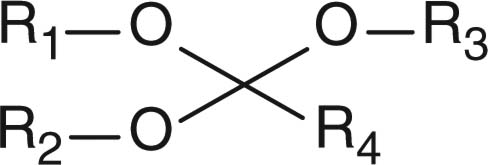
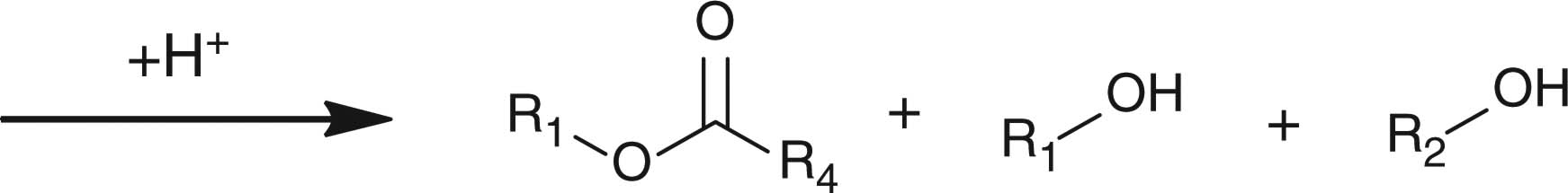
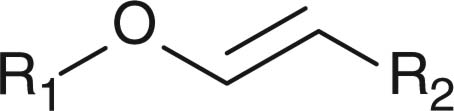
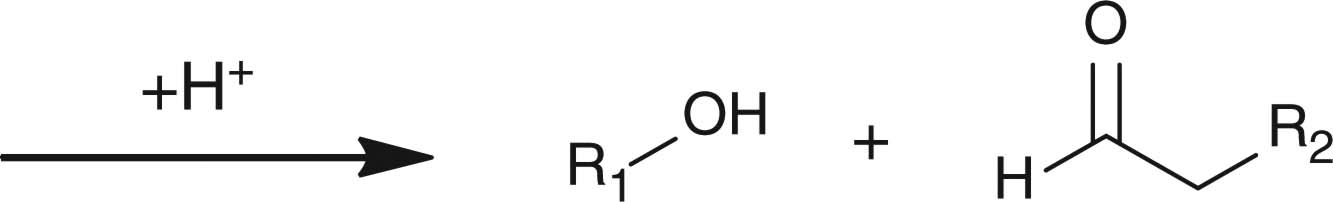
Abstract
Introduction
Drug delivery systems (DDSs) are important for effective, safe, and convenient administration of drugs. pH- and ion-responsive polymers have been widely employed in DDS for site-specific drug release due to their abilities to exploit specific pH- or ion-gradients in the human body.
Areas covered
Having pH-sensitivity, cationic polymers can mask the taste of drugs and release drugs in the stomach by responding to gastric low pH. Anionic polymers responsive to intestinal high pH are used for preventing gastric degradation of drug, colon drug delivery and achieving high bioavailability of weak basic drugs. Tumor-targeted DDSs have been developed based on polymers with imidazole groups or poly(β-amino ester) responsive to tumoral low pH. Polymers with pH-sensitive chemical linkages, such as hydrazone, acetal, ortho ester and vinyl ester, pH-sensitive cell-penetrating peptides and cationic polymers undergoing pH-dependent protonation have been studied to utilize the pH gradient along the endocytic pathway for intracellular drug delivery. As ion-sensitive polymers, ion-exchange resins are frequently used for taste-masking, counterion-responsive drug release and sustained drug release. Polymers responding to ions in the saliva and gastrointestinal fluids are also used for controlled drug release in oral drug formulations.
Expert opinion
Stimuli-responsive DDSs are important for achieving site-specific and controlled drug release; however, intraindividual, interindividual and intercellular variations of pH should be considered when designing DDSs or drug products. Combination of polymers and other components, and deeper understanding of human physiology are important for development of pH- and ion-sensitive polymeric DDS products for patients.
Keywords: colon delivery, controlling drug release, drug delivery system, ion-responsive, pH-responsive, taste-masking, tumor-targeting
1. Introduction
Drug delivery systems (DDSs) and pharmaceutical formulations are important for effective, safe and convenient administration of drugs. Higher efficacy of drugs can be achieved through improving water solubility of drugs [1-3], restricting drug release in a site-specific manner [4,5], or preventing undesired drug degradation in the stomach or during blood circulation [6,7]. DDS formulation can also promote drug safety by reducing systemic side effects [8,9], preventing drug release at stomach and subsequent gastric damage [10], and preventing drug distribution into normal tissues [11-13]. Further, oral disintegrating tablets [14,15] and taste-masking formulations [16-20] are excellent examples of DDS formulations aiming at improving patient adherence.
Stimuli-responsive polymers are one of the most important excipients in DDSs and pharmaceutical formulations for site-specific drug release as they can be designed to produce specific and desired pH- or ionic concentration-triggered response according to the variations in physiological environments in the human body. Oral drugs and formulations travel from the oral cavity to the stomach, the small intestine and eventually the large intestine; each gastrointestinal site has its specific pH and ionic concentration (Table 1). On the other hand, pH or ionic concentration gradients could also exist among the blood, interstitial and intracellular compartments (Table 1), where drugs are distributed after intravenous (i.v.) injection or drug absorption into the blood circulation.
Table 1.
pH and ionic concentration in different physiological fluids.
| Physiological fluid | Volume (l) | pH | [Na+] (mEq/l) | [K+] (mEq/l) | [Cl-] (mEq/l) | [HCO3-] (mEq/l) |
|---|---|---|---|---|---|---|
| Saliva | 1.5 | 6.0 – 7.0 | 30 | 20 | 30 | 15 |
| Gastric fluid | 2.5 | 1.0 – 3.5 (fasting) | 50 (H+, 90) | 10 | 110 | 0 |
| Bile | 0.5 | 7.8 | 140 | 5 | 105 | 40 |
| Pancreatic fluid | 0.7 | 8.0 – 8.3 | 140 | 5 | 60 | 90 |
| Small-intestinal fluid | 1.5 | 7.5 – 8.0 | 120 | 5 | 110 | 35 |
| Large-intestinal fluid | 1.0 – 1.5 | 5.5 – 7.0 | 130 | 10 | 95 | 20 |
| Plasma | 3.0 | 7.4 | 140 | 5 | 100 | 30 |
| Interstitial fluid | 10.0 | - | 150 | 5 | 110 | 30 |
| Intracellular fluid | 30.0 | - | 10 | 160 | 115 | 30 |
To facilitate drug delivery and improve therapeutic effect, prodrugs, liposomes and microchips have also been designed to respond to different stimuli. Prodrugs can be tailored to contain pH-sensitive linkages for responding to a pH gradient; however, introduction of the chemical linkages to the molecular structure of drugs poses a risk of lowering binding constant of drugs to target biomolecules and thus decreasing drug efficacy [21]. Microchips can be produced to generate the ideal responsiveness and be applied to all kinds of drugs; however, safety of microchips should be further verified in the future [22]. pH-sensitive liposomes have been researched intensively, and they are one of the best i.v. formulations for tumor or intracellular delivery [23]. Although new technologies for drug delivery are available, stimuli-responsive polymers still remain as the most popular materials for drug delivery development due to their flexibility in terms of design and synthesis and their relative low cost. Moreover pH-sensitive polymers can be used in various i.v. and oral formulations such as tablet, capsules, microbeads, nanoparticles, micelles and coating layer of liposomes. In this article, recent studies on DDSs based on pH- or ion-responsive polymers are summarized. Progress and prospect of this field are discussed, and author's opinions are described.
2. pH-sensitive polymers
2.1 Polymers responsive to stomach low pH
Cationic polymers with amino groups have higher water solubility at acidic pH than at neutral pH. These polymers with pH-responsive dissolution characteristics are widely employed for taste-masking formulations of drugs, which have unpleasant qualities such as bitterness, sourness, saltiness or causing oral numbness [24-26]. Through suppressing drug release in the oral cavity, taste-masking formulations can prevent the unpleasant tastes of drugs as the polymers are insoluble at higher pH. While being more soluble at lower pH, pH-sensitive polymers can release the drugs in the stomach or intestine for drug absorption or therapeutic purposes at the released sites. The pH difference between the oral cavity (pH 5.8 – 7.4) [27] and the stomach (pH 1 – 3.5) is thus commonly exploited by pH-responsive polymers to control drug release as summarized in Table 2. For example, aminoalkyl methacrylate copolymer (Eudragit E) is a Food and Drug Administration (FDA)-approved cationic polymer having high solubility below pH 5. Microspheres of Eudragit E containing sumatriptan succinate [28] or donepezil hydrochloride [29] have been prepared by spray-drying technique. The microspheres have been shown to be capable of suppressing drug release in phosphate buffer at pH 7.4 [28] or simulated salivary fluid [29] for 1 minute, thus masking the bitter tastes of the drugs. In acidic buffer, both formulations demonstrated immediate drug release and showed plasma concentration–time profiles similar to those of marketed products [28,29]. Moreover, faster drug release at acidic pH than at neutral pH has been observed with pellets containing quinine sulphate [30] and granules containing promethazine [31] prepared using Eudragit E. Coating of Eudragit E on particles containing atorvastatin for oral disintegrating tablets has also been reported [32]. Despite many studies using aminoalkyl methacrylate copolymers as DDSs and good in vitro results demonstrated, there are few launched taste-masking products. Further, slow drug release, low absorption of drug or absence of therapeutic effect could be the risks for patients with achlorhydria or dosed under fed condition. Polyvinylacetal diethylaminoacetate (AEA) is another cationic polymer used in pharmaceutical industry for pH-dependent drug release. AEA has been reported to be insoluble above pH 5.8 [33]. Microspheres of AEA containing trimebutine maleate have been prepared by a water-in-oil-in-water (w/o/w) emulsion solvent evaporation method [26]. The microspheres suppressed drug release at pH 6.8 but immediately released drug at pH 1.2. The taste-masking ability of the AEA microspheres has been confirmed by a sensation test with healthy volunteers [26]. In another work, AEA was coated on sildenafil (Sdn)–montmorillonite (MMT) nanohybrid for taste-masking [34]. The cationic Sdn molecules were intercalated to MMT, an inorganic clay material. The strong interaction between Sdn and MMT prevented drug release at both pH 1.2 and 7.0. While the release of Sdn in simulated salivary fluid was suppressed by an AEA coating on Sdn–MMT, deintercalation of the drug from MMT at acidic pH could be achieved due to the protonation of AEA; therefore, Sdn could be promptly released in the acidic stomach environment. Plasma concentration–time profile of this system has been reported to be similar to those of marketed products in beagle dogs [34]. The two polymers described above are the only FDA-approved cationic polymers; however, combination of the polymers and other components might result in more specific response to gastric pH and better controlled drug release in the oral cavity and stomach.
Table 2.
