Summary
Background
Current pain management is limited, in particular, with regard to chronic pain. In an attempt to discover novel analgesics, we combined the approach developed to characterize traditional Chinese medicine (TCM), as part of the “herbalome” project, with the reverse pharmacology approach aimed at discovering new endogenous transmitters and hormones.
Results
In a plant used for centuries for its analgesic properties, we identify a compound, dehydrocorybulbine (DHCB) that is effective at alleviating thermally induced acute pain. We synthesize DHCB and show that it displays moderate dopamine receptor antagonist activities. By using selective pharmacological compounds and dopamine receptor knockout (KO) mice, we show that DHCB antinociceptive effect is primarily due to its interaction with D2 receptors, at least at low doses. We further show that DHCB is effective against inflammatory pain and injury-induced neuropathic pain and furthermore causes no antinociceptive tolerance.
Conclusion
Our study casts DHCB as a different type of analgesic compound and as a promising lead in pain management.
Keywords: TCM, Corydalis yanhusuo W.T. Wang, antinociception, dehydrocorybulbine, dopamine receptors
Introduction
Pain reduces the quality of life and imparts high health costs and economic loss to society. Pain can be differentiated into mechanical pain, which represents an acute response to a mechanical insult; inflammatory pain, which is associated with tissue damage and the infiltration of immune cells; and neuropathic pain, which is caused by damage to the nervous system [1]. Pain sensation is transmitted by afferent neurons from the periphery to the spinal cord and from there to the brain, with feedback loops modifying the input [2]. Current pain management strategies rely primarily on anti-inflammatory and antinociceptive drugs. The prevalent anti-inflammatory drugs are the nonsteroidal anti-inflammatory drugs (NSAIDs) including the COX-2 selective inhibitors. They are the first line of therapy against low or moderate pain, followed, if unsuccessful, by the more potent opiate drugs. The opiates are the most common antinociceptive drugs and are effective for 70–80% of patients. This class of drugs is however plagued by side effects. They reduce GI motility, affect blood pressure, induce tolerance, dependence, and at high doses respiratory depression [3]. Neuropathic pain is managed poorly; anticonvulsants and antidepressants are sometimes used but with limited results [4]. Therefore the search for new analgesic compounds that present therapeutic alternatives is important.
For over 7000 years, various extracts of natural products, mostly plants, have served as analgesics. These extracts offer an opportunity to identify new analgesic compounds. They contain numerous components but only some display analgesic properties [5]. Identifying new ones requires a strategy that combines analytical purification and pharmacological analyses. We chose an approach that takes advantage of the purification efforts that have been developed to globalize and modernize traditional Chinese medicines (TCMs) as part of the “herbalome” project [6, 7] and of the reverse pharmacology approach developed to identify compounds acting at G protein-coupled receptors (GPCRs) [8, 9]. We applied this approach to plant extracts and receptors known to display antinociceptive properties.
Results
Purification, identification and pharmacological characterization of dehydrocorybulbine (DHCB), an alkaloid from Corydalis yanhusuo W. T. Wang (C. yanhusuo)
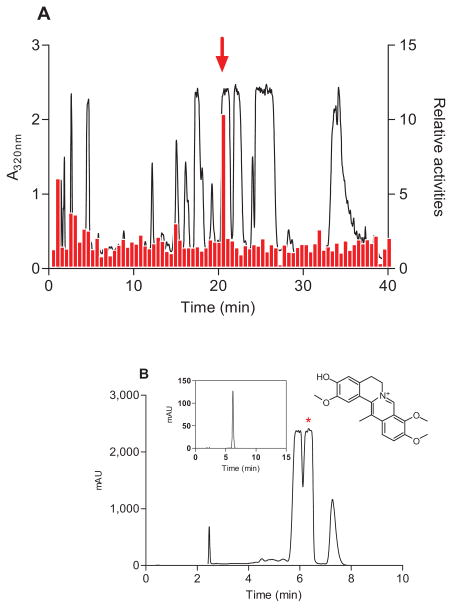
Ten TCMs known for their analgesic properties were screened on cells expressing the μ-opioid receptor. These included Commiphora myrrha (Nees) Engl., Macleaya cordata (Willd.) R.Br., Stephania japonica (Thunb.) Miers, Stephania tetrandra S. Moore, Huperzinaserrata (Thumb.)Trev, Anisodus tanguticus (Maxinowicz) Pascher, Nandina domestica, Carthamus tinctorius L, Lonicera japonica Thunb and C. yanhusuo. Approximately 500 HPLC fractionated samples were tested for their ability to activate the μ-opioid receptor. However, only one fraction in C. yanhusuo was able to induce a reproducible receptor-dependent intracellular Ca2+ mobilization (Figure 1A).
Figure 1. DHCB isolation and characterization.
(A) Elution profile of C. yanhusuo extract on a C18HCE reverse-phase HPLC column (4.6 × 150 mm, 5 μm) and activities of 80 fractions (0.5-min) tested for their abilities to induce intracellular Ca2+ mobilization in HEK293T cells expressing μ-opioid receptor. Elution was performed with a linear gradient which was from 5% to 15% CH3CN in 30 min then from 15% to 95% CH3CN in 10 min. The flow rate was 1 ml/min. The relative activities represent the ratio between the fluorescence intensities obtained with μ- and mock-transfected cells. Fraction 42 (indicated by an arrow) was most active. (B) DHCB purification and structure elucidation. Fraction 42 was further purified by two purification steps. The last step was performed on an analytical C18HCE column (4.6 ×150 mm,5 μm) using an isocratic elution of 15% CH3CN. The insert HPLC profile represents the purity of purified DHCB.
