Abstract
Development of specific inhibitors of allergy has had limited success, in part, owing to a lack of experimental models that reflect the complexity of allergen-IgE interactions. We designed a heterotetravalent allergen (HtTA) system, which reflects epitope heterogeneity, polyclonal response and number of immunodominant epitopes observed in natural allergens, thereby providing a physiologically relevant experimental model to study mast cell degranulation. The HtTA design revealed the importance of weak-affinity epitopes in allergy, particularly when presented with high-affinity epitopes. The effect of selective inhibition of weak-affinity epitope-IgE interactions was investigated with heterobivalent inhibitors (HBIs) designed to simultaneously target the antigen- and nucleotide-binding sites on the IgE Fab. HBI demonstrated enhanced avidity for the target IgE and was a potent inhibitor of degranulation in vitro and in vivo. These results demonstrate that partial inhibition of allergen-IgE interactions was sufficient to prevent mast cell degranulation, thus establishing the therapeutic potential of the HBI design.
Type I hypersensitivity (allergy) is a disorder of the immune system that occurs when the adaptive immune system is directed against substances in the environment that would otherwise be harmless. Immediate hypersensitivity initiates when IgE antibodies bound to their high-affinity receptor (FcεRI) on mast cells are cross-linked by multivalent allergens, leading to mast cell degranulation1,2. Naturally occurring allergens are typically complex, structurally heterogeneous proteins, with multiple allergy-inducing epitopes. Accordingly, the IgE antibodies that are generated against these proteins are polyclonal in nature and bind their respective epitopes with a range of affinities3,4. Typical allergens have two to twelve epitopes that are recognized by polyclonal IgE antibodies5–8. Recent evidence suggests that among the identified epitopes on a given allergen, only one to five are immunodominant, meaning they are involved in the degranulation response observed in the majority of patients with that particular allergy7,9– 11. For example, the peanut allergen Ara h 3, the wheat allergen Tri a 14 and the melon allergen Cuc m 2 were each found to have four distinct epitopes triggering the allergic reaction6,12,13. Additionally, the melon allergen was found to have two high-binding and two low-binding IgE epitopes (Fig. 1a)4,14.
Figure 1. Design of the HtTA and the HBI for the selective inhibition of mast cell degranulation.
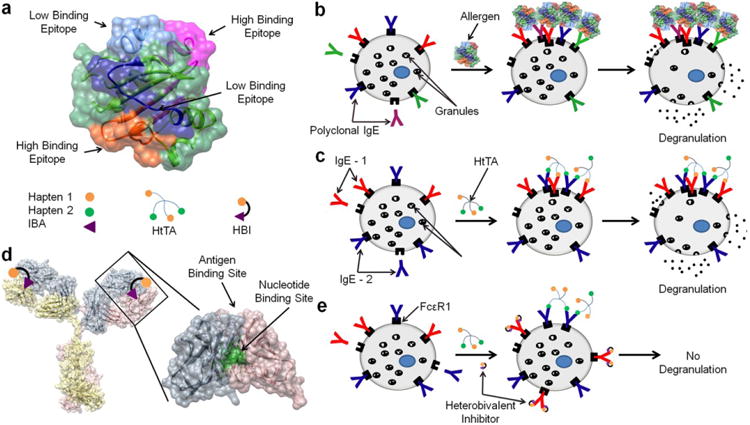
(a) Structure of the melon allergen Cuc m 2 with the four identified epitopes displaying both high and low affinity for IgE antibodies. A HtTA consisting of two distinct haptens each with a valency of 2 was synthesized to model the multiple different epitopes present on a natural allergen such as Cuc m 2. To mimic the high- and low-binding IgE epitopes, the first hapten was chosen to have a high affinity and the second hapten was chosen to have a low affinity for their respective IgEs. (b) In an allergic reaction, the allergen binds the polyclonal IgE antibodies present on the surface of mast cells. Multivalent allergen binding to surface-bound IgE antibodies cause aggregation of the IgE receptor, FcsRI, initiating a signaling cascade that results in mast cell degranulation. (c) The HtTA design mimics multiple distinct epitopes as well as the polyclonal IgE response present in natural allergic responses by using two IgE antibodies, IgE-1 and IgE-2, each with different hapten specifcity. (d) Crystal structure of an IgG antibody with enlarged Fab region to demonstrate the location of the nucleotide- and antigen-binding sites. The HBI was designed to simultaneously bind both the antigen- and the nucleotide–binding sites on an IgE. (e) HBI selectively inhibits the low-affinity hapten/IgE interactions, effectively lowering the valency of the allergen rendering it incapable of causing sufficient IgE cross-linking to stimulate a degranulation response.
Owing to the complexity of natural allergens, it has been a challenge to develop experimental models that mimic natural allergic responses. As a result, most allergy studies are performed using dinitrophenyl (DNP) with DNP-specific IgE (IgEDNP) as the hapten-antibody pair15–19. The DNP-IgEDNP system uses monoclonal IgEDNP to bind the IgE receptor, FcεRI, on mast cells. After the mast cells are primed with IgEDNP, cross-linking is induced with DNP-conjugated synthetic allergens typically synthesized using BSA, human serum albumin or ovalbumin as the scaffold. Despite the prevalence of this model, it has several shortcomings. First, DNP binds IgEDNP with an atypically high affinity, which is not representative of the broad range of affinities IgEs have for natural allergy epitopes10,13,20. Second, the hapten conjugation methods used are nonspecific, resulting in heterogeneous synthetic allergens containing 2 to 25 haptens, which is far more than the number of allergy-inducing epitopes found in natural allergens16–18. Furthermore, the heterogeneity of these synthetic allergens complicates interpretation of results as the precise number and orientation of the haptens are unknown, and some haptens may be unavailable to bind surface-bound IgE owing to steric constraints21,22. Finally, multiple presentations of the same hapten on a synthetic allergen do not accurately represent the multiple distinct epitopes on a natural allergen3,4. Although previous studies using the DNP-IgEDNP model have provided important insight into mast cell degranulation, they fall short in modeling the complexity of natural allergen-IgE interactions.
In this study, we developed a well-defined, multicomponent experimental system that enabled an integrative approach to study mast cell degranulation (Fig. 1b,c). Specifically, in our design, we incorporated (i) epitope heterogeneity, (ii) IgE antibody variability, (iii) range of epitope-IgE affinities and (iv) relevant epitope valency observed in natural allergens to better reflect the complexity of allergen-IgE interactions. This was accomplished through the design of a tetravalent scaffold that allowed four haptens to bind simultaneously to four different IgE antibodies. The flexibility of the design allowed for the simultaneous presentation of four identical haptens, homotetravalent allergens (HmTAs), or two sets of two haptens, HtTAs. In our design, we also controlled the affinities of the IgE-hapten interactions by using multiple IgE-hapten pairs and by chemically modifying the haptens to reflect the range of the epitope-IgE affinities present in physiological systems. The tetravalent allergen design provided a more realistic representation of natural allergens and allowed us to investigate the role of weak-affinity epitopes when presented alone or with high-affinity epitopes on a single allergen. Our results demonstrated that low-affinity epitopes have a major role in eliciting an allergic response when presented in combination with high-affinity epitopes.
