Abstract
Recommendations to consume fish for cardiovascular disease (CVD) prevention and its generally recognized as safe (GRAS) status have had the unanticipated consequence of encouraging long-chain omega-3 (ω-3) fatty acid [(eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] supplementation and fortification practices. While there is evidence supporting a protective role for EPA/DHA supplementation for reducing sudden cardiac events, the safety and efficacy of supplementation with LCω-3PUFA in the context of other disease outcomes is unclear. Recent studies of bacterial, viral, and fungal infections in animal models of infectious disease demonstrate that LCω-3PUFA intake dampens immunity and alters pathogen clearance and result in reduced survival. The same physiological properties of EPA/DHA that are responsible for the amelioration of inflammation associated with chronic cardiovascular pathology or autoimmune states, may impair pathogen clearance during acute infections by decreasing host resistance or interfere with tumor surveillance resulting in adverse health outcomes. Recent observations that high serum LCω-3PUFA levels are associated with higher risk of prostate cancer and atrial fibrillation have heightened the concern for adverse outcomes. Given the widespread use of supplements and fortification of common food items with LCω-3PUFA, this review focuses on the immunomodulatory effects of the dietary LCω-3PUFAs, EPA and DHA, the mechanistic basis for potential negative health outcomes, and calls for biomarker development and validation as rational first steps for setting recommended dietary intake levels.
Keywords: Fenton, JI; Hord, NG; Ghosh, S; Gurzell, EA
Introduction
Increasing the consumption of foods containing omega-3 (ω-3 or n-3) long chain polyunsaturated fatty acids (LCω-3PUFA) from fish oil, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), is widely recommended by public and private health agencies to reduce inflammation and the risk of chronic diseases. Analysis of serum phospholipids in a cohort study of U.S. adults showed that higher plasma levels of LCω-3PUFA biomarkers were associated with lower total mortality which was largely attributable to fewer cardiovascular compared to non-cardiovascular deaths [1]. Significant health benefits are associated with fish consumption including decreased risk of cardiovascular disease (CVD) [2-4]. Yet, fish intake remains low in the U.S. Per capita fish consumption has dropped from a historic high of 16 pounds in 2004 to 15 pounds in 2011 [5]. European Union member nations consumed ~45 pounds (range of 22-97 pounds) per capita in 2006 [6]. With the relatively low dietary intake of EPA and DHA from fish in Western societies, supplementation and fortification of foods is an attractive alternative strategy to increase intake.
Recommendations to consume fish for CVD prevention by the American Heart Association (AHA) are based upon principles of primary and secondary prevention. AHA recommends intake of EPA and DHA for individuals without documented coronary heart disease (CHD) risk, preferably from at least two servings of fatty fish [7] and oils and foods rich in α-linolenic acid ((LNA) flaxseed, canola, and soybean oils; flaxseed and walnuts). In individuals with documented CHD, it is recommended to consume ~ 1 gram of EPA + DHA per day, preferably from oily fish or from EPA + DHA supplements if recommended by a physician. For individuals requiring treatment for hypertriglyceridemia, two to four grams of EPA+DHA per day are recommended under a physician's care.
Approximately 30 million people currently take fish oil supplements in the U.S. [8]. Fish oil supplements generally contain some combination of EPA and DHA, but may contain only EPA or only DHA [9]. Up to 3 grams per day intake of fish oil is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration. In 1997, when GRAS status was granted for fish oil, widespread use of supplements or fortification of common food items with DHA or EPA was not a concern. Now, global consumer spending on LCω-3PUFA fortified foods is projected to jump from 25.4 billion in 2011 to 34.7 billion by 2016 according to research commissioned by the Global Organization for EPA and DHA Omega-3 (GOED) and published by the market research firm ‘Package Facts’ [10]. While this may appear beneficial in the face of the relative lack of DHA/EPA in the Western diet, the effects of long-term supplementation are yet unclear. Foods fortified with ω-3 PUFA from this report included infant formula, fortified foods and beverages, nutritional supplements, pharmaceuticals, medical nutritional products and pet foods.
As consumption continually increases, there is a real risk of consuming excess LCω-3PUFA beyond 3 g/day. On average EPA+DHA represents 30% by volume of fish oil. Each fish oil pill can contain as little as 300 mg to as much as the famous ‘quadruple strength’ 3000 mg of EPA+DHA in each pill (i.e. GRAS limit). In accordance to the ‘more is better’ paradigm, there is a real risk in chronic overconsumption of such supplements. It has been demonstrated recently that a single serving of DHA-fortified yogurt daily (containing 600 mg of DHA) can increase plasma phospholipid DHA levels by 32% in as little as 3 weeks accompanied by a 7% drop in plasma arachidonic acid (AA) [11].
Excessive intakes of all essential dietary nutrients are associated with adverse effects and, in extreme cases, negative health outcomes. Yet, few health risks are ascribed to excessive intakes of LCω-3PUFA in recent calls for the establishment of dietary reference intakes (DRI) [9, 12]. The disparity between data discussed in this review and calls for the establishment of DRI for LCω-3PUFA are striking. We provide evidence in this review for concern for excessive LCω-3PUFA intake and susceptible situations. While these calls for increasing intake are based on epidemiological associations for decreased risk of CVD, there is currently a dearth of validated biomarkers of intake, biological effect and disease risk associated with high dietary LCω-3PUFA intakes. However, as there are insufficient data to establish an upper level where toxic effects of LCω-3PUFA might be observed, the practice has been deemed as safe. Harris and colleagues superbly reviewed the beneficial effects of moderate LCω-3PUFA intake and justification for a DRI for EPA and DHA [12]. Now with recent studies demonstrating increased risk of atrial fibrillation and prostate cancer in the highest quartile of LCω-3PUFA intake the establishment of DRI and tolerable upper limit (TUL) for EPA and DHA should be revisited.
LCω-3PUFA supplementation and immunomodulation: Effects on CVD
Randomized controlled clinical trials have demonstrated that LCω-3PUFA supplementation can reduce cardiac events, decrease progression of atherosclerosis in coronary patients and lower serum triglycerides. Among various types of CVD, ischemic heart disease, characterized by either underlying atherosclerosis or hypertension is the most common form of heart disease in U.S. [13]. Currently, 6% of the entire U.S. population is believed to have some sort of CHD [14]. The primary co-morbidities for ischemic heart disease and stroke are diabetes and/or obesity. Because both of these chronic metabolic disorders are linked to consumption of an improper diet, LCω-3PUFA consumption is often recommended as an adjuvant to pharmacological or behavioral therapy. The AHA recommends a daily intake of 0.5–1.8 g of LCω-3PUFA preferably through increasing fish intake for CHD patients and up to 4g of EPA+DHA per day to lower triglycerides in patients under medical supervision [7]. Additionally, diabetes and obesity are believed to cause systemic inflammation, which may be attenuated by supplementation with LCω-3PUFA.
Inflammation originating from the adipose tissue is believed to be a key initiating event leading to CVD in obesity [15]. Circulating cytokines and acute phase proteins such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP) are significantly associated with expanding visceral adipose stores [16]. LCω-3PUFAs can directly attenuate adipose tissue inflammation [17]. In addition to atherosclerosis, in which the involvement of inflammation is well established, other CVD such as calcific aortic stenosis, aortic aneurysms, and atrial fibrillation are also increased by aberrant inflammation in the obese state [18]. Upon activation, the endothelium increases expression of leukocyte adhesion molecules, including vascular cell adhesion molecule 1 (VCAM-1), intracellular cell adhesion molecule 1 (ICAM-1) and E-selectin [19]. Monocytes bind to the adhesion molecules on endothelial cells, infiltrate the subendothelial space of blood vessels, mature into macrophages and release macrophage chemotactic protein-1 (MCP-1), which allows the recruitment of more macrophages to the area [20]. Macrophages release inflammatory cytokines including TNF-α and IL-6 associated with obesity and obesity-related CVD [21]. Some of the other factors involved in the chronic inflammatory state include the interleukins IL-3, IL-4, IL-5 and IL-10, interferon γ (IFN-γ) and toll-like receptor (TLR)-4 [21]. Transcription factors like NF-κB increase expression of cytokines such as IL-1β, IL-6, and TNF-α as well as the chemokine MCP-1. Activation of the NF-κB pathway has been detected in fibrotic intima atherosclerotic vessel walls [22]. In separate studies, LCω-3PUFA inhibits IL-1β [23], as well as other inflammatory mediators VCAM-1, ICAM-1, TNF-α, IL-6 [24], and TLR-4 [25]. In addition, LCω-3PUFA can have both direct (e.g. inhibition of NF-κB and other proinflammatory transcription factors) [26, 27] and indirect effects (e.g. production of three and five series eicosanoids which are less proinflammatory than eicosanoids derived from arachidonic acid, an ω-6 PUFA) [28, 29].
Apart from the traditional anti-inflammatory functions of LCω-3PUFA, several new classes of compounds have been identified that are generated from LCω-3PUFA. The most important of these compounds are termed as ‘resolution phase interaction products’ or resolvins. Both EPA and DHA generate these molecules and are termed as resolvins of the E series (RvE) and D series (RvD) [30]. Resolvins block the production of pro-inflammatory mediators and regulates leukocyte trafficking to inflammatory sites as well as clearance of neutrophils from mucosal surfaces during the resolution phase of injury/inflammation [31]. In vitro, resolvins limit polymorphonuclear leukocyte (PMN) migration and in vivo limits infiltration to the site of injury. Resolvins are highly potent compounds with only 10 nM concentrations reducing PMN transmigration by half. Most recently resolvin E1 was shown to reduce ischemic heart injury [32]. Another class of anti-inflammatory molecules include protectins which are metabolic products of DHA. These compounds have been mainly characterized in neural tissues [33] and hence described by the prefix neuroprotectin. Generation of neuroprotectin D1 (NPD1) from DHA has been shown to limit both retinal and corneal injury [34] and thus provides an additional functional basis of the high prevalence of DHA in neuronal systems.