By using a novel stationary phase [10, 11], this fraction, which contains a series of structurally- related alkaloids difficult to be resolved by conventional chromatography, was efficiently purified and its active component was successfully isolated (Figure 1B). The structure of this component was elucidated by UV, Mass Spectrometry, NMR, and single X-ray crystallography (see Supplemental Information) and determined to be DHCB (structure shown in Figure 1B insert).
Purified DHCB induced a dose-dependent Ca2+ change in μ-expressing cells (Figure S1A left panel) with a half-maximum response (EC50) of 100 μM, and failed to induce a detectable response in parental cells. This activity is antagonized by naloxone (Figure S1A right panel). DHCB showed marginal activity at δ- and κ-opioid receptors (Table 1).
Table 1.
DHCB in vitro activities at the μ opioid and dopamine receptors and comparison to l-THP
| Opioid Agonist (EC50, μM) | Dopamine Antagonist (IC50, μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| μ | δ | κ | D1 | D2 | D3 | D4 | D5 | |
| DHCB | 100 (74~136) | >500 | >500 | 2.16 (1.4~3.2) | 0.52 (0.24~1.12) | 2.4 (1.3~4.5) | 8.4 (4.6~15) | 0.73 (0.25~2.1) |
| l-THP | N/A | N/A | N/A | 0.51 (0.31~0.82) | 0.32 (0.24~0.41) | 9.7 (4.9~19) | 9.7 (6.8~14) | 0.19 (0.11~0.32) |
Synthesis of DHCB
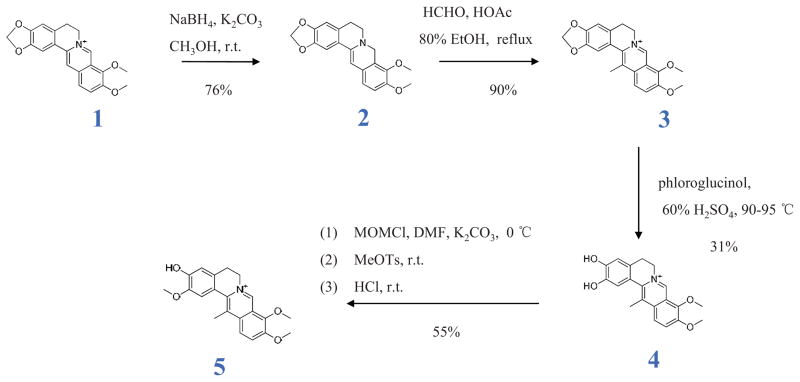
DHCB was originally isolated from Corydalis ambigua var amurensis in 1964 [12], but its biological or medicinal properties have remained unknown. It has also never been synthesized. In order to generate sufficient amounts, we synthesized DHCB through a four-step process (Figure 2). Berberine was used as a starting material for a selective reduction reaction, in which NaBH4 was used as a reducing agent and methanol as the solvent. The key intermediate product 2 was obtained with a 76% yield by adding dropwise NaBH4 in 5% NaOH in 10 min and the total amount of NaBH4 was strictly controlled. Product 2 was allowed to react with 37% formaldehyde in a mixture of EtOH and HOAc and then acidified with 2 N HCl to provide the key intermediate product 3 with a 90% yield. 3 was reacted with phloroglucinol in H2SO4 (60%) at 90–95 °C to yield 4. The concentration of H2SO4 (55–65%) and reaction time (20–30 min) are key factors during this step. Macroporous resins (XAD-4 and D152) were employed to separate 4 from the reaction mixture (31% yield). The desired compound (DHCB) was obtained through a selective methylation of 4, in which chloromethyl methyl ether was used to selectively protect the phenolic hydroxyl at 3- position, and then the phenolic hydroxyl at 2-position was methylated by methyl p-toluenesulfonate, followed by a deprotection with 2N HCl. The final product was purified through preparative HPLC. This approach is efficient and yielded 12.5% purified material from a readily available berberine. The synthesized compound was found to be identical to purified DHCB in chromatographic behavior and spectra data. To our knowledge, this is the first report of DHCB synthesis.
Figure 2.
DHCB synthesis pathway
DHCB is antinociceptive in an acute pain model
C. yanhusuo is used for its antinociceptive properties [13]. Since strong sedation may influence assessment of pain-related behaviors and since most analgesics carry sedative effects, DHCB was first tested for its potential sedative properties by monitoring its effects on locomotor activity and in the rotarod assay. The results show that DHCB is not sedative at doses of 10 mg/kg or lower (Figure S1B). We consequently used 10 mg/kg as the non-sedative threshold dose.
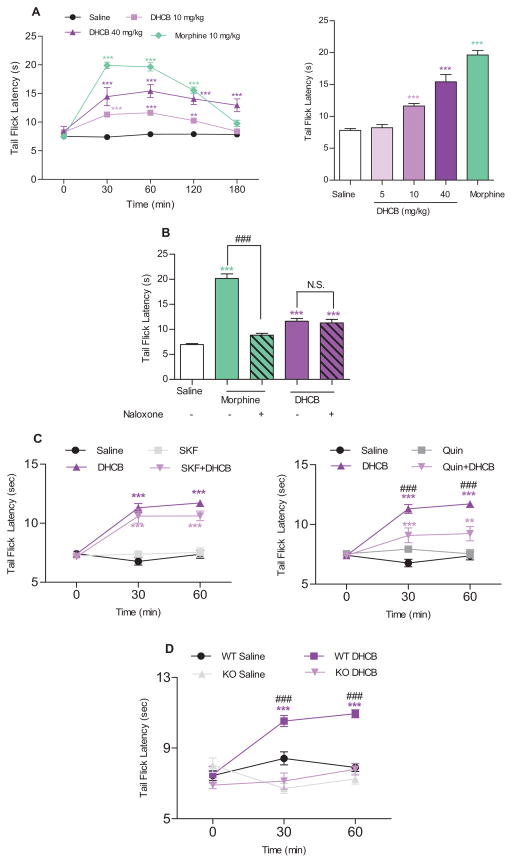
DHCB’s antinociceptive activity was tested in the tail-flick assay which records responses to a thermal stimulus. These experiments were carried out using a moderate 7–8 sec baseline to permit low antinociception detection. As shown in Figure 3A, DHCB was found to induce antinociception in a dose-dependent manner. It shows a potent effect and has a longer lasting antinociceptive property at high doses (at least 3 h) and remained antinociceptive at the non-sedative dose (10 mg/kg). Pharmacokinetic analyses have revealed that DHCB remains in the plasma at relatively high concentrations at least 3 hours after i.p. injection (Figure S1C). In addition, preliminary in vitro metabolism analyses show that DHCB is not metabolized in phase I, but is slowly metabolized into two glucuronidated products in phase II (Figure S1D). These findings indicate that DHCB is able to penetrate the blood-brain barrier and has favorable pharmacokinetic properties.
