Abstract
Objective
Patients in the Carotid Revascularization Endarterectomy versus Stenting Trial had duplex ultrasound (DU) scans prior to treatment (PRE) and during follow-up (FU) to document the severity of carotid disease and the anatomic outcome of carotid endarterectomy (CEA) or carotid artery stenting (CAS). An ultrasound core laboratory (UCL) reviewed DU data from the clinical sites. This analysis was done to determine the agreement between site-reported and UCL-verified DU velocity measurements.
Methods
Clinical site DU worksheets, B-mode images, and Doppler velocity waveforms for the treated carotid arteries were reviewed at the UCL. The highest internal carotid artery peak systolic velocity (PSV) and associated Doppler angle were verified. If the angle was misaligned by >3 degrees, it was re-measured at the UCL and the PSV was recalculated. Agreement for PSV was defined as site-reported PSV within ±5% of UCL-verified PSV. Transcription errors were corrected by the UCL but were not considered as disagreements. FU analysis was limited to patients who received the assigned treatment.
Results
The UCL reviewed 1702 PRE and 1743 12-month FU DU scans (873 CEA, 870 CAS) from 111 clinical sites. Site-reported and UCL-verified PSV agreed in 1124 (66%) of the PRE scans and 1200 (69%) of the FU scans. In those cases with a disagreement, Doppler angle accounted for disagreement in 339 (59%) of the PRE scans and 277 (51%) of the FU scans. Based on a threshold PSV for ≥70% stenosis of ≥230 cm/s on the PRE scans and ≥300 cm/s on the FU scans, UCL review resulted in reclassification of stenosis severity in 75 (4.4%) of the PRE scans and 13 (0.75%) of the FU scans. There is evidence that the proportion of reclassification at FU was greater for CAS (10 scans, 1.2%) than for CEA (3 scans, 0.34%) (P=.057).
Conclusions
There was a high rate of agreement between site-reported and UCL-verified DU results in CREST, and UCL review was associated with a low rate of stenosis reclassification. However, angle alignment errors were quite common and prompted recalculation of velocity in 20% of PRE scans and 18% of FU scans. The use of a UCL provides a uniform process for DU interpretation and can identify sources of error and suggest technical improvements for future studies.
INTRODUCTION
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) was a prospective, randomized, multicenter trial designed to compare the efficacy of carotid artery stenting (CAS) versus carotid endarterectomy (CEA) in patients with symptomatic (≥50%) or asymptomatic (≥60%) extracranial carotid stenosis. The primary outcome endpoint was a composite of any stroke, myocardial infarction, or death during a 30-day periprocedural period, or ipsilateral stroke on follow-up of up to four years.1 Secondary outcomes included restenosis rates associated with CAS and CEA, differential efficacy of CAS and CEA according to symptomatic status, and health-related quality of life. A previously reported analysis found no significant difference between CAS and CEA in the primary endpoint.2 A separate analysis found similarly low rates of a composite endpoint of ≥70% carotid restenosis or occlusion within two years after CAS and CEA.3
Patients in CREST had duplex ultrasound (DU) scans to document the severity of extracranial carotid stenosis prior to randomization and at specified intervals following treatment by CAS or CEA. B-mode ultrasound images and Doppler velocity waveforms from CREST clinical sites were forwarded to the University of Washington Ultrasound Core Laboratory (UCL) for review and verification. The final carotid stenosis classification based on the UCL interpretation of the DU scans was used in the analyses of CREST data. Although it is common practice to utilize a core laboratory for clinical trials involving diagnostic imaging, the role of a core laboratory in this setting has not been investigated. The purpose of this study was to evaluate the agreement between the site-reported DU results and the final UCL-verified stenosis classification.
METHODS
Study Design and Patients
The CREST study design, eligibility criteria, and randomization procedures have been reported.1, 2, 4 The protocol was approved by the ethics committees of all study institutions and administrative sites. Written informed consent was obtained from each study patient. (ClinicalTrials.gov number, NCT00004732). Procedures for review of DU scans performed at CREST clinical sites by the UCL were approved by the Human Subjects Division at the University of Washington. Only the treated carotid sides of patients who underwent their assigned treatment within 30 days of randomization were included in this analysis. Although patients in CREST had DU scans at pre-treatment baseline and then at one, six, 12, and 24 months after treatment, the DU scans were included in this analysis if both site-reported and UCL-verified velocity data were available at the pre-treatment baseline and the 12-month follow-up interval.