Next, we evaluated the therapeutic potential of selective inhibition of weak-affinity epitope-IgE interactions by taking an engineering approach where we designed selective and targeted inhibitors (HBIs) of weak-affinity interactions by using the conserved nucleotide-binding site found on the Fab domain of IgE antibodies (Fig. 1d). Through a combination of molecular modeling and experimental approaches, we have extensively characterized this underused site and demonstrated that this binding site is conserved across all immunoglobulins17,23. In addition, our analysis demonstrated that the conformation of the four residues that make up this binding site, two tyrosine residues on the light chain and one tyrosine and one tryptophan on the heavy chain, are also highly conserved17,24,25. Previously, we described in detail the design, synthesis and characterization of HBIs that selectively inhibited allergen binding to IgE antibody, thereby inhibiting mast cell degranulation in a variant of the commonly used DNP-IgEDNP model17. HBIs were composed of a hapten molecule conjugated to a nucleotide-binding site ligand, which enabled simultaneous targeting of the antigen-binding site as well the nucleotide-binding site located on the Fab domain of all antibodies (Fig. 1d). Simultaneous bivalent binding to both of these sites provided the HBIs with enhanced avidity and selectivity for the target IgE and enabled competitive inhibition of allergen binding. In this study, we demonstrated that an HBI designed to selectively inhibit only the weak-affinity epitope-IgE interactions of a HtTA consisting of high- and low-affinity haptens was sufficient to prevent degranulation in both in vitro and in vivo allergy models (Fig. 1e). Taken together, the HtTA design provided an experimental tool to elucidate formerly unrevealed aspects of mast cell degranulation, and the HBI design provided us with a new antibody-targeting approach with therapeutic potential to selectively inhibit allergic responses.
Results
Design and characterization of tetravalent allergens
Previous methods of synthesizing allergens use nonspecific chemical methods to conjugate haptens to protein scaffolds, resulting in poorly defined allergens that complicate interpretation of results15–18,21,22. To address this problem, we synthesized well-defined and well-characterized tetravalent allergens with the criteria that each of the four haptens bound a different IgE. Through a combination of experimental approaches and molecular modeling, it has been demonstrated that the average distance between the two Fab domains of IgE is 11–13 nm and that, owing to the differences between the extended and in-solution length of ethylene glycol, a PEG3350 linker (extended length of 29 nm) is required to span the two antigen-binding sites on a single IgE26–28. Previously, we identified that ethylene glycol with an extended length of ∼6 nm is optimal for haptens to bind multiple antibodies without bridging the two antigen-binding sites on a single antibody29–33. Consequently, in our tetravalent allergen design, the four hapten moieties were conjugated to the core of the molecule with 8 units of ethylene glycol, which provided an extended length of 3.2 nm, yielding a maximum separation of 6.4 nm between haptens (Fig. 2a,b). The resulting separation distance between haptens was substantially shorter than the length required for bivalent binding to a single IgE, ensuring that the tetravalent allergen cross-linked the neighboring IgE molecules on mast cells rather than the two Fab arms of a single IgE28. Lysine residues were incorporated into the scaffold to provide a means of conjugating each moiety to the ethylene glycol linker as well as to provide a charge to increase the solubility of the synthetic allergens. The flexibility and solubility of the tetravalent scaffold ensured that each hapten was available to bind an IgE antibody, yet the length of the ethylene glycol linker made it sterically unfavorable for a single IgE to bind bivalently to a single tetravalent allergen.
Figure 2. Chemical structures of the haptens and tetravalent synthetic allergens.
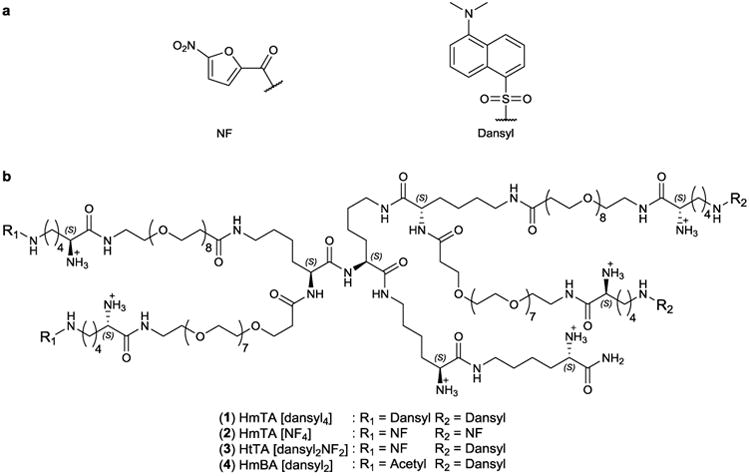
(a) Structures of the haptens NF and dansyl used to synthesize the allergens. (b) The structure of the tetravalent scaffold and the compositions of the HtTA, HmTAs and HmBA are shown.
The next step was the identification of haptens with a broad range of affinities for IgE antibodies to reflect the range of affinities found in natural allergy systems. To identify the high-affinity and low-affinity haptens, we determined the monovalent binding affinities of several hapten-IgE pairs using a previously described fluorescence quenching method17. Out of the screened candidates, dansyl-IgEdansyl was identified as a high-affinity pair with a monovalent Kddansyl = 54 ± 4 nM (Fig. 3a). To identify a hapten-IgE pair that could be used to represent the weak-affinity interactions, we modified the commonly used DNP-IgEDNP pair. Although DNP binds IgEDNP with high affinity, we established that nitrofuran (NF) binds IgEDNP with a monovalent KdNF of 41 ± 4 μM (Fig. 3a). The dansyl-IgEdansyl and NF-IgEDNP pairs provided a 760-fold difference in affinity, allowing us to model both the high- and low-affinity epitopes found in natural allergy systems. Notably, inclusion of two different IgE-hapten pairs in our design provided a closer approximation to physiological conditions, where multiple clones of IgEs are involved in mast cell degranulation (Fig. 1b,c). The HtTA design provided that a degranulation response will only occur if both hapten-specific IgEs are present on the mast cell surface; if only one of the respective IgEs is present, the HtTA will essentially behave as a bivalent ligand, which is insufficient for triggering degranulation34,35. As a first step in demonstrating that both hapten-IgE pairs are necessary for degranulation, we assessed any cross-reactivity between the two haptens; NF did not cross-react with IgEdansyl, and, similarly, dansyl did not cross-react with IgEDNP (Supplementary Results, Supplementary Fig. 1).
Figure 3. Characterization of hapten/IgE binding interactions.