Figure 3. Antinociceptive effects of DHCB in the tail-flick assay and its mode of action.
(A) Antinociceptive effects in the tail-flick assay. CD1 mice were used in this assay. Left panel: Time-course of the tail-flick latencies (n=8–24). Two-way ANOVA revealed significant drug effect (F3,67=145.6, P < 0.0001), time effect (F4,268=72.84, P < 0.0001) and drug × time interaction (F12,268=27.09, P<0.0001); Right panel: Dose-response effect of DHCB (5, 10, 40 mg/kg, i.p.) on tail-flick latency 60 min after administration (n=8–24). One-way ANOVA revealed significant drug effect (F4,80=82.74, P<0.0001). Bonferroni post hoc tests: drug vs. saline: * P<0.05, ** P<0.01, *** P<0.001. (B) Naloxone (1 mg/kg, i.p.) effect on DHCB (10 mg/kg, i.p.)- induced antinociception in the tail-flick assay (n=6–8). Naloxone was injected right before DHCB administration. Tail-flick latency was measured 30 min after DHCB administration. CD1 mice were used in this assay. One-way ANOVA revealed significant drug effect (F4,30=62.44, P < 0.0001). Bonferroni post hoc tests: drug vs. saline: *** P < 0.001; morphine vs morphine+naloxone: ### P < 0.001; N.S., not significant, P>0.05. (C) Time-course of SKF-38393 (1 mg/kg, i.p, left panel) and quinpirole (0.5 mg/kg, i.p. right panel) effects on DHCB (10 mg/kg, i.p.)-induced response in the tail-flick assay (n=7–8). CD1 mice were used in this assay. SKF-38393 or quinpirole was injected 30 min before DHCB. Tail-flick latencies were measured 30 and 60 min following administration of DHCB. Two-way ANOVA revealed significant drug effect (left panel: F3,27=56.21, P<0.0001; right panel:F3,27=21.28, P<0.0001), time effect (left panel: F2,54=39.40, P<0.0001; right panel:F2,54=33.43, P<0.0001) and drug×time interaction (left panel: F6,54=14.19, P<0.0001; right panel: F6,54=16.69, P<0.0001). Bonferroni post hoc tests: drug vs. saline: ** P < 0.01, *** P < 0.001; DHCB vs. DHCB+quinpirole: ### P<0.001; N.S., not significant. (D) Time-course of DHCB (5 mg/kg, i.p.) effects in D2KO mice assessed in the tail-flick assay (n=6–7). The D2KO mice used in this study were backcrossed in a 50% C57Bl/6/50% 129SV background for six consecutive generations with pure C57Bl/6 mice resulting into 98.5% C57Bl/6-1.5%129 SV animals. Age-matched wild-type littermates with the same genetic background were used as control animals. Tail-flick latencies were measured 30 and 60 min following administration of DHCB (5 mg/kg, i.p.). Two-way ANOVA revealed significant drug effect (F3,22=32.99, P<0.0001), time effect (F2,44=10.02, P=0.0003) and drug×time interaction (F6,44=10.59, P<0.0001). Bonferroni post hoc tests: DHCB/WT vs. saline/WT groups: *** P<0.001; DHCB/WT vs. DHCB/D2KO groups: ### P<0.001; N.S., not significant, P>0.05. In all cases, data are means ± S.E.M.
The mechanism of DHCB’s antinociceptive activity
DHCB’s weak activity at the μ receptor prompted us to carry out a survey of the properties of known alkaloids in C. yanhusuo. L-tetrahydropalmatine (l-THP), a primary active constitute in C. yanhusuo, has been shown to display dopamine receptor antagonism and exert analgesic effects [14, 15]. Because DHCB and l-THP (structure shown in Figure S1E) are structurally similar, their activities were compared at the five dopamine receptor subtypes. We found that both DHCB and l-THP behave as antagonists (Figure S1F). As summarized in Table 1, DHCB exhibits micro- or submicro-molar affinities to all five dopamine receptors and displays its highest affinity to the D2 receptor. When compared to l-THP, DHCB shows higher or comparable affinities to the D2-like and lower affinities to the D1-like receptors (Table 1). Consistent with a previous report [16], l-THP showed no activity at the μ-opioid receptor.
Because DHCB exhibits a weak μ-opioid receptor agonist activity, we first ascertained whether its antinociceptive role in vivo can be inhibited by naloxone. As shown in Figure 3B and Figure S1G, the antinociception induced by DHCB (10 mg/kg) in the tail-flick assay was not found to be antagonized by naloxone, which is in accord with its activity at the μ-opioid receptor in vitro.