Carotid Duplex Ultrasound
Because there are a variety of methods for performing and interpreting carotid DU scans, the University of Washington UCL developed a standardized examination protocol for use in CREST, which was distributed to the clinical sites. The ultrasound laboratories at CREST clinical sites were then certified for participation by submitting five acceptable carotid DU scans that were done in accordance with the protocol. The UCL protocol required 16 Doppler velocity waveform samples to be taken for each examination. These included eight samples from each side of the neck: three from the common carotid artery and three from the internal carotid artery taken at 1 to 2 cm intervals, one from the external carotid artery, and one from the vertebral artery.5 A constant 60 degree Doppler angle was recommended for obtaining all velocity waveforms; however, correctly aligned Doppler angles of less than 60 degrees were also acceptable. The UCL protocol defined Doppler angle as the angle between the ultrasound beam and the long axis of the artery with the Doppler angle cursor set parallel to the artery wall.
Ultrasound Review Process
B-mode ultrasound images and Doppler velocity waveforms were forwarded from the CREST clinical sites to the University of Washington UCL along with a worksheet that included the peak systolic velocity (PSV) from each waveform and the associated Doppler angle. At the UCL, the clinical site worksheet data was entered into a database, and a review form was printed which included the site-reported data. The UCL review and verification process consisted of two steps, the first performed by a sonographer “reader” and the second by a supervising “reviewer”. The ultrasound images and velocity waveforms were initially read by a vascular sonographer certified by the American Registry for Diagnostic Medical Sonography who confirmed the images, velocity values, and angle cursor alignments. The images and velocity waveforms were then reviewed by a senior sonographer or the UCL Director. Both reader and reviewer verified the selection, location, and labeling of each B-mode image and waveform pair, and the alignment of the Doppler angle cursor with the long axis of the artery on the B-mode image was confirmed. Two different methods were used in the UCL to measure the Doppler angle. In the early years of the study, images and waveforms were submitted by the clinical sites in hard copy form. In those cases, lines were drawn on the images aligned with the long axis of the artery and the Doppler ultrasound beam, and a handheld protractor was used to measure the angle. When electronic images became available, the images were pasted into PowerPoint and an electronic “screen protractor” (Iconico Inc.) with a resolution of 0.1° was used to make the measurement.5
If the UCL review process determined that the waveform sample used by the site was appropriate to determine the PSV, the UCL used that sample for verification. If another waveform sample was deemed more appropriate, then that waveform sample was used by the UCL. For the waveform sample selected by the UCL, if the angle was acceptable and correctly aligned, the PSV reported by the site was deemed verified and that value was used for both the site value and the UCL value. If the UCL review process determined that the Doppler angle was misaligned by >3 degrees, the angle was re-measured and the PSV was recalculated, as previously described.5 For velocity recalculations involving angles of ≥65 degrees, the cosine of 65 degrees was used to avoid very high (non-physiological) calculated velocity values. All other velocity recalculations used the actual cosine of the new re-measured angle. The equation for recalculation of the velocity was:
(New Velocity) = (Site Velocity) × [cos(Site Angle)/cos(New Angle)].
The ±3 degree range was based on a variability study performed in the University of Washington Vascular Laboratory in which the Doppler angles for 100 carotid DU images were measured independently by two sonographers. The 95% confidence limit on the difference between the two measurements was 3 degrees. Based on this experience, the ±3 degree range has been used in subsequent studies, and this was applied to the DU protocol used by the UCL for CREST. If there was disagreement between the reviewer and reader, the case was returned to the reader for re-evaluation. If the disagreement was not resolved, the case was sent to an adjudicator who rendered a final decision and then presented the case to the readers and reviewers to minimize future interpretation differences. Corrections and changes marked on the review form during the reader/reviewer process were entered into the database. The highest PSV value verified by the UCL was used to classify carotid stenosis at pre-treatment baseline and restenosis following CAS or CEA at the 12-month follow-up interval.3
Definitions of Agreement
The highest PSV reported by the CREST clinical site was compared to the highest PSV verified by the UCL for each baseline and 12-month follow-up DU scan. Agreement was defined as site-reported PSV within ±5% of the UCL-verified PSV. Most but not all of the PSV recalculations resulted in disagreements. Some transcription and decimal point errors were discovered during the review process that involved incorrect transfer of velocity data from the source B-mode images and Doppler velocity waveforms to the clinical site worksheets. These errors were corrected by the UCL prior to further review but were not considered as disagreements in this analysis.