Multiple excellent reviews have discussed the anti-inflammatory and immunomodulatory actions of LCω-3PUFA supplementation in CVD [19, 35, 36] and they will not be discussed in detail here. Overall, it is well recognized that LCω-3PUFA can have profound inhibitory effects on inflammation and immune responses in the context of chronic inflammatory states including the potential to reduce chronic CVD risk. However, a recent systematic review and meta-analysis of the effect of LCω-3PUFA supplementation to major cardiovascular events revealed no overall benefit [37].
Potential negative CVD consequences of LCω-3PUFA intake
The potential negative effects of high LCω-3PUFA intakes, as summarized by the AHA and European Food Standards Agency, include fishy aftertaste, bleeding episodes, impaired immune function, increased lipid peroxidation, and impaired lipid and glucose metabolism. Gastrointestinal disturbances and nausea were the most commonly reported side effects [7]. It is noteworthy that no TUL for LCω-3PUFAs has been set by any authoritative body.
A recently published review concluded that there were inconsistent benefits reported in clinical and experimental studies of LCω-3PUFA and CVD [38]. The authors summarize data and present potential adverse actions on cardiac rhythm noted during myocardial ischemia. In studies conducted in the 80's and 90's in various animal models including rats, dogs and monkeys, LCω-3PUFAs were found to interact with cardiac ion channels and prevent arrhythmias [39-43]. These effects were long believed to be beneficial, yet recent studies have begun to show potential detrimental cardiovascular effects of LCω-3PUFA. A recent review summarized research showing that LCω-3PUFAs led to increased mortality in angina patients and increased rather than decreased malignant arrhythmias during regional myocardial ischemia in animal models of sudden cardiac death [38].
Potential negative cardiovascular effects of high LCω-3PUFA in serum and risk of atrial fibrillation (AF) were demonstrated in a Japanese population [44]. The investigators evaluated the serum concentrations of LCω-3PUFAs in 110 patients with AF, 46 patients with ischemic heart disease (IHD) and no AF, and 36 healthy volunteers. In this study, serum EPA concentrations were associated with the incidence of AF suggesting that an excess of EPA may be a precipitating factor for AF. A Danish cohort study supports the observation from the Japanese study; the Danish cohort study reported a monotonic, negative, dose-response trend for DHA, EPA and DPA and atrial fibrillation [45]. In fact, higher levels of DHA and total LCω-3PUFA in RBC membranes, measured immediately prior to coronary artery bypass grafting and on postoperative day 3, were linearly associated with an increased risk of postoperative AF [46]. These findings contradict the widely held view that LCω-3PUFA exposure decreases risk of ventricular arrhythmias, as well as the prevention and treatment of AF [47]. Further studies are needed to establish which patients are more likely to benefit from LCω-3PUFAs, the timing of treatment, and the dosages. LCω-3PUFA should be prescribed with caution and generalized recommendations to take LCω-3PUFA supplements need to be reconsidered. Recommendations to consume fish or LCω-3PUFA supplements for the secondary prevention of CVD, has recently been rescinded by the National Institute for Health Care Excellence in the United Kingdom [48].
LCω-3PUFA supplementation and immunomodulation: risks during acute inflammation and infection
Calder and Grimble reviewed the anti-inflammatory effects of fish oil intake, concluding that the anti-inflammatory effect fish oil involves impairment of innate immune and lymphocyte responses [49]. In healthy humans above 55 y of age, 1 g per day of EPA+DHA reduced circulating natural killer cell population over 12 weeks [50]. Supplementation with DHA alone (4.9 g/day) for four weeks also lowered T lymphocyte activation in healthy humans [51]. As in adults, the anti-inflammatory effects of prenatal and postnatal supplementation with fish oil are also marked in infants and newborns. Eating two portions of salmon weekly from 20 weeks of gestation through delivery reduced multiple cord blood mononuclear cell-derived cytokines like IL-2, IL-4, IL-5, IL-10, and TNF-α in response to allergens, which is believed to reduce the risk of allergies in children [52]. Similarly, prenatal supplementation with 400mg DHA from 18-22 weeks of gestation to delivery, led to a reduction of general illness in infants at 3 months of age[53]. At 6 months post prenatal supplementation with DHA, infants experienced a significant reduction in fever severity, nasal secretions, difficulty breathing and rash and other-illness [53]. In HIV+ humans, fed fish oil there was a trend toward a decline in CD4 cell numbers [54]. Overall, both EPA and DHA in isolation or in combination are demonstrated to lower inflammation and impair immunity in humans. In a chronic inflammatory state such as rheumatoid arthritis (RA), EPA/DHA supplementation may reduce RA inflammation and symptoms benefiting the patient [55].
While inflammation is commonly portrayed as detrimental, the inflammatory response is absolutely required for survival after infection or injury. Attenuated response to an acute pathogen or injury may be interpreted as an impairment of immune function in the context of an acute inflammation, e.g., infection. Altering innate immune responses to pathogens or tumor surveillance by immune cells results in negative outcomes in animal studies [56-58]. Anderson and Fritsche, in a superb review, summarized that dietary DHA and EPA can both improve and impair host resistance to a number of pathogens [59]. McMurray et al. also presented data in a review article concluding that LCω-3PUFA enrichment alters membrane-mediated functions in macrophages and results in a detrimental outcome, (i.e., loss of antimicrobial resistance) in animal models [60].
Table 1 lists studies published since 2000 where dietary LCω-3PUFA results in adverse outcomes in murine models of pathogen exposure. These adverse outcomes associated with LCω-3PUFA intake were observed with bacterial, fungal, and viral pathogen models. In murine influenza, dietary LCω-3PUFA supplementation resulted in delayed influenza virus clearance and impaired production of IFN-γ and virus-specific immunoglobulin A in the lungs of mice [57] and suppression of virus-specific lung T cell cytotoxicity [61, 62]. In mice supplemented with menhaden oil and then infected with influenza virus, there was a 40% higher mortality rate, a 70% higher lung viral load at 7 days post infection, and a prolonged recovery period following infection [63]. Additionally, the lung tissue from infected fish oil-fed mice had significantly fewer CD8+ T cells and decreased expression of MIP-1α, TNF-α, and IL-6. Dietary LCω-3PUFA supplementation also resulted in increased M. tuberculosis bacterial load [64] and reduced clearance [65]. Endogenous enrichment of n-3 PUFAs in fat-1 mice increased their susceptibility to pulmonary tuberculosis infection as well [66]. L. monocytogenes infection was also influenced by dietary LCω-3PUFA supplementation by increasing bacterial load and reducing survival after infection [67, 68]. Similar results are observed with LCω-3PUFA feeding and reduced early reovirus clearance, S. enteritidis, and P. brasiliensis infections [56, 69, 70]. In addition, reduced survival and impaired wound healing were observed in several colitis models to be discussed in detail later [71-73].
TABLE 1.
Summary of mouse studies where dietary EPA/DHA negatively influenced outcomes related to pathogen exposure (since 2000).
| Model1; Pathogen | (DHA+EPA)/100g2 | Observed Effects | Reference |
|---|---|---|---|
| BALB/c mice; lnfluenzaA/Queensland/6/72 | 5.1% (10.0%en) | ↓ virus-specific T lymphocyte cytotoxicity ↑ Spleen and alveolar lymph proliferation |
Byleveld et al. 2000. [62] |
| BALB/c mice; Herpes simplex virus type 1 Strain F | 4.4% (8.6%en) | ↑ Stromal keratitis ↑ Viral infectivity, DTH |
Courreges et al. 2001. [144] |
| BALB/c mice; L. monocytogenes | 4.8% (9.6%en) | ↓ Survival ↓ Clearance |
Irons et al. 2003. [68] |
| BALB/c mice; P. brasiliensis | 2.7% (5.6%en) | ↓ Antifungal Activity (DHA only) | Oarada et al. 2003. [57] |
| Yorkshire x Landrace piglets; Influenza A/Puerto Rico/ 8/34 | 0.034% (0.1%en) | ↓ antigen-specific T cell response to virus ↓ IL-10 |
Bassaganya-Riera et al. 2007. [63] |
| BALB/c mice; L. monocytogenes ± cyclophosphamide (CPA) | 4.8%3 (9.6%en) | ↓ Survival ↑ Bacterial Load (spleen and liver) Fish oil exacerbates CPA effect |
Cruz-Chamorro et al. 2007. [69] |
| B6C3F1 mice; Reovirus T1L | 4.1% (9.4%en) | ↓ Early viral clearance | Beli et al. 2008. [70] |
| Hartley Strain Guinea Pigs; M. tuberculosis | 0.8% (2.3%en) | N.S. Lung pathology vs. control ↓ DTH; ↑ Bacterial Load (lung) |
McFarland et al. 2008. [65] |
| C57BL/6J mice; Influenza A/Puerto Rico/ 8/34 | 1.0% (2.5%en) | ↓ Survival, Weight Recovery ↓ Lung Histopathology |
Schwerbrock et al. 2009. [64] |
| Fat-1 (C57BL/6) mice; M. tuberculosis | ~0%3 (N/a) | ↑ Bacterial Load (spleen and liver) ↓ Pulmonary inflammation;↓ TNF-α |
Bonilla et al. 2010. [67] |
| J774A.1 cells; M. tuberculosis | 50μM DHA-BSA (N/a) | ↓ ROS, TNF-α, IL-6 ↓ Bacterial clearance |
Bonilla et al. 2010. [67] |
| Wistar rats; S. enteritidis | 5.8% (11.4%en) | ↓ DTH ↓ IFN-y, lgG2b |
Snel et al. 2010. [71] |
| 129-Smad3tm1Par/J mice; H. hepaticus | 0.44% – 3.5% (1.0 – 8.0%en) | ↓ Survival ↑ Colon Inflammation & dysplasia |
Woodworth etal. 2010. [72] |
| C57BL/6J mice; C. rodentium | 0.34% (0.7%en) | ↓ Survival ↓ Colon Inflammation & hyperplasia |
Ghosh et al. 2010. [74] |
| C57BL/6J mice; Dextran Sodium Sulfate | 1% EPA (2.4%en) | ↓ Survival ↓ colitis, cell migration in response to wounding |
Turk et al. 2013. [73] |
| C57BL/6J mice; Dextran Sodium Sulfate | 1% DHA (2.4%en) | = Survival ↓ colitis,delayed activation wound-healing process |
Turk et al. 2013. [73] |
All animals were 3-10 weeks old at the start of study and fed EPA/DHA enriched diets for 2-8 weeks prior to challenge.
