Abstract
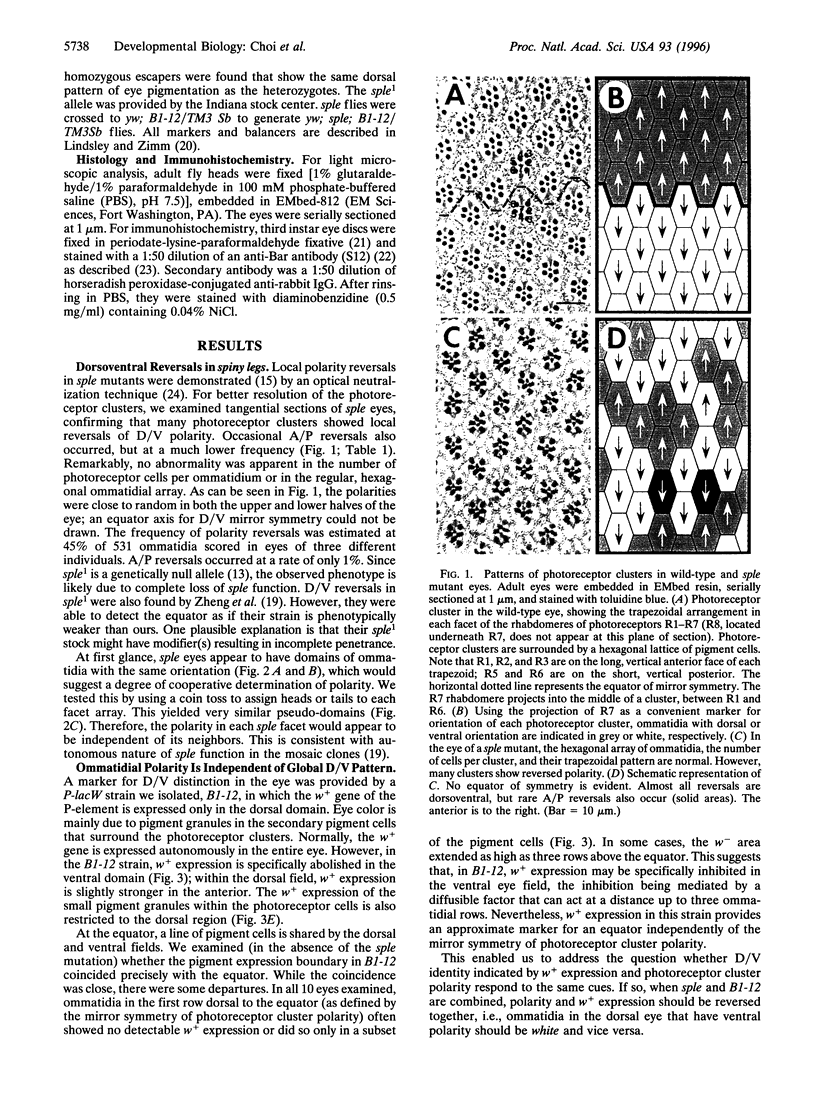
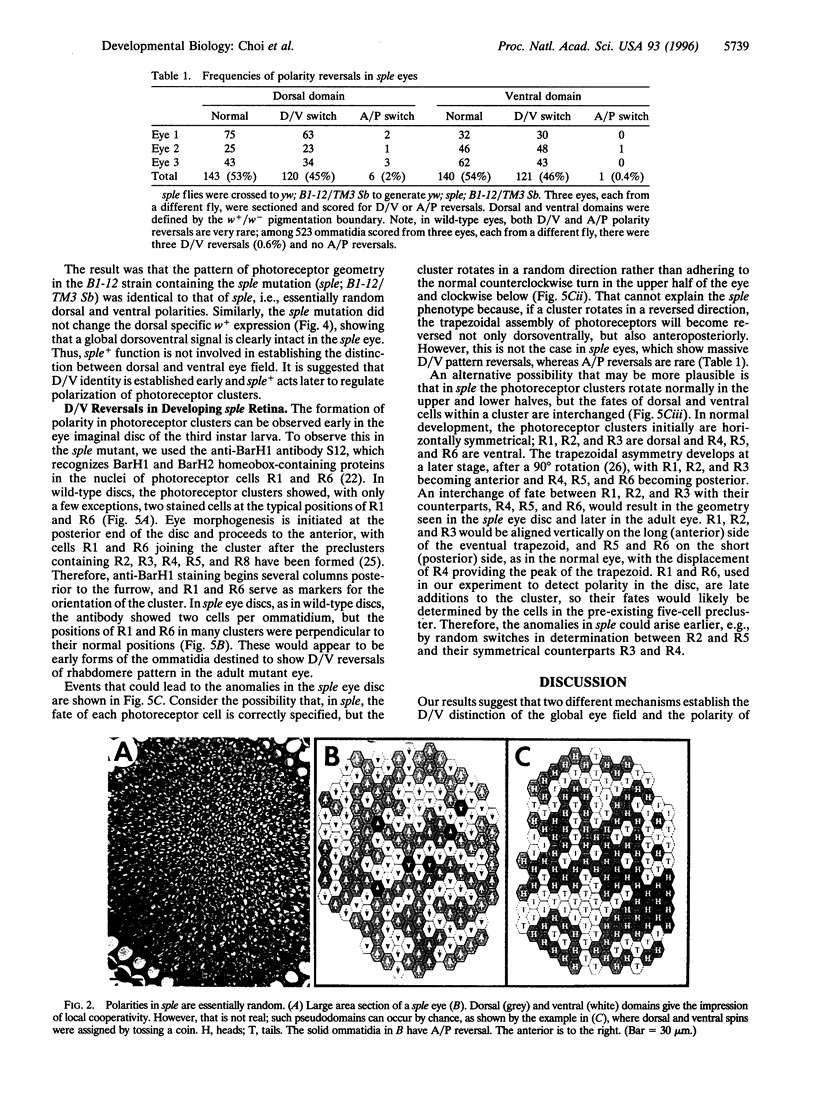
In each facet of the Drosophila compound eye, a cluster of photoreceptor cells assumes an asymmetric trapezoidal pattern. These clusters have opposite orientations above and below an equator, showing global dorsoventral mirror symmetry. However, in the mutant spiny legs, the polarization of each cluster appears to be random, so that no equator is evident. The apparent lack of an equator suggests that spiny legs+ may be involved in the establishment of global dorsoventral identity that might be essential for proper polarization of the photoreceptor clusters. Alternatively, a global dorsoventral pattern could be present, but spiny legs+ may be required for local polarization of individual clusters. Using an enhancer trap strain in which white+ gene expression is restricted to the dorsal field, we show that white+ expression in spiny legs correctly respects dorsoventral position even in facets with inappropriate polarizations; the dorsoventral boundary is indeed present, whereas the mechanism for polarization is perturbed. It is suggested that the boundary is established before the action of spiny legs+ by an independent mechanism.
Full text
PDF
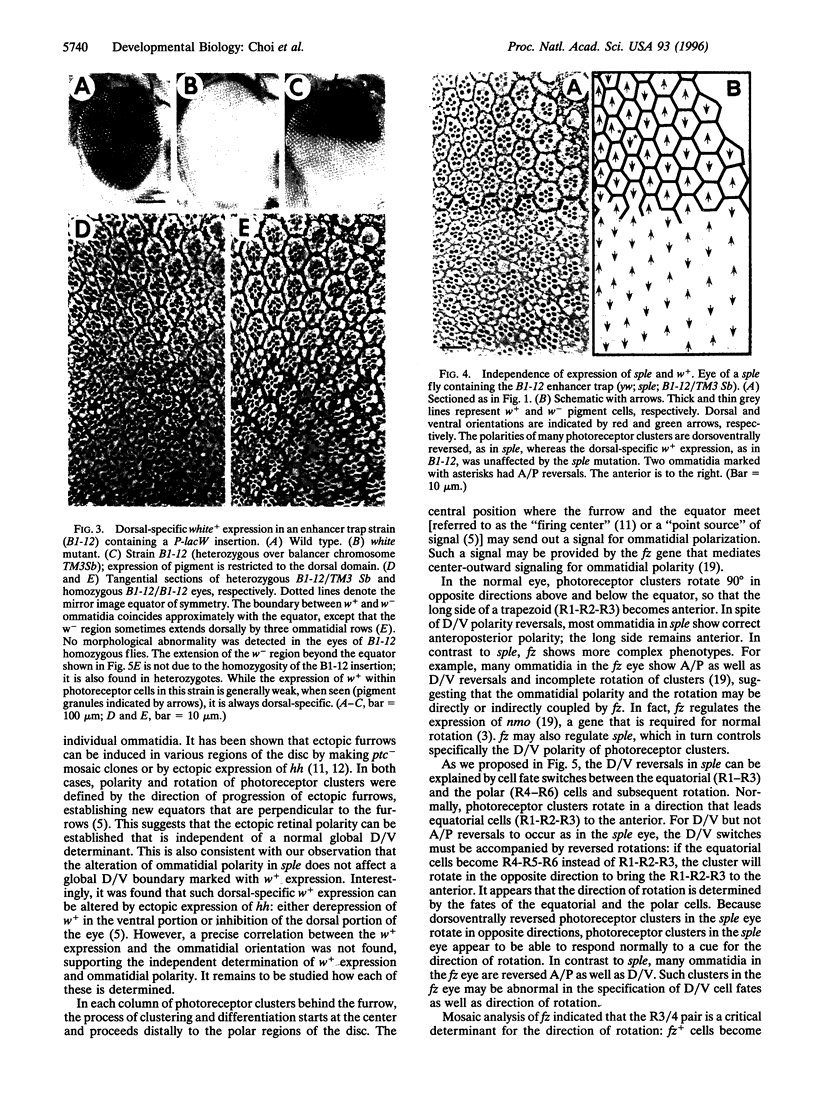

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler P. N. The genetic control of tissue polarity in Drosophila. Bioessays. 1992 Nov;14(11):735–741. doi: 10.1002/bies.950141103. [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Shepherd S., Lee K., McCall K., Barbel S., Ackerman L., Carretto R., Uemura T., Grell E. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989 Sep;3(9):1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- Chanut F., Heberlein U. Role of the morphogenetic furrow in establishing polarity in the Drosophila eye. Development. 1995 Dec;121(12):4085–4094. doi: 10.1242/dev.121.12.4085. [DOI] [PubMed] [Google Scholar]