Common pH-sensitive polymers used in drug delivery.
| Name | Chemical structure | Clinical indication | Refs. |
|---|---|---|---|
| Aminoalkyl methacrylate copolymer (Eudragit E) |
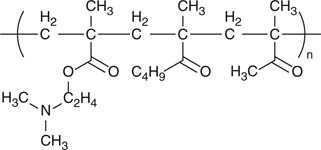
|
Taste-masking | [28-32] |
| Poly(methacrylic acid-co-methyl methacrylate) (Eudragit L/S) |
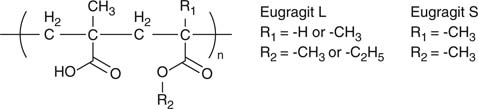
|
Protection of acid-degradable drugs, colon delivery | [6,35-43] |
| Hydroxypropyl-methylcellulose phthalate (HPMC-P) |
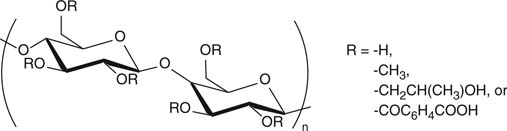
|
Protection of acid-degradable drugs, colon delivery | [6] |
2.2 Polymers responsive to intestinal high pH
Anionic polymers with carboxyl groups have higher water solubility at basic pH than at acidic pH. These polymers can be used for preventing gastric degradation of drug, colon drug delivery and achieving high bioavailability of weakly basic drugs. Poly(methacrylic acid-co-methyl methacrylate) (Eudragit L, S and F), hydroxypropylmethylcellulose phthalate (HPMC-P) and HPMC acetate succinate (HPMC-AS), which possess carboxyl groups on the polymer side chains, are insoluble at stomach low pH but soluble at intestinal neutral pH. The pH value controlling the aqueous solubility of the polymers can be finely tuned by adjusting the amount of carboxyl or other substituent groups on the polymers. The ratio of carboxyl groups to ester groups (carboxyl/ester ratio) of poly(methacrylic acid-co-methyl methacrylate) can be manipulated to control the polymer dissolving pH. For example, Eudragit L has a carboxyl/ester ratio of 1:1 and is soluble at pH 6, while Eudragit S having a ratio of 1:2 is soluble at pH 7. By varying the amount of phthalate groups, HPMC-P can be designed to dissolve at pH 5.0 – 5.5. A high polymer dissolving pH (pH 5.5 – 6.8) can be achieved by HPMCAS through adjusting the amounts of methoxyl, hydroxypropyl, acetyl and succinoyl groups on the polymers. These polymers are the FDA-approved enteric polymers and are widely used for pharmaceutical products on the market.
Enteric polymers are commonly used for protecting acid-degradable drugs, for example, proton pump inhibitors (PPIs) including omeprazole, pantoprazole, rabeprazole, tenatoprazole and esomeprazole, aiming for efficiently delivering drugs to the intestine with high bioavailability. There are several launched products containing enteric polymers for protecting acid-liable drugs. Coating of beads containing rabeprazole with Eudragit L or HPMC-P has been shown to suppress drug release at acidic pH without compromising the release at neutral pH [6]. Further, enteric capsules could preserve the integrity of tenatoprazole until the drug reached the small intestine [35] and were able to obtain almost 2.5 times higher Cmax of blood drug levels compared with free drug solution after oral administration to dogs [36]. Enteric polymers have also been demonstrated to be useful for protein drug delivery by minimizing the drug release at low pH as protein drugs are subject to rapid denaturation at acidic pH in the stomach [37-39]. Microparticles of α-amylase based on HPMC-P and Eudragit L and submicron particles of papain protected by Eudragit L suppressed drug release at pH 6.0 but released drug at pH 6.8 [37,38]. In the same study, through substituting Eudragit L with Eudragit S, the drug-releasing pH of papain from the particles could be shifted to pH 7.0 by while minimizing drug release at both pH 6.0 and 6.8 [38]. Microspheres containing bovine serum albumin prepared by free radical polymerization of methacrylates have also been reported to maintain drug release at pH 6.8 but impede drug release at pH 1.0 [39]. These results indicate that the FDA-approved enteric polymers are sufficiently effective for protecting acid-liable drugs.
Colon drug delivery can also be achieved using enteric polymers. As protein and peptide drugs are easily degraded by enzymes, which are more abundant in the small intestine than in the colon [40-44], colon is considered a target for more efficient delivery of enzyme-liable drugs. In a colon-targeted delivery system (CODES), tablets containing drugs and lactulose were first coated with an acid-soluble polymer and subsequently coated with an additional layer of enteric polymer [40-43]. The outermost enteric polymer coat prevents the dissolution of the acid-soluble polymer in the acidic gastric environment but dissolves in the more basic intestinal environment. Once becomes unprotected, lactulose, a synthetic disaccharide, is degraded to organic acids by the enter-obacteria in the colon. The acidic products then dissolve the acid-soluble polymer; eventually drug release can be obtained specifically in the colon. The CODES using Eudragit L and Eudragit E has been shown to suppress drug release at both pH 1.2 and 6.8 but release drug specifically at pH 5.0 [41,42]. The CODES containing 5-aminosalicylic acid demonstrated high plasma drug concentration from 4 to 12 h after oral administration to dogs [43]. As the colon arrival time of indigestive solids in fasting dogs bas been reported to be 3 h after dosing [45], this indicated that the CODES was able to deliver drug to the lower part of the gastrointestinal tract. The transit and disintegration of radiolabeled CODES were also investigated by gamma scintigraphy after oral dosing to healthy volunteers [40]. The results revealed that the CODES started to disintegrate in the ascending colon in majority of subjects at 7.1 h posttreatment, and the drug was observed to be released from the system within 1 h after arriving at the colon. The CODES system is applicable to some kinds of drugs such as acetaminophen, 5-aminosalycylic acid, mebeverine hydrochloride, insulin and salmon calcitonin, and will contribute to oral absorption of enzyme-liable drugs in human.
Eudragit F is another example of enteric polymers used for colon-targeted DDS [44] due to its solubility at pH higher than 7.0.Štembírek and coworkers developed a multiple-unit dosage system based on drug/chitosan core coated with Eudragit F for colon-specific drug release. As Eudragit F and chitosan are designed to dissolve in the small intestine and the colon, respectively, drug could be protected from undesired release until it reaches the colon after oral administration. In nine healthy volunteers after oral administration of the system containing caffeine as a model drug, caffeine first appeared in the saliva at 7 h post administration. This indicated that the enteric polymer, Eudragit F, prevented the cationic chitosan from dissolving in the stomach, thus facilitating colon drug delivery. Mesalamine colon DDS product, containing an enteric coating layer of Eudragit S, is widely used in patients. Colon DDS using pH-responsive polymers showed some success in human studies; however, the number of launched products is low. Due to the absorption inefficiency of drugs in the colon, studies on absorption enhancement of peptides or antibody drugs in the colon may gather reattention to this area.
2.3 Polymers responsive to tumoral low pH
Nanosized polymers and particles can preferentially accumulate in the tumor tissues by the enhanced permeability and retention (EPR) effect after intravenous administration [46] due to the leaky neovascular vessels and the lack of lymphatic drainage in the tumor tissues. Tumor tissues have been shown to be more acidic (pH 6.5 – 7.0) than healthy tissues (pH 7.4) [47]. The lower pH value of the tumor tissues arises from the rapid growth rate of cancer cells, leading to a high level of glucose consumption and lactic acid accumulation, and insufficient blood supply at the tumor sites [48]. Therefore, pH-sensitive DDSs have been extensively investigated, aiming at tumor-targeted delivery through exploiting the pH gradient existing between the normal and tumor tissues.
2.3.1 Polymers having imidazole groups
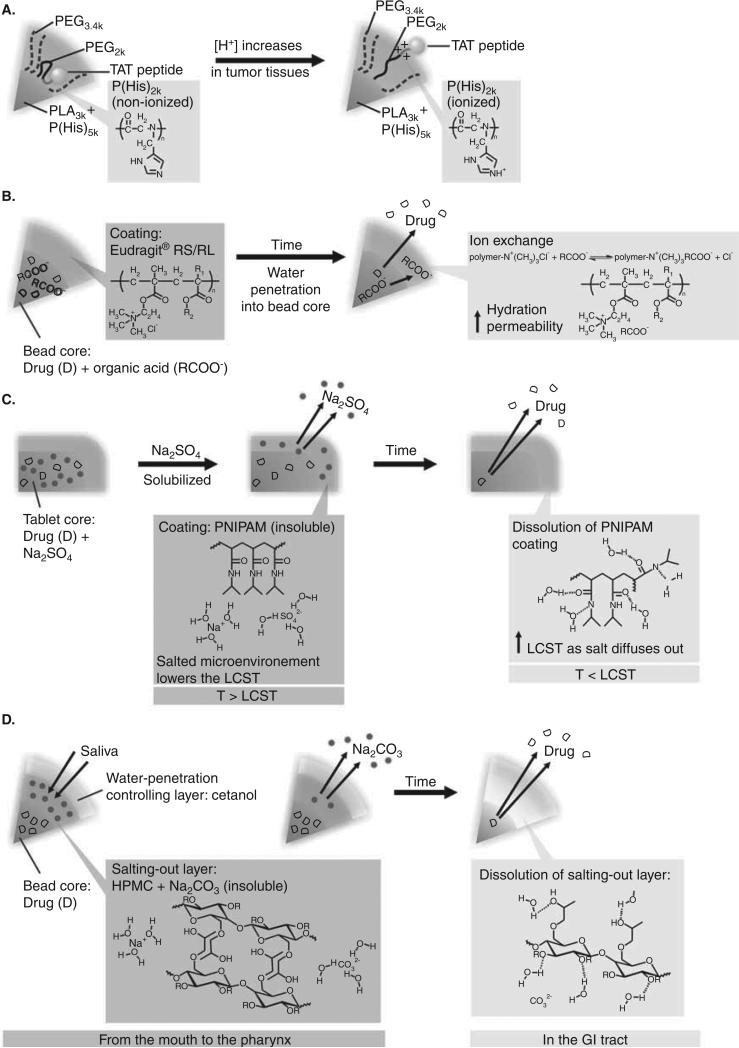
Having a pKa value at around 6, imidazole group is neutral at pH 7.4 but cationic in acidic environments. Poly(l-histidine) (P(His)) with pendant imidazole groups is hydrophobic in the blood and normal tissues but hydrophilic in acidic environments, for example, tumor tissues and intracellular endocytic vesicles. This pH sensitivity has led imidazole-containing polymers to be heavily studied as tumor-targeted DDSs [49-52]. Bae and coworkers prepared polymeric mixed micelles, PHSMpop-up TAT (Figure 1A), capable of pH-specific exposure of Trans-acting activator of transcription (TAT) cell-penetrating peptides using two P(His)-containing polymers, poly(l-lactic acid)-block-poly(ethylene glycol)-block-poly(His)-TAT peptide (PLA-b-PEG-b-P(His)-TAT) and poly(His)-block-poly(ethylene glycol) (P(His)-b-PEG) [50]. As a cationic peptide, TAT (GRKKRRQRRRPQ) has been installed onto the surface of nanoparticles to facilitate the cellular membrane interaction and cellular uptake of the particles. However, the cell-penetrating effect is not restricted to certain cell types, and the cationic charges of the TAT peptides induce aggregation of the particles with the blood components during systemic circulation, thus limiting the in vivo tumor-targeting efficacy. To overcome these obstacles, PHSMpop-up TAT was designed to have triggered-exposure of TAT peptides in acidic tumor tissues. PHSMpop-up TAT micelle core was formed by the hydrophobic segments, PLA block of PLA-b-PEG-b-P(His)-TAT and P (His) block of P(His)-b-PEG, while the surface of the particles was covered by hydrophilic PEG. The P(His) block adjacent to the TAT peptide on PLA-b-PEG-b-P(His)-TAT concealed the TAT peptides during systemic circulation due to the hydrophobicity of P(His) block at neutral pH. Tumor-specific exposure of the TAT-peptide was achieved by the protonation of the P(His) block at acidic tumor pH. The increase in hydrophilicity of the P (His) block at tumor sites allowed the TAT peptide to be more exposed on the particle surface, therefore increasing the cellular uptake of the particles. In the same study, doxorubicin was loaded to PHSMpop-up TAT micelles. The micelles demonstrated faster drug release and higher cytotoxicity at acidic pH than at neutral pH. The micellar doxorubicin showed 4 – 9 times lower IC50 than free doxorubicin in drug-resistant cell lines at acidic pH. Further, in vivo antitumor efficacy of doxorubicin-loaded PHSMpop-up TAT micelles has been shown to be higher than that of free doxorubicin. This DDS possesses almost all functions for DDS-targeting tumor: a polyethylene glycol (PEG) shell protecting the particle from the reticuloendothelial system (RES) and minimizing the toxicity of TAT peptides, tumor-specific enhanced cellular uptake and intracellular drug release. In vitro IC50 and in vivo antitumor efficacy are satisfactory, and drug accumulation at tumors in mice has also been confirmed. However, the complexity of this system may impose an obstacle in the scale-up process for further testing in large-scale studies in the future.
Figure 1. Representative drug delivery systems with stimulus-responsive capability.