Unspecifed fish oil was assumed to be menhaden oil. EPA+DHA was estimated using USDA Nutrient Database. Values are EPA+DHA unless otherwise noted.
Transgenic (expresses n-3 desaturase) mouse capable of endogenously synthesizing LCω-3PUFAfrom n-6 LCPUFAs
These consistent observations associate LCω-3PUFA immunomodulation with altered immune response to infection, including decreased pathogen clearance, decreased host resistance and adverse health outcomes. Potential mechanisms by which LCω-3PUFAs, EPA and DHA, can alter immune cell proliferation, function and cytokine production will be discussed [74].
Dietary LCω-3PUFA and intestinal infection, inflammation and cancer: an illustrative example
Infection-associated cancers are estimated to contribute to more than 20% of cancer cases worldwide [75]. In the context of pathogen-induced chronic inflammation and cancer risk, inhibition or alteration of the initiation of an immune response to a pathogen could be deleterious given the necessity to balance pathogen removal and tissue damage. The etiology of specific human cancers requires the presence of persistent infection and inflammation necessary for development of dysplasia and eventual tumor formation. Examples include hepatitis B virus and liver cancer, H. pylori and stomach cancer, and human papillomavirus and cervical cancer [76]. Other environmental factors, particularly host nutritional status, are proposed to influence infection persistence and the development of dysplasia. Evidence from preclinical animal models indicates that excess LCω-3PUFA intake can result in reduced bacterial and viral clearance leading to persistent infection and/or reduced survival (Table 1) [63, 64, 66-69, 71-73].
New diagnostic techniques are linking previously unidentified bacteria to colon cancer tumors, highlighting an emerging role for bacterially-driven host inflammation and colon cancer risk [77-79]. Individuals with inflammatory bowel disease (IBD) are at higher risk of developing colon cancer than the general population [80]. Although the etiology is poorly understood, there are indications that the immune system of individuals with IBD react abnormally to bacteria in the digestive tract leading to an inappropriately activated immune response, leading to chronic inflammation and increased risk of colon cancer [81]. A combination of genetic susceptibility and environmental factors, of which nutrition plays a key role, can modify host immune response to a pathogen, inflammation (IBD development) and cancer progression [59, 82, 83]. LCω-3PUFAs in fish oil are one such nutritional factor with potent immunomodulatory effects on immune cell function and inflammation.
In humans, fish oil supplementation had no effect on the maintenance and remission of active ulcerative colitis (UC), but was generally safe [84]. However, no clear and consistent effect of fish oil supplementation on colitis initiation and progression has been reported. Several animal studies demonstrate a protective effect of fish oil in chemically-induced colitis [85], however cancer initiation in a chemically-induced colitis model differs substantially from initiation via infection-induced inflammation. The effects of dietary fish oil in models of colitis that incorporate genetic and environmental (bacteria) risk factors are less consistent. For example, 4% dietary fish oil (wt/wt) in the IL-10 −/− mouse model reduced colitis development under non-steroidal anti-inflammatory drug (NSAID) treatment [86]. In contrast, another study using the same IL-10 −/− mouse model reported that 7% dietary fish oil increased spontaneous colitis and associated neoplasia [87]. Furthermore, 8% fish oil increased spontaneous colitis and associated neoplasia in DSS-induced colitis [88].
DHA-enriched fish oil was shown to increase inflammation and dysplasia and reduce survival in a Helicobacter hepaticus-induced colitis model [71]. Our laboratory observed that the addition of 0.75% (w/w) fish oil high in DHA (DFO; 540 mg/g DHA and 50 mg/g EPA fish oil) to the diet did not reduce colitis or increase colitis severity. However, 2.25%, 3.75%, and 6.0% dietary DFO (w/w) caused exacerbated inflammation and dysplasia compared to control colitis scores with 6% DFO having the most severe colitis scores [71]. Our results indicated that DFO as low as 2.25% enhances inflammation and accelerated dysplastic tissue formation in a bacterially-induced colitis model. Further experiments from our laboratory comparing EPA- and DHA-rich fish oils, indicates that a higher dietary concentration of EPA-enriched fish oil (3.75%) is required to enhance inflammation and dysplasia (unpublished data). These data indicate that inconsistent observations in the literature may be due to fish oil type and fatty acid content and composition.
Recently, Ghosh et al. showed that altering the LCω-3PUFA and LCω-6PUFA fatty acid composition of diets significantly affected infection-induced colitis in mice [73]. Overall, they observed that LCPUFA feeding led to dysbiosis (enriched pro-inflammatory microbes in the gut) and augmented colitis. The LCω-6PUFA diet prevented Citrobacter rodentium infection-induced systemic inflammation. In contrast, LCω-3PUFA supplementation reversed the effects of the LCω-6PUFA diet on dysbiosis but impaired infection-induced responses resulting in sepsis and greater mortality [73]. Mice fed LCω-3PUFA enriched diets had higher levels of sepsis-related serum factors such as LPS binding protein, IL-15 and TNF-α whereas intestinal alkaline phosphatase, responsible for neutralizing circulating LPS, were lowered [73]. These authors concluded that LCω-3PUFA supplementation during infection was detrimental when host inflammatory response was critical for survival.
In a colitis wound healing model, DHA and EPA supplementation reduced cell migration in response to wounding [72]. In addition, colonic histological injury scores were increased in EPA- and DHA-fed mice compared with control mice. Interestingly, even though colonic repair was increased in EPA- relative to DHA-fed mice, mortality was increased in mice fed EPA [72]. These authors concluded that in the early response to chemically-induced intestinal wounding, DHA and EPA uniquely delay the activation of key wound-healing processes in the colon.
Recent work by Chapkin and others have illuminated another aspect of how LCω-3PUFA affect immune cells through polarization and wound healing. This work demonstrated that rodent diets containing EPA, DHA, or EPA+DHA reduced Th17-cell polarization by reducing expression of IL-17A and RORγτ [89]. These data show that LCω-3PUFAs can exert a direct effect on the development of Th17 cells to create an anti-inflammatory phenotype via the suppression of the initial development of inflammatory Th17-cell subset. A similar suppression of wound healing was observed in scratch-wound repair assay was carried out in cultured human microvascular endothelial cells (HMEC-1) with and without different concentrations of DHA or EPA [90]. DHA and EPA dose-dependently suppressed HMEC-1 cell proliferation and wound repair, significantly suppressed VEGF mRNA expression and protein secretion under both normoxic and hypoxic culture conditions. The authors concluded that the use of DHA and EPA may have potential side effects to patients undergoing revascularization therapy.
These mouse studies demonstrate that fatty acids can alter response to bacteria in colitis models and suggest mechanisms for increased risk of disease progression. Fatty acid intake can also alter IBD development in humans. A systematic analysis of 19 studies of pre-illness diet and IBD development in humans identified that pre-illness diets high in total fats, PUFAs, omega-6 fatty acids, and meat were associated with an increased risk of developing Crohn's disease (CD) and UC in humans [91]. In addition, four studies included in this analysis demonstrated an association between high fish and seafood consumption and an increased risk of developing UC [91]. It is clear from this analysis that fatty acid intake pre-illness influences the development of IBD, however, the mechanism is not yet understood. Biopsy samples from 69 UC patients and 69 controls showed that inflamed mucosa had higher AA, DPA and DHA levels and lower EPA AA:EPA ratio [92]. There were significant correlations between severity of inflammation and contents of AA, DPA and DHA (positive correlations) and of linoleic acid (LA), α-LNA and EPA (negative correlations). These data suggest that fatty acid metabolism may be altered in the inflamed gut mucosa and/or affect immune cell function resulting in negative health consequences.
Taken together, these data suggest that dietary fatty acids can modulate both host immune cells and the community structure of the microbiota in the host and have dramatic effects on risk of developing IBD. This modulation of immune response may lead to persistent inflammation and subsequent risk for cancer. In support, two recent studies comparing the highest to lowest quartile of LCω-3PUFA intake reported a significant increase in the relative risk of colon cancer in humans [93, 94]. As well, high serum phospholipid DHA was recently positively associated with high-grade prostate cancer [95, 96]. A recent meta-analysis supports these findings and discusses potential mechanisms [97]. Briefly, the authors suggest that the observations could be due to local inflammation and related to how the beta cell metabolizes the fatty acids and/or potential negative effects of increased toxins from fish such as biphenyls or methylmercury compounds. The environmental toxicants, biphenyls and methylmercury, may disrupt androgen and estrogen balance and potentially lead to increased risk of high-grade prostate cancer. However, it is possible that the high DHA intake may perturb the immune system in a way that exacerbates inflammation in the prostate promoting tumors or may alter tumor immunosurveillance. In either case, the immunomodulatory effects may be shown to at least partially explain these observations..