- Choi K. W., Benzer S. Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron. 1994 Feb;12(2):423–431. doi: 10.1016/0896-6273(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Choi K. W., Benzer S. Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell. 1994 Jul 15;78(1):125–136. doi: 10.1016/0092-8674(94)90579-7. [DOI] [PubMed] [Google Scholar]
- Franceschini N., Kirschfeld K. Etude optique in vivo des éléments photorécepteurs dans l'oeil composé de Drosophila. Kybernetik. 1971 Jan;8(1):1–13. doi: 10.1007/BF00270828. [DOI] [PubMed] [Google Scholar]
- Gubb D., García-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982 Apr;68:37–57. [PubMed] [Google Scholar]
- Gubb D. Genes controlling cellular polarity in Drosophila. Dev Suppl. 1993:269–277. [PubMed] [Google Scholar]
- Heberlein U., Wolff T., Rubin G. M. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993 Dec 3;75(5):913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- Heitzler P., Coulson D., Saenz-Robles M. T., Ashburner M., Roote J., Simpson P., Gubb D. Genetic and cytogenetic analysis of the 43A-E region containing the segment polarity gene costa and the cellular polarity genes prickle and spiny-legs in Drosophila melanogaster. Genetics. 1993 Sep;135(1):105–115. doi: 10.1093/genetics/135.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S., Kojima T., Michiue T., Ishimaru S., Emori Y., Saigo K. Dual Bar homeo box genes of Drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes Dev. 1992 Jan;6(1):50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- Ma C., Moses K. Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995 Aug;121(8):2279–2289. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- Ma C., Zhou Y., Beachy P. A., Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993 Dec 3;75(5):927–938. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Pan D., Rubin G. M. cAMP-dependent protein kinase and hedgehog act antagonistically in regulating decapentaplegic transcription in Drosophila imaginal discs. Cell. 1995 Feb 24;80(4):543–552. doi: 10.1016/0092-8674(95)90508-1. [DOI] [PubMed] [Google Scholar]
