Abstract
A major contributor to impaired locomotion post-stroke is abnormal phasing of muscle activity. While inappropriate paretic muscle phasing adapts to changing body orientation, load, and speed, it remains unclear whether paretic muscle phasing adapts to reversal of locomotor direction. We examined muscle phasing in backward pedaling, a task that requires shifts in biarticular but not uniarticular muscle phasing relative to forward pedaling. We hypothesized that if paretic and neurologically intact muscle phasing adapt similarly, then paretic biarticular but not paretic uniarticular muscles would shift phasing in backward pedaling. Paretic and neurologically intact individuals pedaled forward and backward while recording electromyograms (EMGs) from vastus medialis (VM), soleus (SOL), rectus femoris (RF), semimembranosus (SM), and biceps femoris (BF). Changes in muscle phasing were assessed by comparing the probability of muscle activity in forward and backward pedaling throughout 18 pedaling cycles. Paretic uniarticular muscles (VM and SOL) showed phase-advanced activity in backward versus forward pedaling, whereas the corresponding neurologically intact muscles showed little to no phasing change. Paretic biarticular muscles were less likely than neurologically intact biarticular muscles to display phasing changes in backward pedaling. Paretic RF displayed no phase change during backward pedaling, and paretic BF displayed no consistent adaptation to backward pedaling. Paretic SM was the only muscle to display backward/forward phase changes that were similar to the neurologically intact group. We conclude that paretic uniarticular muscles are more susceptible and paretic biarticular muscles are less susceptible to direction-dependent phase shifts, consistent with altered sensory integration and impaired cortical control of locomotion.
INTRODUCTION
A major factor contributing to impaired locomotor ability in individuals with post-stroke hemiparesis is abnormal phasing of lower-limb muscle activity (Kautz and Brown 1998; Knutsson and Richards 1979), where phasing refers to a period of muscle activity with respect to the phase of the locomotor cycle. Kautz and Brown (1998) identified two distinct types of muscle phasing abnormalities in paretic limbs while pedaling: prolonged excitation in vastus medialis (VM) and soleus (SOL) and phase-advanced excitation in rectus femoris (RF) and two hamstring muscles. Muscle phasing abnormalities, particularly those present in paretic VM, RF, and hamstrings, were significantly correlated with reduced net mechanical work performed by the paretic limb during pedaling (Kautz and Brown 1998).
Despite inappropriate phasing under forward pedaling conditions, paretic locomotor muscle activity does adapt appropriately to a number of mechanical task constraints (Brown and Kautz 1998, 1999; Brown et al. 1997). For example, when paretic individuals pedal against higher mechanical loads, there is an appropriate increase in muscle activity and force output from the paretic leg (Beneche et al. 1983; Brown and Kautz 1998). Also, at progressively faster pedaling speeds, the duration of the prolonged VM burst in paretic limbs is reduced in time appropriately as indicated by termination of VM activity at the same phase of the crank cycle (Brown and Kautz 1999). Furthermore, with increasing vertical antigravity postures, EMG activity in paretic lower limb muscles shows appropriate amplitude modulation, similar to that observed in age-matched individuals without stroke (Brown et al. 1997). These observations suggest that even though locomotor muscle activity phasing is abnormal in persons with hemiparesis, the ability to modify muscle timing and amplitude in response to task demands remains largely intact.
The purpose of the present experiment was to further test the ability of paretic muscle activity to adapt to task requirements by examining lower-limb muscle activity during backward pedaling. The comparison of forward and backward pedaling is important because it examines the adaptability of locomotor pattern-generating networks. Previous work has shown that changes in muscle phasing during backward pedaling and walking represent neural adaptations to changing task demands, not a fundamentally different neurophysiological control scheme (Duysens et al. 1996; Ting et al. 1999). Hence, it has been suggested that locomotor patterns in forward pedaling and forward walking are reconfigured to produce backward locomotion. It remains unclear whether or not the same locomotor control mechanism is preserved post-stroke.
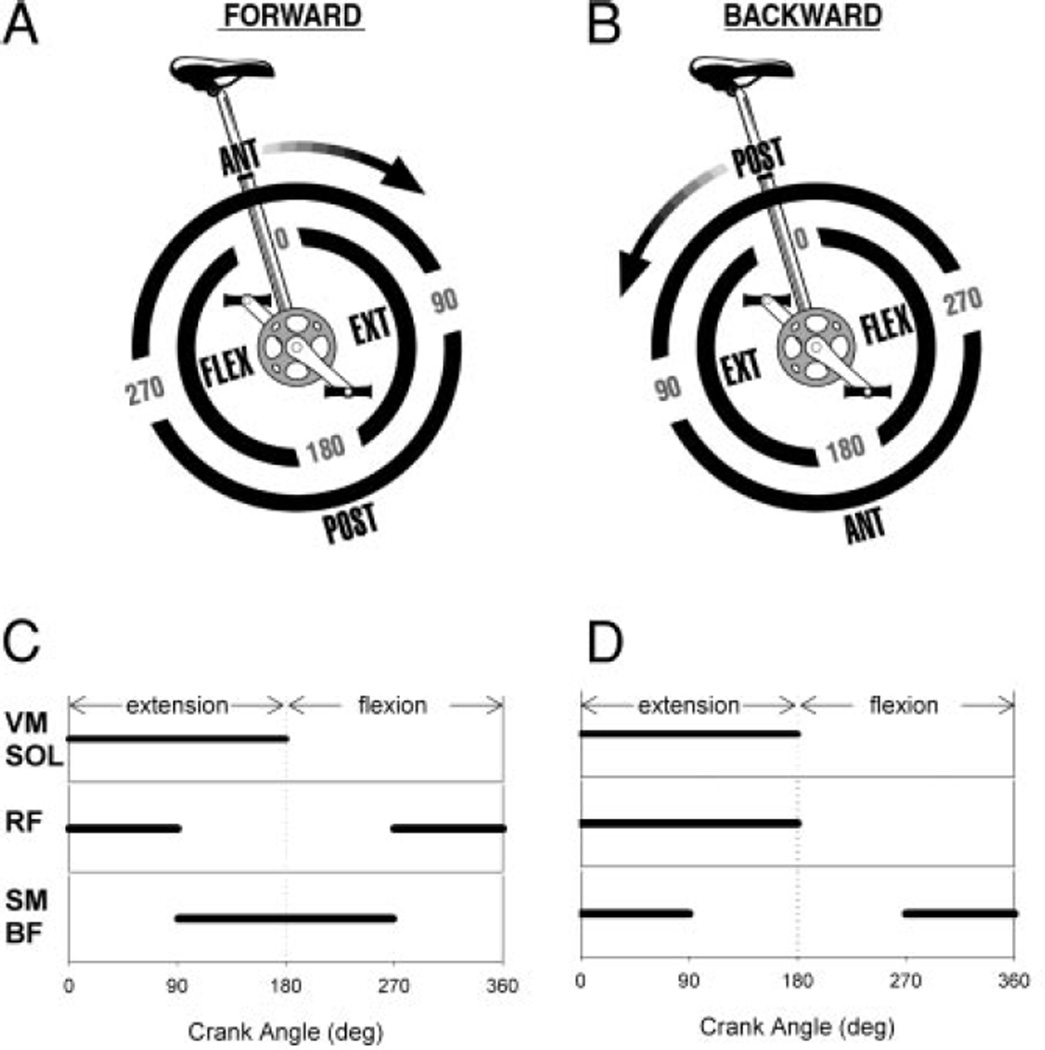
The comparison of forward and backward pedaling is a robust test for task-dependant adaptability of paretic muscle activity and reconfiguration of locomotor pattern-generating networks. In individuals who are neurologically intact, reversal of pedaling direction is associated with predictable changes in the phasing of biarticular muscles that cross the hip and knee joints and no change in the phasing of uniarticular muscles (Raasch and Zajac 1999; Ting et al. 1999). Figure 1 (A and B) illustrates the biomechanical components of pedaling and corresponding muscle activation patterns as predicted by a forward dynamics simulation of an uninterrupted pedaling cycle (Raasch and Zajac 1999). When driving the model, uniarticular extensor muscles such as VM and SOL are active predominantly during the extension phase of the crank cycle and do not change phasing with reversal of pedaling direction. However, biarticular muscles such as rectus femoris (RF), semimembranosis (SM), and biceps femoris (BF) are active during the transitions between flexion and extension phases of the pedaling cycle and display phase changes in backward pedaling. During forward pedaling, the SM and BF are active during the transition between extension and flexion, when the foot is moving posteriorly with respect to the pelvis. However, to drive the leg posteriorly during backward pedaling, the phasing of hamstrings changes such that they are active during the flexion-to-extension transition. The forward dynamics simulation predicts RF activity during anterior movement of the foot with respect to the pelvis such that the RF is active during the flexion-to-extension transition in forward pedaling and extension-to-flexion transition in backward pedaling.
