Abstract
Rap1 signaling is important for both major processes of vessel formation: vasculogenesis, or de novo vessel formation, and angiogenesis, sprouting of new vessels from pre-existing ones. We provide an overview of genetic studies in mice and zebrafish and discuss some of the proposed underlying mechanisms derived from cellular models, with particular emphasis on Rap1's role in angiogenesis, maintenance of endothelial barrier and connection with cerebral cavernous malformation (CCM), a neurological deficit that leads to seizures and lethal stroke. Lastly, we provide a brief summary of studies in cardiac and smooth muscle cells, where the Epac-Rap1 signaling axis is emerging as an important regulator of contractility.
Genetic models
Rap1 proteins are highly evolutionarily conserved, with 97% and 99% identity between zebrafish, mouse and human proteins. Genetic models of Rap1 implicate its involvement in multiple functions during development [1]. The role of two Rap1 proteins, Rap1A and Rap1B in vascular development has been studied in mouse and zebrafish models. Because signaling pathways governing vessel development are preserved across species, these models are likely to provide insight into Rap1 function in human vascular development and vascular dysfunction in disease.
Vasculogenesis
Both Rap1 isoforms are ubiquitously expressed, with Rap1B the predominant isoform in endothelial cells (ECs) [2]. Deletion of either Rap1 isoform leads to some degree of embryonic lethality, although adult homozygous animals can be obtained with low efficiency (Table) [3, 4]. While no lethality or hematopoietic or homing defects were described in Rap1A−/− mice on a mixed genetic background [5], deletion of Rap1A in mice in a C57Bl6 background leads to a partial embryonic lethal phenotype that includes bleeding and edema; however, specific vascular defects have not been described [6]. Rap1B−/− mice develop normally until embryonic day (E)12.5, following which up to 50% of embryos succumb to interspersed hemorrhage and hemorrhage on the side of the head [3] (and M.C.-W., unpublished data). Rap1A and Rap1B double knockout mice die due to major malformation between E8.5 and E10.5, consistent with Rap1's major role in promoting adhesion (M.C.-W. unpublished data). Interestingly, endothelial-lineage restricted knockouts of either Rap1B and Rap1A do not exhibit embryonic lethal phenotype observed in total knockouts of each isoform [7]. Deletion of both isoforms leads to lethality due to hemorrhage at various times between E10.5 and E13.5, with 50% of the embryos having normal vasculature at E10.5 (M.C.-W. and Kevin Whitehead, University of Utah, unpublished data). It is not clear if death is due to a primary vascular problem or if it is secondary to a hemodynamic or physiologic abnormality. Interestingly, Tie2-Cre mediated deletion of all but one Rap1A allele phenocopies the embryonic pathology observed in total Rap1B−/− mice (M.C.-W., unpublished data). These observations suggest Rap1 in endothelium is not absolutely required for early vasculogenesis and that there is redundancy of functions between two Rap1 isoforms. However, at least one Rap1 allele is required for normal vascular development and function. Since Tie2 promoter is active in hematopoietic and ECs [8], the function of Rap1 in both these cellular compartments has to be considered as potentially critical for development.
Table.
Phenotypes of total and tissue-specific murine knockouts of Rap1 isoforms, Rap1-GEFs and Rap1 effector proteins in the cardiovascular system.
| Mouse genotype | Mouse phenotype |
|---|---|
| Rap1b−/− | Partial embryonic lethality with bleeding and edema after E12.5 [3]; impaired: angiogenesis [7, 26], platelet function [3], B-cell development and homing to lymph nodes [30, 31] in surviving adults; otherwise normal life span. |
| Rap1a−/−;(on C57Bl6 background) | Partial embryonic lethality with bleeding and edema [4], defect in FGF-mediated angiogenesis [6], normal T-cell and B-cell development but altered myeloid function [4]. |
| Rap1a−/− (on mixed genetic background) | Normal life span, impaired T-cell and B-cell adhesion [5]. |
| Rap1a−/−Rap1b−/−; | Embryonic lethality due to major malformation between E8.5-E10.5 (M.C.-W. unpublished data) |
| Tie2-Cre+/0;Rap1bf/f | Impaired angiogenesis, otherwise normal lifespan [7]. |
| Tie2-Cre+/0; Rap1af/+Rap1bf/f | Partial embryonic lethality with bleeding after E12.5; defect in angiogenesis [7]. |
| Tie2-Cre+/0; Rap1af/fRap1bf/f | Embryonic lethality due to hemorrhage between E10.5-E13.5 (M.C.-W. and Kevin Whitehead, unpublished data). |
| PDZ-GEF-1−/− | Embryonic lethality after E8.5-E9.5, likely due to failure in yolk sac primary vascular plexus formation [16] |
| C3G−/− | Embryonic lethality before E7.5; defective adhesion and spreading of embryonic fibroblasts [23]. |
| C3Ggt/gt | Embryonic lethality around E11.5 due to hemorrhage and vascular integrity defects; defective pericyte development [24]. |
| Afadin−/− | Embryonic lethality after E9.5 due to developmental defects and loss of structures derived from ectoderm and mesoderm [93]. |
| Tie2-Cre+/0; Afadinf/f | Impaired postnatal angiogenesis in response to VEGF and S-1-P; reduced postnatal viability suggestive of other vascular defects [34] |