Velocity Thresholds for Stenosis and Restenosis
For both the pre-treatment baseline and 12-month follow-up DU scans, PSV thresholds were selected to correspond to a ≥70% diameter-reducing lesion. A threshold of ≥230 cm/s is commonly used to identify ≥70% diameter-reducing stenosis in native extracranial carotid arteries, and this was applied for the baseline scans.6 Reported experience indicates that velocities in widely patent carotid stents are often higher than those in widely patent native carotid arteries, and use of velocity threshold criteria for stenosis in native carotid arteries could result in overestimation of restenosis severity in carotid stents.7–11 Based on these observations, a threshold of ≥300 cm/s was used to identify ≥70% diameter-reducing restenosis after CAS on the 12-month follow-up scans.3 Since no specific velocity thresholds have been established for carotid restenosis after endarterectomy, the threshold of ≥300 cm/s was also used in this analysis on the 12-month follow-up scans after CEA.
Statistical Analysis
Patient and clinical characteristics of those included and not included in the current study were performed using chi-square and t-tests. Comparisons of PSV measurements and angle measurements between CREST clinical sites and the UCL were conducted using chi-square tests.
RESULTS
The University of Washington UCL reviewed 1702 pre-treatment baseline and 1743 12-month follow-up DU scans from 111 CREST clinical sites. The baseline characteristics of the CREST pre-treatment patients are listed in Table 1. In addition to the 1702 pre-treatment baseline patients included in this analysis, there were 800 patients that did not have DU scans reviewed by the UCL. The only significant difference between these two baseline patient groups with respect to the characteristics listed in Table 1 was a higher prevalence of severe carotid stenosis among patients who had a DU scan reviewed by the