Defining the mechanistic basis of immunomodulation by LCω-3PUFA
Several potential mechanisms for the immunomodulatory effects of LCω-3PUFAs have been elucidated [49, 98]. These potentially interrelated mechanisms include disruption of lipid rafts, inhibiting activation of the NLRP3 inflammasome, activation of the anti-inflammatory PPAR-γ transcription factor, and ligand binding of LCω-3PUFAs (particularly DHA) to the G protein-coupled receptor GPR120 [98, 99]. One central mechanistic theme that relates these disparate phenomena has emerged from studies using model membrane systems, cells in culture, and animal models is direct incorporation of LCω-3PUFAs into phospholipids of the plasma membrane. These studies identified both EPA and DHA as disruptors to the biophysical and biochemical organization of the plasma membrane ultimately modulating membrane architecture and potentially functional outcomes (e.g. altered membrane-mediated signaling). Incorporation of LCω-3PUFAs into the plasma membrane is thought to primarily disrupt/reorder specialized cell membrane domains called lipid rafts [100, 101]. Manipulation of lipid domains (i.e. rafts, signalosomes) with LCω-3PUFA is a central, upstream mechanism by which the numerous immunomodulatory effects of downstream cellular activities (e.g. generation of bioactive lipids, gene activation, protein trafficking, cytokine secretion, etc) are observed.
Recent studies have demonstrated that LCω-3PUFA acyl chains (DHA in particular), due to their unique molecular structure, can disrupt lipid raft molecular organization [102, 103]. DHA, which can adopt multiple conformational states, does not interact favorably with cholesterol and saturated acyl chains (Fig. 1) [104]. A recent hypothesis purports that exposure of ordered saturated acyl chains and cholesterol molecules in rafts to LCω-3PUFA acyl chains promotes changes in lateral organization of cholesterol, that then promote further disruption of protein clustering and thereby altering downstream biological responses (Fig. 1) [105-109]. The theoretical framework through which LCω-3PUFAs incorporate into phospholipids and disrupt membrane organization eliciting downstream, functional consequences has been demonstrated in various models. LCω-3PUFA incorporation alters innate and adaptive immune responses, including dendritic cell maturation, macrophage function, and B and T cell polarization/activation [60, 110-114].
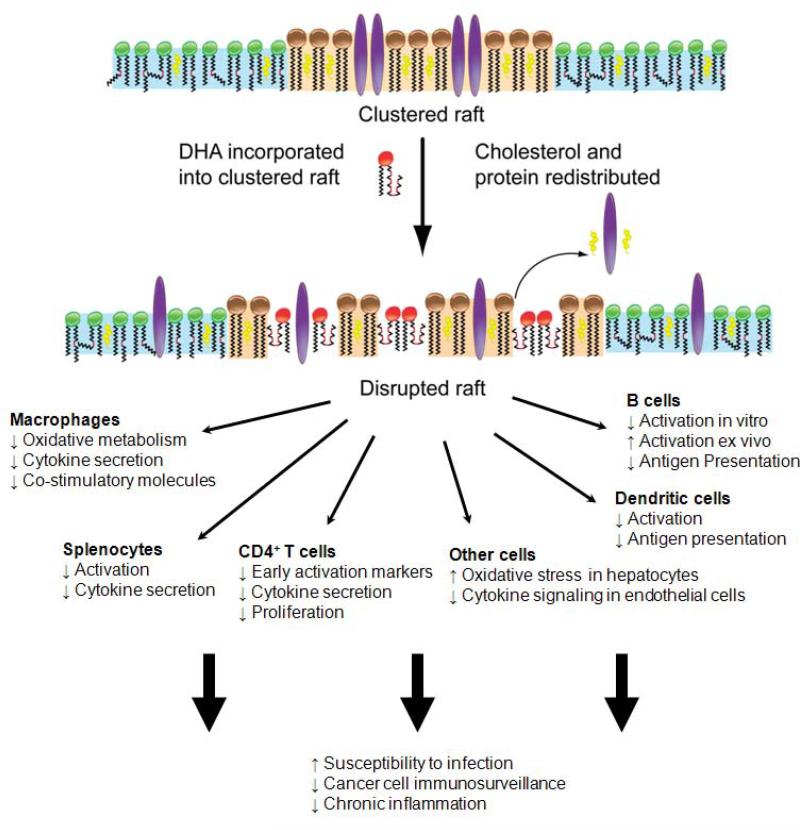
Figure 1.
The model shows a proposed mechanism by which DHA acyl chains disrupt the physical clustering of lipid rafts and associated proteins. Upon LCω-3PUFA incorporation into the membrane, and possibly through changes in lipid raft organization the following could result. LCω-3PUFAs disrupt the molecular organization of the plasma membrane, which initiates changes downstream on generation of bioactive lipids, cell signaling, and gene expression. A disruption in raft domains with n-3 PUFAs will have downstream effects on immune cell function. To date, the function of several immune cells (i.e. CD4+ T, B, dendritic cells, macrophages) in addition to other cells are disrupted by incorporation of LCω-3PUFAs into the plasma membrane. Suppression of function would have utility for treatment of symptoms associated with chronic inflammation. However, a suppression of immune cell function, perhaps at high doses of LCω-3PUFAs, could have negative side effects on susceptibility to infection and targeting of cancer cells by specific immune cells. LCω-3PUFA feeding may also influence microbial community structure and risk of inflammation. It is not known whether dietary fatty acids affect microbial cell wall structure directly, whether these changes influence eukaryotic-prokaryotic signaling or whether fatty acid-induced changes in the overall balance of microbial populations contribute to increased risk of inflammation.
Research has primarily investigated lipid raft-associated proteins of T and B cells involved at the immunological synapse, the physical junction through which immune cells propagate signals, where membrane protein aggregation and signaling occur. The work of Chapkin et al. demonstrates that LCω-3PUFA are capable of suppressing T cell activation by altering the functional outcomes of signaling proteins (e.g. PLCγ1 and PKCθ) and transcription factors (e.g. AP1 and NF-κB) [115, 116]. More recently they have demonstrated that DHA is capable of decreasing levels of PtdIns(4,5)P2 and recruitment of WASP to the immunological synapse, two outcomes that serve to inhibit PtdIns (4,5)P2-dependent actin remodeling [117]. This exciting observation links a novel mechanism by which dietary LCω-3PUFAs mediate cytoskeletal organization.
Shaikh et al. have shed light on LCω-3PUFA-induced immunomodulation by demonstrating DHA affects clustering and size of lipid rafts in B cells in vivo and ex vivo by altering the lateral organization and surface expression of MHC class I molecules [109]. Furthermore, they were able to verify observations from in vitro cholesterol depletion studies with recent in vivo data on LCω-3PUFA-induced disruption of MHC class II organization within the immunological synapse [118]. Depending on the B cell lineage, changes in lipid composition with LCω-3PUFA in high-fat diets promoted pro-inflammatory responses as well [113]. Indeed, recent research from the Fenton lab corroborates increased B cell activation after feeding mice a diet prepared with DHA-enriched fish oil [119]. Depending on the cell type, animal model, and condition under study, these effects may be considered beneficial (e.g., anti-inflammatory) or detrimental (e.g., loss of anti-microbial immunity) [60].
In addition to the aforementioned mechanism of membrane reorganization, incorporation of LCω-3PUFAs into the plasma membrane provides a substrate/ligand reservoir for LCω-3PUFA-derived lipid mediators, such as resolvins, or LCω-3PUFA-binding interactions, such as with GPR120. These lipid mediators were described in brief earlier and will not be discussed in further; however, to complicate our understanding of the mechanisms by which LCω-3PUFA exert their effect, resolvin E1 and D1 are agonists against various to G protein-coupled receptors [31, 120-122]. Recent studies have illustrated LCω-3PUFA metabolite-independent interactions with GPRs, such as the LCPUFA interactions with GPR120. Indeed, GPR120 has been shown to recognize LCω-3PUFAs, including DHA, resulting in GPR120 activation and subsequent inhibition of pro-inflammatory TAK1 signaling and downstream NF-κB responses [122]. Interactions between GPRs and LCω-3PUFAs have recently been reviewed and warrant further investigation [123].
It is clear that more research is required to determine the optimal dose and duration of LCω-3PUFAs in the diet in order to maintain physiologic control of the functional status of a healthy immune system and optimal heath [60]. For example, it is possible that LCω-3PUFA deficiency resulting in low membrane EPA/DHA concentrations in the plasma membranes of immune cells may be associated with inflammatory sequelae of atherosclerosis (e.g., IL-6, CRP, etc.) identified in observational epidemiologic studies. We propose that immunmodulation by high LCω-3PUFA intake can potentially negatively influence infection-associated inflammation and cancer risk by affecting acute response to pathogens leading to pathogen persistence, altering dynamics of infection-resolving inflammation, and thereby increasing the opportunity for dysplasia. It is critical to understand what levels of LCω-3PUFA intake may be optimal for human health.
Given the potent anti-inflammatory and immunomodulatory potential of DHA and EPA, we believe that the dietary requirement for DHA and EPA exists as a continuum represented by a normal, Gaussian distribution that, similar to other essential nutrients, characterizes dietary states of deficiency, sufficiency, and excess. There is a potential for dietary deficiency and adverse symptoms associated with lower DHA and EPA intakes and an optimal intake level where health benefits are observed. However, there may exist an excess intake level where adverse effects are observed. The demonstration that LCω-3PUFA intakes can be associated with health benefits and risks provides a strong rationale for the development of biomarkers.
Interpreting LCω-3PUFA exposures across study types
While the aforementioned potential effects are heterogeneous and individualized, it is necessary to provide guidance for potential dose requirements for the immunomodulatory effects reviewed herein. Guidance for interpreting intake levels of dietary LCω-3PUFAs is described below given the heterogeneity of exposures in various human and animal studies. For patients without documented heart disease or dyslipidemia, 250 mg EPA+DHA approximates the LCω-3PUFA content from the current recommendation of two servings of fish a week. In animal studies, the medium and high LCω-3PUFA exposure levels may model the 1000-1500 mg EPA+DHA recommendation for patients with documented heart disease and 4000 mg EPA+DHA prescription (Lovaza®) for patients with high triglycerides. Concentrations of 250 mg, 1500 mg, and 4000 mg EPA+DHA, based upon a 2000 kcal human diet composed of ~30% energy from fat translates to dietary energy from EPA + DHA of 0.001%, 0.675%, and 1.8%, respectively from EPA + DHA in the human diet. These reference values are helpful in the interpretation of exposure levels in rodent studies of LCω-3PUFA intakes represented in Table 1. The table includes the percentage of energy (en%) calculation for each study so that one can make basic dosing comparisons between human and mouse model dosing in those studies.