FIG. 1.
A and B: the biomechanical components of pedaling as described by Raasch and Zajac (1999). In forward and backward pedaling, the limbs flex and extend in alternation. At the transitions between flexion and extension, the limb must move anteriorly or posteriorly with respect to the pelvis to maintain smooth crank progression. In backward pedaling, the location of the anterior and posterior transitions shifts 180°. Forward dynamics simulation predicts that uniarticular extensor muscles [vastus medialis (VM) and soleus (SOL)] will be active during limb extension and biarticular muscles [rectus femoris (RF) and hamstring (HAMS)] will be active during phase transitions (Raasch and Zajac 1999). C and D: idealized muscle phasing in forward and backward pedaling as described by Ting et al. (1999) for neurologically intact adults. VM and SOL are active during limb extension and do not change their phasing in backward pedaling. RF and HAMS are active during the anterior and posterior limb transitions, respectively, and shift their phasing in backward pedaling. The RF does not shift as much as the model predicts. POST, posterior; ANT, anterior; EXT, extension; FLEX, flexion; BF, biceps femoris.
The predictions of the forward dynamics simulation are supported by electromyographic (EMG) data recorded from neurologically intact individuals during forward and backward pedaling (Ting et al. 1999). Figure 1 (C and D) displays idealized versions of “on-off” muscle activity phasing that were reported by Ting et al. (1999) during forward and backward pedaling. There was no change in VM or SOL phasing in backward compared with forward pedaling, while there was a nearly 180° phase shift in SM and BF activity (Ting et al. 1999). The RF displayed approximately a 90° phase delay in backward versus forward pedaling (Ting et al. 1999); this was statistically significant although smaller than the RF phase shift predicted by the model (Raasch and Zajac 1999).
In the present study, individuals with and without chronic post-stroke hemiparesis performed motor-assisted pedaling in the forward and backward direction while EMG activity was recorded from five lower extremity muscles (VM, SOL, RF, SM, and BF). First, we replicated the observation that neurologically intact VM and neurologically intact SOL show no change in their phasing of activity, whereas neurologically intact RF and neurologically intact hamstring activity change their phasing with reversal of pedaling direction. Most importantly, we hypothesized that if paretic muscle activity adapts similarly to neurologically intact muscle during backward pedaling, direction-dependent changes in lower limb muscle phasing would not occur in paretic uniarticular muscles (VM and SOL) but would occur in the paretic biarticular muscles (RF, SM, and BF). Moreover, we hypothesized that direction-dependent phase changing of paretic biarticular muscle activity (RF, SM, and BF) would be the same as neurologically intact individuals after accounting for the phase advance that is present in these paretic muscles during forward pedaling. Instead, we found that in contrast to neurologically intact muscles, uniarticular paretic muscles showed direction-dependent shifts, whereas some biarticular paretic muscles lost their characteristic phase shifting. Portions of this work have been presented previously in abstract (Brooke et al. 2003; Schindler-Ivens et al. 2003).
METHODS
Subjects
Fourteen individuals with chronic post-stroke hemiparesis (paretic; 12 male, 2 female) and 13 neurologically intact individuals (10 male, 3 female) volunteered to participate. The mean age of paretic and neurologically intact subjects was 56.3 ± 10 and 47.3 ± 8.6 (SD) years, respectively. Although effort was made to match the groups with respect to gender and age, the paretic group was older than the control group (independent t-test, P < 0.05). Because of this disparity, we examined all the data for age effects and found similar patterns of muscle phasing in older and younger subjects. Paretic subjects had sustained a single, unilateral cerebrovascular accident (CVA) ≥2 yr earlier with residual lower limb paresis, had no serious perceptual, cognitive or language deficits, and no cardiovascular impairment contraindicative to pedaling. All subjects could sit on a bicycle seat while strapped to a backboard for 1 h. On average, subjects had sustained their stroke 8.3 ± 4.4 years before participating in this study. There were nine subjects with left hemiparesis and five subjects with right hemiparesis. Neurologically intact individuals showed no sign of neurological disease and had no significant past medical history for neurological disease or injury. All subjects participated voluntarily and gave informed consent according to the declaration of Helsinki and as approved by the Institutional Review Board at Northwestern University.
Paretic individuals underwent the lower limb portion of the Fugl-Meyer test (Fugl-Meyer et al. 1975) for assessment of global motor function. The average motor and sensory scores were 24.2 ± 4.1 of 34 possible and 10.9 ± 1.7 of 12 possible, respectively. All but one subject with hemiparesis who was wheelchair-dependent used walking as their primary mode of ambulation; however, substantial gait deviations and decreased walking velocity were evident with all paretic individuals tested.