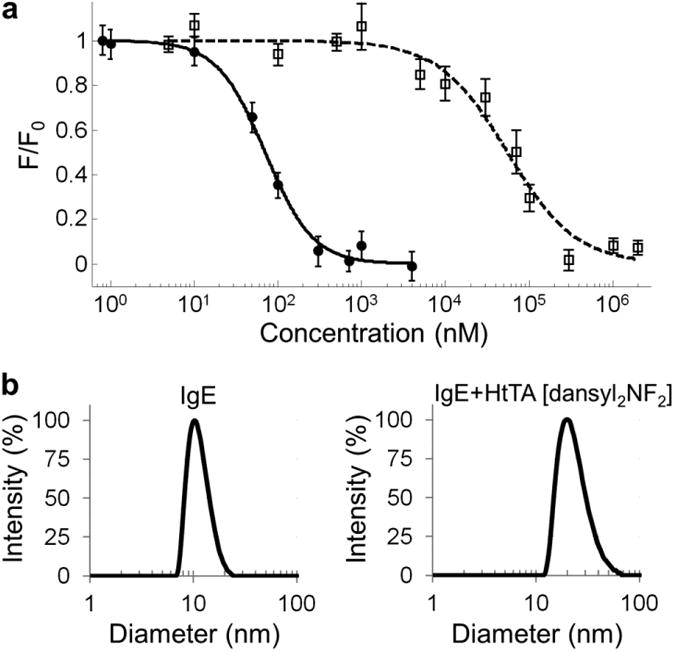
(a) Binding curves for monovalent dansyl (●) binding to IgEdansyl and monovalent NF (□) binding to IgEDNP. Binding was observed by monitoring the fluorescence quenching of the tryptophan residues in the IgE antibodies. Values for the binding constants are Kd,dansyl = 54 ± 4 nM and Kd,NF = 41 ± 4 μM. Control experiments with NF-IgEdansyl and dansyl-IgEDNP did not show any cross-reactivity in the hapten IgE pairs (Supplementary Fig. 1). Data represent the mean ± s.d. of triplicate experiments. (b) Dynamic light scattering data demonstrate the aggregation of IgEDNP (left) and IgEdansyl (right) in response to the addition of a stoichiometric concentration of HtTA. The size of the equimolar solution of IgEDNP and IgEdansyl was 10 nm and was increased to 20 nm upon the addition of HtTA [dansyl2NF2].
Next, we synthesized a series of tetravalent synthetic allergens using Fmoc chemistry on a solid support (Fig. 2b). Conjugation of dansyl to the tetravalent scaffold, with a valency of 4, provided us with a high-affinity HmTA (HmTA [dansyl4]; 1), whereas conjugation of NF to the tetravalent scaffold, with a valency of 4, provided us with a low-affinity HmTA (HmTA [NF4]; 2). Notably, conjugation of both dansyl and NF to the tetravalent scaffold, each with a valency of 2, provided us with HtTA [dansyl2NF2] (3) to elucidate the role weak-affinity epitopes have in mast cell degranulation when presented in combination with high-affinity epitopes on the same allergen. Homobivalent allergen dansyl (HmBA [dansyl2]; 4), which has the same structure as HtTA [dansyl2NF2] except for two acetylated arms instead of NF, was also synthesized for use as a control.
After synthesizing the tetravalent allergens, it was necessary to characterize the molecules to ensure that each hapten was available for simultaneous binding to a separate IgE antibody. This was accomplished by analyzing the complexes formed by a stoichiometric mixture of IgE antibodies to tetravalent allergens (2:1) using dynamic light scattering. The results of a representative experiment performed by mixing HtTA [dansyl2NF2] with 2 molar equivalents of IgE (50:50 mixture of IgEDNP and IgEdansyl) are shown in Figure 3b. Although IgE antibodies had an average hydrodynamic diameter of 10 nm (Fig. 3b), addition of HtTA [dansyl2NF2] to the antibody mixture rapidly resulted in the formation of complexes with a hydrodynamic diameter of 20 nm (Fig. 3b). Markedly, the signal at 10 nm completely disappeared, indicating that all of the monomeric IgEs were sequestered by the tetravalent allergen. Thermodynamic equilibrium was reached in less than 1 min, and complexes were stable over several hours. Similarly, HmTA [dansyl4] formed complexes with a hydrodynamic diameter of 20 nm with IgEdansyl and, as expected, did not form complexes with IgEDNP (Supplementary Fig. 2). The allergen HmTA [NF4] did not form complexes with IgEDNP owing to the low affinity of the NF-IgEDNP interaction (Supplementary Fig. 2). On the basis of these results and the design of the tetravalent scaffold, we concluded that the structure formed was a bicyclic tetramer consisting of four IgEs and two tetravalent allergens (IgE4HtTA2 or IgE4HmTA2). The only other possible structure with a hydrodynamic diameter of 20 nm with complete sequestration of IgE is two IgEs binding a single tetravalent allergen (IgE2HtTA or IgE2HmTA); however, this was eliminated as a possibility on the basis of the design of the tetravalent allergens. Efficient cross-linking of two Fabs on a single IgE requires a hapten separation distance of 11–13 nm, which is substantially longer than the maximum separation distance between haptens in our design28,33,36. Additionally, the binding of HtTA [dansyl2NF2] to IgEdansyl, IgEDNP and an equimolar solution of both IgEs was evaluated using the previously described fluorescence quenching technique17. There was a 3.3-fold increase in the affinity of HtTA [dansyl2NF2] for IgEDNP in the presence of IgEdansyl compared to IgEDNP alone, thus providing further evidence for the formation of a bicyclic tetramer consisting of four IgEs and two tetravalent allergens (Supplementary Fig. 3). The complexes were not stable for the ∼10 min required for size-exclusion chromatography analysis owing to the weak-affinity haptens. Combined, these results indicated that each hapten on the tetravalent allergen was simultaneously bound to a different IgE, demonstrating that binding of four IgE molecules to the tetravalent allergen was not sterically hindered.
Tetravalent allergens induce degranulation in vitro
Hapten-conjugated BSA is a poor model of natural allergens. However, BSA conjugates have been shown to be potent stimulators of allergic responses15–17. Therefore, we first used hapten-conjugated BSA to optimize our system and assess any cross-reactivity between the antibody-hapten pairs by using the well-established rat basophilic leukemia (RBL) mast cell degranulation model. RBL cells were primed with IgEDNP, IgEdansyl or an equimolar solution of both antibodies. The relative amounts of each IgE on the surface of the mast cells reflected the relative IgE ratios in solution, as determined using a fluorescein-DNP ligand (Supplementary Fig. 4). Our results with BSA allergens dansyl14-BSA and NF17-BSA (14 and 17 average haptens per BSA respectively) demonstrated that both allergens triggered degranulation when the RBL cells were primed with the hapten-specific IgE or the equimolar solution of both antibodies (Fig. 4a). The lack of a response by cells primed with IgEDNP using dansyl14-BSA as the allergen or cells primed with IgEdansyl using NF17-BSA as the allergen demonstrated the specificity of each hapten for the corresponding IgE. These results were consistent with the monovalent affinity assays and further demonstrated that there was no cross-reactivity between the hapten-IgE pairs. This result indicates that (i) both antibodies were able to bind the FcεRI receptors on the cell surface, (ii) the presence of either antibody did not inhibit the other from binding to its hapten and (iii) maximum degranulation does not require complete cross-linking of mast cell–bound IgE. These control experiments validated that RBL cells primed with both IgEDNP and IgEdansyl antibodies provide a suitable experimental system to test the tetravalent synthetic allergens.