UCL. Of the baseline patients included in this analysis, approximately one-third were female, just over half had symptomatic carotid artery disease, and carotid stenosis of ≥70% diameter-reduction was present in 90%. The follow-up scans included 873 CEA cases and 870 CAS cases. Prior to analysis, transcription and decimal point errors were corrected by the UCL in 30 baseline and 40 follow-up DU scans.
Table 1.
Baseline characteristics of the pre-treatment patients in CREST.
| Baseline Characteristics | Analysis of Agreement Between Clinical Sites and the Ultrasound Core Lab |
||
|---|---|---|---|
| Included N=1702 |
Not included N=800 |
Total N=2502 |
|
| Age (mean±SD) | 68.9±8.8 | 69.4±8.9 | 69.0±8.9 |
| Female | 34.4% | 35.9% | 34.9% |
| Symptomatic | 52.8% | 52.8% | 52.8% |
| Diabetes | 30.5% | 30.4% | 30.5% |
| Hypertension | 85.4% | 87.1% | 85.9% |
| Dyslipidemia | 84.3% | 84.5% | 84.4% |
| Smoke | 27.3% | 24.0% | 26.3% |
| History of CVD or CABG | 44.9% | 45.0% | 45.0% |
| Severe stenosis (≥70%) | 89.8% | 78.0%* | 86.0% |
P<.001
CVD = Cardiovascular Disease
CABG = Coronary Artery Bypass Graft
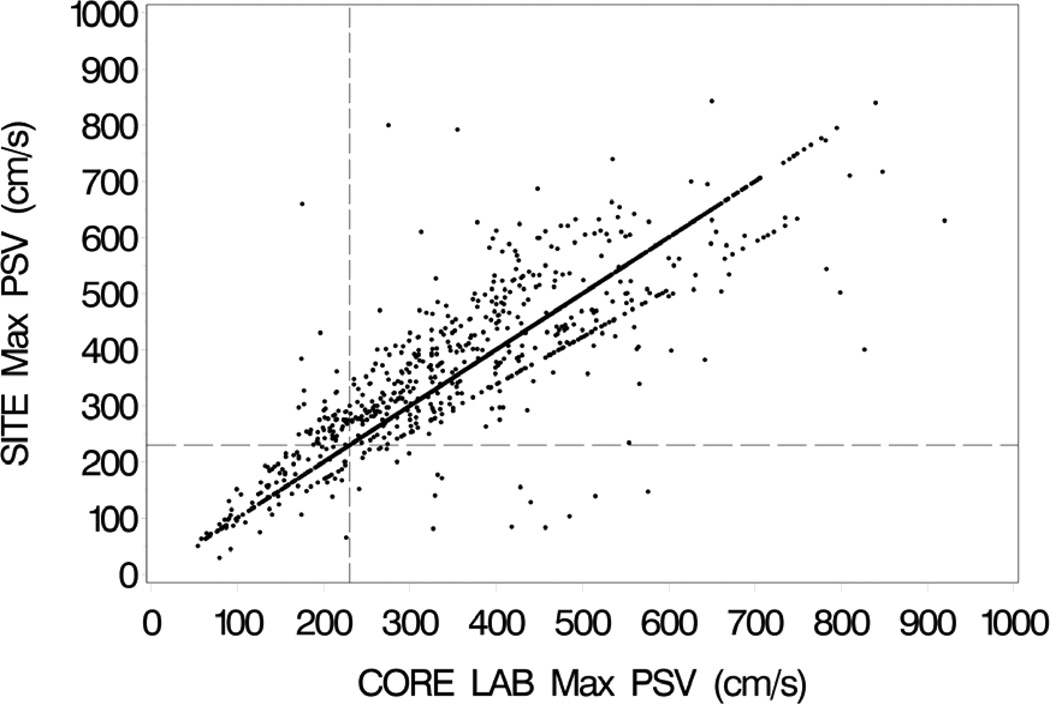
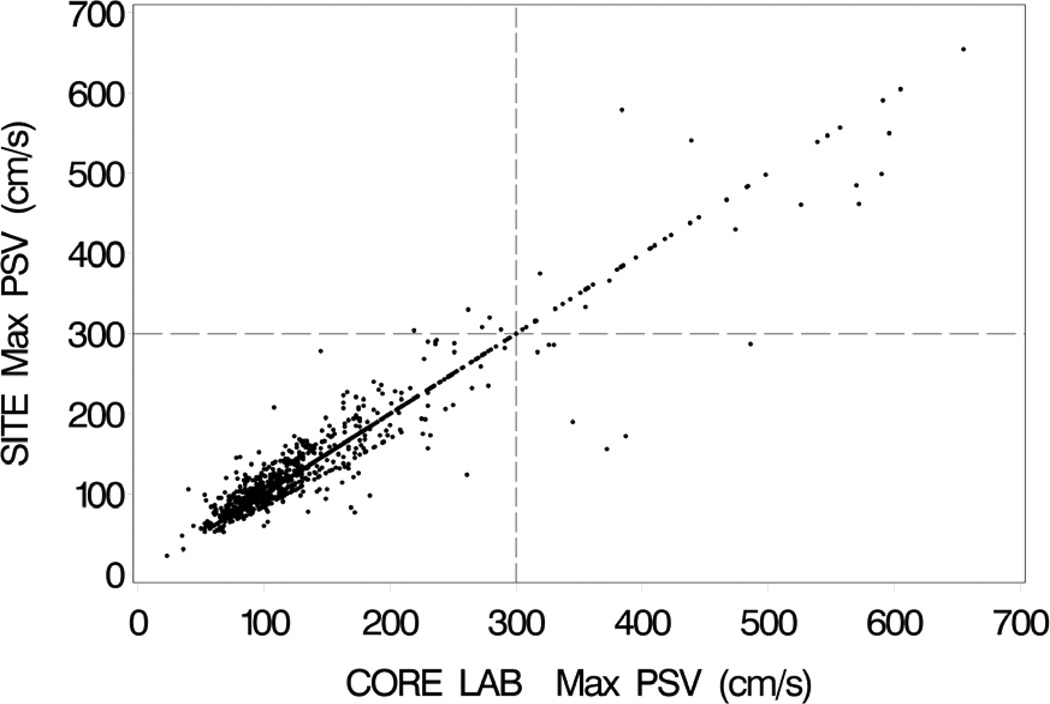
Site-reported and UCL-verified velocity measurements agreed in 1124 (66%) of the pretreatment scans and 1200 (69%) of the follow-up scans. There was not a significant difference in the rate of agreement for the CAS and CEA cases on the 12-month follow-up scans (P=0.61). Among those cases with a disagreement, Doppler angle accounted for disagreement in 339 (59%) of the pre-treatment and 277 (51%) of the follow-up scans. Angle errors prompted recalculation of velocity in 346 (20%) of the pre-treatment and 307 (18%) of the follow-up scans. Figure 1 shows the clinical site and UCL-verified PSV pairs for the pre-treatment baseline scans. The PSV pairs for the 12-month follow-up scans are shown in Figure 2, including both CAS and CEA cases.