Next, we tested whether dopamine D1 and/or D2 receptors are involved in DHCB antinociception. As shown in Figure 3C (left panel), SKF-38393, a selective D1 agonist, was unable to block the effect of DHCB (10 mg/kg) in the tail-flick assay. In contrast, quinpirole, a selective D2 agonist, significantly reversed DHCB antinociception (Figure 3C right panel). To further study the role of dopamine D2 receptor in mediating DHCB antinociception, we tested its effects in D2 receptor knockout (D2KO) mice. Wild-type (WT) and D2KO animals display similar tail-flick latency baselines (Figure 3D). DHCB at a non-sedative dose (5 mg/kg, shown in Figure S1H) induced an antinociceptive response in WT animals, but not in D2KO mice (Figure 3D). This shows that DHCB antinociceptive effect is primarily, at least at low doses, due to its interaction with D2 receptors, also raising the possibility that this may also be the site of l-THP action [14, 15].
DHCB is antinociceptive in inflammatory and neuropathic pain models
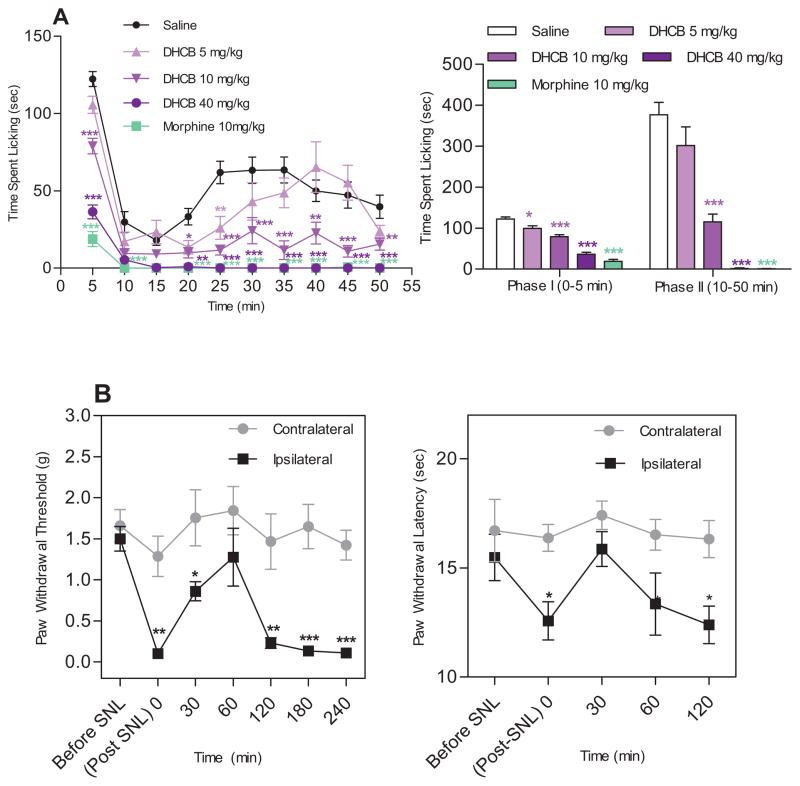
To ascertain and extend our understanding of its antinociceptive efficacy, DHCB was tested in the formalin assay, a test designed to assess both acute and persistent inflammatory pain responses. The assay produces a distinct biphasic response. Phase I (early phase) occurs within the first 5 min after formalin injection and corresponds to acute neurogenic pain. Phase II (10–50 min, late phase) corresponds to inflammatory pain and is inhibited by nonsteroidal anti-inflammatory drugs. As shown in Figure 4A, DHCB caused a significant reduction in the time spent licking in the early (P<0.05) and late phase (P<0.001). This effect is dose dependent, effective at a non-sedative dose and is comparable to that of morphine at high doses.
Figure 4. Antinociceptive effects of DHCB in inflammatory pain and neuropathic pain models.
(A) Effects of DHCB in the formalin assay (n=9–24). CD1 mice were used in this assay. Formalin (25 μl, 5% in saline) was injected into the dorsal surface of the right hind paw 15 min after DHCB administration. Left panel: time-course effects. Two-way ANOVA revealed significant drug effect (F4,74=63.47, P<0.0001), time effect (F9,666=44.54, P<0.0001) and drug×time interaction (F36,666=5.09, P<0.0001). Bonferroni post hoc tests: drug vs. saline: * P<0.05, ** P<0.01, *** P<0.001. Right panel: Cumulative effects in Phase I (0–5 min) and Phase II (10–50 min) of the formalin assay. One-way ANOVA indicated significant drug effect in both Phase I (F4,76 =71.93, P<0.0001) and Phase II (F4,76=45.96, P<0.0001). Dunnett’s post hoc tests: drug vs. saline: * P<0.05, ** P<0.01, *** P<0.001. (B) Effects of DHCB (10 mg/kg, i.p.) after SNL. 129/sv mice were used in this assay. Mice were injected with DHCB about two weeks post SNL when all injured mice have developed hindpaw mechanical and thermal hypersensitivies on the injured side. Left panel: Effects of DHCB in Von Frey assay. Two-way ANOVA revealed significant drug effect (F1,14=18.88, P=0.0007), time effect (F6,84=10.53, P< 0.0001) and drug × time interaction (F6,84=4.11, P=0.0012). Bonferroni post hoc tests: contralateral vs. ipsilateral: * P<0.05, ** P<0.01, *** P<0.001. Right panel: Effects of DHCB in hot box assay (n=8). Two-way ANOVA indicated only significant main effect of treatments (F1,14=17.44, P=0.0009). Bonferroni post hoc tests: contralateral vs. ipsilateral: * P<0.05. In all cases, data are means ± S.E.M.