Figure 4. Mast cell degranulation in response to tetravalent synthetic allergens.
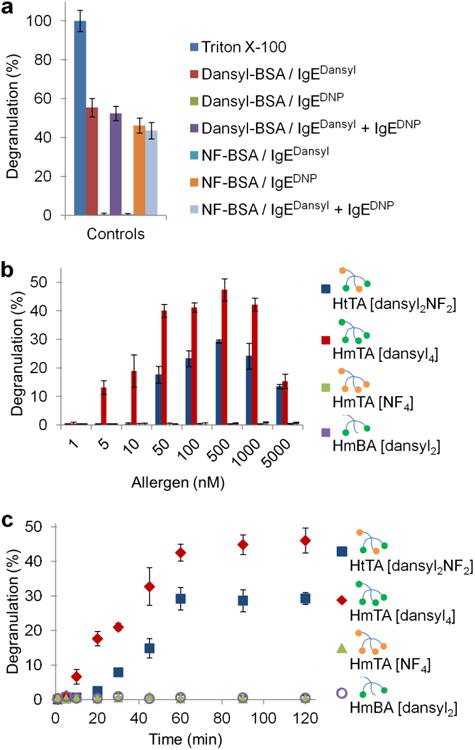
(a) RBL cells were primed with IgEDNP, IgEdansyl or an equimolar solution of both antibodies. Dansyl14-BSA and NF17-BSA both stimulated a response when the hapten-specific IgE was present on the surface of the mast cells, but did not stimulate a response if it was absent. (b) RBL cells were primed with an equimolar solution of IgEDNP and IgEdansyl and then exposed to increasing concentrations of the indicated synthetic allergen. (c) RBL cells were primed with an equimolar solution of IgEDNP and IgEdansyl followed by exposure to a constant allergen concentration for the indicated period of time. HtTA [dansyl2NF2] and HmTA [dansyl4] were used at 500 nM, whereas HmTA [NF4] and HmBA [dansyl2] were used at 10 μM. Triton X (1%) was used to determine the percent degranulation. Data represent the mean ± s.d. of triplicate experiments.
Next, we evaluated HmTA [dansyl4], HmTA [NF4] and HtTA [dansyl2NF2]. HmTA [dansyl4] proved to be the most potent allergen, stimulating a bell-shaped dose response from 5 nM to 5 μM with a maximum response at 500 nM (Fig. 4b). Conversely, HmTA [NF4] did not initiate a degranulation response. Markedly, HtTA [dansyl2NF2], which is composed of both dansyl and NF haptens, each with a valency of 2, stimulated a bell-shaped degranulation response from 50 nM to 5 μM, similar to HmTA [dansyl4], with a maximum response occurring at 500 nM. These results were in agreement with our previous work performed with homotetravalent allergens, where we determined that a hapten must have a Kd <235 nM to stimulate a response29. These results suggested a mechanism of activation where a single hapten binds a single IgE on the surface of a mast cell, and then the allergen–IgE complex diffuses laterally across the surface of the mast cell until a second IgE is encountered, and the hapten binds the second IgE, thus cross-linking the receptors. Accordingly, a HmTA with low-affinity haptens will not stay bound to an IgE long enough for complexes to form29,33. Control experiments were performed with HmBA [dansyl2], which did not stimulate a response under any condition. These results were consistent with previous literature reports that bivalent allergens are incapable of stimulating degranulation34,35. Combined, these results indicate that the response observed with HtTA [dansyl2NF2] was not due to the dansyl moieties alone (Fig. 4b). Additional control experiments were performed with increased allergen exposure time and increased allergen concentrations. HmTA [dansyl4] and HtTA [dansyl2NF2] reached their maximum degranulation potential at ∼60 min, whereas HmTA [NF4] and HmBA [dansyl2] did not induce degranulation even when the allergen exposure time was increased to 120 min (Fig. 4c). These observations did not change even when the HmTA [NF4] and HmBA [dansyl2] concentration was increased to 10 μM (Supplementary Fig. 5). Given that the individual components of HtTA (HmTA [NF4] and HmBA [dansyl2]) failed to elicit a degranulation response, these results illustrate the importance of low-affinity epitopes in mast cell degranulation, particularly when presented simultaneously with high-affinity epitopes on the same allergen.
HBI inhibits mast cell degranulation in vitro
Previously, we described HBIs that selectively inhibited allergen binding to IgE antibody, thereby inhibiting mast cell degranulation17. HBI (5) was composed of the NF hapten (Kd of 41 ± 4 μM for IgEDNP) conjugated to a nucleotide-binding site ligand, indole-3-butyric acid (IBA; Kd of 4.5 ± 0.6 μM for IgE), with an ethylene glycol linker (Fig. 5a). This design enabled simultaneous targeting of the antigen-binding site as well as of the adjacent nucleotide-binding site located in the Fab of antibodies (Fig. 1d). Simultaneous bivalent binding to both sites provided HBI with greater than 120-fold enhancement in avidity for IgEDNP compared to monovalent NF17. In this study, we investigated the potential of HBI to inhibit mast cell degranulation stimulated by HtTA [dansyl2NF2] by selectively and exclusively inhibiting the weak-affinity epitope interactions, specifically the NF-IgEDNP interactions. We predicted that HBI would partially inhibit the binding of HtTA [dansyl2NF2] to mast cell-bound IgE by blocking the NF-IgEDNP interaction and that this partial inhibition of allergen binding would effectively lower the valency of the allergen, decreasing its potential to stimulate a response. To test our hypothesis, RBL cells were primed with an equimolar solution of IgEDNP and IgEdansyl and then were exposed to HtTA [dansyl2NF2] with increasing concentrations of the HBI (Fig. 5b). HBI inhibited the degranulation response with a half-maximum effective concentration of 2 μM. The individual components of HBI, ethylene glycol-conjugated NF as a monovalent inhibitor (MI, 6; Fig. 5a) or IBA did not inhibit degranulation (Fig. 5c,d). These results demonstrated that both moieties, IBA and NF, were required for the enhanced avidity of HBI for IgEDNP that enabled the competitive inhibition of HtTA [dansyl2NF2] binding to IgEDNP. An additional experiment was performed using the weak-affinity allergen HmTA [NF4] as the inhibitor, and, as expected, HmTA [NF4] did not inhibit HtTA [dansyl2NF2] degranulation as the tetravalent allergen design only allows monovalent binding to a single IgE and therefore does not provide avidity enhancement for IgE (Supplementary Fig. 6). Next, to demonstrate the specificity of the HBI for the NF-IgEDNP interaction, HmTA [dansyl4] was used in place of HtTA [dansyl2NF2]. As NF does not bind IgEdansyl, HBI did not inhibit HmTA [dansyl4]–induced degranulation (Fig. 5e). Combined, these studies demonstrated that partial inhibition of HtTA [dansyl2NF2]–IgE binding by HBIs, which exclusively inhibit only the weak affinity NF-IgEDNP interactions, was sufficient to prevent mast cell degranulation.