Figure 1.
Clinical site (SITE) maximum peak systolic velocities (PSV) on the vertical axis vs. UCL-verified (CORE LAB) maximum peak systolic velocities on the horizontal axis for the pretreatment baseline duplex scans. Vertical and horizontal dashed lines mark the velocity threshold of 230 cm/s. The concentration of data points along the “line of unity” represents cases where the site PSV value was verified by the UCL and used as the UCL value (correct waveform sample and Doppler angle). The data points forming a line just below the line of unity is an artifact created by using the cosine of 65 degrees for all PSV recalculations involving angles of ≥65 degrees; this was done to avoid very high (non-physiological) calculated PSV values.
Figure 2.
Clinical site (SITE) maximum peak systolic velocities (PSV) on the vertical axis vs. UCL-verified (CORE LAB) maximum peak systolic velocities on the horizontal axis for the 12-month follow-up duplex scans. Both carotid stent and carotid endarterectomy cases are included. Vertical and horizontal dashed lines mark the velocity threshold of 300 cm/s. The concentration of data points along the “line of unity” represents exact agreements between the clinical sites and the UCL.
Based on a threshold PSV of ≥230 cm/s for ≥70% stenosis on the pre-treatment scans, UCL review resulted in reclassification of stenosis severity in 75 (4.4%) of the cases (Table 2). These included 20 scans that were reclassified as ≥70% stenosis and 55 scans that were reclassified as <70% stenosis. Using a threshold PSV of ≥300 cm/s for ≥70% stenosis on the follow-up scans, 13 (0.75%) of the cases were reclassified by the UCL; 8 scans were reclassified as ≥70% stenosis and 5 scans were reclassified as <70% stenosis. As shown in Table 3, the proportion of reclassifications at follow-up was greater for CAS (10 scans, 1.2%) than for CEA (3 scans, 0.34%) (P=.057). In Figures 1 and 2 the threshold velocity values for ≥70% stenosis are indicated by horizontal and vertical dashed lines, with reclassified cases falling in the left upper and right lower quadrants.
Table 2.
Agreement between CREST site-reported and UCL-verified peak systolic velocity (PSV) based on the threshold of 230 cm/s for ≥70% stenosis on the pre-treatment baseline duplex scans.
| UCL-Verified PSV | |||
|---|---|---|---|
| <230 cm/s | ≥230cm/s | ||
|
CREST Site-Reported PSV |
<230 cm/s | 204 | 20 |
| ≥230cm/s | 55 | 1423 | |
Table 3.
Agreement between CREST site-reported and UCL-verified peak systolic velocity (PSV) based on the threshold of 300 cm/s for ≥70% stenosis on the 12-month follow-up duplex scans.
| UCL-Verified PSV | ||||
|---|---|---|---|---|
| <300 cm/s | ≥300 cm/s | |||
|
CREST Site-Reported PSV |
Total | <300 cm/s | 1683 | 8 |
| ≥300 cm/s | 5 | 47 | ||
| CAS | <300 cm/s | 834 | 5 | |
| ≥300 cm/s | 5 | 26 | ||
| CEA | <300 cm/s | 849 | 3 | |
| ≥300 cm/s | 0 | 21 | ||
CAS = Carotid Artery Stent
CEA = Carotid Endarterectomy
DISCUSSION
The goals of carotid imaging for routine diagnosis are different from those for clinical trials. When DU is used in a clinical setting, the primary concern is the severity of carotid disease in an individual patient and the need for intervention. However, in a multicenter clinical trial, DU data from many patients at numerous clinical sites must be combined to support analyses of outcomes and risk factors. These research applications also often require serial measurements to document changes in disease severity over time. Although DU has been used for the evaluation of carotid artery disease for more than 30 years, specific details of testing protocols and interpretation criteria are known to vary from one laboratory to another.6,12,13 This variability could be a source of error in clinical trials when DU is used to identify endpoints and provide parameters for analysis. In multicenter clinical trials a core laboratory can be used to improve compliance with a standard protocol to reduce variability. This study examined the role of the UCL in CREST by determining the rate of agreement and sources of disagreement between the site-reported and UCL-verified DU results.