Instrumentation
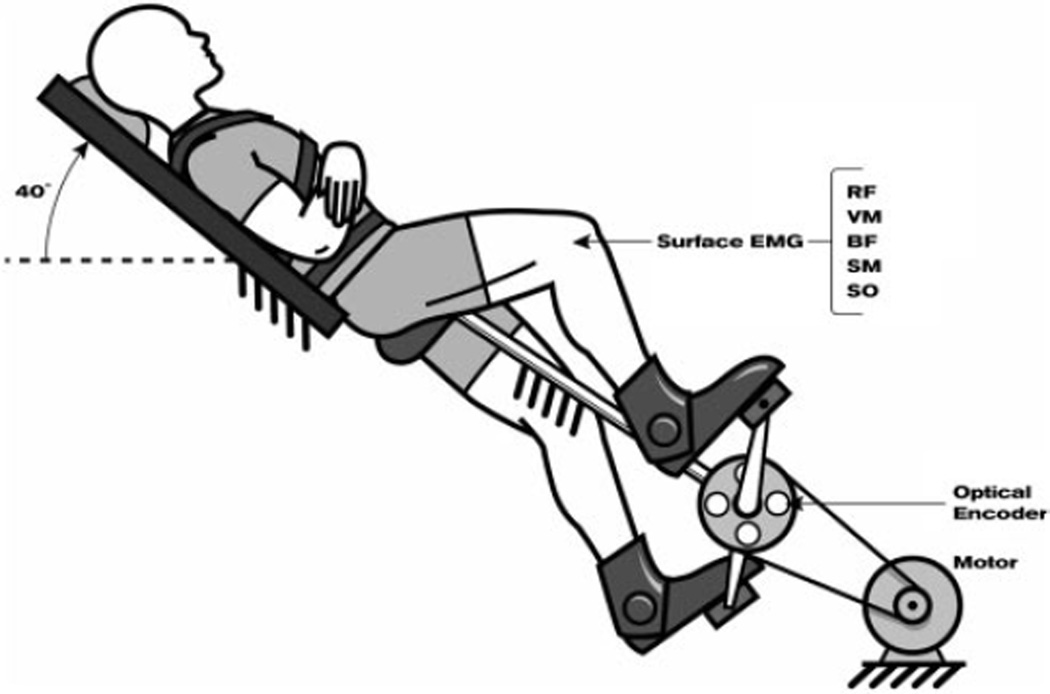
As depicted in Fig. 2, subjects were positioned on a custom-designed bicycle ergometer that was equipped with a motorized crank and velocity controller. A rigid backboard was connected to the pedaling mechanism to support the subject’s pelvis, trunk, and head. Subjects were secured to the backboard with nylon straps. The head and trunk were oriented 40° from horizontal. In this position, participants completed the entire experiment without discomfort from the bicycle seat. Subjects’ feet were coupled to the pedals using the bottom portion of a rigid walking brace that kept the foot on the pedal during the upstroke phase of pedaling. The ankle was free to dorsiand plantar flex.
FIG. 2.
Subjects were positioned on a custom-designed bicycle ergometer. See text for details.
Optical encoders (BEI Model EX116-1024-2) were used to measure the angular position of the crank to an accuracy of 0.3°. Bipolar silver surface electrodes (DelSys, 10 mm length, 1 mm width, 1 cm interelectrode distance) were used to record EMG activity from VM, SOL, RF, SM, and BF. EMG signals were amplified 10 times at the electrode site before remote differential amplification (common mode rejection ratio: 92 dB, gain range: 100–10,000 times, frequency response: 20–450 Hz) and low-pass filtering (500 Hz, custom-designed filter). The digital optical encoder signals were converted to analog with a digital-to-analog converter module before sampling. Position and EMG data were sampled on-line at 1000 Hz via a 12-bit analog-to-digital converter (National Instruments) and Labview software.
In preparation for placement of the EMG electrodes, the skin over each muscle was cleaned and gently abraded with an alcohol swab. Surface EMG electrodes were placed over the distal half of the VM, SOL, RF, SM, and BF (short head) muscles of the right leg of neurologically intact subjects and both legs of paretic subjects. A common reference electrode was placed over the tibia on the anterior aspect of the leg. Electrodes were secured with adhesive tape and with a tensor bandage to prevent electrode movement during the experiment.
Protocol
The bicycle motor rotated the legs in the forward and then the backward direction at a constant velocity of 39 rotations/min. Subjects were instructed to assist the motor by pedaling forward or backward with a moderate amount of effort using both legs. Muscle activity was monitored throughout the pedaling cycle to ensure that subjects with stroke used their paretic leg. If paretic muscle activity was absent or very low, subjects were reminded to pedal with their paretic leg, and data collection began when the subject was pedaling with both legs. Each bout of pedaling lasted 30 s, during which time 18 complete crank revolutions were recorded.
Data processing and analysis
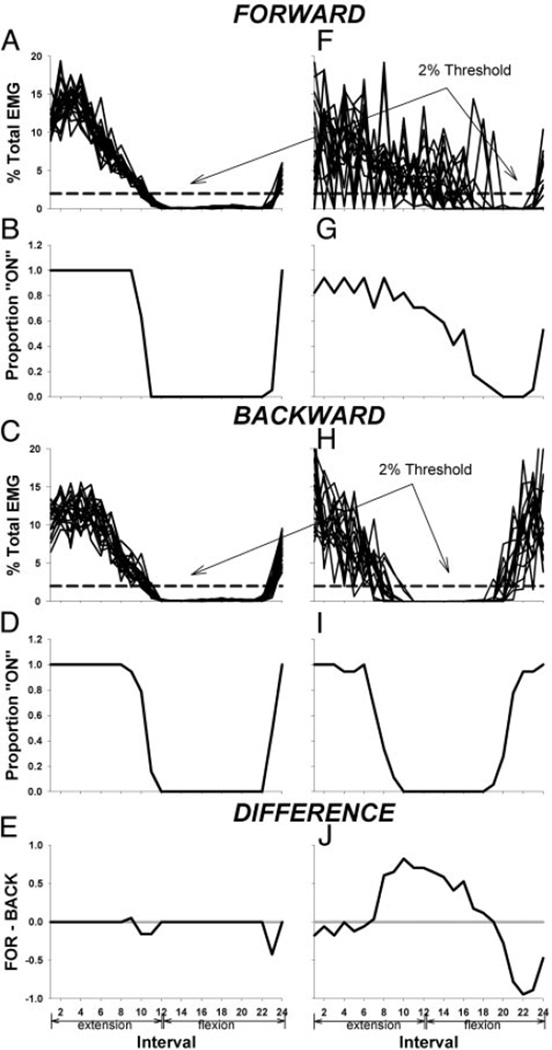
This study focused on muscle activity phasing, or the relative period of muscle activation with respect to the crank angle, in forward and backward pedaling. Therefore we used an EMG processing technique that allowed comparison of forward and backward muscle phasing that was minimally influenced by EMG amplitude. First, to compare muscle activity at the same point in the pedaling cycle for every crank revolution, we rectified the EMG signals and referenced them to the crank position in 1° increments. Data that were sampled within any 1° increment were averaged and assigned to the crank position that represented the middle value of the range. For example, EMG data sampled between 0.5 and 1.5° in the crank cycle were averaged and assigned to the 1° position of the crank cycle.