Figure 5. Inhibition of mast cell degranulation via the HBI design.
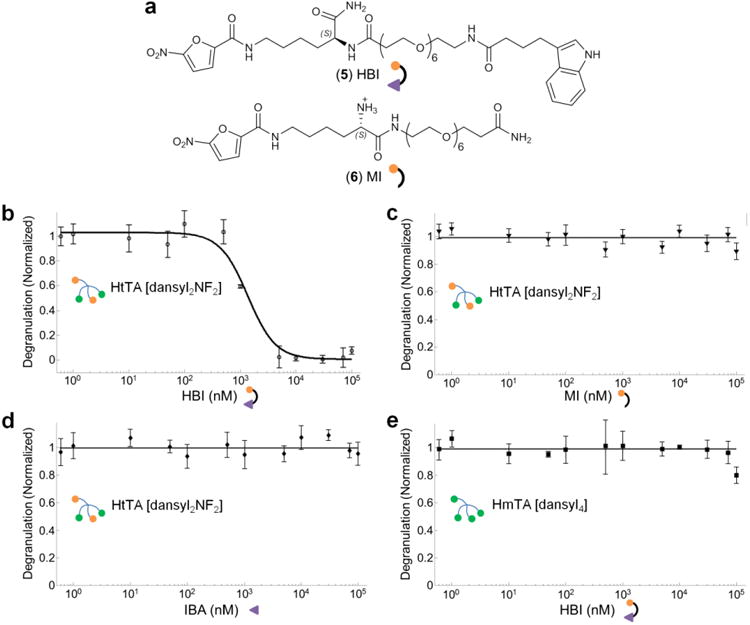
(a) Structures of the HBI and the control molecule MI are shown. HBI is composed of the NF hapten molecule conjugated to IBA with an ethylene glycol linker, whereas MI is composed of the ethylene glycol– conjugated NF hapten only. (b) The inhibitory potential of HBI was evaluated using mast cells primed with an equimolar solution of IgEDNP and IgEdansyl and stimulated with 500 nM of HtTA [dansyl2NF2]. HBI effectively inhibited mast cell degranulation with a half-maximum inhibitory concentration of 2 μM. (c,d) In control experiments performed with MI (c) or IBA (d), no inhibition of mast cell degranulation was observed. (e) In another control experiment, HmTA [dansyl4], was used in place of HtTA [dansyl2NF2] to stimulate degranulation, and HBI was unable to inhibit the response. Triton X (1%) was used to determine the percent degranulation. Data represent the mean ± s.d. of triplicate experiments.
HtTA induced degranulation in PCA mouse allergy model
The next step in validating the HtTA experimental system was to demonstrate the ability of HtTA to induce an allergic response in vivo. We selected the passive cutaneous anaphylaxis (PCA) mouse allergy model, a well-established model for studying anaphylaxis in vivo19,37. The HtTA design requires the presence of two IgE antibodies each specific for a different hapten to stimulate degranulation. Consequently, a mixture of 50 ng of IgEDNP and IgEdansyl (right ear) or PBS (left ear) was injected intradermally into C57BL/6 mice. Twenty-four hours later, 1.5 nmol of HtTA [dansyl2NF2] was injected intravenously, causing swelling in the ear that received the IgE injection. Then, by comparing the tissue thickness of the ear that received the IgE injection to the ear that received the PBS injection using both micrometer measurements and histology, the severity of the allergic reaction was quantified. The 1.5-nmol dose of HtTA [dansyl2NF2] corresponded to an initial blood concentration of ∼1,000 nM, slightly above the concentration that elicited the maximum degranulation response in vitro (assuming mouse blood volume of 1.5 mL). HtTA [dansyl2NF2] significantly increased tissue swelling 2 h after challenge when compared with the naive group (P<0.05, Fig. 6). Mice treated with DNP-BSA were used as a control and showed a similar increase in ear thickness (Supplementary Fig. 7). These results validated the utility of the HtTA design as an experimental model system that enables both in vitro and in vivo evaluation of mast cell activation.
Figure 6. Inhibition of PCA via the HBI in an in vivo mouse allergy model.
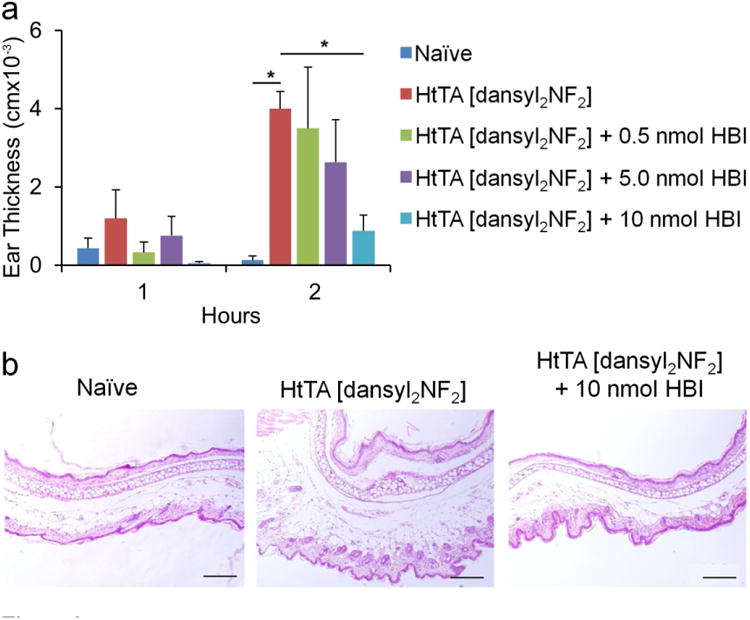
(a) Ear swelling during PCA was determined 1 h and 2 h after systemic HtTA [dansyl2NF2] challenge in mice receiving no HBI treatment or 0.5 nmol, 5 nmol or 10 nmol of HBI. The data was expressed as change in thickness compared with values before HtTA [dansyl2NF2] inoculation, measured with a micrometer caliper. Naive mice did not receive injection of IgE, HtTA [dansyl2NF2] or HBI. (b) Ear tissue was stained with hematoxylin and eosin stain at 2 h after systemic challenge. Representation of dermal thickness in naive (left), HtTA [dansyl2NF2]–challenged (middle) and HtTA [dansyl2NF2]–challenged (plus 10 nmol HBI) (right) mice with HBI are shown. Scale bar, 200 μm. Data represent the mean ± s.e.m. of five mice from two independent experiments. *P < 0.05.