A. Polymeric mixed micelles prepared with poly(l-lactic acid)-block-poly(ethylene glycol)-block-poly(His)-TAT peptide and poly(His)-block-poly(ethylene glycol) for exposure of TAT cell-penetrating peptides responsive to the tumoral acidic pH [50]. B. A bead system containing drug and organic acid in the core and coated with a Eudragit RS layer for ion-sensitive sigmoidal drug release [100,101]. C. Tablets containing drug and Na2SO4 and coated with a PNIPAM layer for ion-sensitive colon drug delivery [108]. D. ‘Salting-out taste-masking systems’ consisting of a drug core, a salting-out layer containing salts and water-soluble polymers, and a water-penetration-control layer of water-insoluble materials for ion-sensitive taste-masking of oral disintegrating tablets [16-19].
Imidazole groups have also been grafted onto an amphiphilic derivative based on a poly (aspartamide) backbone conjugated with PEG and octadecylamine, aiming at generating a pH-sensitive polymeric micelle system for paclitaxel delivery [52]. Paclitaxel was loaded into the nanoparticles using a pH-changing method. The particle size and drug release kinetics were observed to be pH-dependent. The size of the particles was maintained at 80 nm at neutral pH, whereas the particles dissociated at acidic pH due to the ionization of the imidazole groups. Parallel with the observed pH-responsive particle dissociation, the drug release kinetics has been demonstrated to be three times faster at pH 6.0 than that at pH 7.4. Tumor progression was suppressed comparably by the paclitaxel-loaded pH-sensitive nanoparticles (1 mg/kg) and a clinically approved paclitaxel formulation, Taxol (2 mg/kg). Comparison of the survival time of tumor-bearing animals treated with different formulations would provide valuable information about judging if the pH-sensitive polymeric micelle system has a higher anti-cancer activity than does Taxol. pH-dependent particle dissociation and drug release are interesting phenomena. The pH-responsiveness shown was sufficiently sharp; however, in vivo antitumor efficacy of this system was not significantly higher than that of control. Cellular uptake of the nanoparticles and intracellular drug release could be further optimized to improve antitumor efficacy.
2.3.2 Poly (β-amino ester)
PEG-b-poly (β-amino ester), a synthetic, hydrolytically degradable polymer, has been reported to be used for preparation of a pH-sensitive PbAEM micelle DDS [53-55]. Camptothecin-loaded PbAEM micelles showed a sharp micellization/demicellization transition in a slightly acidic environment (pH 6.4 – 6.8) and approximately four times faster drug release at pH 6.4 compared with that at pH 7.4 [53]. Camptothecin-encapsulated PbAEM micelles have been demonstrated to have higher ability in accumulating in tumors, suppression of tumor growth, and prolonging the survival of tumor-bearing mice than free camptothecin. In another study, doxorubicin was loaded into PbAEM micelles, showing pH-dependent drug release and higher antitumor efficacy relative to free doxorubicin [55]. The pH-responsiveness of drug release and amount of drug leaked at pH 7.4 of this poly (β-amino ester) system are similar to those of the hydrazone PHSMpop-up TAT system.
Numerous studies on pH-sensitive nanoparticle drug DDS have shown that the relatively lower pH at tumor tissues is exploitable to achieve desired physicochemical properties and drug release kinetics under acidic conditions [46-55]. Currently, DDSs targeting the tumoral low pH are still in an infant stage; more preclinical studies on the safety, stability, and manufacturability and accumulation of results from clinical studies are expected to facilitate the progress of development of these polymeric DDSs in the future.
2.4 Polymers responsive to intracellular low pH
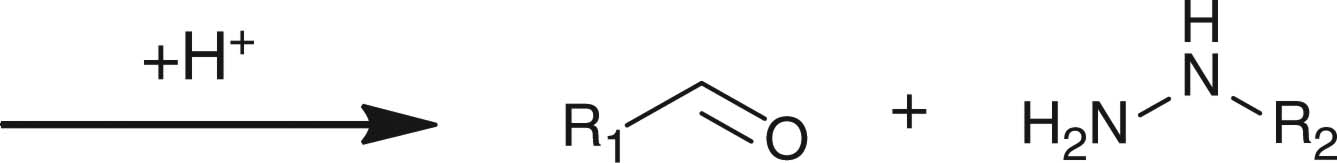
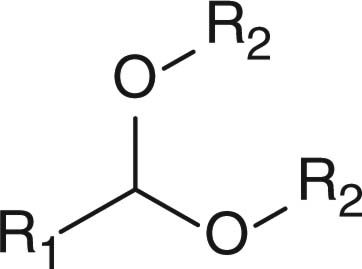
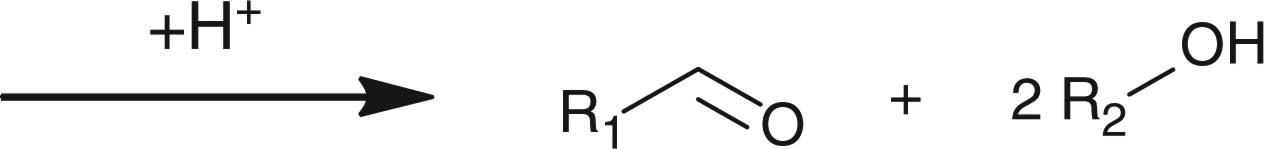
In contrast to small molecules travelling across the cellular membrane by diffusion, macromolecules, such as polymer–drug conjugates, polymeric micelles and liposomes, usually internalize into the cells through endocytosis. During the cell internalizing process, the pH value gradually decreases from physiological pH of 7.4 to endosomal pH of 6 and ultimately to pH 5 in the lysosomal compartments. Nanosized DDSs capable of responding to the acidic endo/lysosomal pH have been extensively investigated to utilize the pH gradient along the endocytic pathway, aiming at eliciting intracellularly specific effects, for example, intracellular release of therapeutic agents and endosomal escape. Different pH-sensitive chemical linkages, including hydrazone, acetal, ortho ester and vinyl ester, have been employed for constructing pH-responsive drug delivery vehicles (Table 3). Cell-penetrating peptides and cationic polymers responsive to intracellular pH are also discussed in this section.
Table 3.
Common pH-responsive functional groups used in drug delivery.
2.4.1 Polymers with pH-responsive hydrazone linkages
Hydrazone bond is one of the most widely used pH-sensitive linkages for drug delivery applications due to its faster hydrolytic rate at acidic pH relative to neutral physiological pH. Hydrazone linkage is frequently used for conjugating drugs to polymer backbones or antibodies, intending to improve the circulation time of the therapeutic agent and minimize systemic toxicity by restricting drug release inside the target cells. Several pH-sensitive polymer–drug conjugates based on N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers using hydrazone linkages for drug conjugations have been reported [56-59]. HPMA–drug conjugate systems have been developed for paclitaxel and docetaxel delivery [57]. Both conjugates released drug 3 – 5 faster under acidic conditions relative to neutral pH. Moreover, they were able to suppress tumor progression and prolong survival time in tumor-bearing mice. Recently, several kinds of HPMA–drug conjugates without pH-sensitivity have been confirmed to be safe in Phase I clinical trials [58]. Among these conjugates, HPMA-copolymer platinates have demonstrated clinically meaningful stabilization of disease in some recurrent ovarian cancer patients in a Phase II study [59]. Higher efficacy and lower side effects can be expected by the pH-sensitive HPMA–drug conjugates.
Hydrazone linkage has also been employed for conjugating PEG to proteins and anticancer drugs [60]. Zhong and coworkers have developed conjugates with multiple doxorubicin molecules linked to a PEG polymer backbone [48]. The conjugates demonstrated four times faster drug release at pH 6.0 than at pH 7.4. Intracellular release of doxorubicin caused by the cleavage of the hydrazone linkages was demonstrated by nuclear accumulation of the drug. In the same study, the PEG–doxorubicin conjugates showed longer plasma half-life and approximately 2 times higher tumor accumulation in mice than did free drug after intravenous administration. In this study, about 30% of drug was rapidly cleared from the bloodstream, implying the ability of the conjugates to escape from the RES might be insufficient.
Star-like polymer–drug conjugates have received considerable attention as drug delivery vehicles due to their long blood circulation and efficient drug accumulation at tumors [61]. Ulbrich and coworkers compared the physicochemical and biological properties of doxorubicin-conjugated HPMA copolymer via hydrazone bonds to those of a delivery system based on poly (amindoamine) (PAMAM) dendrimers linked to the same HPMA copolymer–doxorubicin conjugates [61]. Both the linear and star-like constructs showed 10 – 20 times faster release of doxorubicin at pH 5 than at pH 7.4 [61,62]. In vivo studies demonstrated that the dendrimer-based conjugates had a longer circulating half-life, higher drug accumulation at tumor, and superior suppression of tumor progression in tumor-bearing mice than did the linear conjugates. The larger size and more rigid structure of the star conjugates probably caused more efficient tumor accumulation by the EPR effect. Moreover, this star conjugates showed no acute toxicity in the animal studies. Clinical studies of this system will be needed for proper indications.
pH-sensitive polymeric micelles with therapeutic agents conjugated through hydrazone linkages are another class of drug vehicles widely studied for anticancer applications. Kataoka and coworkers have developed a pH-sensitive polymeric micelle DDS for doxorubicin based on block copolymers of PEG-block-poly(aspartate) (PEG-b-P(Asp)). Doxorubicin molecules were conjugated to the polymer backbone through hydrazone linkage formation [63]. The polymeric micelles showed pH-dependent drug release with faster release at pH 5.3 than at pH 7.4. It has also been shown that doxorubicin molecules were able to accumulate in the nucleus of the cells after micelle treatment, suggesting that intracellular drug release could be achieved presumably due to the acidic endo/lysosomal pH. In another study, the authors conjugated folate as a targeting ligand onto the surface of the same micelle system [64]. They observed that both folate-conjugated micelles and the non-targeted micelles delivered similar amount of drugs to the tumors of mice; however, the anti-tumor efficacy was shown to be superior with the folate-conjugated micelles. This indicates that the folate-conjugated micelles probably accumulated at the tumor sites passively by the EPR effect, and the folate molecules catalyzed the internalization of the micelles into the folate receptor-overexpressing cancer cells.
Temporary PEGylation through hydrazone linkage has been shown to promote transfection efficiency of gene delivering vehicles [7]. PEGylation improved the colloidal stability and was expected to prolong the blood circulation at neutral pH. Once internalized into the cells, PEG detachment from the particles could be achieved due to the hydro-lysis of hydrazone bonds in the acidic endo/lysosomal compartments as evident by the increase in zeta potential of the particles and the faster PEG release kinetics under acidic conditions. Intracellular release of PEG from the particles is beneficial to the endosomal escape or decondensation process and ultimately the transfection activity [65]. Although improvement in transfection efficiency was observed with this temporary PEGylation strategy in in vitro evaluation, the stability of hydrazone linkage might not be sufficient for the particles to remain PEGylated under in vivo conditions. Therefore, further optimization of hydrazone stability might be necessary for obtaining in vivo biological effects.
2.4.2 Polymers with pH-responsive acetal, ortho ester or vinyl ether linkages
Apart from hydrazone linkage, other pH-responsive chemical linkages such as acetal, ortho ester and vinyl ester have also been used for constructing drug/gene delivery carriers aiming at endosomal release of materials. Polyacetals, which display pH-dependent degradation and produce biocompatible degradation products of alcohols and aldehydes, have been conjugated to hydrophilic polymers to form polymeric micelles for drug-loading purposes. For example, PEG-b-poly(ethyl glyoxylate)-b-PEG [66], polyacetal-b-Pluronic [67], and PEG-b-poly (2,4,6-trimethoxybenxylidenepentaerythritol carbonate) [68], have been used for forming drug-loaded polymeric micelles showing faster drug lease at acidic pH than at neutral pH. Protein-based vaccines have been synthesized by copolymerizing benzylidene acetal cross-linking monomers and acrylamide in the presence of the protein payloads [69,70]. Using ovalbumin as the model protein, ovalbumin-loaded polymeric particles with diameters of 35 nm to 3.5 μm were generated. Moreover, the particles demonstrated faster hydrolysis at acidic pH than at neutral pH [69]. Results from animal studies showed that, compared with free ovalbumin, ovalbumin-loaded polymeric particles stimulated T-cell proliferation and protected animal from tumor development more efficiently, indicating that the polymeric particles dissociated in the endosomes after cellular uptake due to acetal hydrolysis and released the protein intracellularly. More direct observation such as intracellular trafficking of the payload or the particles is necessary for verification of the concept.