Next, we characterized the EMG throughout every pedaling cycle as either on or off and calculated the probability that a muscle was on during a given interval of each crank cycle. To do this, we calculated the sum of the rectified and crank referenced EMG during each revolution for every 15° interval of crank rotation (24 intervals), and we normalized each value to the sum of the total EMG over the entire revolution. See Fig. 3, A, C, F, and H. If the EMG in an interval was ≥2% of total EMG for the VM or ≥5% of total EMG for the other muscles studied, the muscle was considered on, and the interval was assigned the value of 1. If the EMG in an interval was <2% of total EMG for the VM or <5% of total EMG for the other muscles studied, the muscle was considered off, and the interval assumed the value of 0. These threshold values (2 and 5% of total EMG for VM and other muscles, respectively) were based on the average minimum amplitude of the normalized EMG. In all muscles except the VM, the average minimum EMG was ~2% of total EMG. Minimum VM EMGs were ~1% of total EMG. Minimum EMG values were not different in forward and backward pedaling. The threshold for on-off muscle activity was set at approximately two times the minimum value in all muscles, and each trace was visually inspected to ensure that on-off processed EMG accurately reflected EMG phasing in the raw data.
FIG. 3.
Stages of electromyographic (EMG) processing used to identify differences in phasing between forward and backward pedaling. Examples for VM. Left: neurologically intact. Right: paretic. A, C, F, and H: sum of the rectified EMG in each 15° interval of crank rotation normalized to the sum of the total EMG over the entire revolution. All 18 revolutions in forward and backward pedaling are overlayed. B, D, G, and I: mean probability of VM activity at each interval. E and J: difference between probability of VM activity in forward and backward pedaling at each interval.
For each subject, the mean probability that a muscle was on was calculated by averaging the 0 and 1 values at each interval over all 18 crank revolutions for the forward and backward condition, as shown in Fig. 3, B, G, D, and I. For each subject, we then calculated the difference between forward and backward pedaling with respect to the probability of being on for each 15° interval by subtracting the mean on probability in the backward condition from the mean on probability in the forward condition. See Fig. 3 (E and J). Positive differences indicated a greater probability of activity in forward compared with backward pedaling and vice versa for negative differences.
Differences between forward and backward probability of activity at each 15° interval were averaged across all subjects in each group. To test whether the difference between forward and backward pedaling was different from zero within each group (paretic and neurologically intact), we used one-sample t-test (P ≤ 0.05) adjusted for multiple comparisons using Holm’s step-down procedure (Holm 1979). To test for between-group effects (P ≤ 0.05, paretic vs. neurologically intact) in the difference between forward and backward pedaling, the interaction term of a two-way repeated-measures ANOVA model was used. In the presence of a significant interaction, the appropriate simple effects analyses (independent t-test) were used to identify between-group differences at each interval of the pedaling cycle.
To determine whether direction-dependent changes in muscle activity in the paretic limb were appropriate given abnormal initial phasing during forward pedaling, we did the following: for paretic muscles that displayed a phase advance in forward pedaling as compared with neurologically intact muscles (RF, SM, and BF), a cross-correlation analysis was performed on the group mean data for forward pedaling in the paretic and neurologically intact groups to find the phasing of maximum correlation between the two groups. The phasing of the mean difference values was then adjusted for the phase advance observed during forward pedaling, and statistical analyses on the difference values were carried out as in the preceding text.
Group analysis on the VM, SOL, and RF data was based on all subjects. Group analysis of hamstring data was based on a subset of subjects. BF or SM data were excluded from group analysis when the muscle failed to display activity during the extension-to-flexion transition of forward pedaling. Our data were consistent with previous work showing that some people do not display hamstring activity during the extension-to-flexion transition of forward pedaling (Gregor et al. 1985; Ryan and Gregor 1992). Rather, some individuals display bursts of activity in either the SM or BF during extension and/or flexion. When muscles are not active during a transition phase of forward pedaling, there is no expectation that they will change their phasing during backward pedaling (Raasch and Zajac 1999). The number of subjects included in SM and BF analysis is indicated in results, and all subjects are accounted for.