HBI inhibits degranulation in PCA mouse allergy model
To evaluate the therapeutic potential of HBI, we next evaluated its inhibitory potential in the PCA mouse allergy model. C57BL/6 mice were injected intradermally with an equimolar mixture of IgEdansyl and IgEDNP (right ear) and PBS (left ear). Twenty-four hours later, mice were challenged intravenously with 1.5 nmol HtTA [dansyl2NF2] alone or in combination with HBI as indicated in Figure 6. The amounts of HBI, 0.5 nmol, 5 nmol and 10 nmol per mouse, were chosen on the basis of the in vitro inhibition data and correspond to initial blood concentrations of ∼0.3–7 μM (1.5-ml blood volume), approximating the range where inhibition was observed in vitro. HBI significantly inhibited ear swelling when compared to nontreated mice in a dose-dependent manner, reaching near-complete inhibition at 10 nmol (P<0.05, Fig. 6). This result indicated that HBI blocks the effects of HtTA [dansyl2NF2] on tissue inflammation and edema induced by mast cell degranulation. Additionally, these results provided further evidence that inhibition of all of the IgE epitopes on an allergen is not required to effectively inhibit the degranulation response. In fact, inhibition of only a few low-binding epitopes may be sufficient to prevent mast cell degranulation, thus highlighting the therapeutic potential of the HBI design.
Discussion
Previous studies have addressed the role of high-affinity epitopes in mast cell degranulation by using simplified allergen models; however, the importance of low-affinity epitopes have not been sufficiently or properly evaluated. In this study, we established a well-defined synthetic tetravalent allergen system that models the epitope heterogeneity and polyclonal response of natural allergy systems through the multivalent presentation of high- and low-affinity hapten molecules on the same scaffold. The bivalent presentation of each hapten on the HtTA required the presence of two distinct IgE antibodies to elicit a mast cell degranulation response. As such, the HtTA system better reflected the complexity of IgE-epitope interactions observed in natural allergy systems and allowed for the elucidation of the role weak-affinity epitopes have in mast cell degranulation. The tetravalency of the synthetic allergens provided a realistic approximation of the number of immunodominant epitopes present in several common allergens, and having precise control over the valency and structure of the scaffold enabled us to study the effect of affinity on mast cell degranulation without other confounding factors.
Using the HtTA allergen system, we demonstrated that low-affinity epitopes have an important role in mast cell degranulation when presented in combination with high-affinity epitopes on a multivalent allergen. The importance of low-affinity epitopes was demonstrated with the synthetic allergen HtTA [dansyl2NF2], which was a potent stimulator of mast cell degranulation, whereas a tetravalent version of NF, HmTA [NF4], and a bivalent version of dansyl, HmBA [dansyl2], did not elicit a response. These results challenge the previous theory that due to the extremely low IgE serum concentration, IgE must have a moderate to high affinity for the specific allergen to stimulate mast cell degranulation38. Combined, these experiments illustrate the importance of valency, affinity and cooperativity in allergen-IgE binding interactions in mast cell degranulation, thus highlighting the importance of weak-affinity epitopes in the induction of an allergic response.
To evaluate the therapeutic potential of selective inhibition of weak-affinity epitopes for the inhibition of an allergic response, we used a molecular engineering approach to design the HBI. The HBI was engineered to selectively inhibit only the weak-affinity hapten-IgE interactions by simultaneously targeting the antigen- and nucleotide-binding sites present on the Fab arm of antibodies. The simultaneous bivalent binding provided the HBI with 120-fold enhancement in affinity for IgEDNP compared to monovalent NF, making it a potent inhibitor of mast cell degranulation. Markedly, HBI was a potent inhibitor of HtTA-induced mast cell degranulation in both the in vitro RBL mast cell model and the in vivo PCA mouse model of allergy. Using the HBI design in conjunction with the HtTA allergy model, we demonstrated that inhibition of every epitope-IgE interaction on an allergen was not required for complete inhibition of mast cell degranulation. Rather, partial inhibition of allergen binding to the mast cell–bound IgEs to reduce the allergen valency was sufficient to inhibit the overall mast cell degranulation response. Combined, the results presented here demonstrate the utility of the conserved nucleotide-binding site for the inhibition of allergic reactions and established the HBI design as an effective inhibitor of mast cell degranulation. A major challenge in the current therapies used to treat allergic responses involves the nonspecific suppression of the immune system that puts patients at risk for infections and generates other side effects. Therefore more selective treatment options are needed. Given that most allergens have one to five immunodominant epitopes and that selective inhibition of only a few epitopes are sufficient to inhibit mast cell degranulation, short peptide sequences that mimic an IgE epitope can be used in the HBI design as potential therapeutics for the selective inhibition of food allergies, environmental allergies and asthmas.
Online Methods
Synthesis of the tetravalent and bivalent synthetic allergens
The synthetic allergens were synthesized using Fmoc solid phase synthesis as previously described33. Fmoc-protected residues were activated with HBTU or HATU in DMF with DIEA for 3 min, and coupling was monitored with Kaiser tests. Fmoc protecting groups were removed by three exposures to 20% piperidine in DMF for 3 min. The synthetic allergens were synthesized using multiple lysine derivatives to achieve branching while N-Fmoc-amido-dPEG8-acid was used to provide the EG8 linkers. The syntheses of the synthetic allergens were identical except for the conjugation of the different haptens. The synthetic strategy was as follows: Boc-Lys(Fmoc)-OH was conjugated to NovaPEG Rink Amide resin. After removal of the Fmoc group, a second Boc-Lys(Fmoc)-OH group was added to the molecule, and Fmoc was removed. Next, Fmoc-Lys(ivDde)-OH was conjugated to the molecule. After removal of the Fmoc group, Fmoc-Lys(Fmoc)-OH was conjugated to the ligand to facilitate branching. Both Fmoc groups were removed simultaneously, providing two primary amines for the conjugation of the ethylene glycol linkers. After removing the Fmoc groups on the ethylene glycol linkers, Boc-Lys(Fmoc)-OH was conjugated to each chain followed by the removal of the Fmoc groups. This provided two primary amines for the conjugation of the first set of haptens, NF, dansyl or acetyl. The next step was the removal of the orthogonal protecting group, ivDde, from the previously conjugated lysine residue using 2% hydrazine in DMF. The same branching process was repeated by sequentially conjugating Fmoc-Lys(Fmoc)-OH, N-Fmoc-amindo-dPEG8-acid and Boc-Lys(Fmoc)-OH to the molecule, thus providing two additional primary amines for the conjugation of the second set of haptens, dansyl or NF. Conjugation of the haptens was achieved using: dansyl chloride (5-(dimethylamino)naphthalene-1-sulfonyl chloride) for dansyl, 5-nitro-2-furoic acid for NF and acetic anhydride to create the acetyl group. Following synthesis, all of the molecules were cleaved from the resin by two exposures to 92:4:4 TFA/H2O/TIS for 30 min. The TFA solution was removed under vacuum, and the molecule was purified using RP-HPLC with a semipreparative Zorbax C18 column (9.4 mm × 250 mm) with a linear solvent gradient of 2.5% min–1 increments in acetonitrile at a 4.0 mL/min flow rate. The purified molecules were characterized with a Bruker micrOTOF II mass spectrometer. Purity was determined with the described HPLC system using a Zorbax C18 analytical column (4.6 mm × 150 mm). The purity of all of the synthesized ligands was estimated to be >97% (Supplementary Fig. 9). The calculated exact mass of HtTA [dansyl2NF2] (C164H283N27O57S2) was 3,606.9518 Da; found 3,607.9477 Da. The calculated exact mass of HmTA [dansyl4] (C178H303N27O53S4) was 3,795.0727 Da; found 3,796.0856 Da. The calculated exact mass of HmTA [NF4] (C150H263N27O61) was 3,418.8308 Da; found 3,419.84091 Da. The calculated exact mass of HmBA [dansyl2] (C158H285N25O51S2) was 3,412.9918 Da; found 3,414.0026 Da. Characterization matched literature values29,33.