| Ccm1tm1Dmar/tm1Dmar(Ccm1−/−) | Embryonic lethality at mid-gestation; dilatation of brain vessels and vascular defects in branchial arches [48] |
| Ccm1tm1Dmar/+(Ccm1+/−) | Normal cardiovascular phenotype [48]. |
| Ccm1tm1Dmar/+(Ccm1+/−);Trp53−/− | Vascular lesions in the brains similar to CCM lesions [45]. |
| Ccm2−/− | Embryonic lethality prior to E10.5, cardiovascular defects [49, 94]. |
| Tie2-Cre+/0; Ccm2f/f | Defective vascular lumen formation and arteriogenesis; pericardial edema and arrested heart development [49, 74, 94, 95]. |
| Nestin-Cre+/0; Ccm2f/f | Normal cerebrovascular phenotype [95]. |
| MX1-Cre+/0; Ccm2f/f | Brain hemorrhages in 7-8 month old adults, following induction of Cre expression at 6-8 weeks of age [49]. |
| Cdh5(PAC)-CreERT2; Ccm2f/Del; Rosa26-St -Stopf-LacZ | Vascular lesions mimicking human CCM lesions, following induction of Cre expression at P1 [96]. |
In zebrafish, morpholino-mediated inhibition of Rap1B expression leads to vascular malformations [7, 9] (and M.C.-W., Sribalaji Lakshmikanthan and Ramani Ramchandran, unpublished data). We found that morpholino-mediated knockdown of Rap1B did not interfere with early development in the majority of embryos; however, it significantly impaired angiogenesis at 20-28 hours post fertilization (hpf), as described below. At 48 hpf head bleeding and heart defects were observed in Rap1B morphants [10, 11] (and M.C.-W., S.L. and R.R., unpublished data).
Several Rap1 GEFs regulate Rap1 function in vascular cells [12, 13], but only a few of them have been implicated in vasculogenesis in vivo: PDZ-GEF-1 and CalDAG-GEF1/RasGRP2 in endothelium and C3G in pericyte recruitment (Table). PDZ-GEF-1 is an adaptor protein with (PSD-95/DlgA/ZO-1) PDZ and Ras/Rap-association (RA) domains [14] and an evolutionarily conserved GEF specific for Rap1 [14, 15]. In mice, PDZ-GEF1 is transiently expressed at E8.5-E9.5 and PDZ-GEF-1−/− mouse embryos die shortly after that, most likely due to failure in yolk sac vasculature, as blood islands fail to form a primary vascular plexus [16]. Consistent with the yolk sac failure are extended range in the severity of vascular phenotypes and other observed defects: growth retardation, defective neural tube closure and incomplete embryonic turning. In 25% of PDZ-GEF-1−/− embryos allantois is not connected to the chorion, a developmental defect similar to that in mice lacking integrin α4[17] or its receptor, VCAM-1 [18], both required for the formation of chorioallantois. Thus, Rap1, activated by PDZ-GEF-1, may be involved in integrin α4-VCAM-1-dependent chorioallantoic fusion. The in vitro allantois explant culture of PDZ-GEF1-deficiency led to reduced accumulation of VE-cadherin at cell-cell junctions and abnormal blood vessel formation, defects that were suppressed by ectopic expression of constitutively activated Rap1 [19].
In the Xenopus embryo, XRASGRP2 (an ortholog of human CalDAG-GEF1/RasGRP2), induced by VEGF, is required for endothelial differentiation of hemangioblast cells and its knockdown leads to delayed vascular development [20]. In human ECs, CalDAG-GEF1 has been implicated in both Ras and Rap1 pathways: its overexpression leads to increased expression of Ras, but increased activity only of Rap1, through which it promotes EC adhesion and migration [21].
Crk SH3-binding Guanine Nucleotide Release Protein (C3G), a ubiquitously expressed adaptor protein and a GEF for Rap1, Rap2 and R-Ras is essential for early embryonic development [22, 23]. Mice with hypomorphic C3G mutation die around E11.5 due to hemorrhage and vascular integrity defects resulting from defective pericyte development and abnormal PDGF response necessary for vascular myogenesis [24]. It remains to be determined if Rap1 is the C3G effector regulating this process.
Angiogenesis
During the second half of gestation, the vascular bed is formed through angiogenesis, a process of sprouting new capillaries from existing vasculature [25]. Prompted by the smaller size of surviving Rap1B−/− mice, suggestive of a cardiovascular developmental defect, we investigated the effect of Rap1B-deficiency on developmental angiogenesis and found that both retinal angiogenesis and neoangiogenesis were inhibited [26]. Similar studies in Rap1A−/− mice also demonstrated a defect in hindlimb ischemia and in a Matrigel plug neovascularization model, indicating that both Rap1 isoforms promote angiogenesis [6, 7, 26, 27]. Deletion of Rap1 in endothelium leads to similar angiogenesis defects, in a dose-dependent manner [7]. In zebrafish, Rap1B is essential for angiogenesis, as Rap1B loss-of-function (LOF) leads to a severe defect in intersomitic vessel (ISV) sprouting [7], a process dependent on Vascular Endothelial Growth Factor (VEGF) signaling [28]. Furthermore, a combination of genetic and pharmacological studies indicate that Rap1 and VEGF Receptor 2 (VEGFR2) act in the same pathway in ISV formation [7]. This result, combined with the observation that all responses to VEGF are inhibited in Rap1-deficient ECs [29], prompted us to hypothesize that Rap1 regulation of VEGF responses occurs at the level of its receptor, VEGFR2 (discussed in the next chapter).