To examine the possibility that differences in EMG magnitude in forward and backward pedaling contributed to direction-dependent phasing differences in paretic and neurologically intact limbs, we compared the sum of the EMG recorded from each muscle during forward and backward pedaling in both groups. Two-way repeated-measures ANOVA (split-plot design, P < 0.05) was used to examine the effect of pedaling direction on EMG magnitude and to identify any group by direction interactions in EMG magnitude.
RESULTS
Phasing of uniarticular muscle activity in backward versus forward pedaling
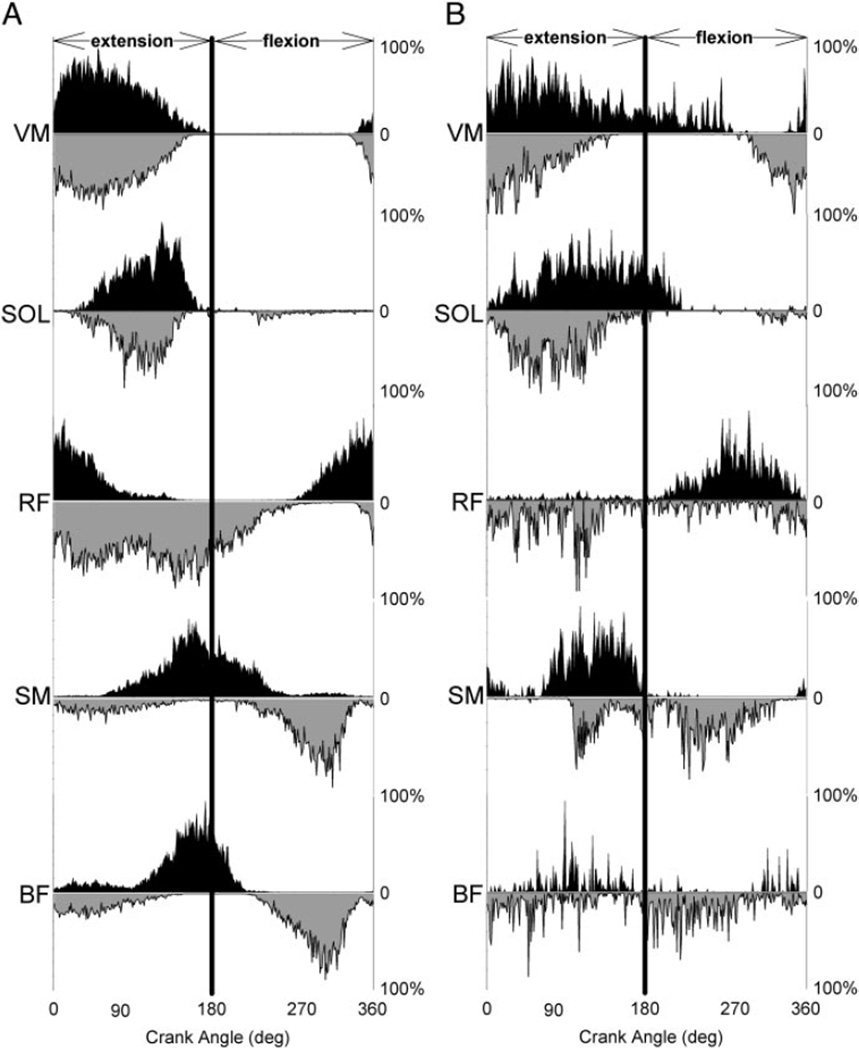
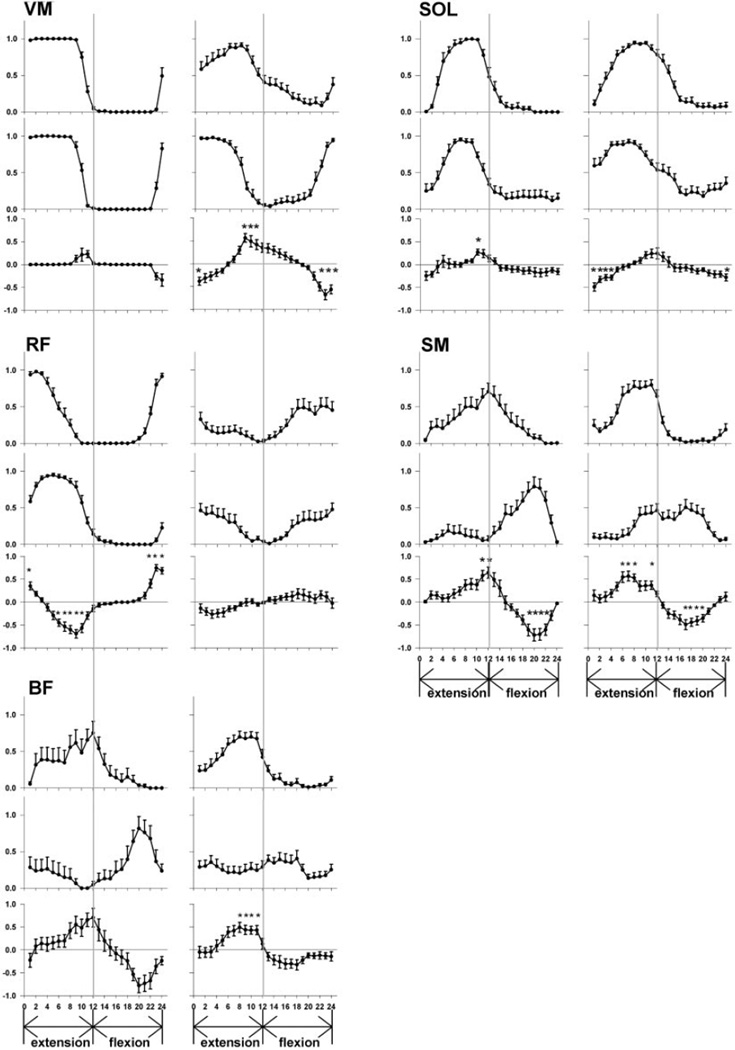
In the neurologically intact group, there was no difference in the phasing of VM activity in backward versus forward pedaling (Figs. 4, left representative subject, and 5, group data). Figure 5 (VM, left) demonstrates that the probability of neurologically intact VM activity was not significantly different in backward versus forward pedaling at any interval in the pedaling cycle (P > 0.05). In contrast, paretic VM activity during backward pedaling was advanced in phase compared with forward pedaling. (Figs. 4, right representative subject, and 5, group data.) As illustrated in Fig. 5 (VM, right), paretic VM activity during backward compared with forward pedaling was more likely to occur during the transition from flexion to extension and less likely to occur during the transition from extension to flexion. Significant backward/forward differences in the probability of paretic VM activity were evident at intervals 1, 9, 10, 11, 22, 23, and 24 (P < 0.05).
FIG. 4.
Representative data from a neurologically intact (A) and a paretic (B) subject. Arranged from top to bottom, each panel displays a different muscle. The top of each panel shows forward pedaling muscle activity in black. The bottom of each panel shows backward pedaling muscle activity in gray and on a reflected axis. All 18 trials recorded during each condition are averaged, and EMG is displayed as a percent of peak activity during the pedaling cycle.
FIG. 5.
Muscle phasing in forward and backward pedaling for the VM, SOL, RF, SM, and BF. Group means for neurologically intact data are on the left, and group means for paretic data are on the right. Figures represent the probability of muscle activity during forward (top) and backward (middle) pedaling. The mean difference between forward and backward probability of muscle activity is on the bottom. The ordinate represents the mean probability of muscle activity, and the abscissa displays the interval of the pedal cycle. Extension and flexion phases of the pedaling cycle are indicate by arrows below the abscissa. The vertical line in the middle of each graph represents the transition point between limb flexion and extension. Error bars are standard error of the mean, and asterisks represent significant within-group differences (P < 0.05, adjusted for multiple comparisons).
The SOL muscle response to reversal of pedaling direction was similar to that seen in the VM. In the neurologically intact group, there was little difference in the phasing of SOL activity in backward versus forward pedaling (Figs. 4, left representative subject, and 5, group data.). Figure 5 (SOL, left) shows a significant difference in the likelihood of neurologically intact SOL activity at only one interval in the pedaling cycle (interval 10, P < 0.05). However, Fig. 5 (SOL, right) shows that the paretic SOL was significantly more likely to be active during the late flexion and early extension phases of backward compared with forward pedaling. Significant differences in the probability of SOL activity were present at intervals 1, 2, 3, 4, and 24 (P < 0.05).
Phasing of biarticular muscle activity in forward versus backward pedaling
As shown in Figs. 5 (RF, left) and 4A, we observed a phase delay in neurologically intact RF activity in backward compared with forward pedaling. In comparison to forward pedaling, neurologically intact RF activity during backward pedaling was less likely to occur during the transition from flexion to extension and more likely to occur during late limb extension. A significant direction-dependent difference in the likelihood of neurologically intact RF activity was seen at intervals 1, 6, 7, 8, 9, 10, 22, 23, and 24 (P < 0.05). In contrast, Fig. 5 (RF, right) shows that the paretic RF did not change its phasing with reversal of pedaling direction. The probability of activity in the paretic RF at any interval in the cycle was not significantly different in backward versus forward pedaling (P > 0.05).