Synthesis of HBI
The HBI was synthesized using Fmoc solid phase synthesis as previously described in detail17. The purity was estimated to be >97% (Supplementary Fig. 9) by an analytical injection using the previously described HPLC system and was characterized with a Bruker micrOTOF II mass spectrometer. The calculated exact mass of HBI (C38H56N6O13) was 804.3905 Da; found 805.3928 Da. Characterization matched literature values17.
Synthesis of BSA-conjugated allergens
NF was conjugated to BSA using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), and dansyl was conjugated to BSA using dansyl chloride as previously described17. BSA conjugates were purified using 0.5 mL 10-kDa molecular weight cut-off spin concentrators (Millipore), and purity was determined using RP-HPLC and found to be >95% (Supplementary Fig. 8)
Fluorescence quenching assay for determination of IgE-hapten binding affinities
The monovalent binding constants of NF and dansyl for IgEDNP and IgEdansyl were determined using the previously described fluorescence quenching assay17. Briefly, NF and dansyl quench the fluorescence from the IgE tryptophan residues, occurring at 335 nm, only when the two molecules are in proximity to each other (<10 nm). The monovalent haptens were titrated into a 96-well plate containing a 200-μL solution of 10 nM IgE in PBS. All of the experiments were repeated in at least triplicate.
IgE-HtTA complex formation
Dynamic light scattering experiments were performed with a Malvern Zetasizer Nano S. Monomeric IgE was evaluated at a concentration of 0.8 μM (0.4 μM IgEDNP and 0.4 μM IgEdansyl) in PBS. IgE complex formation was evaluated with an IgE concentration of 0.8 μM (0.4 μM IgEDNP and 0.4 μM IgEdansyl) with a stoichiometric amount of HtTA (0.4 μM) in PBS with an estimated refractive index of 1.45.
RBL degranulation assay
RBL cells and IgEDNP were kindly provided by Dr. B Wilson (University of New Mexico), and IgEdansyl (clone 27-74) was purchased from BD Biosciences. RBL cells were maintained as described previously16. For the degranulation assays, 100 μL of cells were plated at 0.5 × 106 cells/mL in a 96-well plate and were incubated for 24 h followed by a 2-h incubation with the IgE antibodies as indicated in Figures 4 and 5 at a total IgE concentration of 1 μg/mL. Cells were washed immediately before experiments and were stimulated with the concentration of allergen as indicated in Figures 4 and 5 for 60 min unless otherwise stated. When testing the allergy inhibitors, the inhibitor was added to cells 30 min before the allergen. Degranulation was detected spectroscopically by measuring the activity of the granule-stored enzyme β-hexosaminidase secreted into the supernatant on the substrate p-nitrophenyl-N-actyl-β-O-glucosamine. All of the degranulation assays were repeated in at least triplicate. In all of the experiments, the total IgE concentration was kept constant at 1 μg/mL.
Animals
C57BL/6 female mice (7–8 weeks) were obtained from Harlan Biosciences (Indianapolis, IN). Mice were maintained in pathogen-free conditions, and studies were approved by the Indiana University Institutional Animal Care and Use Committee.
PCA
PCA was performed as previously described19. Briefly, mice were injected intradermally with PBS (left ear) and an equimolar solution of IgEDNP and IgEdansyl (100 ng total IgE) (right ear). After 24 h, mice were injected intravenously with HtTA [dansyl2NF2] (1.5 nmol) and treated with the amount of HBI indicated in Figure 6. The thickness of ears was measured before HtTA [dansyl2NF2] and at 1 h and 2 h after challenge. The data were expressed as change in thickness compared with values before HtTA [dansyl2NF2] inoculation.
Statistical analysis
A one-way analysis of variance (ANOVA) for multiple comparisons was performed, and P < 0.05 was considered statistically significant as indicated in Figure 6.
Supplementary Material
Acknowledgments
We thank Dr. B Wilson (University of New Mexico) for generously providing us with IgEDNP and the RBL cells and P. Bryce (Northwestern University) for providing protocols and advice on the PCA model. We thank Dr. B Boggess at the Mass Spectrometry and Proteomics Facility in the University of Notre Dame for the use of MS instrumentation. This work was supported by the National Institutes of Health–National Institute of Allergy and Infectious Diseases (grant number R03 AI085485 (to B.B.) and R01 AI095282 (to M.H.K.)).
Footnotes
Author contributions: B.B. supervised and coordinated all of the research activities, designed the experiments and funded the study. M.W.H designed the experiments, synthesized and characterized all of the molecules, performed the in vitro experiments and analyzed all of the data. T.K. designed and supervised the in vitro assays. M.H.K. designed and supervised the in vivo studies. A.P.S. performed the in vivo experiments. M.W.H., T.K. and B.B. wrote the manuscript.
Competing financial interests: The authors declare no competing financial interests.