Chronic neuropathic pain is a common clinical problem affecting over 50 million people in the United States [17]. The management of neuropathic pain remains a major clinical challenge due to the poor efficacies and severe side effects of conventional analgesics. The assay commonly used to model neuropathic pain is spinal nerve ligation (SNL) which induces allodynia as measured by mechanical sensitivity in the von Frey hair stimulation assay and hyperalgesia as measured by thermal sensitivity in the Hargreave-type hot box. We show that DHCB at a non-sedative dose (10 mg/kg determined in Figure S2A) significantly attenuates mechanical allodynia in response to von Frey stimulation (Figure 4B left panel) and hyperalgesia by hot box (Figure 4B right panel).
Taken together, these data demonstrate that DHCB is effective at suppressing responses to both chemically induced, inflammatory-derived and injury–induced pain. Further studies will be needed to determine whether these effects also depend on DHCB dopamine D2 receptor antagonist activity.
DHCB does not cause antinociceptive tolerance
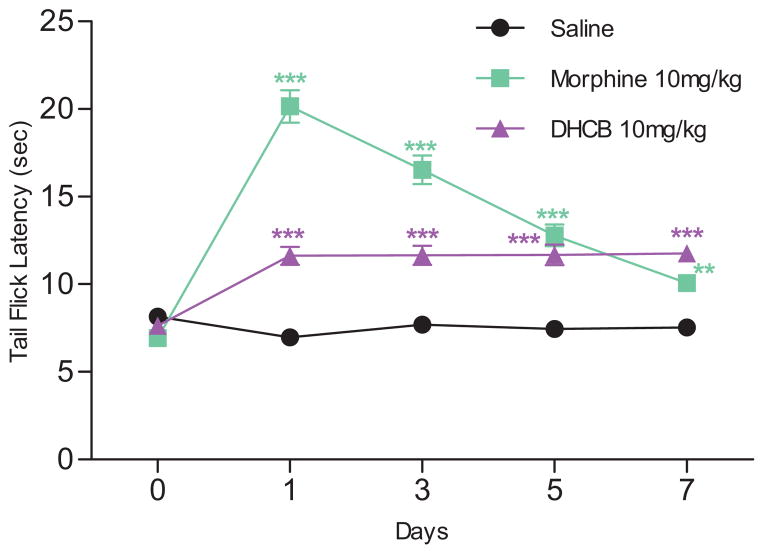
The fact that DHCB exerts its antinociceptive effects through dopamine D2 receptors but not the μ-opioid receptor led us to test it for antinociceptive tolerance. Mice were subjected to daily DHCB administrations over a seven-day period and monitored for their responses in the tail-flick assay (Figure 5). Unlike morphine (10 mg/kg), mice did not develop tolerance to DHCB (10 mg/kg).
Figure 5. Lack of antinociceptive tolerance of DHCB (10 mg/kg, i.p.).
Repeated injections of DHCB for 7 consecutive days induced no antinociceptive tolerance (n=6–8). CD1 mice were used in this study. Two-way ANOVA revealed significant drug effect (F2,17=125.3, P<0.0001), time effect (F4,68=55.46, P<0.0001) and drug × time interaction (F8,68=36.4, P<0.0001). Bonferroni post hoc tests: drug vs. saline: * P<0.05, ** P<0.01, *** P<0.001. Data are means ± S.E.M.
Discussion
Management of chronic pain is an unmet medical need. Use of conventional drugs is often neither effective nor free of side effects. To find new natural analgesics, we combined the “herbalome” and the “reverse pharmacology” approaches. Out of 500 HPLC fractions derived from 10 TCMs, only one fraction, isolated from C. yanhusuo, was found active at the opioid receptor. Of the 10 TCMs that were screened, C. yanhusuo is the only one belonging to the Papaveraceae family, which includes opium (Papaver somniferum) and thus may be the only one that has retained compounds that act at opioid receptors. It is also worthwhile to mention that through this approach, we may have missed several analgesic compounds that do not behave as μ receptor agonists.
We then took advantage of novel separation techniques to purify this fraction and to identify DHCB as its active component. C. yanhusuo preparations have been historically employed for the treatment of various pains and are officially listed in the Chinese Pharmacopoeia [13]. DHCB has however never been synthesized, although a few studies have been carried out on berberine analogues [18, 19]. As shown in Figure 2, we synthesized the key intermediate products 3 and 4 based on previous reports describing the synthesis of berberine analogues [18, 19]. The major challenge was to selectively methylate the phenolic hydroxyl at 2-position but not at 3-position in the intermediate product 4. The issue resides with the phenolic hydroxyl at 3-position which is easy to methylate because the N+ renders the hydroxyl at 3-position more acidic and thus more able to accept -CH3. Our strategy was to find a reagent able to protect the phenolic hydroxyl at 3-position. Several reagents were tried and chloromethyl methyl ether was found efficient. After methylation of the hydroxyl at 2-position, the deprotection at 3-position was performed using 2 N HCl. DHCB synthesis proceeds in 4 steps and leads to 12.5% overall yield from a readily available berberine.
Synthetic DHCB was used to show that it is effective at alleviating thermally- as well as chemically-induced acute pain and inflammatory-induced persistent tonic pain. It is effective at doses which do not induce sedation and at high doses exerts an antinociceptive response similar to that obtained with morphine. Furthermore DHCB is effective at relieving injury-induced neuropathic pain. Because DHCB antinociceptive effects are displayed in both the acute and the inflammatory phases of the formalin assay, its activity may result from direct effects on the central nervous system [20, 21]. Indeed our pharmacokinetic and preliminary in vitro metabolism analyses indicate that DHCB is able to penetrate the blood-brain barrier. DHCB remains present in the brain and plasma at least 3 h which correlates with the duration of its antinociceptive action in the tail-flick assay. It should be mentioned that it did not exhibit such a long lasting duration in the neuropathic pain model which may reflect differences in sensitivity between the mouse strains used in these two assays.