In both groups, most subjects displayed a significant phase delay in SM activity in backward compared with forward pedaling. Figure 5 (SM, left) shows that the majority of neurologically intact subjects (10 of 13) displayed a high probability of SM activity during the extension-to-flexion transition of forward pedaling. During backward pedaling in these individuals, the peak likelihood of neurologically intact SM activity shifted to the flexion phase of the cycle. Hence, there was a significant difference in the probability of neurologically intact SM activity at intervals 11, 12, 19, 20, 21, and 22 (P < 0.05). In three neurologically intact subjects (not shown), the SM had a high probability of activity exclusively during the limb flexion phase of forward pedaling and did not shift its phasing in backward pedaling. In contrast, all paretic subjects displayed a consistent pattern of SM activity in forward pedaling that was characterized by peak likelihood of activity just before the extension-to-flexion transition. (see Fig. 5, SM, right.) In backward compared with forward pedaling, the likelihood of paretic SM activity was increased during limb flexion and was decreased during limb extension. Significant backward/forward differences in paretic SM activity were evident at intervals 6, 7, 8, 11, 17, 18, 19, and 20 (P < 0.05).
Because the neurologically intact and paretic groups both displayed a phase delay in SM activity during backward pedaling, we were interested in determining whether the backward/forward difference in SM phasing was the same in both groups. Hence, we used two-way ANOVA with repeated measures to compare the SM backward/forward difference values between groups. There was a significant group by interval interaction (P < 0.0001), indicating that forward/backward phasing differences were not the same in both groups. The two groups were significantly different from each other at intervals 12, 13, 21, and 22. However, after the paretic SM difference values were phase delayed by 30° to account for phase advanced paretic SM activity during forward pedaling, there was no significant group by interval interaction (P = 0.31). Thus neurologically intact and paretic backward/forward difference values were not significantly different from each other once paretic SM phasing in forward pedaling was adjusted for its inappropriate phase advance in forward pedaling.
In approximately half of neurologically intact subjects (6 of 13), the BF was active mainly during the transition between the extension and flexion phases of forward pedaling. In these individuals, there was a trend to suggest that neurologically intact BF phasing was delayed in backward pedaling as shown in Fig. 5 (BF, left). However, there were no significant differences in the probability of BF activity in backward versus forward pedaling at any point in the cycle. In contrast, most subjects in the paretic group (12 of 14) had a high likelihood of BF activity during the late extension phase of forward pedaling and the extension-to-flexion transition. This pattern was similar to the one displayed by neurologically intact subjects but was advanced in phase. As shown in Fig. 5 (BF, right), when these paretic subjects pedaled backward, there was a significant difference in BF phasing as indicated by a decrease in the probability of BF activity at intervals 8–11. However, this result must be considered cautiously because further inspection of backward pedaling data revealed that the paretic BF displayed no consistent pattern between subjects. Paretic BF activity in backward pedaling was advanced, delayed, or unchanged in phase or was active throughout the pedaling cycle. These observations explain the relatively flat group profile of paretic BF muscle activity in backward pedaling. Subjects whose BF activity was phase advanced, phase delayed, and present throughout the entire backward pedaling cycle contributed to the significant forward/backward paretic BF effect.
The BF muscle of the remaining subjects (7 of 13 neurologically intact and 2 of 14 paretic) displayed two bursts of activity during forward pedaling, one during mid extension and another during mid flexion. When pedaling direction was reversed, there was no direction-dependent change in BF phasing in either group.
Changes in EMG amplitude in forward versus backward pedaling
Total EMG (in millivolts*degrees) during backward compared with forward pedaling was elevated in the VM, RF, and BF (P < 0.05), was reduced in the SOL and SM (P < 0.05), and was unchanged in the BF (P > 0.05). Changes in the magnitude of muscle activity in forward versus backward pedaling were not different in the paretic and neurologically intact limbs (group by direction interaction, P > 0.05).
DISCUSSION
Our data indicate that paretic lower limb muscle activity does not adapt to reversal of pedaling direction in the same way as neurologically intact muscle. We reject our hypothesis that paretic muscle activity phasing adjusts to the mechanical task demands of backward pedaling similarly to neurologically intact muscle. The paretic VM and SOL showed significant changes in their activation pattern with reversal of pedaling direction that were not expected from these uniarticular muscles (Raasch and Zajac 1999; Ting et al. 1999). The paretic RF showed no significant change in its pattern of activation in backward versus forward pedaling when a change in RF phasing was expected (Raasch and Zajac 1999; Ting et al. 1999). The paretic BF displayed no consistent pattern of activity in backward pedaling. A variety of paretic BF responses to backward pedaling were observed including no change, earlier activation, later activation, and activation throughout the pedaling cycle. The paretic SM was the only muscle to display an expected change in its phasing when pedaling direction was reversed. These data suggest that paretic uniarticular muscles may be more susceptible and paretic biarticular muscles may be less susceptible to direction-dependent phase shifts.
Our observations of neurologically intact and paretic muscle activity during pedaling are in agreement with previous studies (Kautz and Brown 1998; Raasch and Zajac 1999; Ting et al. 1999). The neurologically intact patterns of direction-dependent muscle activity that we report have been predicted from biomechanical models of pedaling (Raasch and Zajac 1999) and are consistent with the results of Ting et al. (1999), who recorded muscle activity during forward and backward pedaling in young healthy adults. Our observations of paretic muscle activity phasing agree with those of Kautz and Brown (1998), who showed prolonged VM and SOL activity and phase advanced RF and hamstring activity during forward pedaling post-stroke. One difference between the present results and those reported previously is that we did not detect a significant shift in the neurologically intact BF phasing in backward compared with forward pedaling. This result likely was due to the large number of individuals (7 of 13) who did not show BF activity during the extension-to-flexion transition of forward pedaling. Instead, these subjects showed two bursts of hamstring activity, one during limb extension and another during limb flexion. In these subjects, a backward/forward change in hamstring phasing was neither predicted nor detected (Raasch and Zajac 1999), and their data were not included in group analysis. Others have reported a two-burst pattern of hamstring activity during forward pedaling (Gregor et al. 1985; Ryan and Gregor 1992) and have suggested that some components of the hamstring complex are uniarticular, meaning that they have fiber bundles that effectively cross only one joint (Raasch et al. 1997). Such fiber bundles act as either pure hip extensors or pure knee flexors (Raasch et al. 1997). However, the number of subjects who displayed the two-burst pattern in this study was higher than reported in previous studies. Perhaps the placement of some of our electrodes was closer to these uniarticular elements than in previous work.
Similarities between our findings and those of previous studies suggest that the use of a motorized crank in the present study did not confound our results. We used a motorized crank so that all hemiparetic subjects, even those with seriously limited locomotor performance, could be studied. The motor also propelled the crank at a constant speed in forward and backward pedaling. A potential problem with using a motorized crank was that subjects were not required to use their muscle activity to successfully drive the crank. To avoid this problem, subjects were asked to pedal “normally” as if they were pedaling a bicycle, and muscle activity was monitored throughout the experiments. All subjects displayed pedaling-related activity in all muscles that exceeded that which was present when they were asked to relax completely. While it is possible that muscle activity patterns that were produced during motorized pedaling were not the same as patterns used during non-motorized pedaling, the similarities between our results and the results of previous studies of non-motorized pedaling argue strongly against this possibility (Kautz and Brown 1998; Ting et al. 1999). Moreover, others who have used motorized cranks have reported results that are not different from those observed during non-motorized pedaling (Kautz et al. 2002; Ting et al. 2000).