Additional information: Supplementary information and chemical compound information is available in the online version of the paper. Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Metzger H. Transmembrane signaling—the joy of aggregation. J Immunol. 1992;149:1477–1487. [PubMed] [Google Scholar]
- 2.Blank U, Rivera J. Assays for regulated exocytosis of mast cell granules. Curr Protoc Cell Biol. 2006:15.11–15.11.18. doi: 10.1002/0471143030.cb1511s32. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, et al. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J Allergy Clin Immunol. 2010;125:695–702. doi: 10.1016/j.jaci.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Torrejón G, et al. An experimental and modeling-based approach to locate IgE epitopes of plant profilin allergens. J Allergy Clin Immunol. 2007;119:1481–1488. doi: 10.1016/j.jaci.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Rougé P, et al. Mapping and conformational analysis of IgE-binding epitopic regions on the molecular surface of the major Ara h 3 legumin allergen of peanut (Arachis hypogaea) Mol Immunol. 2009;46:1067–1075. doi: 10.1016/j.molimm.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Pacios LF, et al. Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: peach Pru p 3 allergen as a model. Mol Immunol. 2008;45:2269–2276. doi: 10.1016/j.molimm.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Stanley JS, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997;342:244–253. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe S. Epitope peptides and immunotherapy. Curr Protein Pept Sci. 2007;8:109–118. doi: 10.2174/138920307779941569. [DOI] [PubMed] [Google Scholar]
- 10.Cerecedo I, et al. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J Allergy Clin Immunol. 2008;122:589–594. doi: 10.1016/j.jaci.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Cong Y, Lou F, Xue W, Li L, Chen M. Characterisation of the IgE-binding immunodominant epitopes on Ara h1. Food Agric Immunol. 2008;19:175–185. [Google Scholar]
- 12.Denery-Papini S, et al. Immunoglobulin-E-binding epitopes of wheat allergens in patients with food allergy to wheat and in mice experimentally sensitized to wheat proteins. Clin Exp Allergy. 2011;41:1478–1492. doi: 10.1111/j.1365-2222.2011.03808.x. [DOI] [PubMed] [Google Scholar]
- 13.Tordesillas L, et al. Characterization of IgE epitopes of Cuc m 2, the major melon allergen, and their role in cross-reactivity with pollen profilins. Clin Exp Allergy. 2010;40:174–181. doi: 10.1111/j.1365-2222.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- 14.Guex N, Peitsch M. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 15.Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- 16.Andrews NL, et al. Small, mobile FcεR1 receptor aggregates are signaling competent. Immunity. 2009;31:469–479. doi: 10.1016/j.immuni.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handlogten MW, Kiziltepe T, Moustakas DT, Bilgicer B. Design of a heterobivalent ligand to inhibit IgE clustering on mast cells. Chem Biol. 2011;18:1179–1188. doi: 10.1016/j.chembiol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Collins AM, Basil M, Nguyen K, Thelian D. Rat basophil leukaemia (RBL) cells sensitized with low affinity IgE respond to high valency antigen. Clin Exp Allergy. 1996;26:964–970. [PubMed] [Google Scholar]
- 19.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS ONE. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James LC, Tawfik DS. The specificity of cross-reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Sci. 2003;12:2183–2193. doi: 10.1110/ps.03172703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hlavacek WS, Posner RG, Perelson AS. Steric effects on multivalent ligand-receptor binding: exclusion of ligand sites by bound cell surface receptors. Biophys J. 1999;76:3031–3043. doi: 10.1016/S0006-3495(99)77456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu K, Goldstein B, Holowka D, Baird B. Kinetics of multivalent antigen DNP-BSA binding to IgE-FcεRI in relationship to the stimulated tyrosine phosphorylation of FcsRI. J Immunol. 1998;160:3225–3235. [PubMed] [Google Scholar]
- 23.Rajagopalan K, et al. Novel unconventional binding site in the variable region of immunoglobulins. Proc Natl Acad Sci USA. 1996;93:6019–6024. doi: 10.1073/pnas.93.12.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves NJ, et al. Selective photocrosslinking of functional ligands to antibodies via the conserved nucleotide binding site. Biomaterials. 2013;34:5700–5710. doi: 10.1016/j.biomaterials.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 25.Alves NJ, et al. A small molecule based affinity chromatography method for antibody purification via nucleotide binding site targeting. Anal Chem. 2012;84:7721–7728. doi: 10.1021/ac300952r. [DOI] [PubMed] [Google Scholar]
- 26.Schweitzer-Stenner R, Licht A, Luscher I, Pecht I. Oligomerization and ring-closure of immunoglobulin-E class antibodies by divalent haptens. Biochemistry. 1987;26:3602–3612. doi: 10.1021/bi00386a053. [DOI] [PubMed] [Google Scholar]
- 27.Hunt J, et al. A fluorescent biosensor reveals conformational changes in human immunoglobulin E Fc implications for mechanisms of receptor binding, inhibition, and allergen recognition. J Biol Chem. 2012;287:17459–17470. doi: 10.1074/jbc.M111.331967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baird EJ, Holowka D, Coates GW, Baird B. Highly effective polyethylene glycol) architectures for specific inhibition of immune receptor activation. Biochemistry. 2003;42:12739–12748. doi: 10.1021/bi034884l. [DOI] [PubMed] [Google Scholar]
- 29.Handlogten MW, Kiziltepe T, Alves NJ, Bilgicer B. Synthetic allergen design reveals the significance of moderate affinity epitopes in mast cell degranulation. ACS Chem Biol. 2012;7:1796–1801. doi: 10.1021/cb300193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefanick JF, Kiziltepe T, Handlogten MW, Alves NJ, Bilgicer B. Enhancement of antibody selectivity via bicyclic complex formation. J Phys Chem Lett. 2012;3:598–602. [Google Scholar]
- 31.Bilgiçer B, Moustakas DT, Whitesides GM. A synthetic trivalent hapten that aggregates anti-2,4-DNP IgG into bicyclic trimers. J Am Chem Soc. 2007;129:3722–3728. doi: 10.1021/ja067159h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilgiçer B, et al. A non-chromatographic method for the purification of a bivalently active monoclonal IgG antibody from biological fluids. J Am Chem Soc. 2009;131:9361–9367. doi: 10.1021/ja9023836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handlogten MW, Kiziltepe T, Bilgicer B. Design of a heterotetravalent synthetic allergen that reflects epitope heterogeneity and IgE antibody variability to study mast cell degranulation. Biochem J. 2013;449:91–99. doi: 10.1042/BJ20121088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sil D, Lee JB, Luo D, Holowka D, Baird B. Trivalent ligands with rigid DNA spacers reveal structural requirements for IgE receptor signaling in RBL mast cells. ACS Chem Biol. 2007;2:674–684. doi: 10.1021/cb7001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Posner RG, et al. Trivalent antigens for degranulation of mast cells. Org Lett. 2007;9:3551–3554. doi: 10.1021/ol071175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baird B, Zheng Y, Holowka D. Structural mapping of IgE-Fc-ε-RI, an immunoreceptor complex. Acc Chem Res. 1993;26:428–434. [Google Scholar]
- 37.Dinneswara Reddy G, Park S, Cho HM, Kim T, Lee ME. Antiallergic activity profile in vitro RBL-2H3 and in vivo passive cutaneous anaphylaxis mouse model of new sila-substituted 1,3,4-oxadiazoles. J Med Chem. 2012;55:6438–6444. doi: 10.1021/jm300421h. [DOI] [PubMed] [Google Scholar]
- 38.Lund G, et al. Antibody repertoire complexity and effector cell biology determined by assays for IgE-mediated basophil and T-cell activation. J Immunol Methods. 2012;383:4–20. doi: 10.1016/j.jim.2012.05.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