Ortho ester, a functional group with three alkoxy groups attached to one single carbon atom, is readily hydrolyzed in mildly acidic aqueous environments to form esters. The hydrolytic rates of the ortho ester side chains of PEG-b-polymethacrylate diblock copolymer derivatives have been investigated and shown to increase with acidity of the environment [71,72]. In the same study, doxorubicin was loaded into polymeric micelles formed with the PEG-b-polymethacrylate bearing ortho ester side chains. The drug release was observed to be faster at acidic pH. In vitro experiments demonstrated that doxorubicin delivered by the micelles was able to accumulate in the cell nucleus within 1 h, indicating a rapid intracellular drug release process [72]. Ortho ester linkage has also been used for constructing gene delivery vector. Aiming at overcoming the PEG dilemma, PEG was conjugated to poly(2-(dimethylamino)ethyl methacrylate) cationic polymers for pDNA condensation [73]. The transfection efficiency of the PEG-detachable polyplex particles was 100 times higher at pH 5 than at pH 7.4.
pH-sensitive PEG-phospholipids have been synthesized with the PEG block linked to the hydrophobic lipids, for example, dioleoylphosphatidyl ethanolamine, via vinyl ether bonds [74,75]. The PEG–phospholipid conjugates were used to form liposomes, and which demonstrated pH-dependent release of the encapsulated calcein, giving faster release under acidic conditions [74]. Further, lipoplex particles were generated using pDNA and pH-sensitive PEG-phospholipids. The pH-responsive lipoplexes released pDNA faster at pH 5.5 than at pH 7.4. It has also been shown that the lipoplexes could deliver pDNA to the cellular cytoplasm and nuclear regions and achieve efficient transfection [75].
Many drug/gene delivery systems capable of responding to intracellular pH have been developed and showed intracellular specific release of drug or nucleic acid cargoes, producing promising therapeutic effect or transfection efficiency. Compared with the mildly acidic extracellular pH in the tumor microenvironment (pH 6.5 – 7), the more acidic pH in the intracellular endo/lysosomal compartments (pH 5 – 6) provides a more significant pH difference relative to the physiological pH for DDS to utilize to generate specific pH-dependent responses. The investigations on pH-responsive nanoparticles for intracellular release of therapeutic agents are still at the preclinical stage; however, pH-sensitive DDS should definitely improve the therapeutic effects of the drugs having narrow therapeutic window due to high toxicity and accelerate the clinical translation.
2.4.3 Cell-penetrating peptides responsive to intracellular pH
Cell-penetrating peptides are short peptide sequences that are capable of efficiently cellular internalization. In general, they employ a random coil structure at pH 7. As the pH decreases in the endo/lysosomes along the endocytic pathway, certain domains of the amino acids sequences become protonated. This leads to a transition from a random coil conformation to an amphipathic α-helical conformation, allowing the peptide to interact with and destroy the endo/lysosomal membrane and eventually escape to the cytoplasm [76]. Hemagglutinin is one of major glycoproteins on influenza virus. Under the acidic pH inside the endosomes, conformational change of the hemagglutinin from random coil to α-helix allows the protein to be inserted into the endosomal membrane, resulting in fusion between the virus envelope and the endosomal membrane and the release of the nucleocapsid of the virus into the cytoplasm of the infected cell [77]. N-terminus of the hemagglutinin HA2(1-23) (GLFGAIAGFIENGWEGMIDGWYG) is a pH-sensitive cell-penetrating peptide useful for endosomal escape. Moreover, in a glutamic acid-enriched analogue (INF7) (GLFEAIEGFIENGWEGMIDGWYG), two glutamic acid moieties were introduced into the HA2 structure to extend the α-helix structure, thereby increasing the pH sensitivity and improving endosomal escape [78]. Amphipathic cell-penetrating peptides GALA (WEAALAEALAEALAEHLAEALAEALEALAA) was designed based on the HA2 peptide for achieving artificial pH-sensitive endosomal escape [79]. This peptide is soluble at pH 7.5 and can destabilize the lipid bilayers at pH lower than 6.0 [79]. The membrane-disruptive property of this peptide has been demonstrated in several drug or gene delivery systems [80]. Moreover, cationic KALA peptide has been produced by replacing some alanine residues by lysines for gene delivery purposes [81]. This cationic peptide undergoes a conformational change from pH 7.5 to 5.0, resulting in effectively destabilization of the endosomal membranes and facilitating delivery of genetic materials to the cytoplasm [82].
Composed of three LAEL amino acid sequence units, 43E peptide can disrupt the endosomal membrane via a conformational change from a random coil structure at pH 7.4 to an α-helix structure at pH 5.0 [83]. L2 peptide, which is located at the C terminus of the minor capsid protein of Papillomavirus, has shown a strong membrane-disrupting activity at low pH, allowing release of the viral genomes from endosomes and causing cytolysis of bacteria and eukaryotic cells [84]. More-over, the major envelope protein (E) of the West Nile virus exerts its endosomal disruptive activity by rapid conformational change (within seconds) at an upper threshold of pH 7.0 and has a maximum activity at pH 6.4 and below [79]. As a synthetic analog of penetratin, EB1 destabilizes the endosomal membrane by forming an amphipathic α-helix upon protonation at the endosomal pH. It has been shown that EB1 was more far more effective than penetratin in forming complexes with siRNA and delivering siRNA [85].
Although some cell-penetrating peptides have been applied in preclinical studies, the lack of specificity toward target cells is a major obstacle for clinical development. Modification of the peptide sequences or introduction of non-natural amino acids can improve their specificity; however, it can also affect the pH-sensitiveness and thus efficiency of endosomal escape.
2.4.4 Cationic polymers responsive to intracellular pH
The proton sponge effect has laid the foundation of the development pH-responsive DDS that are capable of endosomal escape based on the buffering capacity. Polymers with functional groups exhibit pKa values between physiological and lysosomal pH, such as polyethylenimine (PEI) [86-89], poly(l-histidine) [90-94], poly(amidoamine)s [95,96], and poly(propylacrylic acid), can protonate at the acidic endo/lysosomal compartments. Protonation of the polymers induces an extensive influx of ions and water into the endo/lysosomes, which subsequently leads to rupture of the endo/lysosomal membrane and release of the entrapped materials [79]. PEI is one of the most successful and widely studied gene delivery polymers. PEI is rich in secondary and tertiary amines that can be protonated at the acidic pH of endosomes/lysosomes. Upon protonation, PEI results in the rupture of endosomes by the proton sponge effect as summarized in reference [86]. Although PEI shows high transfection efficiency, significant toxicity is usually observed [87]. Some novel polymers based on PEI showed higher transfection efficiency and low toxicity in vivo. The toxicity profiles of branched PEI has been improved by full deacylation of the polymer. While branched PEI caused significant animal death, the fully deacylated PEI allowed safe delivery of gene and produced dramatically higher transfection efficiency [88]. It has been shown that the biocompatibility issue of high-molecular PEI (e.g., PEI 25 kDa) could be solved by using low molecular weight PEIs linked with β-cyclodextrin. Moroever, high transfection efficiency could be obtained with the PEI-cyclodextrin system in cultured neurons and in the central nervous system of mice [89]. These newly designed polymers are excellent examples of gene delivery vehicles which are able to maintain the pH-responsive endosomal/lysosomal escape ability of PEI while minimizing the toxic effects.
Poly(l-histidine) is another heavily studied polymers possessing pH-buffering capacity. Protonation of the imidazole groups under acidic conditions in endo/lysosomes leads to the proton sponge effect, followed by rupture of endo/lysosomes and release of materials to the cytoplasm. [90,91]. LAH4 peptide (KKALLALALHHLAHLALHLALALKKA) contains four histidine residues with imidazole groups having pKa values of 5.4, 5.8, 5.9 and 6.0. Solid-state NMR studies indicated the peptide disrupted anionic lipids on model membranes, and caused destabilization of the membrane at pH 5 but not at pH 7.5 [92]. The transfection efficiency of complexes formed with the peptide and pDNA was 10 times higher than that of lipofectamine, and it was suggested that the peptide efficiently transported pDNA to the cytoplasm [90]. It has been shown that the gene transfer ability of imidazole-modified chitosan was 100 times higher relative to that of native chitosan. The higher transfection ability was probably caused by the proton sponge effect and efficient endo/lysosomal escape. The toxicity accompanied this polymer was low as minimal cytotoxicity was detected at a polymer concentration 10 times higher than the concentration used in transfection study [93]. The imidazole-modified chitosan has also been used for siRNA delivery. After intravenous administration of the imidazole-modified chitosansiRNA complexes to mice, significant knockdown of a target enzyme in both lung and liver was achieved at a siRNA dose of 1 mg/kg siRNA. Further, intranasal administration of the complexes to mice showed significant silencing of the target protein expression in the lungs with a siRNA dose of 0.5 mg/kg/day over 3 consecutive days. These results indicate that pH-responsive imidazole polymers are potentially effective for intracellular DDS and nucleic acid delivery [94].