Our results indicate that while paretic lower extremity muscles may be capable of scaling their overall amplitude and duration (Brown and Kautz 1998 1999; Brown et al. 1997), they do not similarly modify their phasing according to task demands. Previous studies in which speed (Brown and Kautz 1999), load (Brown and Kautz 1998), and antigravity posture (Brown et al. 1997) were manipulated during pedaling showed that paretic muscle activity was inappropriately phased under initial conditions but adjusted similarly to neurologically intact muscles with changing task demands. For example, when pedaling speed increased, the duration of VM activity was reduced in terms of the absolute time that it was active (Brown and Kautz 1999). This is thought to be an appropriate response to increasing speed because peak forces are produced at equivalent regions of the crank cycle. When an increase in load was encountered, the amplitude of VM activity increased (Brown and Kautz 1998). This response is thought to be appropriate because force output by the paretic limb was enhanced at higher workloads. In each of these cases, task-dependent adaptations in muscle activity can be described as scaling adjustments. EMG amplitude was scaled upward with increasing load, and EMG duration was scaled down with increasing pedaling speed.
Whereas changes in muscle activity scaling were expected in previous studies, the current study expected phasing changes and examined the ability of the paretic locomotor pattern-generating networks to reconfigure their output in response to biomechanical task demands. Because paretic muscle phasing did not adapt to changes in locomotor direction similarly to neurologically intact muscle, we conclude that the paretic backward pedaling strategy is not a reconfiguration of the forward pedaling control strategy adapted to achieve the biomechanical demands of backward pedaling. Rather the post-stroke nervous system appears to have a different locomotor control scheme in forward and backward pedaling. However, we cannot rule out the possibility that, unlike the neurologically intact system, paretic locomotor control may be optimized for task demands other than crank propulsion. For example, the post-stroke locomotor strategy may be attempting to achieve stability, and task-dependent adaptations may be a congruent with this goal.
Potential mechanisms for forward/backward phasing differences
Although this study was not designed to identify mechanisms contributing to abnormal muscle phase shifts in forward versus backward pedaling post-stroke, it is possible to make inferences about mechanisms from the design of this study. Backward versus forward muscle phase changes that are normally observed in biarticular muscles during pedaling may be triggered by afferent cues. There is a substantial body of literature suggesting that sensory information influences the phasing of locomotor muscle activity (Van de Crommet et al. 1998). In the cat, hindlimb flexor muscle stretch (Hiebert et al. 1996) and limb unloading (Whelan et al. 1995) during the stance phase of locomotion reset the locomotor pattern to swing. In human subjects, vibration of quadriceps during the stance phase of walking induces an earlier onset of tibialis anterior activity (Verschueren et al. 2003), suggesting that Group I afferent discharge is involved in triggering locomotor phase transitions. Biomechanical modeling of lower extremity joint kinematics during pedaling using a four-bar linkage reveals that while the hip and knee undergo the same excursion in forward and backward pedaling, the relative trajectory of the hip with respect to the knee is different in backward versus forward pedaling. Hence, differences in sensory discharge associated with altered limb kinematics in forward and backward pedaling may trigger phase changes in neurologically intact biarticular muscles. There are extensive heteronymous Group I projections from proximal thigh muscles to motor neurons supplying other leg muscles (Baldissera et al. 1981), and hip position alters reflex excitability in distal muscles (Brooke et al. 1993; Knikou and Rymer 2002), further supporting the assertion that the relative position of the hip with respect to the knee affects muscle phasing.
In individuals post-stroke, integration of sensory input may be altered such that uniarticular muscles are more sensitive and biarticular muscles are less sensitive to altered sensory cues during backward versus forward pedaling. Evidence to suggest that sensory information is integrated differently in the post-stroke nervous system stems from observations that cutaneous (Zehr et al. 1998) and H-reflex (Garrett and Caulfield 2001) modulation during locomotion is abnormal post-stroke and tendon reflexes are elevated. However, it remains to be demonstrated whether uniarticular and biarticular muscles respond to sensory information differently post-stroke.
Differences in the way muscle phasing adapts to backward versus forward pedaling in paretic and neurologically intact limbs may be caused by altered cortical control of locomotor muscle activity. While cortical activity is not essential for initiating or maintaining the rhythmic alternating pattern of flexion and extension muscle activity that is characteristic of locomotion (Grillner 1981), the cortex is involved in adapting locomotion to environmental and motivational demands (Drew et al. 2002). When switching from forward to backward pedaling, the cortex may have a role in altering the forward locomotion muscle activation pattern to accomplish backward pedaling. Loss of cortical control of locomotion caused by stroke may account for unexpected phase changes in backward pedaling. Future studies will examine the hypothesis that abnormal sensory and cortical control of locomotion post-stroke contributes to inappropriate muscle phasing in forward and backward pedaling.
Limitations of experimental model
Although pedaling is a locomotor activity, there are differences between pedaling and walking that are important to consider when interpreting the present results. During the pedaling task used in this study, the head and trunk were supported on a rigid backboard that provided postural stability. This set-up allowed us to study in relative isolation the reciprocal pattern of flexion and extension movement that is characteristic of locomotion without confounding effects of posture. Although the interaction between posture and locomotion is important during walking (Massion 1992), studying locomotion in isolation can shed light on neural control mechanisms of post-stroke locomotion (Hesse and Werner 2003). Moreover, the similarities between post-stroke muscle phasing during pedaling and walking (Kautz and Brown 1998; Knutsson and Richards 1979), suggest that pedaling is an appropriate model.
Implications for rehabilitation
Although not related to our initial hypotheses, our data suggest that certain aspects of paretic VM phasing may be more appropriate in backward compared with forward pedaling. This observation may have important implications for rehabilitation. When paretic individuals pedaled backward, the likelihood of observing extraneous paretic VM activity during early knee flexion was nearly zero. Because extraneous VM activity during early limb flexion is strongly associated with reduced work performed during pedaling (Kautz and Brown 1998), the absence of paretic VM activity during early limb flexion in backward pedaling may indicate a more appropriate activation strategy. Cross-correlation analysis on the group mean data for backward pedaling in the paretic and neurologically intact groups indicated that the maximum between-group correlation (r = 0.99) occurred when paretic VM activity during backward pedaling was phase delayed by 45°. This suggests that the pattern of paretic VM phasing in backward pedaling is similar to neurologically intact VM phasing except that it is phase advanced. However, these observations should be interpreted cautiously. The present study did not assess the net mechanical work performed during the pedaling cycle, and we do not know whether more work was done by paretic limbs during backward compared with forward pedaling. Furthermore, despite improvements in VM phasing in backward pedaling, VM phasing during backward pedaling is not the same as neurologically intact VM phasing as indicated by the presence of extraneous VM activity during late limb flexion. Future studies will be directed at exploiting this potentially favorable VM phase shift to improve locomotor performance post-stroke.