Our results also indicate that DHCB displays advantageous activities over l-THP, which is another antinociceptive component of C. yanhusuo extract. DHCB is more analgesic than l-THP in the tail-flick at a non-sedative dose (Figure S1I) and is less sedative (Figure S1B). L-THP has been previously thought to account for most of the analgesic properties of C. yanhusuo. Because both DHCB and l-THP are present at comparable levels in C. yanhusuo (0.018% and 0.025%, respectively), it is reasonable to believe that not only l-THP, but also DHCB, is responsible for the analgesic effects of C. yanhusuo extracts.
To gain further insight on the mechanism by which DHCB exerts its antinociceptive responses, we first show that in vitro it behaves as a weak agonist at the μ opioid receptor and an antagonist at the dopamine receptors. We then show that in vivo DHCB displays a naloxone resistant antinociceptive response in the tail-flick test, as well as in formalin assay (Figure S2B). This is expected because of its low affinity for the μ-opioid receptor, which indicates that DHCB cannot reach an effective concentration to activate this receptor in vivo. The affinities of DHCB to the dopamine receptors are more than one hundred times higher than that to the μ-opioid receptor. Therefore the role of DHCB in vivo was first analyzed by using selective dopamine D1 and D2 receptor agonists at doses that do not affect antinociception in the tail-flick assay. We found that quinpirole, a D2 receptor agonist, antagonizes DHCB antinociceptive response while SKF-38393, a D1 receptor agonist, does not. This result was extended and confirmed by using D2KO mice. In these mice, the antinociceptive effects of DHCB are strongly decreased which indicates that its action is mediated primarily through its inhibition of the dopamine D2 receptor. This experiment was carried out using a non-sedative dose of DHCB and thus does not reflect a locomotion-related response. It has been reported that dopamine D2 receptor activity in the striatum is associated with pain sensitivity and pain modulatory capacity in healthy subjects [22, 23]. Interestingly, both dopamine D2 receptor agonists [24–28] and antagonists [29–31] have been reported to exert analgesic properties. Preferential presynaptic dopamine D2 receptor binding by some of the D2 antagonists might explain these seemingly contradictory propositions [31, 32]. Antagonists selective to the presynaptic D2 receptors would increase dopamine release and in turn increase antinociception. In this respect, low doses of amisulpride, a selective antagonist of dopamine D2/D3 receptor with selective preference for presynaptic dopamine autoreceptors, significantly increase tail-flick latency in mice [31]. Further studies will be needed to determine whether the antinociceptive effects of DHCB are regulated by presynaptic dopamine D2 receptors.
Because one of the major drawbacks of the narcotic analgesics is development of tolerance, we tested DHCB behavior versus antinociceptive tolerance. We show that repeated DHCB administrations do not lead to development of tolerance, and thus that DHCB may present advantages over morphine in chronic pain treatment. Due to its interaction with dopamine receptors, further studies should be carried out to determine whether DHCB exhibits the other side effects of the neuroleptics.
The strategy that we adopted to ultimately isolate DHCB can be applied to any natural source and to many receptors. It therefore has the potential to discover not only new analgesics but also numerous other naturally-occurring active compounds. In view of the interest in pharmacotherapy for finding compounds with multipharmacological profiles, this study suggests that natural products still hold great promise.
Experimental Procedures
Purification, identification and synthesis of DHCB
The fractionation of the crude n-butanol extract was performed on an analytical C18HCE column (4.6×150 mm, 5 μm) with an Alliance HPLC system (Milford, MA, USA) that consisted of a Waters 2695 HPLC pump and a Waters 2996 photodiode array detector (PAD). The mobile phases were composed of CH3CN (phase A) and 0.1% formic acid aqueous (phase B), with a gradient of 5% A to 15% A in 30 min and then to 95% A in 10 min. The flow rate was 1.0 ml/min and the column temperature was maintained at 30 °C. The amount of sample loading is 6 mg per injection. The fractions were collected with a Waters Fraction Collector III (Milford, MA, USA) at intervals of 0.5 min/fraction. After the active fraction (F42) was found, 1.0 g of the crude sample was fractionated on a semi-preparative C18HCE column (20 × 150 mm, 10 μm), with a similar HPLC method mentioned above but at 20.0 ml/min. The active fraction was pooled and it was further separated on the analytical C18HCE column with an isocratic elution of 15% CH3CN. Finally, 5.8 mg (yield 0.01%, dry weight) of the single compound was purified.
Structure elucidation of the isolated active compound was carried out by using various spectral techniques. HRESIMS were recorded on a Q-TOF system (Waters Co., UK). NMR spectra were obtained on Bruker AV400 spectrometer. Single-crystal was obtained in the perchlorate form of DHCB, by slowly volatilizing its methanol solution. X-ray diffraction was performed by using a Bruker Apex II CCD diffractometer equipped with a fine-focus sealed-tube X-ray source (MoKα radiation, graphite monochromated). Structures were solved by direct methods using SHELXTL and were refined by full-matrix least-squares on F2 using SHELX-97. Non-hydrogen atoms were refined with anisotropic displacement parameters during the final cycles. Hydrogen atoms were placed in calculated positions with isotropic displacement parameters set to 1.2×Ueq of the attached atom.