A comparison of the Doppler velocity measurements used to classify the severity of carotid stenosis or restenosis showed that the site-reported value was within ±5% of the UCL-verified value in almost 70% of the DU scans for both the native (pre-treatment) and follow-up (treated) carotid arteries. The role of the UCL started with the development of a standardized DU protocol designed to provide the data required for the clinical trial, distributing the protocol to the clinical sites, and certifying the sites by an initial review of DU scans performed in accordance with the protocol. The UCL corresponded with the clinical sites during the certification process to resolve protocol issues and provide feedback. After the trial was underway, the UCL was available to provide advice and support to the clinical sites as needed. Comments returned to the sites served to promote continued adherence to the protocol. These steps likely resulted in more uniform and consistent results than would have been obtained if the clinical sites had been allowed to apply their individual protocols. The strict two-step reader-reviewer process employed by the UCL verified adherence to the prescribed DU protocol and the method used for measurement of PSV. Disagreements between reader and reviewer were rare, and a third review by an adjudicator has been necessary in less than 3% of cases in the University of Washington UCL.5
Incorrect alignment of the Doppler angle cursor was the most common error identified and corrected in the UCL review process. The optimal angle for obtaining Doppler velocity measurements has been a topic of debate, with some authorities recommending a constant angle of 60 degrees and others accepting any angle of 60 degrees or less.6 The process of “angle correction” involves setting the Doppler angle cursor at the intended angle to the ultrasound beam and using the B-mode image to align the cursor parallel to the arterial wall (the long axis of the vessel). The instrumentation then uses the set angle and the Doppler equation to compute a velocity, assuming that the direction of flow is also parallel to the arterial wall. These angle corrected velocities increase with Doppler angle from zero to 90 degrees. If the Doppler angle is changed from 40 degrees to 60 degrees, the computed Doppler velocity increases by 42% or 2.1% per degree.5 This is a result of hemodynamic factors including helical flow and turbulence. The UCL protocol for CREST recommended a constant 60 degree Doppler angle for all velocity measurements; however, during the review process angles between 57 and 63 degrees were considered acceptable since it is unlikely that angle measurements could be reproduced with greater precision. Correctly measured and aligned Doppler angles of less than 57 degrees were also considered acceptable by the URC. Other sources of error in determination of PSV at the clinical sites that were identified by the UCL included: 1. improper placement of the velocity measurement cursor on the Doppler waveform; 2. not selecting the waveform with the highest PSV for classification of stenosis severity; 3. a waveform with a correctly aligned angle was identified by the UCL and used in place of the waveform selected by the clinical site which contained an incorrectly aligned angle, and 4. in the presence of an arrhythmia, an atypical waveform was selected by the clinical site but UCL review identified a series of waveforms with more normal morphology for assessment of PSV.
In spite of disagreement between the site-reported and UCL-verified velocities in more than 30% of the cases, when specific clinically relevant velocity thresholds were applied to classify carotid stenosis and restenosis, the UCL review process rarely resulted in reclassification of stenosis severity. This occurred in 4.4% of the pre-treatment baseline scans and 0.75% of the follow-up scans. For the follow-up scans, reclassification was required in 1.2% of the CAS scans and 0.34% of the CEA scans (P=.057). This suggests that DU follow-up after CAS may be more difficult or variable than after CEA. The DU scans performed at pre-treatment baseline and one follow-up interval were selected for this analysis to detect any differences in agreement between the clinical sites and UCL for native carotid arteries versus carotid arteries treated by CAS or CEA. The 12-month follow-up interval was selected to provide a large number of cases and still allow enough time for the effects of treatment to become apparent.
Velocity threshold criteria for ≥70% diameter-reducing stenosis in native carotid arteries and following treatment by CAS or CEA were selected for this analysis based on the clinical relevance of this stenosis severity.3,6 Specific DU velocity thresholds for classifying the severity of stenosis in native carotid arteries are well-established, and the value of ≥230 cm/s for ≥70% stenosis is commonly used.6,12,14 The CREST threshold of ≥300 cm/s for ≥70% stenosis following treatment has been applied in a previous report showing a restenosis rate of 5.8% after both CAS and CEA.3 The present study focused on the agreement between the site-reported and UCL-verified DU velocity measurements, and it did not address correlations between DU and other imaging methods or the management of recurrent stenosis following endarterectomy or stenting.