When trying to interpret dose, one limitation is that negative results from rodent studies could result from high doses of LCω-3PUFAs, which are not readily achieved in many clinical studies. Rodent diets are typically lower in fat than human diets so comparison by % of energy is a better approach. Expressing LCω-3PUFA intake as a percentage of energy (en%) in the diet removes the need to measure food intake in rodent studies and allows for meaningful comparisons between human and animal-based studies [124]. Another limitation that can muddle the dose issue is how the subject's genetic background (including age, SNPs, epigenetics, oncogenes) can influence fatty acid levels in tissue. A recent study found that levels of all four n-3 PUFAs were associated with genetic markers in known desaturation and elongation genes [125]. Specifically, the authors observed a weaker association between ALA and EPA among carriers of the minor allele of a representative SNP in FADS2 (rs1535), suggesting a lower rate of ALA-to-EPA conversion in these subjects. Their findings show that common variation in ω-3 metabolic pathway genes influence plasma phospholipid levels of LCω-3PUFAs in populations of European ancestry and, for the FADS1 SNP, in other ancestries. The results have important implications for genes/diet interaction and how they can influence circulating levels of fatty acids.
A continuum of LCω-3PUFA-induced immunomodulation: anti-inflammatory to anergic
The immunomodulatory effects of DHA and EPA may be beneficial, as reflected in the ostensibly beneficial term ‘anti-inflammatory’ or may reflect an anergic-type response, defined as a reduction in or inability to mount an immune response to a specific antigen, detrimental to health depending on the pathogen burden and the disease-specific microenvironment [60]. The continuum of immunomodulatory effects of LCω-3PUFAs depending upon dose and microenvironmental context is blurred by the heterogeneity of LCω-3PUFA sources for dietary exposures, animal model and disease condition under study and study designs.
It has also been noted that the immunomodulatory effects of DHA and EPA are dependent on the age of the individual and the health status in humans. As an example, Rees et al provided various doses of EPA between 1.65 and 4.95 g EPA/d for 12 wk in young and older healthy men [126]. Whereas immunomodulation was noted in younger men only at 3.3 g of EPA and above, older individuals demonstrated a dose-dependent decrease in neutrophil respiratory burst at all doses of EPA [126]. In a later authoritative review by Sijben and Calder, it was concluded that a depletion of the natural buffering capacity present in healthy subjects, due to a higher turnover rate of immune cells in disease states and augmented production of proinflammatory eicosanoid synthesis, makes diseased individuals more sensitive to immunomodulation with LCω-3PUFA [127]. Most safety studies with large doses of EPA or DHA have been performed in healthy individuals, yet increasingly, older individuals with chronic diseases are being recommended to increase intakes of LCω-3PUFA, thus there is an ongoing concern of improper or excessive immunosuppression in older patients especially under acute inflammation or infection.
Numerous studies demonstrate suppression of various aspects of human immune function in vitro or ex vivo in peripheral blood mononuclear cells, or in isolated neutrophils or monocytes in individuals provided n-3 polyunsaturated fatty acids as a supplement or as an experimental diet compared with baseline values before the intervention [128]. Single treatment studies comparing baseline versus post LCω-3PUFA supplementation and immune function indicate that LCω-3PUFA at levels 7 to 15 times greater than typical current U.S. intakes, diminish the potential of the immune system to attack pathogens [128]. However, this diminished ability is associated with suppression of inflammatory responses, suggesting benefits for individuals suffering from autoimmune diseases.
These conclusions, however, have been criticized by the European Food Standards Agency (EFSA) for being based primarily upon ex vivo and in vitro studies [129]. It is noteworthy that reviews citing potential beneficial or negative effects of LCω-3PUFA do not stipulate specific biomarkers of exposure or risk that may result from specific LCω-3PUFA intake levels. The issue of biomarker development and validation is best approached from the perspective of potential mechanisms by which LCω-3PUFA may influence host immunological responses.
Dietary deficiency and excess of LCω-3PUFA: a call for the establishment of Dietary Reference Intakes
There is a strong consensus on the recommendation of consuming foods or supplements containing LCω-3PUFA in children and adults (reviewed extensively in [9]). Westernized societies, the United States in particular, are generally deficient in EPA and DHA. Americans consume an average of approximately 1.6 g total LCω-3PUFA per day, of which EPA and DHA accounts for only 0.1 to 0.2 g and the balance is made up of ALA from plant sources [130]. However, the conversion rate from ALA to EPA/DHA in most parts of the body is very low, including the heart. As such, suboptimal intakes of LCω-3PUFAs by Americans may place them at a higher risk for CVD [131].
The Panel on Dietetic Products, Nutrition and Allergies of the European Commission recently delivered a scientific opinion on the Tolerable Upper Intake Level (UL) of the LCω-3PUFAs EPA, DHA and DPA [129]. This group found the available data insufficient to establish a UL for LCω-3PUFA (individually or combined) for any population group. They noted that, at observed intake levels, consumption of LCω-3PUFA has not been associated with adverse effects in healthy children or adults. Furthermore, long-term supplemental intakes of EPA and DHA combined up to about 5 g/day do not appear to increase the risk of spontaneous bleeding episodes or bleeding complications, or affect glucose homeostasis immune function or lipid peroxidation, provided the oxidative stability of the LCω-3PUFAs is guaranteed. Other conclusions of this body were that supplemental intakes of EPA and DHA, at combined doses of 2.6 g/day, and of DHA at doses of 2.4 g/day, were found to induce an increase in LDL-cholesterol concentrations which may adversely affect CVD risk. However, EPA at doses up to 4 g/day had no significant effect on LDL cholesterol.
The International Society for the Study of Fatty Acids and Lipids recommends intakes of at least 0.5 g per day of EPA+DHA for cardiovascular benefits in healthy adults (http://www.issfal.org.uk/lipid-matters/issfal-policystatements/.html) [132]. These recommendations may reflect adequate dietary intake levels for dietary LCω-3PUFA. Beneficial health outcomes attributed to adequate LCω-3PUFA intake other than CVD-associated include hemostasis [133], improved visual acuity [134], and the reduced risk for certain cancers [135]. Post-recommendation, there has been an exponential growth in the fish oil supplement consumption creating a real concern for over dosing. However, as there are insufficient data to establish an upper level where the toxicity of LCω-3PUFA is observed, the practice has been deemed as safe.
Necessity for the discovery and validation of biomarkers of LCω-3PUFA intake and effect
With current secular trends in LCω-3PUFA supplementation and fortification of processed foods in the U.S., characterization of potential adverse effects of excessive intakes on disease risk is timely and highly relevant. The demonstration that LCω-3PUFA intakes can be associated with health benefits and risks, provides a strong rationale for the development of biomarkers. According to the IOM , the development of new biomarkers require a three step biomarker evaluation process that includes analytical validation (reliability, reproducibility), qualification (association of biomarker with the disease and evidence of efficacy that interventions targeting the biomarker impact the clinical endpoints) and utilization (strong evidence and a compelling context are needed for the use of a biomarker as a surrogate endpoint) [136]. There is evidence to support the consideration for the establishment of DRIs for LCω-3PUFAs but the lack of biomarkers of dietary exposure or biomarkers of disease susceptibility hamper the validity with which exposure can be linked to potential health effects. Since cell membrane phospholipids reflect stable, recent intakes of LCω-3PUFA, researchers have developed dietary ω-3 fatty acid intake-dependent and tissue-specific biomarkers.
The Omega-3 Index serves as one example of a tissue-specific biomarker of LCω-3PUFA intakes. This index is defined as the sum of EPA and DHA in erythrocyte membranes expressed as a percentage of total fatty acids. [137]. The index was originally suggested as a marker of increased risk for death from CHD and is purported to be serve as a surrogate biomarker of CHD risk [138]. The index is responsive to dietary LCω-3PUFA intakes but dietary DHA + EPA intakes explained only 12% of its variability (P < 0.001) in a Mediterranean population [139]. The Omega-3 Index is associated with biomarkers of effect (e.g., plasma IL-6, CRP, thrombotic factors and ventricular fibrillation) [140]. Yet, less work has correlated the Omega-3 Index with tissue LCω-3PUFA levels related to stage of disease or prognosis. We acknowledge the difficulty and expense necessary to collect human tissue samples prospectively for the purpose of pre-diagnostic risk characterization. This limitation highlights the need to validate biomarkers of LCω-3PUFA intakes that are associated with deficient, adequate, and excess intake levels and how these biomarkers relate to tissue phenotypes, including inflammatory microenvironments, and/ or disease risk. The relevance of the necessity to validate biomarkers associated with disease risk is highlighted by the recent observations that high serum phospholipid DHA was positively associated with high-grade prostate cancer [95, 96].
The purported health benefits of LCω-3PUFA have led two prominent groups of researchers to propose the establishment of LCω-3PUFA DRIs by the Food and Nutrition Board of the National Academy of Sciences [9, 12]. The establishment of DRIs for EPA and DHA will entail, based on the available evidence, the determination of the Estimated Average Requirement (EAR), Recommended Daily Allowance (RDA), Adequate Intake (AI), and Upper Level (UL) that define, in broad terms, dietary intakes associated deficiency, sufficiency, and upper limits for these nutrients. These calls for the establishment of DRI for LCω-3PUFA adequately addressed the high prevalence of low dietary intakes in Western countries as well as the anti-atherogenic efficacy of adequate LCω-3PUFA intakes. We support these efforts and provide biologically plausible evidence in support of an UL intake limit for LCω-3PUFA DRI recommendations in this review. We have presented evidence that high dietary intakes of LCω-3PUFAs may be associated with an increased risk of certain diseases due to LCω-3PUFAs modulation of immune cell response to bacterial and viral pathogens. Figure 2 builds on the DRI paradigm and ascribes phenotypes to deficiency, sufficiency, and toxicity associated with LCω-3PUFA intake overlaid a potential biomarker, i.e. red blood cell EPA + DHA phospholipid content.