The synthesis pathway is shown in Figure 2
Cell culture, cDNA constructs and transfection
All GPCRs used in this study were amplified from human cDNA library (Clontech, Palo Alto, CA) and cloned into pcDNA 3.1 (−) (Invitrogen, Carlsbad, CA). Human embryonic kidney-293 T cells (HEK293T) were cultured in Dulbecco’s Minimum Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The stable cell lines expressing human opioid receptors μ, δ and κ, respectively were created as previously reported [33]. The Individual dopamine receptors D1, D2, D3, D4 or D5 were transiently co-transfected with Gα15 in HEK293T cells using lipofectamine as described in Invitrogen’s protocol.
Ca2+ response monitored by Fluorometric Imaging Plate Reader Assay (FLIPR)
The assay was performed as reported earlier [34]. The samples, which were re-dissolved in dimethyl sulphoxide (DMSO) and stored in 96-well drug plates, were diluted with FLIPR buffer and then automatically added into the cells within 4 sec. For agonist tests, the intracellular Ca2+ concentration was monitored at 520 nm with excitation wavelength at 488 nm over a period of 4 min. For antagonist tests, the compound was first incubated with the cell for 10 min, before the addition of dopamine with EC50 dose determined in individual dopamine receptor expressing cell lines. Data were expressed as fluorescence (arbitrary units) versus time.
Antinociceptive assays
Male CD1 mice (30–40 g), age 9–11 weeks were used in the majority of the experiments. Male 129/sv mice (20–30 g), age 9–12 weeks were used in Von Frey filament and hot box assays. D2KO mice (25–35 g), age 12–13 weeks were also used in the tail-flick assay. Age-matched wild-type littermates with the same genetic background were used as control animals. The generation of D2KO mice was reported previously [35]. Saline, morphine (10 mg/kg), DHCB (5–40 mg/kg) or l-THP (5–40 mg/kg) was injected intraperitoneally (5 ml/kg, i.p). Naloxone (1 mg/kg, 2.5 ml/kg, i.p.) was injected right before drug administration. SKF-38393 (1 mg/kg, 5 ml/kg, i.p.) or quinpirole (0.5 mg/kg, 5 ml/kg, i.p.) was injected 30 min before DHCB. Inflammation was evoked by injecting 25 μl of 5 % formalin solution into the dorsal surface of the right hind paw 15 min after drug administration. The tail-flick, formalin paw test, Von Frey filaments test and hot box assay experiments were carried out as previously reported [20, 36, 37]. Detailed experimental procedures were described in Supplemental Experimental Procedures. All experimental procedures were approved by the Institutional Animal Care and Use Committee of University of California, Irvine and were performed in compliance with national and institutional guidelines for the care and use of laboratory animals.
The tolerance study was performed by a repeated-injection schedule. Mice were injected (5 ml/kg, i.p.) with saline, morphine or DHCB at dose of 10 mg/kg once daily for 7 consecutive days. The loss of the antinociceptive effects of drugs in the tail-flick test was used to assess the degree of tolerance. The tail-flick latency was assessed on days 1, 3, 5, and 7 at 30 min after the drug injections.
Data analysis
Graphpad Prism 5.0 (GraphPad Software, Inc., San Diego, CA) was used for statistical analysis. Data are means ± S.E.M.. Results were analyzed by ANOVA (one-way or two-way) followed by the appropriate post hoc comparisons, and P<0.05 was considered statistically significant.
Supplementary Material
Highlights.
DHCB is isolated from C. yanhusuo, a plant used for centuries for pain relief
DHCB is antinociceptive against acute, inflammatory and neuropathic pains
DHCB’s antinociception relies on its inhibitory activity at the dopamine D2 receptor
DHCB administration does not induce antinociceptive tolerance
Acknowledgments
We would like to thank Drs. Richard Chamberlin, James D. Belluzzi, Jim Carolan and Amal Alachkar for helpful discussions and Lily Tamura for proof reading. This work was supported by National Institute of Health Grants MH60231, DA024746, an Established Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD) and a Tourette Syndrome Association award to Olivier Civelli. This work was also supported by the Key Program of National Natural Science Foundation of China (Grant No. 21135005) and the Major Project of National High Technology Research and Development Program of China (863 Program, 2012AA020203).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 3.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol. 2010;6:191–197. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- 4.Hama A, Sagen J. Combination Drug Therapy for Pain following Chronic Spinal Cord Injury. Pain Res Treat. 2012 doi: 10.1155/2012/840486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCurdy CR, Scully SS. Analgesic substances derived from natural products (natureceuticals) Life Sci. 2005;78:476–484. doi: 10.1016/j.lfs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Stone R. Biochemistry. Lifting the veil on traditional Chinese medicine. Science. 2008;319:709–710. doi: 10.1126/science.319.5864.709. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Liu Y, Guo Z, Feng J, Dong J, Fu Q, Wang C, Xue X, Xiao Y, Liang X. The herbalome--an attempt to globalize Chinese herbal medicine. Anal Bioanal Chem. 2012;402:573–581. doi: 10.1007/s00216-011-5533-y. [DOI] [PubMed] [Google Scholar]
- 8.Civelli O, Nothacker HP, Saito Y, Wang Z, Lin SH, Reinscheid RK. Novel neurotransmitters as natural ligands of orphan G-protein-coupled receptors. Trends Neurosci. 2001;24:230–237. doi: 10.1016/s0166-2236(00)01763-x. [DOI] [PubMed] [Google Scholar]