The role of the UCL in CREST involved all aspects of carotid DU scanning, and the results reported by the clinical sites most likely represent a “best case scenario” for overall consistency and accuracy. In routine clinical practice where there may be less emphasis on adherence to a strict protocol and interpretations are not independently reviewed and verified, more variability would be expected. However, the DU results that would have been reported by the clinical sites if a UCL had not been used in CREST cannot be inferred from this study. Given the rigorous data requirements and complexity of multicenter clinical trials, the use of a UCL is a means to insure consistency and reduce variability. In conclusion, there was a high rate of agreement between site-reported and UCL-verified DU results in CREST. Incorrect Doppler angle alignment was the most common source of disagreement. Based on commonly used velocity threshold criteria for ≥70% diameter-reducing carotid stenosis, the standardized protocol and strict review process provided by the UCL resulted in a low rate of stenosis or restenosis reclassification in CREST.
Acknowledgments
Grant Sponsorship: NIH – NINDS, U01 NS 038384
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Vascular Annual Meeting May 31 – June 1, 2013, San Francisco, CA.
REFERENCES
- 1.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, et al. Design of the Carotid Revascularization Endarterectomy vs. Stenting trial (CREST) Int J Stroke. 2010;5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WL, Malas MB, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomized controlled trial. Lancet. 2012;11:755–763. doi: 10.1016/S1474-4422(12)70159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lal BK, Brott TG. The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: lessons learned and anticipated results. J Vasc Surg. 2009;50:1224–1231. doi: 10.1016/j.jvs.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach KW, Bergelin RO, Leotta DF, Primozich JF, Sevareid PM, Stutzman ET, et al. Standardized ultrasound evaluation of carotid stenosis for clinical trials: University of Washington Ultrasound Reading Center. Cardiovasc Ult. 2010;8:39. doi: 10.1186/1476-7120-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: Gray-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 7.Lal BK, Hobson RW, II, Goldstein J, Chakhtoura EY, Duran WN. Carotid artery stenting: is there a need to revise ultrasound velocity criteria? J Vasc Surg. 2004;39:58–66. doi: 10.1016/j.jvs.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Lal BK, Hobson RW, II, Tofighi B, Kapadia I, Cuadra S, Jamil Z. Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg. 2008;47:63–73. doi: 10.1016/j.jvs.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Setacci C, Chisci E, Setacci F, Iacoponi F, de Donato G. Grading carotid intrastent restenosis. Stroke. 2008;39:1189–1196. doi: 10.1161/STROKEAHA.107.497487. [DOI] [PubMed] [Google Scholar]
- 10.AbuRahma AF, Abu-Halimah S, Bensenhaver J, Dean LS, Keiffer T, Emmett M, et al. Optimal carotid duplex velocity criteria for defining the severity of carotid in-stent restenosis. J Vasc Surg. 2008;48:589–594. doi: 10.1016/j.jvs.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Felkai DD, Evans M, McCoy SA, Lin PH, Kougias P, et al. Ultrasound criteria for severe in-stent restenosis following carotid artery stenting. J Vasc Surg. 2008;47:74–80. doi: 10.1016/j.jvs.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Moneta GL, Mitchell EL, Esmonde N, Rumwell C, Primozich JP. Extracranial carotid and vertebral arteries. In: Zierler RE, editor. Strandness’s duplex scanning in vascular disorders. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. pp. 87–100. [Google Scholar]
- 13.Beach KW, Leotta DF, Zierler RE. Carotid Doppler velocity measurements and anatomic stenosis: Correlation is futile. Vasc Endovasc Surg. 2012;46:466–474. doi: 10.1177/1538574412452159. [DOI] [PubMed] [Google Scholar]
- 14.Moneta GL, Edwards JM, Chitwood RW, Taylor LM, Lee RW, Cummings CA, et al. Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 99% stenosis with duplex scanning. J Vasc Surg. 1993;17:152–159. doi: 10.1067/mva.1993.42888. [DOI] [PubMed] [Google Scholar]