, Gerson SL, Hawkins N, Stellato TA. Splenectomy and the induction of murine colon cancer. Arch Surg. 1988;123(4):462–4 [DOI] [PubMed] [Google Scholar]
- 29.Yamagishi H, Pellis NR, Kahan BD. Effect of splenectomy upon tumor growth: characterization of splenic tumor-enhancing cells in vivo. Surgery. 1980;87(6):655–61 [PubMed] [Google Scholar]
- 30.Soda K, Kawakami M, Takagi S, Kashii A, Miyata M. Splenectomy before tumor inoculation prolongs the survival time of cachectic mice. Cancer Immunol Immunother. 1995;41(4):203–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellemkjoer L, Olsen JH, Linet MS, Gridley G, McLaughlin JK. Cancer risk after splenectomy. Cancer. 1995;75(2):577–83 [DOI] [PubMed] [Google Scholar]
- 32.Linet MS, Nyren O, Gridley G, Mellemkjaer L, McLaughlin JK, Olsen JH, et al. Risk of cancer following splenectomy. Int J Cancer. 1996;66(5):611–6 [DOI] [PubMed] [Google Scholar]
- 33.Richardson C, Waldrop J. Veterans: 2000 Census 2000 Brief, Bureau UC Washington DC: Department of Commerce, 2003 [Google Scholar]
- 34.Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107(3):904–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyko EJ, Koepsell TD, Gaziano JM, Horner RD, Feussner JR. US Department of Veterans Affairs medical care system as a resource to epidemiologists. Am J Epidemiol. 2000;151 (3):307–14 [DOI] [PubMed] [Google Scholar]
- 36.Page WF, Mahan CM, Kang HK. Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration. Ann Epidemiol. 1996;6(2):102–9 [DOI] [PubMed] [Google Scholar]
- 37.Breslow N, Langholz B. Nonparametric estimation of relative mortality functions. J Chronic Dis. 1987;40 (Suppl 2):89S–99S [DOI] [PubMed] [Google Scholar]
- 38.Karanika M, Vasilopoulou VA, Katsioulis AT, Papastergiou P, Theodoridou MN, Hadjichristodoulou CS. Diagnostic clinical and laboratory findings in response to predetermining bacterial pathogen: data from the Meningitis Registry. PLoS One. 2009;4(7): e6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374(9700):1543–56 [DOI] [PubMed] [Google Scholar]
- 40.Landgren O, Bjorkholm M, Konradsen HB, Soderqvist M, Nilsson B, Gustavsson A, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin's lymphoma. J Intern Med. 2004;255(6):664–73 [DOI] [PubMed] [Google Scholar]
- 41.Cherif H, Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. 2006;24(1):75–81 [DOI] [PubMed] [Google Scholar]
- 42.Davies JM, Barnes R, Milligan D. Update of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Clin Med. 2002;2(5): 440–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldin LR, Landgren O. Autoimmunity and lymphomagenesis. Int J Cancer. 2009;124(7): 1497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delaitre B, Maignien B, Icard P. Laparoscopic splenectomy. Br J Surg. 1992;79(12):1334. [DOI] [PubMed] [Google Scholar]
- 45.Park A, Marcaccio M, Sternbach M, Witzke D, Fitzgerald P. Laparoscopic vs open splenectomy. Arch Surg. 1999;134(11):1263–9 [DOI] [PubMed] [Google Scholar]
- 46.Ron E, Gridley G, Hrubec Z, Page W, Arora S, Fraumeni JF., Jr Acromegaly and gastrointestinal cancer. Cancer. 1991;68(8):1673–7 [DOI] [PubMed] [Google Scholar]
- 47.Gridley G, Klippel JH, Hoover RN, Fraumeni JF., Jr Incidence of cancer among men with the Felty syndrome. Ann Intern Med. 1994;120(1):35–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