3. Ion-sensitive polymers
3.1 Ion-exchange resins
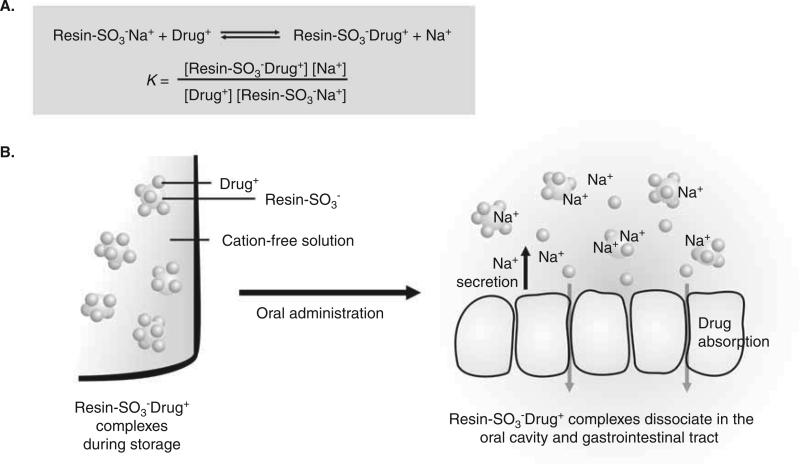
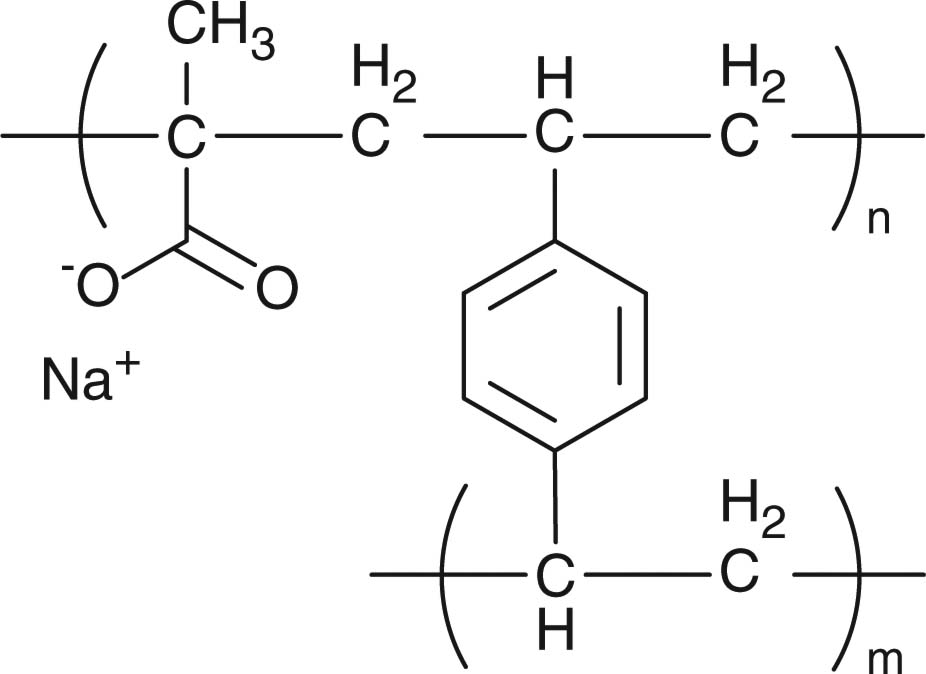
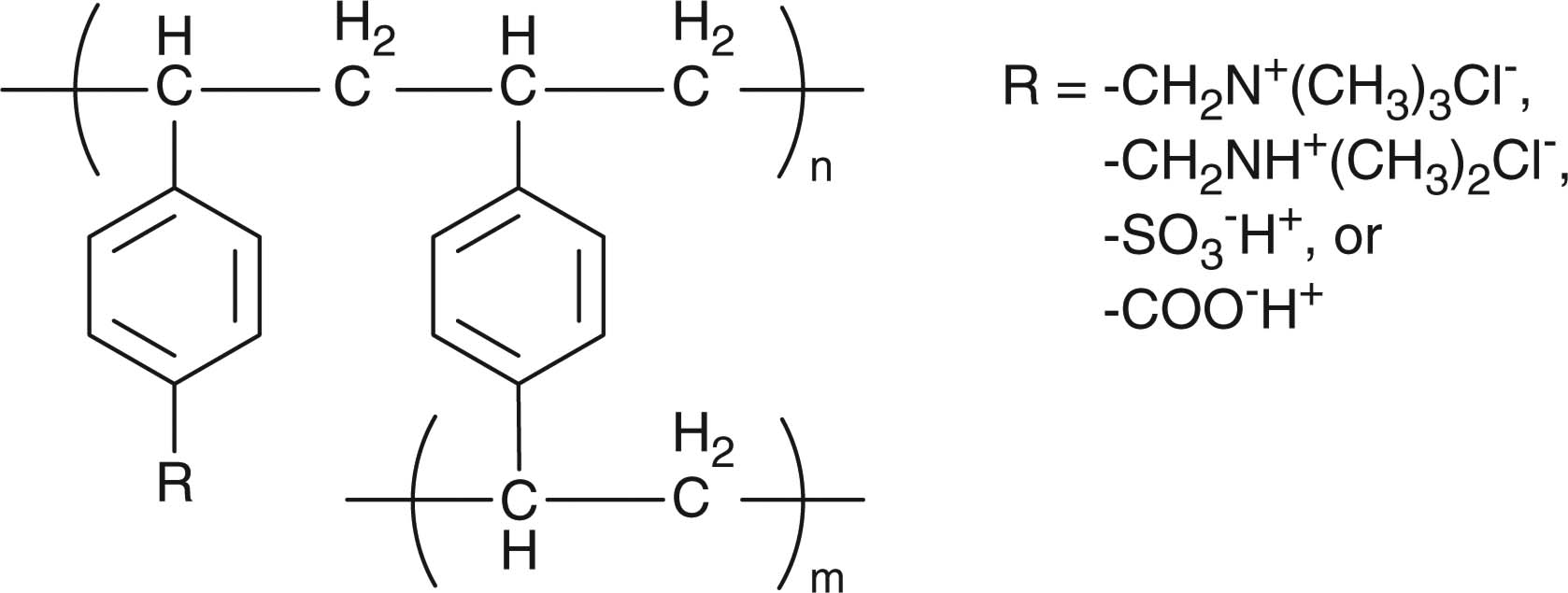
Ion-exchange resins are frequently used for taste-masking, counterion-responsive drug release, and sustained drug release. Ion-exchange resins are insoluble polymers comprising a polystyrene backbone cross-linked with divinylbenzene and side chains of ion-active groups (Table 4). The ion-active groups are predominantly tertiary amine substitutes, quarternary ammonium, sulfonic acid and carboxylic acid. Drug loading to the ion-exchange resins is achieved by complexation based on electrostatic interaction between the drugs and the resins. The drug–resin complexes can be carried in oral formulations such as liquid suspensions, tablets, and beads. Drug release from the resins is governed by an equilibrium exchange reaction when placed in contact with a solution containing the corresponding counterions (Figure 2). Therefore, during storage, the drug–resin binding can be maintained by keeping the drug–resin complexes in liquid free of the counterions of the resins. In contrast, after oral administration, the ions present in the saliva and gastrointestinal fluids promote drug release from the complexes. Secretion of the saliva and gastrointestinal fluids and absorption of the released drug into the body drive the equilibrium forward and promote the drug release.
Table 4.
Common ion-sensitive resins/polymers used in drug delivery.
| Name | Chemical structure | Clinical indication | Refs. |
|---|---|---|---|
|
|||
| Ion-exchange resins |
|
Taste-masking | [97,98] |
| Poly(ethylacrylate-methylmethacrylate-trimethylammonioethyl methacrylate chloride) copolymers (Eudragit RS/RL) |
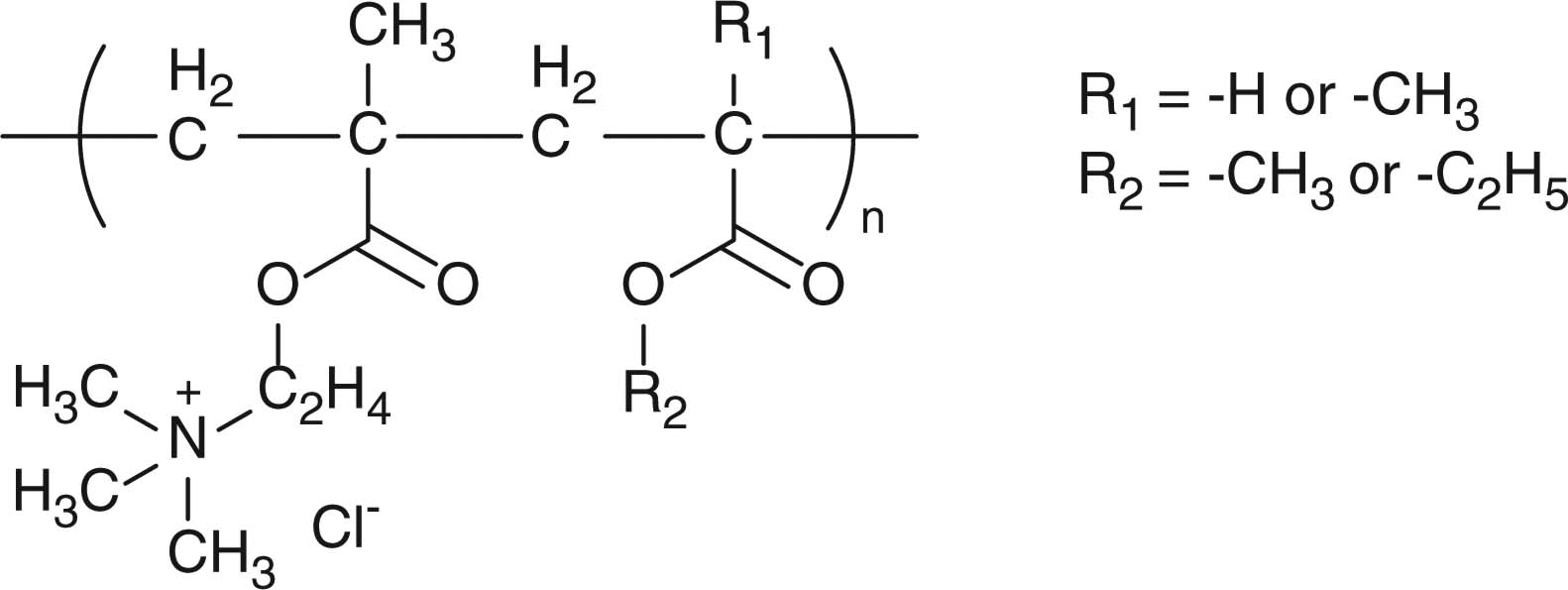
|
Sustained release, Taste-masking | [99-101] |
| Poly(N-isopropylacrylamide) (PNIPAM) |
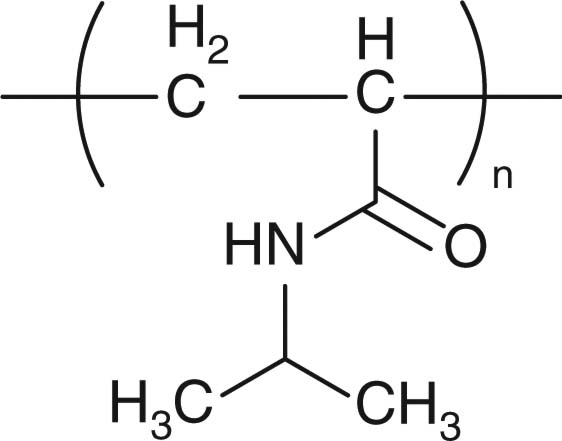
|
Not used clinically | [102,105,108] |
Figure 2. Drug release from ion-exchange resins.
A. Drug release from the resins is governed by an equilibrium exchange reaction. B. During storage, the drug-resin complexes can be maintained in liquid free of the counterions of resins. After oral administration, drug release is promoted by the ions present in the saliva and gastrointestinal fluids. Secretion of the saliva and gastrointestinal fluids and absorption of the released drug promote the drug release.
A polymethacrylic acid-based ion-exchange resin is used for taste-masking of pseudoephedrine in chewable tablet to mask bitterness of the drug [97]. The product contains complexes of pseudoephedrine and the ion-exchange resin, further coated with ethylcellulose/HPMC membrane. Due to the lower cation concentration in saliva than in the gastric fluid (Table 1), drug release from the product is minimized in saliva for taste-masking while achieving immediate drug release in simulated gastric juice for high bioavailability. A carboxylic acid-based ion-exchange resin, is used for sustained release of nicotine from chewing gum [97]. The resin efficiently suppressed nicotine release to only 5% in salt-free pure water; to the contrary, 70 – 90% drug release has been observed in saliva within 30 min. The sustained release of dextromethorphan from liquid suspension is achieved by using a sulfonic acid ion-exchange resin. A part of the drug–resin complexes in the product is coated with ethylcellulose. The product contains ethylcellulose-coated drug–resin complexes and uncoated complexes at an approximate ratio of 2:1, producing a constant plasma drug concentration for 12 h [98].
Coating of water-insoluble polymers on drug's particles without resin has no driving force for promoting drug release and cannot release drugs completely in the gastrointestinal fluids. The drug–resin complexed DDSs have the significant advantage of complete drug release caused by the ionic equilibrium. On the other hand, the drug release rate from drug–resin complexes depends on the strength of drug–resin interaction. Nicotine has sufficiently strong interaction with the resin to achieve sustained drug release. However, pseudoephedrine and dextromethorphan have insufficient drug–resin interaction; therefore, coating of the drug–resin complexes with water-insoluble polymers is necessary for taste-masking and sustained drug release. The combined use of drug–resin complexes and polymer coatings is very useful for balancing suppression of drug release and obtaining complete drug release. As oral disintegrating tablets and pediatric liquid formulations are getting more popular, ion-exchange resins will definitely play a more important role in taste-masking and sustained drug release in those formulations.