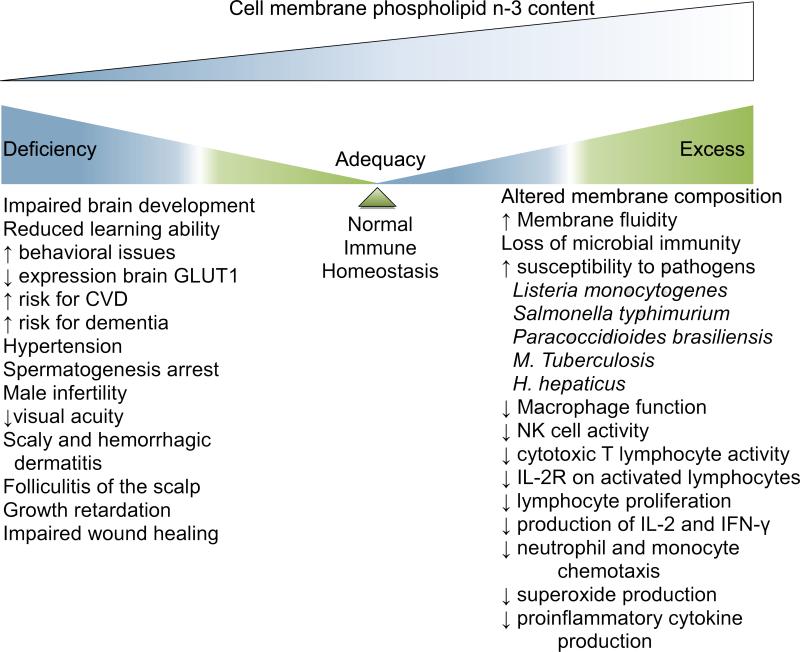
Figure 2.
Observations associated with very low and high dietary intake of the LCω-3PUFA fatty acids, EPA and DHA. Deficiency of LCω-3PUFA intakes are associated with negative neurological, cardiovascular and reproductive outcomes. Excess LCω-3PUFA intakes are associated with an immune anergic phenotype in antigen-driven models of pathologies. Cell membrane phospholipid content has been proposed as a potential biomarker of dietary fatty acid intake and associated phenotypes. These require validation in support of the calls for Dietary Reference Intakes for these proposed nutrients.
Our call for validation of biomarkers of exposure, effect, and risk is harmonious with the recently announced Biomarker of Nutrition for Development (BOND) Program of the NIH. This program was launched to discover and develop valid biomarkers for all essential nutrients with the goal of generating evidence-based policies. It meets the growing need for discovery, development, and implementation of reliable and valid biomarkers to assess nutrient exposure, status, function, and effect. The initial plan is to take five case nutrients (iron, zinc, vitamin A, folate, vitamin B-12) and then expand to all 40 essential nutrients [141]. We view the development and validation of biomarkers for LCω-3PUFA (EPA + DHA) exposure as relevant as for established nutrients in the NIH BOND program.
When setting recommendations based upon the DRI paradigm, considerations must address, if possible, definitions of dietary deficiency, sufficiency, and excess. The increasing prevalence of supplementation and prescription of LCω-3PUFAs for health benefits must be balanced against their potentially adverse effects. These trends reinforce previous recommendations for the establishment of the DRI for LCω-3PUFAs.
Conclusion
This review has discussed the underappreciated but highly relevant and consistent evidence for immunomodulatory effects of dietary ω-3 PUFA (EPA + DHA) intakes. High LCω-3PUFA consumption may alter the immune response to microbes in the gut, alter the community structure of the microbiota and enhance susceptibility to IBD and infection-induced inflammation and cancer. Antigenic stimulation (e.g. pathologies associated with persistence of viral, bacterial, and, perhaps, tumor antigens) may require optimal, but not excessive, dietary intake of EPA and DHA. In the physiological contexts of these disease conditions, pathogenesis appears to be driven by alterations in normal immune responses that result in pathogen persistence and chronic inflammation. Given the increasing prevalence of dietary supplementation of LCω-3PUFA, there is a necessity to develop robust, valid biomarkers of the physiological effects and disease risks associated with LCω-3PUFA intakes. Valid biomarkers in relevant eukaryotic tissues that assess the overall balance in the composition of the gut microbial community may assist in the prediction of negative health outcomes associated with excessive LCω-3PUFA exposure and establishment of a DRI for LCω-3PUFA intake. Valid biomarkers, such as quantitative measures of the cell membrane composition, will also assist in the development of LCω-3PUFA-based approaches wherein the immunomodulatory properties of these food components may be properly harnessed for disease prevention and therapeutics. Use of the IOM DRI framework, guided by validated biomarkers of exposure and risk, could assist our understanding of upper limits for LCω-3PUFA intake and potential disease risk with overconsumption.
Acknowledgements
The authors acknowledge Dr. Drew Rockett for creating and illustrating figure 1.
Financial support for this research was provided by NIH R03CA162427 and the Canadian Diabetes Association
Abbreviations Used
- TLR
toll-like receptor
- RA
rheumatoid arthritis
- CHD
coronary heart disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Authors’ Contributions to Manuscript
J.I.F, N.G.H, S.G and E.A.G. wrote the paper. JIF developed Figures 1 and 2. JIF and EAG developed Table 1. JIF, NGH and SG edited and revised the paper. JIF had primary responsibility for final content. All authors read and approved the final manuscript
Author Disclosure: Fenton, JI, Hord NG, Ghosh S and Gurzell EA, have no conflict of interest to report.
REFERENCES
- 1.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Annals of internal medicine. 2013;158:515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 3.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 4.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 5.Lowther A, editor. Fisheries of the United States 2011. National Oceanic and Atmospheric Organization; Silverspring, MD: 2012. [Google Scholar]
- 6.Bank S. Trends and Developments in the European Fishery Market. Center for the promotion of imports from developing countries. 2009 [Google Scholar]
- 7.Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association C. Nutrition, Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. [PubMed] [Google Scholar]
- 9.Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81:99–104. doi: 10.1016/j.plefa.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Facts P. The Global Market for EPA/DHA Omega-3 Products in. Packaged Facts. 2012:2012. [Google Scholar]
- 11.McCowen KC, Ling PR, Decker E, Djordjevic D, Roberts RF, Coupland JN, Bistrian BR. A simple method of supplementation of omega-3 polyunsaturated fatty acids: use of fortified yogurt in healthy volunteers. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2010;25:641–645. doi: 10.1177/0884533610385699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. The Journal of nutrition. 2009;139:804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang KSJ, Keenan NL. Prevalence of Coronary Heart Disease --- United States, 2006--2010, in. 2011 [Google Scholar]
- 15.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American College of Cardiology. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Despres JP. Inflammation and cardiovascular disease: is abdominal obesity the missing link? International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(Suppl 3):S22–24. doi: 10.1038/sj.ijo.0802495. [DOI] [PubMed] [Google Scholar]
- 17.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2:304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Despres JP. Visceral obesity and the heart. The international journal of biochemistry & cell biology. 2008;40:821–836. doi: 10.1016/j.biocel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. The American journal of clinical nutrition. 2008;87:2003S–2009S. doi: 10.1093/ajcn/87.6.2003S. [DOI] [PubMed] [Google Scholar]
- 20.Lyngso D, Simonsen L, Bulow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. The Journal of physiology. 2002;543:379–386. doi: 10.1113/jphysiol.2002.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bousserouel S, Brouillet A, Bereziat G, Raymondjean M, Andreani M. Different effects of n-6 and n-3 polyunsaturated fatty acids on the activation of rat smooth muscle cells by interleukin-1 beta. Journal of lipid research. 2003;44:601–611. doi: 10.1194/jlr.M200092-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. The American journal of clinical nutrition. 2000;71:213S–223S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- 25.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Jump DB. Dietary polyunsaturated fatty acids and regulation of gene transcription. Current opinion in lipidology. 2002;13:155–164. doi: 10.1097/00041433-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Denys A, Hichami A, Khan NA. n-3 PUFAs modulate T-cell activation via protein kinase C-alpha and -epsilon and the NF-kappaB signaling pathway. Journal of lipid research. 2005;46:752–758. doi: 10.1194/jlr.M400444-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.von Schacky C, Fischer S, Weber PC. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest. 1985;76:1626–1631. doi: 10.1172/JCI112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kris-Etherton PM, Harris WS, Appel LJ. C. for the Nutrition, Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:e20–30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 33.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calder PC, Yaqoob P. Marine omega-3 fatty acids and coronary heart disease. Curr Opin Cardiol. 2012;27:412–419. doi: 10.1097/HCO.0b013e328353febd. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 37.Urquhart R, Chan RY, Li QT, Tilley L, Grieser F, Sawyer WH. omega-6 and omega-3 fatty acids: monolayer packing and effects on bilayer permeability and cholesterol exchange. Biochemistry international. 1992;26:831–841. [PubMed] [Google Scholar]
- 38.Billman GE. The effects of omega-3 polyunsaturated fatty acids on cardiac rhythm: A critical reassessment. Pharmacology & therapeutics. 2013 doi: 10.1016/j.pharmthera.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 40.McLennan PL, Abeywardena MY, Charnock JS. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. American heart journal. 1988;116:709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- 41.McLennan PL, Abeywardena MY, Charnock JS. Reversal of the arrhythmogenic effects of long-term saturated fatty acid intake by dietary n-3 and n-6 polyunsaturated fatty acids. The American journal of clinical nutrition. 1990;51:53–58. doi: 10.1093/ajcn/51.1.53. [DOI] [PubMed] [Google Scholar]
- 42.McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. American heart journal. 1992;123:1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- 43.Pepe S, McLennan PL. Dietary fish oil confers direct antiarrhythmic properties on the myocardium of rats. The Journal of nutrition. 1996;126:34–42. doi: 10.1093/jn/126.1.34. [DOI] [PubMed] [Google Scholar]
- 44.Tomita T, Hata T, Takeuchi T, Oguchi Y, Okada A, Aizawa K, Koshikawa M, Otagiri K, Motoki H, Kasai H, Izawa A, Koyama J, Hongo M, Ikeda U. High concentrations of omega-3 fatty acids are associated with the development of atrial fibrillation in the Japanese population. Heart Vessels. 2013;28:497–504. doi: 10.1007/s00380-012-0264-3. [DOI] [PubMed] [Google Scholar]