- Park W. J., Liu J., Adler P. N. The frizzled gene of Drosophila encodes a membrane protein with an odd number of transmembrane domains. Mech Dev. 1994 Feb;45(2):127–137. doi: 10.1016/0925-4773(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976 Oct 15;53(2):217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Strutt D. I., Wiersdorff V., Mlodzik M. Regulation of furrow progression in the Drosophila eye by cAMP-dependent protein kinase A. Nature. 1995 Feb 23;373(6516):705–709. doi: 10.1038/373705a0. [DOI] [PubMed] [Google Scholar]
- Sun Y. H., Tsai C. J., Green M. M., Chao J. L., Yu C. T., Jaw T. J., Yeh J. Y., Bolshakov V. N. White as a reporter gene to detect transcriptional silencers specifying position-specific gene expression during Drosophila melanogaster eye development. Genetics. 1995 Nov;141(3):1075–1086. doi: 10.1093/genetics/141.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen H., Purcell J., Bennett M., Kansagara D., Syed A., Marsh J. L. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994 Feb;120(2):347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Ready D. F. Neuronal differentiation in Drosophila ommatidium. Dev Biol. 1987 Apr;120(2):366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Tomlinson A. The cellular dynamics of pattern formation in the eye of Drosophila. J Embryol Exp Morphol. 1985 Oct;89:313–331. [PubMed] [Google Scholar]
- Vinson C. R., Adler P. N. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987 Oct 8;329(6139):549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Conover S., Adler P. N. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989 Mar 16;338(6212):263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wehrli M., Tomlinson A. Epithelial planar polarity in the developing Drosophila eye. Development. 1995 Aug;121(8):2451–2459. doi: 10.1242/dev.121.8.2451. [DOI] [PubMed] [Google Scholar]
- Wolff T., Ready D. F. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991 Nov;113(3):841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhang J., Carthew R. W. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995 Sep;121(9):3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]