3.2 Polymers responding to the ions in the saliva and gastrointestinal fluids
Anion-responsive drug release can be achieved using cationic polymers bearing quaternary ammonium groups. Poly(ethylacrylate–methylmethacrylate–trimethylammonioethyl methacrylate chloride) copolymers (Table 4, Eudragit RS and RL) are practically insoluble in water but can be hydrated with and swell in water. Therefore, the polymers can be coated on beads and tablets containing drugs for oral formulations to control drug release through the polymer membrane depending on the swelling status of the polymer coat. Strong interaction between the quaternary ammonium groups and counterions in the media induces lesser degree of hydration or swelling and contributes to slower drug release. Buffer species-dependent drug release kinetics of diltiazem from Eudragit RS/RL-coated beads has been reported. Due to the different strength of interaction between the buffer anions and the quaternary ammonium groups of Eudragit RS/RL, the order of drug release in various buffer media was found as follows: acetate > formate > chloride [99]. To achieve a sigmoidal drug release profile, a bead system has been prepared by coating Eudragit RS onto a drug core containing drug and organic acid (Figure 1B) [100,101]. The orders of hydration and drug release rates were reported as follows: acetic acid > succinic acid > glutalic acid > malic acid > tartaric acid > citric acid [100]. The drug release lag time and Tmax in plasma concentration–time profiles after oral administration to dogs were shown to be controllable by the bead system [101]. This system made good use of salt formation of the polymers; however, functional groups of drug or the counterions might interact with the polymers or organic acid. After optimization of polymers and organic acid for each drug, this system is considered to be effective for the applications of taste-masking and site-specific drug delivery in the gastrointestinal tract.
Polymers exhibiting lower critical solution temperature (LCST) transitions can be used as ion-sensitive DDS. LCST transitions have been observed in aqueous solution of several polymers, such as poly(N-isopropylacrylamide) (PNI-PAM) [102], cellulose derivatives [103], poly(vinyl ether), poly (N-vinyl caprolactam) [104]. The polymers are soluble in water at T (temperature) < LCST by favorable hydration but insoluble at T > LCST due to the entropy-driven dehydration and collapse of the hydrophobic polymers. Highly water-soluble substances, such as electrolytes and sugars, surrounding the polymers remove water molecules from the polymers and, therefore, decrease the LCST of the polymers. This is known as the salting-out effect. The salting-out effects of various ions on PNIPAM, HPMC, PEG and polyvinylpyrrolidone have been reported [105]. As is known as the Hofmeister series [106], the effect of anions on decreasing the LCST of the polymers is generally in the following order: citrate3– > SO42– > tartrate2– > HPO42– > CrO42– > acetate– > HCO3– > Cl– > NO3– > CiO3–, while the order of cationic species is Mg2+ > Li+ > Na+ = K+ > NH4+. The sensitivity of water-soluble polymers to the salting-out effect has been suggested to be in the following order: HPMC > Povidone K90 > Copolypidone > Povidone K30 > PEG6000 [20]. Various oral formulations containing these polymers and salts have been reported to effectively control drug release [16-19,104,107,108]. In one study, tablets containing terbutaline sulfate and Na2SO4 and coated with a PNIPAM layer were prepared, aiming for colon drug delivery by generating long drug release lag time (Figure 1C) [108]. Compared with a control tablet containing no salt, which demonstrated a 90 minute lag time, tablets containing Na2SO4 generated a lag time of 130 min in the drug release profile. Tablet containing the ion-sensitive polymers and ions is an interesting concept; however, its in vitro drug release performance can be achieved in simpler tablet formulations containing the FDA-approved polymers such as HPMC and polyethylene oxide.
‘Salting-out taste-masking systems’ for general taste-masking of oral disintegrating tablets are microbeads consisting of a drug core, a salting-out layer containing salts and water-soluble polymers, and a water-penetration-control layer of water-insoluble materials (Figure 1D) [16-19]. The water-penetration-control layer regulates the saliva intake rate when the microbeads are in the mouth. Dissolved salts, such as Na2CO3, in the saliva prevent the water-soluble polymers, for example, HPMC, from dissolving based on the salting-out effect. The insolubilized water-soluble polymers can, therefore, suppress drug release and mask drug numbness. In the gastrointestinal tract, most of the salts in the salting-out layer dissolve and release from the system, minimizing the salting-out effect. The water-soluble polymers in the same layer then dissolve; immediate drug release is achieved. This system generates long lag time in drug release and subsequent immediate release. Studies have proved that the major mechanism of the controlled drug release of this system is the salting-out effect [16,19]. This salting-out taste-masking system is used for taste-masking of solifenacin succinate in a launched oral disintegrating tablet. The concept, in vitro drug release data, taste-masking sensory tests, and verification in the clinical studies indicate that this system is versatile and applicable to other drugs that require rapid release for absorption and therapeutic effect after taste-masking.
Ion-responsive polymers can be used not only in oral DDS but also in local DDS, such as ophthalmic, subcutaneous depot, and transdermal formulations. Integrated use of polymers, ions, and other materials might lead to higher sensitivity to small changes in ionic concentrations, allowing DDS to have higher precision in site-specific drug release at target sites.
4. Conclusions
pH-responsive polymers are utilized for taste-masking, protection of acid-liable drugs, and colon delivery in various products on the market. Combination of the FDA-approved polymers and other components, such as MMT and lactulose, is also useful for pH-sensitive oral DDS.
Polymeric systems responsive to tumoral or intracellular pH have been extensively studied; however, the validation of their effectiveness in human is insufficient. The smart polymeric systems summarized in this article showed satisfactory performance in vitro and in mice. After evaluating immunogenicity and hemolytic property of those systems, human clinical studies are expected in the near future.
Ion-exchange resins and polymeric coatings are very useful for achieving both taste-masking and complete drug release for therapeutic effects. DDS containing both ion-responsive polymers and organic acids or salting-out ions have been reported. Use of interaction between ion-responsive polymers and other components might produce more effective pH-responsive DDS.
5. Expert opinion
To develop pH-responsive DDS and formulations, inter-individual variation in gastrointestinal pH of patients are one of the most important factors should be accounted for. In general, gastric pH in patients is 1.0 – 3.5 (Table 1); however, the pH value is observed to be higher than 4.0 for patients with achlorhydria. There are approximately 60% of Japanese with age over 50, 20% of Norwegian, and 11% of North American are with achlorhydria [109]. Use of acid soluble polymers for taste-masking should be avoided as higher gastric pH results in no or slower drug release or low therapeutic effect in patients with achlorhydria. This is especially important for the drugs used in therapeutic areas with high proportion of achlorhydria patients.
Intra-individual variability of gastrointestinal pH should also be considered when developing pH-responsive DDS. Under fasted conditions, gastric pH is 1.0 – 3.5, and pH in the oral cavity is 6.0 – 7.0 (Table 1). In contrast, gastric pH increases to 4.0 – 5.0, and pH in the oral cavity decreases to 4.0 under fed conditions. These physiological variations indicate that taste-masking formulation using polymers responsive to stomach low pH should be administered under fasted conditions. The two FDA-approved cationic polymers, Eudragit E and AEA, are soluble below pH 5 and 5.8, respectively, and thus are useful for taste-masking. However, strong interaction between drugs and the polymers is a major factor for slow drug release and low stability of drugs during storage. Other cationic polymers having different amounts of amino groups or other cationic groups and low immunogenicity are needed for further improvement of DDS and formulations. As another approach, combination of the FDA-approved polymers and other materials, including MMT, lactulose, organic acids and salting-out ions, is useful for DDS design such as the colon-targeted delivery system [40-43] and salting-out taste-masking system [16-19]. The combination of FDA-approved materials has several advantages, such as good safety, no immunogenicity, and short time and low cost for development by bypassing safety tests of the materials. There is limited number of FDA-approved materials; however, their combinations are very diverse and have infinite potential for DDS development.
The variability in pH in the endocytic pathway among different cells also imposes a challenge to DDS designed to intracellular release therapeutic agents based on the acidic endosomal pH. Studies have shown that vacular ATPase (V-ATPase) is a major controller in endosomal acidification and responsible for pumping protons from the cytoplasm to the endosomes/lysosomes [110,111]. Subunits of the V-ATPase (a1, a2 and a3) have been identified to be playing a role in acidifying endosomal pH. Investigation on correlating cellular level of a3 and a4 subunits to endosomal pH has been done using MDA-MB-231 and MCF-7 breast cancer cell lines. MDA-MB-231 cells was demonstrated to express higher levels of a3 and a4 subunits than did MCF-7 cells; this might contribute to the relatively lower endo/lysosomal pH observed in MDA-MB-231 cells [112]. Moreover, raft-domains have been reported to regulate activity of the V-ATPase, and lipid distribution or composition is suggested to affect endosomal pH [110]. Therefore, the endosomal pH variations across different cell types should be considered for pH-responsive DDS targeting specific cells or tissues. Similarly, it is expected that the pH values of tumor tissues are also different among cancer types and cancer stages. Due to this variation, one single DDS specific pH-responsive element may not be suitable for delivering drugs to all types of tumors. Present connection between the understanding of the physiology of tumors and the development of DDS is insufficient. Emerging pH imaging technologies are expected to provide more valuable information about tumoral and endo/lysosomal pH in humans, which will be useful for improving the performance of DDS.
Substantial progress in DDS development has been made based on ion-responsive polymers. Drug release can be controlled not only by interaction of ion-exchange resins and drugs but also by polymer coating [97,98]. The drug release from ion-exchange resins is promoted by an equilibrium exchange reaction; however, some drugs should be released faster for fast or high systemic exposure. For example, some drugs against heart attack or diabetes should be exposed immediately after the attack or meal. In other drugs that are metabolized extensively in the gut or liver, drug release rates should be faster than metabolism rate for sufficient systemic exposure and efficacy of the drugs. DDS containing ion-exchange resins, their counter ions, and drug might be useful for immediate drug release from ion-exchange resins and precise control of the ion-sensitivity. In our opinion, combination of polymers and other components, and deeper understanding of human body are important for pH- and ion-sensitive polymeric DDS products for patients.
Article highlights.
Cationic polymers with amino groups are useful for taste-masking and achieving high bioavailability of drugs by their low water solubility in the oral cavity (pH 5.8 – 7.4) and high solubility in the stomach (pH 1 – 3.5), respectively.
Anionic polymers with carboxyl groups having higher water solubility at basic pH than at acidic pH are used for protecting drugs from acid degradation in the stomach (enteric DDS) or enzyme digestion in the intestine (colon-targeted DDS).
Many tumor-targeted micelles have been investigated using polymers with imidazole groups (pKa ~ 6) or poly(β-amino ester) for more rapid drug release and more efficient cellular uptake at tumors (pH 6.5 – 7.0) than at normal tissues (pH 7.4).
Polymers having pH-sensitive chemical linkages, such as hydrazone, acetal, ortho ester, and vinyl, pH-sensitive cell-penetrating peptides, and cationic polymers undergoing pH-dependent protonation can respond to intracellular acidic endo/lysosomal compartments (pH 5 – 6) and achieve intracellular release of drug, gene, or protein.
Ion-exchange resins control drug release by responding to ions in the saliva and gastrointestinal fluids and are frequently used for taste-masking and sustained drug release.
Ion-responsive controlled drug release has been achieved by utilizing the lower critical solution temperature (LCST) transitions of ion-sensitive polymers.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
Takayuki Yoshida, and Kazuhiro Sako are employees of Astellas Pharma, Inc.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Alam MA, Ali R, Al-Jenoobi FI, et al. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9:1419–40. doi: 10.1517/17425247.2012.732064. [DOI] [PubMed] [Google Scholar]
- 2.Alani AW, Rao DA, Seidel R, et al. The effect of novel surfactants and Solutol HS 15 on paclitaxel aqueous solubility and permeability across a Caco-2 monolayer. J Pharm Sci. 2010;99:3473–85. doi: 10.1002/jps.22111. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Kurimoto I, Yoshihara K, et al. Aminoalkyl methacrylate copolymers for improving the solubility of tacrolimus I: evaluation of solid dispersion formulations. Int J Pharm. 2012;428:18–24. doi: 10.1016/j.ijpharm.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Sawada T, Kondo H, Nakashima H, et al. Time-release compression-coated core tablet containing nifedipine for chronopharmacotherapy. Int J Pharm. 2004;280:103–11. doi: 10.1016/j.ijpharm.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Katsuma M, Watanabe S, Kawai H, et al. Effects of absorption promoters on insulin absorption through colon-targeted delivery. Int J Pharm. 2006;307:156–62. doi: 10.1016/j.ijpharm.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Tirpude RN, Puranik PK. Rabeprazole sodium delayed-release multiparticulates: effect of enteric coating layers on product performance. J Adv Pharm Technol Res. 2011;2:184–91. doi: 10.4103/2231-4040.85539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai TC, Bae Y, Yoshida T, et al. pH-sensitive multi-PEGylated block copolymer as a bioresponsive pDNA delivery vector. Pharm Res. 2010;27:2260–73. doi: 10.1007/s11095-010-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda A, Shinoda T, Ito T, et al. Evaluating tamsulosin hydrochloride-released microparticles prepared using single-step matrix coating. Int J Pharm. 2011;408:84–90. doi: 10.1016/j.ijpharm.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Nakanishi K, Maeda A, et al. Pharmaceutical composition containing lipophilic IL-2 production inhibitor. 2009 WO2009054463.