- 45.Rix TA, Joensen AM, Riahi S, Lundbye-Christensen S, Overvad K, Schmidt EB. Marine n-3 fatty acids in adipose tissue and development of atrial fibrillation: a Danish cohort study. Heart. 2013 doi: 10.1136/heartjnl-2013-304385. [DOI] [PubMed] [Google Scholar]
- 46.A.D. Bjorgvinsdottir L, Indridason OS, Heidarsdottir R, Skogstrand K, Torfason B, Hougaard DM, Palsson R, Skuladottir GV. Do High Levels of n-3 Polyunsaturated Fatty Acids in Cell Membranes Increase the Risk of Postoperative Atrial Fibrillation? Cardiology. 2013;126:107–114. doi: 10.1159/000351432. [DOI] [PubMed] [Google Scholar]
- 47.Wu JH, Lemaitre RN, King IB, Song X, Sacks FM, Rimm EB, Heckbert SR, Siscovick DS, Mozaffarian D. Association of plasma phospholipid long-chain omega-3 fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2012;125:1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institute for Health and Care Services. 2013 [Google Scholar]
- 49.Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr. 2002;56(Suppl 3):S14–19. doi: 10.1038/sj.ejcn.1601478. [DOI] [PubMed] [Google Scholar]
- 50.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long- chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. The American journal of clinical nutrition. 2001;73:539–548. doi: 10.1093/ajcn/73.3.539. [DOI] [PubMed] [Google Scholar]
- 51.Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. The American journal of clinical nutrition. 2004;79:674–681. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- 52.Noakes PS, Vlachava M, Kremmyda LS, Diaper ND, Miles EA, Erlewyn-Lajeunesse M, Williams AP, Godfrey KM, Calder PC. Increased intake of oily fish in pregnancy: effects on neonatal immune responses and on clinical outcomes in infants at 6 mo. Am J Clin Nutr. 2012;95:395–404. doi: 10.3945/ajcn.111.022954. [DOI] [PubMed] [Google Scholar]
- 53.Imhoff-Kunsch B, Stein AD, Martorell R, Parra-Cabrera S, Romieu I, Ramakrishnan U. Prenatal docosahexaenoic acid supplementation and infant morbidity: randomized controlled trial. Pediatrics. 2011;128:e505–512. doi: 10.1542/peds.2010-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell SJ, Chavali S, Bistrian BR, Connolly CA, Utsunomiya T, Forse RA. Dietary fish oil and cytokine and eicosanoid production during human immunodeficiency virus infection. JPEN. Journal of parenteral and enteral nutrition. 1996;20:43–49. doi: 10.1177/014860719602000143. [DOI] [PubMed] [Google Scholar]
- 55.Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. The American journal of clinical nutrition. 2000;71:349S–351S. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]
- 56.Oarada M, Tsuduki T, Suzuki T, Miyazawa T, Nikawa T, Hong-quan G, Kurita N. Dietary supplementation with docosahexaenoic acid, but not with eicosapentaenoic acid, reduces host resistance to fungal infection in mice. Biochimica et biophysica acta. 2003;1622:151–160. doi: 10.1016/s0304-4165(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 57.Byleveld PM, Pang GT, Clancy RL, Roberts DC. Fish oil feeding delays influenza virus clearance and impairs production of interferon-gamma and virus-specific immunoglobulin A in the lungs of mice. The Journal of nutrition. 1999;129:328–335. doi: 10.1093/jn/129.2.328. [DOI] [PubMed] [Google Scholar]
- 58.Fritsche KL, Shahbazian LM, Feng C, Berg JN. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin Sci (Lond) 1997;92:95–101. doi: 10.1042/cs0920095. [DOI] [PubMed] [Google Scholar]
- 59.Anderson M, Fritsche KL. (n-3) Fatty acids and infectious disease resistance. The Journal of nutrition. 2002;132:3566–3576. doi: 10.1093/jn/132.12.3566. [DOI] [PubMed] [Google Scholar]
- 60.McMurray DN, Bonilla DL, Chapkin RS. n-3 Fatty acids uniquely affect anti microbial resistance and immune cell plasma membrane organization. Chem Phys Lipids. 2011 doi: 10.1016/j.chemphyslip.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byleveld M, Pang GT, Clancy RL, Roberts DC. Fish oil feeding enhances lymphocyte proliferation but impairs virus-specific T lymphocyte cytotoxicity in mice following challenge with influenza virus. Clinical and experimental immunology. 2000;119:287–292. doi: 10.1046/j.1365-2249.2000.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassaganya-Riera J, Guri AJ, Noble AM, Reynolds KA, King J, Wood CM, Ashby M, Rai D, Hontecillas R. Arachidonic acid-and docosahexaenoic acid-enriched formulas modulate antigen-specific T cell responses to influenza virus in neonatal piglets. The American journal of clinical nutrition. 2007;85:824–836. doi: 10.1093/ajcn/85.3.824. [DOI] [PubMed] [Google Scholar]
- 63.Schwerbrock NM, Karlsson EA, Shi Q, Sheridan PA, Beck MA. Fish oil-fed mice have impaired resistance to influenza infection. The Journal of nutrition. 2009;139:1588–1594. doi: 10.3945/jn.109.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McFarland CT, Fan YY, Chapkin RS, Weeks BR, McMurray DN. Dietary polyunsaturated fatty acids modulate resistance to Mycobacterium tuberculosis in guinea pigs. The Journal of nutrition. 2008;138:2123–2128. doi: 10.3945/jn.108.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonilla DL, Ly LH, Fan YY, Chapkin RS, McMurray DN. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to Mycobacterium tuberculosis. PloS one. 2010;5:e10878. doi: 10.1371/journal.pone.0010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonilla DL, Fan YY, Chapkin RS, McMurray DN. Transgenic mice enriched in omega-3 fatty acids are more susceptible to pulmonary tuberculosis: impaired resistance to tuberculosis in fat-1 mice. J Infect Dis. 2010;201:399–408. doi: 10.1086/650344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irons R, Anderson MJ, Zhang M, Fritsche KL. Dietary fish oil impairs primary host resistance against Listeria monocytogenes more than the immunological memory response. The Journal of nutrition. 2003;133:1163–1169. doi: 10.1093/jn/133.4.1163. [DOI] [PubMed] [Google Scholar]
- 68.Cruz-Chamorro L, Puertollano MA, Puertollano E, Alvarez de Cienfuegos G, de Pablo MA. Examination of host immune resistance against Listeria monocytogenes infection in cyclophosphamide-treated mice after dietary lipid administration. Clin Nutr. 2007;26:631–639. doi: 10.1016/j.clnu.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Beli E, Li M, Cuff C, Pestka JJ. Docosahexaenoic acid-enriched fish oil consumption modulates immunoglobulin responses to and clearance of enteric reovirus infection in mice. The Journal of nutrition. 2008;138:813–819. doi: 10.1093/jn/138.4.813. [DOI] [PubMed] [Google Scholar]
- 70.Snel J, Born L, van der Meer R. Dietary fish oil impairs induction of gamma-interferon and delayed-type hypersensitivity during a systemic Salmonella enteritidis infection in rats. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2010;118:578–584. doi: 10.1111/j.1600-0463.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 71.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer research. 2010;70:7960–7969. doi: 10.1158/0008-5472.CAN-10-1396. [DOI] [PubMed] [Google Scholar]
- 72.Turk HF, Monk JM, Fan YY, Callaway ES, Weeks B, Chapkin RS. Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing. American journal of physiology. Cell physiology. 2013;304:C905–917. doi: 10.1152/ajpcell.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh S, DeCoffe D, Brown K, Rajendiran E, Estaki M, Dai C, Yip A, Gibson DL. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PloS one. 2013;8:e55468. doi: 10.1371/journal.pone.0055468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 75.De Flora S, Bonanni P. The prevention of infection-associated cancers. Carcinogenesis. 2011;32:787–795. doi: 10.1093/carcin/bgr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bertout J, Thomas-Tikhonenko A. Infection & neoplastic growth 101: the required reading for microbial pathogens aspiring to cause cancer. Cancer treatment and research. 2006;130:167–197. [PubMed] [Google Scholar]
- 77.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2011 doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2011 doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. The ISME journal. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 81.Hisamatsu T, Kanai T, Mikami Y, Yoneno K, Matsuoka K, Hibi T. Immune aspects of the pathogenesis of inflammatory bowel disease. Pharmacol Ther. 2012 doi: 10.1016/j.pharmthera.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 82.Gruber L, Lichti P, Rath E, Haller D. Nutrigenomics and nutrigenetics in inflammatory bowel diseases. J Clin Gastroenterol. 2012;46:735–747. doi: 10.1097/MCG.0b013e31825ca21a. [DOI] [PubMed] [Google Scholar]
- 83.Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160:29–44. doi: 10.1016/j.trsl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Blach-Olszewska Z. [Innate nonspecific antiviral immunity] Postepy Hig Med Dosw. 1999;53:3–21. [PubMed] [Google Scholar]
- 85.Gil A. Polyunsaturated fatty acids and inflammatory diseases. Biomed Pharmacother. 2002;56:388–396. doi: 10.1016/s0753-3322(02)00256-1. [DOI] [PubMed] [Google Scholar]
- 86.Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. The Journal of nutrition. 2007;137:200S–204S. doi: 10.1093/jn/137.1.200S. [DOI] [PubMed] [Google Scholar]
- 87.Hegazi RA, Saad RS, Mady H, Matarese LE, O'Keefe S, Kandil HM. Dietary fatty acids modulate chronic colitis, colitis-associated colon neoplasia and COX-2 expression in IL-10 knockout mice. Nutrition. 2006;22:275–282. doi: 10.1016/j.nut.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 88.Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K, Miura S. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflammatory bowel diseases. 2008;14:1348–1357. doi: 10.1002/ibd.20491. [DOI] [PubMed] [Google Scholar]
- 89.Monk JM, Hou TY, Turk HF, McMurray DN, Chapkin RS. n3 PUFAs Reduce Mouse CD4+ T-Cell Ex Vivo Polarization into Th17 Cells. The Journal of nutrition. 2013 doi: 10.3945/jn.113.178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhuang W, Wang G, Li L, Lin G, Deng Z. Omega-3 polyunsaturated fatty acids reduce vascular endothelial growth factor production and suppress endothelial wound repair. Journal of cardiovascular translational research. 2013;6:287–293. doi: 10.1007/s12265-012-9409-0. [DOI] [PubMed] [Google Scholar]