- 9.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 10.Long Z, Wang C, Guo Z, Zhang X, Nordahl L, Liang X. Strong cation exchange column allow for symmetrical peak shape and increased sample loading in the separation of basic compounds. J Chromatogr A. 2012;1256:67–71. doi: 10.1016/j.chroma.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Guo Z, Zhang J, Zeng J, Zhang X, Liang X. High-performance purification of quaternary alkaloids from Corydalis yanhusuo W. T. Wang using a new polar-copolymerized stationary phase. J Sep Sci. 2011;34:53–58. doi: 10.1002/jssc.201000625. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi H, Imaseki I. Studies on the Components of Corydalis Spp. 3 Alkaloids of Corydalis Ambigua Cham Et Schlecht Var Amurensis Maxim (2) On Tertiary Alkaloids (Suppl) and Quaternary Alkaloids. Yakugaku Zasshi. 1964;84:773–775. [PubMed] [Google Scholar]
- 13.China Pharmacopoeia committee. First Div. 2005. Beijing: China Chemical Industry Press; 2005. Pharmacopoeia of People’s Republic of China. [Google Scholar]
- 14.Chu H, Jin G, Friedman E, Zhen X. Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol Neurobiol. 2008;28:491–499. doi: 10.1007/s10571-007-9179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu JY, Jin GZ. Supraspinal D2 receptor involved in antinociception induced by l-tetrahydropalmatine. Zhongguo Yao Li Xue Bao. 1999;20:715–719. [PubMed] [Google Scholar]
- 16.Zhang ZD, Jin GZ, Xu SX, Yu LP, Chen Y, Jiang FY, Zhang YR, Sun Z, Ding YL, Bian CF, et al. Effects of l-stepholidine on the central nervous and cardiovascular systems. Zhongguo Yao Li Xue Bao. 1986;7:522–526. [PubMed] [Google Scholar]
- 17.Mitka M. “Virtual textbook” on pain developed: effort seeks to remedy gap in medical education. JAMA. 2003;290:2395. doi: 10.1001/jama.290.18.2395. [DOI] [PubMed] [Google Scholar]
- 18.Li YH, Yang P, Kong WJ, Wang YX, Hu CQ, Zuo ZY, Wang YM, Gao H, Gao LM, Feng YC, et al. Berberine analogues as a novel class of the low-density-lipoprotein receptor up-regulators: synthesis, structure-activity relationships, and cholesterol-lowering efficacy. J Med Chem. 2009;52:492–501. doi: 10.1021/jm801157z. [DOI] [PubMed] [Google Scholar]
- 19.Iwasa K, Nishiyama Y, Ichimaru M, Moriyasu M, Kim HS, Wataya Y, Yamori T, Takashi T, Lee DU. Structure-activity relationships of quaternary protoberberine alkaloids having an antimalarial activity. Eur J Med Chem. 1999;34:1077–1083. [Google Scholar]
- 20.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 21.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 22.Hagelberg N, Martikainen IK, Mansikka H, Hinkka S, Nagren K, Hietala J, Scheinin H, Pertovaara A. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain. 2002;99:273–279. doi: 10.1016/s0304-3959(02)00121-5. [DOI] [PubMed] [Google Scholar]
- 23.Pertovaara A, Martikainen IK, Hagelberg N, Mansikka H, Nagren K, Hietala J, Scheinin H. Striatal dopamine D2/D3 receptor availability correlates with individual response characteristics to pain. Eur J Neurosci. 2004;20:1587–1592. doi: 10.1111/j.1460-9568.2004.03622.x. [DOI] [PubMed] [Google Scholar]
- 24.Barasi S, Duggal KN. The effect of local and systemic application of dopaminergic agents on tail flick latency in the rat. Eur J Pharmacol. 1985;117:287–294. doi: 10.1016/0014-2999(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Zhang YQ, Wu GC. Effects of dopaminergic agents on carrageenan hyperalgesia after intrathecal administration to rats. Eur J Pharmacol. 2001;418:73–77. doi: 10.1016/s0014-2999(01)00930-x. [DOI] [PubMed] [Google Scholar]
- 26.Morgan MJ, Franklin KBJ. Dopamine receptor subtypes and formalin test analgesia. Pharmacol Biochem Behav. 1991;40:317–322. doi: 10.1016/0091-3057(91)90560-o. [DOI] [PubMed] [Google Scholar]
- 27.Taylor BK, Joshi C, Uppal H. Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res. 2003;987:135–143. doi: 10.1016/s0006-8993(03)03318-3. [DOI] [PubMed] [Google Scholar]
- 28.Magnusson JE, Fisher K. The involvement of dopamine in nociception: the role of D-1 and D-2 receptors in the dorsolateral striatum. Brain Res. 2000;855:260–266. doi: 10.1016/s0006-8993(99)02396-3. [DOI] [PubMed] [Google Scholar]
- 29.Freedenfeld RN, Murray M, Fuchs PN, Kiser RS. Decreased pain and improved quality of life in fibromyalgia patients treated with olanzapine, an atypical neuroleptic. Pain Pract. 2006;6:112–118. doi: 10.1111/j.1533-2500.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 30.Jurna I, Heinz G. Anti-nociceptive effect of morphine, opioid analgesics and haloperidol injected into the caudate-nucleus of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1979;309:145–151. doi: 10.1007/BF00501222. [DOI] [PubMed] [Google Scholar]
- 31.Weizman T, Pick CG, Backer MM, Rigai T, Bloch M, Schreiber S. The antinociceptive effect of amisulpride in mice is mediated through opioid mechanisms. Eur J Pharmacol. 2003;478:155–159. doi: 10.1016/j.ejphar.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 32.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8:781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Wang Z, Cox DP, Civelli O. Study on the activation of the opioid receptors by a set of morphine derivatives in a well-defined assay system. Neurochem Res. 2012;37:410–416. doi: 10.1007/s11064-011-0627-7. [DOI] [PubMed] [Google Scholar]
- 34.Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 35.Mansikka H, Erbs E, Borrelli E, Pertovaara A. Influence of the dopamine D2 receptor knockout on pain-related behavior in the mouse. Brain Res. 2005;1052:82–87. doi: 10.1016/j.brainres.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 36.D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- 37.Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.