- 10.Babish J, Tripp M, Howell T, Bland JS. Anti-inflammatory pharmaceutical compositions for reducing inflammation and the treatment or prevention of gastric toxicity. 2010 US7811610.
- 11.Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci. 2003;92:1343–55. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H. The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci. 2013 doi: 10.1111/cas.12152. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumura Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev. 2011;63:184–92. doi: 10.1016/j.addr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Mizumoto T, Tamura T, Kawai H, et al. Formulation design of an oral, fast-disintegrating dosage form containing taste-masked particles of famotidine. Chem Pharm Bull (Tokyo) 2008;56:946–50. doi: 10.1248/cpb.56.946. [DOI] [PubMed] [Google Scholar]
- 15.Mizumoto T, Tamura T, Kawai H, et al. Formulation design of taste-masked particles, including famotidine, for an oral fast-disintegrating dosage form. Chem Pharm Bull (Tokyo) 2008;56:530–5. doi: 10.1248/cpb.56.530. [DOI] [PubMed] [Google Scholar]
- 16••.Yoshida T, Tasaki H, Maeda A, et al. Mechanism of controlled drug release from a salting-out taste-masking system. J Control Release. 2008;131:47–53. doi: 10.1016/j.jconrel.2008.07.009. [It describes the new concept using salting-effect for taste-masking in the launched product.] [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Tasaki H, Maeda A, et al. Salting-out taste-masking system generates lag time with subsequent immediate release. Int J Pharm. 2009;365:81–8. doi: 10.1016/j.ijpharm.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Tasaki H, Maeda A, et al. Optimization of salting-out taste-masking system for micro-beads containing drugs with high solubility. Chem Pharm Bull (Tokyo) 2008;56:1579–84. doi: 10.1248/cpb.56.1579. [DOI] [PubMed] [Google Scholar]
- 19.Tasaki H, Yoshida T, Maeda A, et al. Effects of physicochemical properties of salting-out layer components on drug release. Int J Pharm. 2009;376:13–21. doi: 10.1016/j.ijpharm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Kurimoto I, Kasashima Y, Kawai H, et al. Drug-containing coated microparticle for orally disintegrating tablet. 2005 WO2005039542.
- 21.Scaffidi-Domianello YY, Legin AA, Jakupec MA, et al. Synthesis, characterization, and cytotoxic activity of novel potentially pH-Sensitive nonclassical platinum(II) complexes featuring 1,3-dihydroxyacetone oxime ligands. Inorg Chem. 2011;50:10673–81. doi: 10.1021/ic2010612. [DOI] [PubMed] [Google Scholar]
- 22.Richards AC, Santini JRJT, Cima MJ, et al. Microchip devices for delivery of molecules and methods of fabrication thereof. 2002 US20020107470.
- 23.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71:431–44. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douroumis D. Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin Drug Deliv. 2011;8:665–75. doi: 10.1517/17425247.2011.566553. [DOI] [PubMed] [Google Scholar]
- 25.Douroumis D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin Drug Deliv. 2007;4:417–26. doi: 10.1517/17425247.4.4.417. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto Y, Tanaka M, Kishimoto H, et al. Preparation, characterization and taste-masking properties of polyvinylacetal diethylaminoacetate microspheres containing trimebutine. J Pharm Pharmacol. 2002;54:1323–8. doi: 10.1211/002235702760345383. [DOI] [PubMed] [Google Scholar]
- 27.Randale SA, Dabhi CS, Tekade AR, et al. Rapidly disintegrating tablets containing taste masked metoclopramide hydrochloride prepared by extrusion-precipitation method. Chem Pharm Bull (Tokyo. 2010;58:443–8. doi: 10.1248/cpb.58.443. [DOI] [PubMed] [Google Scholar]
- 28.Sheshala R, Khan N, Darwis Y. Formulation and optimization of orally disintegrating tablets of sumatriptan succinate. Chem Pharm Bull (Tokyo) 2011;59:920–8. doi: 10.1248/cpb.59.920. [DOI] [PubMed] [Google Scholar]
- 29.Yan YD, Woo JS, Kang JH, et al. Preparation and evaluation of taste-masked donepezil hydrochloride orally disintegrating tablets. Biol Pharm Bull. 2010;33:1364–70. doi: 10.1248/bpb.33.1364. [DOI] [PubMed] [Google Scholar]
- 30.Kayumba PC, Huyghebaert N, Cordella C, et al. Quinine sulphate pellets for flexible pediatric drug dosing: formulation development and evaluation of taste-masking efficiency using the electronic tongue. Eur J Pharm Biopharm. 2007;66:460–5. doi: 10.1016/j.ejpb.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Haware RV, Chaudhari PD, Parakh SR, Bauer-Brandl A. Development of a melting tablet containing promethazine HCl against motion sickness. AAPS PharmSciTech. 2008;9:1006–15. doi: 10.1208/s12249-008-9133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasaki H, Ishii T, Kasashima Y, et al. Orally disintegrating tablet. 2011 WO2011121824.
- 33.Shimamo K, Kondo O, Miwa A, et al. Evaluation of uniform-sized microparticles containing a vibration nozzle method. Drug Dev Ind Pharm. 1995;21:331–47. doi: 10.3109/03639049509048114. [DOI] [PubMed] [Google Scholar]
- 34•.Lee JH, Choi G, Oh YJ, et al. A nanohybrid system for taste masking of sildenafil. Int J Nanomedicine. 2012;7:1635–49. doi: 10.2147/IJN.S28264. [It describes the interesting nanohybrid system using the cationic pH-sensitive polymer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson DA, Roach AC, Carlsson AS, et al. Stability of esomeprazole capsule contents after in vitro suspension in common soft foods and beverages. Pharmacotherapy. 2003;23:731–4. doi: 10.1592/phco.23.6.731.32181. [DOI] [PubMed] [Google Scholar]
- 36.Liu P, Sun B, Lu X, et al. HPLC determination and pharmacokinetic study of tenatoprazole in dog plasma after oral administration of enteric-coated capsule. Biomed Chromatogr. 2007;21:89–93. doi: 10.1002/bmc.724. [DOI] [PubMed] [Google Scholar]
- 37.Sharma M, Sharma V, Panda AK, Majumdar DK. Development of enteric submicron particles formulation of α-amylase for oral delivery. Pharm Dev Technol. 2013;18(3):560–9. doi: 10.3109/10837450.2011.604782. [DOI] [PubMed] [Google Scholar]
- 38.Sharma M, Sharma V, Panda AK, Majumdar DK. Development of enteric submicron particle formulation of papain for oral delivery. Int J Nanomedicine. 2011;6:2097–111. doi: 10.2147/IJN.S23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iemma F, Spizzirri UG, Puoci F, et al. pH-sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int J Pharm. 2006;312:151–7. doi: 10.1016/j.ijpharm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 40••.Katsuma M, Watanabe S, Takemura S, et al. Scintigraphic evaluation of a novel colon-targeted delivery system (CODES) in healthy volunteers. J Pharm Sci. 2004;93:1287–99. doi: 10.1002/jps.20063. [It describes the human data of the colon-targeted delivery system using pH-sensitive polymers.] [DOI] [PubMed] [Google Scholar]
- 41.Li J, Yang L, Ferguson SM, et al. In vitro evaluation of dissolution behavior for a colon-specific drug delivery system (CODES) in multi-pH media using United States Pharmacopeia apparatus II and III. AAPS PharmSciTech. 2002;3:E33. doi: 10.1208/pt030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Watanabe S, Li J, et al. Effect of colonic lactulose availability on the timing of drug release onset in vivo from a unique colon-specific drug delivery system (CODES). Pharm Res. 2003;20:429–34. doi: 10.1023/a:1022660305931. [DOI] [PubMed] [Google Scholar]
- 43.Katsuma M, Watanabe S, Kawai H, et al. Studies on lactulose formulations for colon-specific drug delivery. Int J Pharm. 2002;249:33–43. doi: 10.1016/s0378-5173(02)00429-5. [DOI] [PubMed] [Google Scholar]
- 44.Zimová L, Vetchý D, Muselík J, Stembírek J. The development and in vivo evaluation of a colon drug delivery system using human volunteers. Drug Deliv. 2012;19:81–9. doi: 10.3109/10717544.2011.644350. [DOI] [PubMed] [Google Scholar]
- 45.Takaya T, Sawada K, Suzuki H, et al. Application of a colon delivery capsule to 5-aminosalicylic acid and evaluation of the pharmacokinetic profile after administration to beagle dogs. J Drug Targeting. 1997;4:271–6. doi: 10.3109/10611869708995842. [DOI] [PubMed] [Google Scholar]
- 46.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 47.Rowinsky EK, Rizzo J, Ochoa L, et al. A phase I and pharmacokinetic study of pegylated camptothecin as a 1-hour infusion every 3 weeks in patients with advanced solid malignancies. J Clin Oncol. 2003;21:148–57. doi: 10.1200/JCO.2003.03.143. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Cheng R, Tao H, et al. Endosomal pH-activatable poly(ethylene oxide)-graft-doxorubicin prodrugs: synthesis, drug release, and biodistribution in tumor-bearing mice. Biomacromolecules. 2011;12:1460–7. doi: 10.1021/bm101340u. [DOI] [PubMed] [Google Scholar]
- 49.Kim D, Gao ZG, Lee ES, Bae YH. In vivo evaluation of doxorubicin-loaded polymeric micelles targeting folate receptors and early endosomal pH in drug-resistant ovarian cancer. Mol Pharm. 2009;6:1353–62. doi: 10.1021/mp900021q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Lee ES, Gao Z, Kim D, et al. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J Control Release. 2008;129:228–36. doi: 10.1016/j.jconrel.2008.04.024. [It describes the smart micelle system for pH-responsive cellular uptake in the tumor, and its in vivo effect.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao ZG, Tian L, Hu J, et al. Prevention of metastasis in a 4T1 murine breast cancer model by doxorubicin carried by folate conjugated pH sensitive polymeric micelles. J Control Release. 2011;152:84–9. doi: 10.1016/j.jconrel.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo K, Chung SW, Byun Y, Kim D. Paclitaxel loaded nano-aggregates based on pH sensitive polyaspartamide amphiphilic graft copolymers. Int J Pharm. 2012;424:26–32. doi: 10.1016/j.ijpharm.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 53.Min KH, Kim JH, Bae SM, et al. Tumoral acidic pH-responsive MPEG-poly(beta-amino ester) polymeric micelles for cancer targeting therapy. J Control Release. 2010;144:259–66. doi: 10.1016/j.jconrel.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Wu XL, Kim JH, Koo H, et al. Tumor-targeting peptide conjugated pH-responsive micelles as a potential drug carrier for cancer therapy. Bioconjug Chem. 2010;21:208–13. doi: 10.1021/bc9005283. [DOI] [PubMed] [Google Scholar]
- 55.Devalapally H, Shenoy D, Little S, et al. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol. 2007;59:477–84. doi: 10.1007/s00280-006-0287-5. [DOI] [PubMed] [Google Scholar]
- 56.Etrych T, Mrkvan T, Chytil P, et al. N-(2-Hydroxypropyl)methacrylamide-Based Polymer Conjugates with pH-Controlled Activation of Doxorubicin. I. New Synthesis, Physicochemical Characterization and Preliminary Biological Evaluation. J Appl Polym Sci. 2008;109:3050–61. [Google Scholar]
- 57.Etrych T, Sírová M, Starovoytova L, et al. HPMA copolymer conjugates of paclitaxel and docetaxel with pH-controlled drug release. Mol Pharm. 2010;7:1015–26. doi: 10.1021/mp100119f. [DOI] [PubMed] [Google Scholar]