Conclusion
This study demonstrates that the phasing of paretic muscle activity adapts differently than neurologically intact muscle when pedaling direction is reversed. This result was surprising given that paretic muscles have been shown to make appropriate scaling adjustments during pedaling. These observations suggest that the post-stroke nervous system does not reconfigure locomotor output the same way as the unimpaired nervous system. Differences between neurologically intact and paretic muscle phasing changes during backward pedaling may be associated with altered sensory signals and impaired cortical control of locomotion in the post-stroke nervous system. Further study will be required to determine whether paretic muscle phase changes are appropriate adaptations to backward pedaling, given that paretic muscle phasing is inappropriate during forward pedaling.
ACKNOWLEDGMENTS
The authors thank K. Barnes and O. Cockrell for help in data collection. We gratefully acknowledge S. Strauch for help with the illustrations and S. Brayden for help with the four-bar linkage model of pedaling.
GRANTS
This work was supported by National Institute of Child Health and Human Development/National Center for Medical Rehabilitation Research Grant 5 R01HD-39406-02 awarded to D. A. Brown. S. Schindler-Ivens was supported by postdoctoral fellowships from Grants 2T32HD-007418-10 and 1 F32 HD-044299-01.
REFERENCES
- Baldissera F, Hultborn H, Illert M. Integration of spinal neuronal systems. In: Greiger S, editor. Handbook of Physiology. The Nervous System. Motor Control. sect. 1. vol. II. Bethesda, MD: Am. Physiol. Soc.; 1981. pp. 509–596. [Google Scholar]
- Beneche R, Conrad B, Meinch H, Hohne J. Electromyographic analysis of bicycling on an ergometer for evaluation of spasticity of lower limbs in man. In: Desmedt J, editor. Motor Control Mechanisms in Health and Disease. New York: Raven; 1983. pp. 1035–1046. [PubMed] [Google Scholar]
- Brooke JD, Misiaszek JE, Cheng J. Locomotor-like rotation of either hip or knee inhibits soleus H reflexes in humans. Somatosens Mot Res. 1993;10:357–364. doi: 10.3109/08990229309028843. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Schindler-Ivens S, Brown DA. Quantifying changes in EMG patterns during pedaling post-stroke. Soc Neurosci Abstr. 2003;29:824.12. [Google Scholar]
- Brown DA, Kautz SA. Increased workload enhances force output during pedaling exercise in persons with poststroke hemiplegia. Stroke. 1998;29:598–606. doi: 10.1161/01.str.29.3.598. [DOI] [PubMed] [Google Scholar]
- Brown DA, Kautz SA. Speed-dependent reductions of force output in people with poststroke hemiparesis. Phys Ther. 1999;79:919–930. [PubMed] [Google Scholar]
- Brown DA, Kautz SA, Dairaghi CA. Muscle activity adapts to antigravity posture during pedalling in persons with post-stroke hemiplegia. Brain. 1997;120:825–837. doi: 10.1093/brain/120.5.825. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Brain Res Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Murrer L, Dietz V. Backward and forward walking use different patterns of phase-dependent modulation of cutaneous reflexes in humans. J Neurophysiol. 1996;76:301–310. doi: 10.1152/jn.1996.76.1.301. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Garrett M, Caulfield B. Increased H(max):M(max) ratio in community walkers poststroke without increase in ankle plantarflexion during walking. Arch Phys Med Rehabil. 2001;82:1066–1072. doi: 10.1053/apmr.2001.23880. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Cavanagh PR, Lafortune M. Knee flexor moments during propulsion in cycling-A creative solution to Lombard’s paradox. J Biomechan. 1985;18:307–316. doi: 10.1016/0021-9290(85)90286-6. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods and fish. In: Geiger S, editor. Handbook of Physiology. The Nervous System. Motor Control. sect. 1. vol. II. Bethesda, MD: Am. Physiol. Soc.; 1981. pp. 1179–1236. [Google Scholar]
- Hesse S, Werner C. Partial body weight supported treadmill training for gait recovery following stroke. Adv Neurol. 2003;92:423–428. [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Kautz SA, Brown DA. Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain. 1998;121:515–526. doi: 10.1093/brain/121.3.515. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Brown DA, Van der Loos HF, Zajac FE. Mutability of bifunctional thigh muscle activity in pedaling due to contralateral leg force generation. J Neurophysiol. 2002;88:1308–1317. doi: 10.1152/jn.2002.88.3.1308. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer Z. Effects of changes in hip joint angle on H-reflex excitability in humans. Exp Brain Res. 2002;143:149–159. doi: 10.1007/s00221-001-0978-4. [erratum appears in Exp Brain Res 144: 558, 2002]. [DOI] [PubMed] [Google Scholar]
- Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain. 1979;102:405–430. doi: 10.1093/brain/102.2.405. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Raasch CC, Zajac FE, Ma B, Levine WS. Muscle coordination of maximum-speed pedaling. J Biomechan. 1997;30:595–602. doi: 10.1016/s0021-9290(96)00188-1. [DOI] [PubMed] [Google Scholar]
- Raasch CC, Zajac FE. Locomotor strategy for pedaling: muscle groups and biomechanical functions. J Neurophysiol. 1999;82:515–525. doi: 10.1152/jn.1999.82.2.515. [DOI] [PubMed] [Google Scholar]
- Ryan MM, Gregor RJ. EMG profiles of lower extremity muscles during cycling at constant workload and cadence. J Electromyogr Kinesiol. 1992;2:69–80. doi: 10.1016/1050-6411(92)90018-E. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Brooke JD, Brown DA. Direction-dependent changes in locomotor muscle activity in individuals with post-stroke hemiparesis. Soc Neurosci Abstr. 2003;29:824.11. [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Zajac FE. Phase reversal of biomechanical functions and muscle activity in backward pedaling. J Neurophysiol. 1999;81:544–551. doi: 10.1152/jn.1999.81.2.544. [DOI] [PubMed] [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Zajac FE. Contralateral movement and extensor force generation alter flexion phase muscle coordination in pedaling. J Neurophysiol. 2000;83:3351–3365. doi: 10.1152/jn.2000.83.6.3351. [DOI] [PubMed] [Google Scholar]
- Van de Crommet H, Mulder T, Duysens J. Neural control of locomotion: sensory control of the central pattern generator and its relation to treadmill training. Gait Posture. 1998;7:251–263. doi: 10.1016/s0966-6362(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Swinnen SP, Desloovere K, Duysens J. Vibration-induced changes in EMG during human locomotion. J Neurophysiol. 2003;89:1299–1307. doi: 10.1152/jn.00863.2002. [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Hiebert GW, Pearson KG. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res. 1995;103:20–30. doi: 10.1007/BF00241961. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Fujita K, Stein RB. Reflexes from the superficial peroneal nerve during walking in stroke subjects. J Neurophysiol. 1998;79:848–858. doi: 10.1152/jn.1998.79.2.848. [DOI] [PubMed] [Google Scholar]