- 91.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. The American journal of gastroenterology. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 92.Pearl DS, Masoodi M, Eiden M, Brummer J, Gullick D, McKeever TM, Whittaker MA, Nitch-Smith H, Brown JF, Shute JK, Mills G, Calder PC, Trebble TM. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. Journal of Crohn's & colitis. 2013 doi: 10.1016/j.crohns.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 93.Chiu BC, Ji BT, Dai Q, Gridley G, McLaughlin JK, Gao YT, Fraumeni JF, Jr., Chow WH. Dietary factors and risk of colon cancer in Shanghai, China. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2003;12:201–208. [PubMed] [Google Scholar]
- 94.Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, Yang G, Cai H, Wen W, Gao YT, Zheng W. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2283–2291. doi: 10.1158/1055-9965.EPI-08-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, Thompson IM, King IB, Albanes D, Kristal AR. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. American journal of epidemiology. 2011;173:1429–1439. doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng TY, King IB, Barnett MJ, Ambrosone CB, Thornquist MD, Goodman GE, Neuhouser ML. Serum phospholipid fatty acids, genetic variation in myeloperoxidase, and prostate cancer risk in heavy smokers: a gene-nutrient interaction in the carotene and retinol efficacy trial. American journal of epidemiology. 2013;177:1106–1117. doi: 10.1093/aje/kws356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chua ME, Sio MC, Sorongon MC, Morales ML., Jr. The relevance of serum levels of long chain omega-3 polyunsaturated fatty acids and prostate cancer risk: A meta-analysis. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2013;7:E333–343. doi: 10.5489/cuaj.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? British journal of clinical pharmacology. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 100.Katagiri YU, Kiyokawa N, Fujimoto J. A role for lipid rafts in immune cell signaling. Microbiology and immunology. 2001;45:1–8. doi: 10.1111/j.1348-0421.2001.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 101.Yaqoob P. The nutritional significance of lipid rafts. Annual review of nutrition. 2009;29:257–282. doi: 10.1146/annurev-nutr-080508-141205. [DOI] [PubMed] [Google Scholar]
- 102.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochimica et biophysica acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88:43–47. doi: 10.1016/j.plefa.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 105.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. The Journal of nutrition. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 106.Teague H, Ross R, Harris M, Mitchell DC, Shaikh SR. DHA-fluorescent probe is sensitive to membrane order and reveals molecular adaptation of DHA in ordered lipid microdomains. The Journal of nutritional biochemistry. 2013;24:188–195. doi: 10.1016/j.jnutbio.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams S.E.B. Justin A., Harris Mitchel, Rockett Benjamin Drew, Shaikh Saame Raza, Stillwell William, Wassall Stephen R. Docosahexaenoic and Eicosapentaenoic Acids Segregate Differently between Raft and Nonraft Domains. Biophysical Journal. 2012;103:228–237. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaikh SR. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. The Journal of nutritional biochemistry. 2012;23:101–105. doi: 10.1016/j.jnutbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rockett BD, Franklin A, Harris M, Teague H, Rockett A, Shaikh SR. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonraft organization in EL4 and B cells. The Journal of nutrition. 2011;141:1041–1048. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shaikh SR, Edidin M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. The American journal of clinical nutrition. 2006;84:1277–1289. doi: 10.1093/ajcn/84.6.1277. [DOI] [PubMed] [Google Scholar]
- 111.Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25:872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Progress in lipid research. 2010;49:250–261. doi: 10.1016/j.plipres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. Journal of lipid research. 2010;51:1284–1297. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shaikh SR, Edidin M. Polyunsaturated fatty acids and membrane organization: elucidating mechanisms to balance immunotherapy and susceptibility to infection. Chem Phys Lipids. 2008;153:24–33. doi: 10.1016/j.chemphyslip.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. Journal of immunology. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 116.Kim W, Fan YY, Barhomni R, Smith R, McMurray DN, Chapkin RS. n-3 Polyunsaturated Fatty Acids Suppress the Localization and Activation of Signaling Proteins at the Immunological Synapse in Murine CD4(+) T Cells by Affecting Lipid Raft Formation. Journal of immunology. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hou TY, Monk JM, Fan YY, Barhoumi R, Chen YQ, Rivera GM, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress phosphatidylinositol 4,5-bisphosphate-dependent actin remodelling during CD4+ T-cell activation. The Biochemical journal. 2012;443:27–37. doi: 10.1042/BJ20111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rockett BD, Melton M, Harris M, Bridges LC, Shaikh SR. Fish oil disrupts MHC class II lateral organization on the B-cell side of the immunological synapse independent of B-T cell adhesion. The Journal of nutritional biochemistry. 2013 doi: 10.1016/j.jnutbio.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gurzell EA, Teague H, Harris M, Clinthorne J, Shaikh SR, Fenton JI. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. Journal of leukocyte biology. 2013;93:463–470. doi: 10.1189/jlb.0812394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of immunology. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 121.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Current topics in medicinal chemistry. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. The Journal of clinical investigation. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Progress in lipid research. 2012;51:232–237. doi: 10.1016/j.plipres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 124.Fritsche K. Important differences exist in the dose-response relationship between diet and immune cell fatty acids in humans and rodents. Lipids. 2007;42:961–979. doi: 10.1007/s11745-007-3106-9. [DOI] [PubMed] [Google Scholar]
- 125.Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, Kabagambe EK, Nettleton JA, King IB, Weng LC, Bhattacharya S, Bandinelli S, Bis JC, Rich SS, Jacobs DR, Jr., Cherubini A, McKnight B, Liang S, Gu X, Rice K, Laurie CC, Lumley T, Browning BL, Psaty BM, Chen YD, Friedlander Y, Djousse L, Wu JH, Siscovick DS, Uitterlinden AG, Arnett DK, Ferrucci L, Fornage M, Tsai MY, Mozaffarian D, Steffen LM. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS genetics. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, Calder PC. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. The American journal of clinical nutrition. 2006;83:331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 127.Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. The Proceedings of the Nutrition Society. 2007;66:237–259. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- 128.Institute of Medicine Report. The National Academies Press; Washington, D.C.: 2002. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) [DOI] [PubMed] [Google Scholar]
- 129.Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA) European Food Safety Journal. 2012;10:2815. [Google Scholar]
- 130.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 131.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540–550. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 132.Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults. International Society for the Study of Fatty Acids and Lipids. 2004 [Google Scholar]
- 133.Kris-Etherton P, Daniels SR, Eckel RH, Engler M, Howard BV, Krauss RM, Lichtenstein AH, Sacks F, St Jeor S, Stampfer M, Grundy SM, Appel LJ, Byers T, Campos H, Cooney G, Denke MA, Kennedy E, Marckmann P, Pearson TA, Riccardi G, Rudel LL, Rudrum M, Stein DT, Tracy RP, Ursin V, Vogel RA, Zock PL, Bazzarre TL, Clark J. Summary of the scientific conference on dietary fatty acids and cardiovascular health: conference summary from the nutrition committee of the American Heart Association. Circulation. 2001;103:1034–1039. doi: 10.1161/01.cir.103.7.1034. [DOI] [PubMed] [Google Scholar]
- 134.Stough C, Downey L, Silber B, Lloyd J, Kure C, Wesnes K, Camfield D. The effects of 90-day supplementation with the Omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 135.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Glauber H, Wallace P, Griver K, Brechtel G. Adverse metabolic effect of omega-3 fatty acids in non-insulin-dependent diabetes mellitus. Annals of internal medicine. 1988;108:663–668. doi: 10.7326/0003-4819-108-5-663. [DOI] [PubMed] [Google Scholar]
- 137.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive medicine. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 138.Harris WS. The omega-3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep. 2010;12:503–508. doi: 10.1007/s11886-010-0141-6. [DOI] [PubMed] [Google Scholar]
- 139.Sala-Vila A, Harris WS, Cofan M, Perez-Heras AM, Pinto X, Lamuela-Raventos RM, Covas MI, Estruch R, Ros E. Determinants of the omega-3 index in a Mediterranean population at increased risk for CHD. The British journal of nutrition. 2011;106:425–431. doi: 10.1017/S0007114511000171. [DOI] [PubMed] [Google Scholar]
- 140.Aarsetoey H, Aarsetoey R, Lindner T, Staines H, Harris WS, Nilsen DWT. Low Levels of the Omega-3 Index are Associated with Sudden Cardiac Arrest and Remain Stable in Survivors in the Subacute Phase. Lipids. 2011;46:151–161. doi: 10.1007/s11745-010-3511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Raiten DJ, Namaste S, Brabin B, Combs G, Jr., L'Abbe MR, Wasantwisut E, Darnton-Hill I. Executive summary--Biomarkers of Nutrition for Development: Building a Consensus. The American journal of clinical nutrition. 2011;94:633S–650S. doi: 10.3945/ajcn.110.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]