- 58.Duncan R, Vicent MJ. Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Adv Drug Deliv Rev. 2010;62:272–82. doi: 10.1016/j.addr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Nowotnik DP, Cvitkovic E. ProLindac (AP5346): a review of the development of an HPMA DACH platinum Polymer Therapeutic. Adv Drug Deliv Rev. 2009;61:1214–19. doi: 10.1016/j.addr.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5:113–28. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 61.Etrych T, Kovář L, Strohalm J, et al. Biodegradable star HPMA polymer-drug conjugates: biodegradability, distribution and anti-tumor efficacy. J Control Release. 2011;154:241–8. doi: 10.1016/j.jconrel.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 62.Etrych T, Strohalm J, Chytil P, et al. Novel star HPMA-based polymer conjugates for passive targeting to solid tumors. J Drug Target. 2011;19:874–89. doi: 10.3109/1061186X.2011.622402. [DOI] [PubMed] [Google Scholar]
- 63.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42:4640–3. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 64.Bae Y, Nishiyama N, Kataoka K. In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjug Chem. 2007;18:1131–9. doi: 10.1021/bc060401p. [DOI] [PubMed] [Google Scholar]
- 65.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 66.Kim JK, Garripelli VK, Jeong UH, et al. Novel pH-sensitive polyacetal-based block copolymers for controlled drug delivery. Int J Pharm. 2010;401:79–86. doi: 10.1016/j.ijpharm.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garripelli VK, Kim JK, Namgung R, et al. A novel thermosensitive polymer with pH-dependent degradation for drug delivery. Acta Biomater. 2010;6:477–85. doi: 10.1016/j.actbio.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen W, Meng F, Cheng R, Zhong Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: a comparative study with micelles. J Control Release. 2010;142:40–6. doi: 10.1016/j.jconrel.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 69.Cohen JA, Beaudette TT, Tseng WW, et al. T-cell activation by antigen-loaded pH-sensitive hydrogel particles in vivo: the effect of particle size. Bioconjug Chem. 2009;20:111–19. doi: 10.1021/bc800338n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Standley SM, Mende I, Goh SL, et al. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem. 2007;18:77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 71.Tang R, Ji W, Wang C. Amphiphilic block copolymers bearing ortho ester side-chains: pH-dependent hydrolysis and self-assembly in water. Macromol Biosci. 2010;10:192–201. doi: 10.1002/mabi.200900229. [DOI] [PubMed] [Google Scholar]
- 72.Tang R, Ji W, Panus D, et al. Block copolymer micelles with acid-labile ortho ester side-chains: synthesis, characterization, and enhanced drug delivery to human glioma cells. J Control Release. 2011;151:18–27. doi: 10.1016/j.jconrel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin S, Du F, Wang Y, et al. An acid-labile block copolymer of PDMAEMA and PEG as potential carrier for intelligent gene delivery systems. Biomacromolecules. 2008;9:109–15. doi: 10.1021/bm7008747. [DOI] [PubMed] [Google Scholar]
- 74.Shin J, Shum P, Thompson DH. Acid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG-lipids. J Control Release. 2003;91:187–200. doi: 10.1016/s0168-3659(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 75.Xu Z, Gu W, Chen L, et al. A smart nanoassembly consisting of acid-labile vinyl ether PEG-DOPE and protamine for gene delivery: preparation and in vitro transfection. Biomacromolecules. 2008;9:3119–26. doi: 10.1021/bm800706f. [DOI] [PubMed] [Google Scholar]
- 76.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–60. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 77.El-Sayed A, Futaki S, Harashima H. Delivery of Macromolecules Using Arginine-Rich Cell-Penetrating Peptides: ways to Overcome Endosomal Entrapment. AAPS J. 2009;11:13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esbjörner EK, Oglecka K, Lincoln P, et al. Membrane binding of pH-sensitive influenza fusion peptides. positioning, configuration, and induced leakage in a lipid vesicle model. Biochemistry. 2007;46:13490–504. doi: 10.1021/bi701075y. [DOI] [PubMed] [Google Scholar]
- 79.Li W, Nicol F, Szoka FC, Jr, et al. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:967–85. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki K, Kogure K, Chaki S, et al. An artificial virus-like nano carrier system: enhanced endosomal escape of nanoparticles via synergistic action of pH-sensitive fusogenic peptide derivatives. Anal Bioanal Chem. 2008;391:2717–27. doi: 10.1007/s00216-008-2012-1. [DOI] [PubMed] [Google Scholar]
- 81.Deshayes S, Morris MC, Divita G, et al. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–49. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Min SH, Lee DC, Lim MJ, et al. A composite gene delivery system consisting of polyethylenimine and an amphipathic peptide KALA. J Gene Med. 2006;8:1425–34. doi: 10.1002/jgm.973. [DOI] [PubMed] [Google Scholar]
- 83.Pujals S, Fernández-Carneado J, López-Iglesias C, et al. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim Biophys Acta. 2006;1758:264–79. doi: 10.1016/j.bbamem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Kaämper N, Day PM, Nowak T, et al. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J Virol. 2006;80:759–68. doi: 10.1128/JVI.80.2.759-768.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lundberg P, El-Andaloussi S, Sutlu T, et al. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J. 2007;21:2664–71. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 86.Pack DW, Hoffman AS, Pun S, et al. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 87.Kim YH, Park JH, Lee M, et al. Polyethylenimine with acid-labile linkages as a biodegradable genecarrier. J Control Release. 2005;103:209–19. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 88.Thomas M, Lu JJ, Ge Q, et al. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci. 2005;102:5679–84. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang GP, Guo HY, Alexis F, et al. Low molecular weight polyethylenimines linked by beta-cyclodextrin for gene transfer into the nervous system. J Gene Med. 2006;8:736–44. doi: 10.1002/jgm.874. [DOI] [PubMed] [Google Scholar]
- 90.Kichler A, Leborgne C, Danos O, et al. Characterization of the gene transfer process mediated by histidine-rich peptides. J Mol Med. 2007;85:191–200. doi: 10.1007/s00109-006-0119-4. [DOI] [PubMed] [Google Scholar]
- 91.Hatefi A, Megeed Z, Ghandehari H. Recombinant polymer--protein fusion: a promising approach towards efficient and targeted gene delivery. J Gene Med. 2006;8:468–76. doi: 10.1002/jgm.872. [DOI] [PubMed] [Google Scholar]
- 92.Mason AJ, Martinez A, Glaubitz C, et al. The antibiotic and DNA transfecting peptide LAH4 selectively associates with, and disorders, anionic lipids in mixed membranes. FASEB J. 2006;20:320–2. doi: 10.1096/fj.05-4293fje. [DOI] [PubMed] [Google Scholar]
- 93.Ghosn B, Kasturi SP, Roy K. Enhancing polysaccharide-mediated delivery of nucleic acids through functionalization with secondary and tertiary amines. Curr Top Med Chem. 2008;8:331–40. doi: 10.2174/156802608783790947. [DOI] [PubMed] [Google Scholar]
- 94.Ghosn B, Singh A, Li M, et al. Efficient gene silencing in lungs and liver using imidazole-modified chitosan as a nanocarrier for small interfering RNA. Oligonucleotides. 2010;20:163–72. doi: 10.1089/oli.2010.0235. [DOI] [PubMed] [Google Scholar]
- 95.Lin C, Zhong ZY, Lok MC, et al. Novel bioreducible poly(amido amine)s for highly efficient gene delivery. Bioconjug Chem. 2007;18:138–45. doi: 10.1021/bc060200l. [DOI] [PubMed] [Google Scholar]
- 96.Mateos-Timoneda MA, Lok MC, Hennink WE, et al. Poly(amido amine)s as gene delivery vectors: effects of quaternary nicotinamide moieties in the side chains. ChemMedChem. 2008;3:478–86. doi: 10.1002/cmdc.200700279. [DOI] [PubMed] [Google Scholar]
- 97.Guo X, Chang RK, Hussain MA. Ion-exchange resins as drug delivery carriers. J Pharm Sci. 2009;98:3886–902. doi: 10.1002/jps.21706. [DOI] [PubMed] [Google Scholar]
- 98.Nadkar S, Lokhande C. Current trends in novel drug delivery - An OTC perspective. Pharma Times. 2010;42:17–23. [Google Scholar]
- 99.Bodmeier R, Guo X, Sarabia RE, Skultety PF. The influence of buffer species and strength on diltiazem HCl release from beads coated with the aqueous cationic polymer dispersions, Eudragit RS, RL 30D. Pharm Res. 1996;13:52–6. doi: 10.1023/a:1016021115481. [DOI] [PubMed] [Google Scholar]
- 100.Narisawa S, Nagata M, Hirakawa Y, et al. An organic acid-induced sigmoidal release system for oral controlled-release preparations. 2. Permeability enhancement of Eudragit RS coating led by the physicochemical interactions with organic acid. J Pharm Sci. 1996;85:184–8. doi: 10.1021/js950180o. [DOI] [PubMed] [Google Scholar]
- 101.Narisawa S, Nagata M, Danyoshi C, et al. An organic acid-induced sigmoidal release system for oral controlled-release preparations. Pharm Res. 1994;11:111–16. doi: 10.1023/a:1018910114436. [DOI] [PubMed] [Google Scholar]
- 102.Furyk S, Zhang Y, Ortiz-Acosta D, et al. Effects of end group polarity and molecular weight on the lower critical solution temperature of poly(N-isopropylacrylamide). J Polym Sci Part A: Polym Chem. 2006;44:1492–501. [Google Scholar]
- 103.Karewicz A, Zasada K, Szczubiałka K, et al. “Smart” alginate-hydroxypropylcellulose microbeads for controlled release of heparin. Int J Pharm. 2010;385:163–9. doi: 10.1016/j.ijpharm.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 104.Crespy D, Rossi RM. Temperature-responsive polymers with LCST in the physiological range and their applications in textiles. Polymer Int. 2007;56:1461–8. [Google Scholar]
- 105.Yoshida T, Tasaki H, Katsuma M, et al. Timed-limited release type granular pharmaceutical composition for oral administration and intraoral rapid disintegration tablet containing the composition. 2007 EP1787640A1.
- 106.Hofmeister F. Zur Lehre von der Wirkung der Salze. Arch Exp Pathol Pharmakol. 1888;24:247–60. [Google Scholar]
- 107.Park KH, Song HC, Na K, et al. Ionic strength-sensitive pullulan acetate nanoparticles (PAN) for intratumoral administration of radioisotope: ionic strength-dependent aggregation behavior and (99m)Technetium retention property. Colloids Surf B Biointerfaces. 2007;59:16–23. doi: 10.1016/j.colsurfb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 108.Eeckman F, Moës AJ, Amighi K. Evaluation of a new controlled-drug delivery concept based on the use of thermoresponsive polymers. Int J Pharm. 2002;241:113–25. doi: 10.1016/s0378-5173(02)00198-9. [DOI] [PubMed] [Google Scholar]
- 109.Morihara M, Aoyagi N, Kaniwa N, et al. Assessment of gastric acidity of Japanese subjects over the last 15 years. Biol Pharm Bull. 2001;24:313–15. doi: 10.1248/bpb.24.313. [DOI] [PubMed] [Google Scholar]
- 110.Lafourcade C, Sobo K, Kieffer-Jaquinod S, et al. Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization. PLoS ONE. 2008;3:e2758. doi: 10.1371/journal.pone.0002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marshansky V. The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem Soc Trans. 2007;35:1092–9. doi: 10.1042/BST0351092. [DOI] [PubMed] [Google Scholar]
- 112.Hinton A, Sennoune SR, Bond S, et al. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem. 2009;284:16400–8. doi: 10.1074/jbc.M901201200. [DOI] [PMC free article] [PubMed] [Google Scholar]