Lymphatics
Deficiency of either Rap1 isoform leads to edema [3, 6] (and M.C.-W., unpublished data), however Rap1 function in the development of lymphatic vessels has not been studied. Although the function of Rap1A−/− myeloid cells is altered, with increased haptotaxis and decreased neutrophil adhesion and decreased superoxide production, the development of Rap1A−/− T-cells and B-cells is normal [4]. Nonetheless, Rap1B, which is the predominant isoform in B-cells, is critically required for the formation of lymphatic organs. Rap1B deficiency leads to markedly reduced marginal zone B-cells in the spleen and decreased numbers of mature B-cells in peripheral and mucosal lymph nodes, without affecting early B-cell development. Underlying these defects are decreased adhesion and chemotaxis and lessened homing to lymph nodes [30, 31].
Hemostasis
Platelet function
Rap1B promotes integrin αIIbβ3 activation and platelet function and its deficiency leads to a mild hemostatic defect in adult mice without spontaneous bleeding [3]. As discussed previously, hemorrhage has been observed in up to 50% of Rap1B−/− embryos and therefore Rap1B-deficiency in platelets might be considered as a cause of this phenotype. However, conditional deletion of Rap1B using a Tie2-driven Cre/flox system, which in addition to deleting Rap1B in endothelium also deletes Rap1B in hematopoietic cells and, specifically, in platelets [7], does not lead to embryonic bleeding. Therefore, defective hemostasis in Rap1B−/− embryos is likely caused by loss of Rap1B in additional tissues.
Vascular integrity & connection with cerebral cavernous malformations (CCM)
Data from Rap1-knockout mice suggest that Rap1 function in both endothelial and nonendothelial compartments is required for normal vasculogenesis. Of the two, the role of Rap1 in endothelium is better understood, and, as suggested by cellular models, involves stabilization of cell-cell junctions. Genetic models of two of the Rap1 effectors regulating this aspect of vascular function have been reported: Afadin and CCM1/KRIT1. Furthermore, connection with CCM implicates defective Rap1 signaling in vascular malformations.
Afadin (similar to AF6 gene product) is a scaffolding protein that links Ig-like adhesion molecules, nectins, with actin cytoskeleton, other adapter molecules, and, via its RA domain binds Rap1 and other small GTPases [32]. Afadin/AF6-Rap1 interactions play a well documented role in facilitating adherens junction (AJ) formation, particularly in epithelial cells [13, 33]. Endothelial knockout of Afadin leads to decreased Matrigel plug neovascularization and decreased capillary density in a hindlimb ischemia model [34]. In the retina, retarded retinal angiogenesis at postnatal day (P)4 is accompanied by a decreased number of fine vascular meshes, and the discontinuous VE-cadherin localization in AJs, consistent with Afadin's role in promoting their formation. Postnatal viability of Afadin homozygous knockout mice is dramatically reduced, suggesting that Afadin is involved also in other aspects of vasculogenesis [34] (Table).
CCMs are vascular defects of the central nervous system consisting of abnormally enlarged capillary cavities without intervening brain parenchyma [35]. Susceptible to hemorrhaging, these brain angiomas result in focal neurological deficits that cause seizures and stroke. Three genetic loci have been implicated in autosomal forms of CCM: CCM1/KRIT1 [36, 37], CCM2/OSM/malcavernin [38] and CCM3/PDCD10 [39], and proteins encoding them form a functional complex. While other loci are likely to be involved, autosomal dominant LOF mutations in any of the three CCM proteins have a causative role in CCM.
KRIT1 (Krev/Rap1 Interaction Trapped 1) was originally identified as a Rap1-binding partner in a yeast two-hybrid screen [40]. In humans, KRIT is expressed in neuronal and vascular tissues [35]. In the endothelial compartment, KRIT1 is predominantly expressed in capillaries and arterioles, with higher expression levels in organs with specialized blood-organ barriers and lower expression in fenestrated capillaries in organs where ECs do not form cell-cell junctions [41]. CCM2 is a scaffolding protein whose binding partners include actin, Rac1 and p38 kinase activating MEKK3 and MKK3, with a function in osmoregulation [42].
CCM genes are evolutionarily conserved and several genetic models of CCM have been generated, some of which, particularly the zebrafish models, share similarity with Rap1-deficient phenotypes, suggesting functional interaction. Cardiac development in zebrafish embryos with the recessive lethal mutations of ccm1 (santa) and ccm2 (valentine) orthologs is abnormal, leading to enlarged, thin-wall hearts [43]. A similar phenotype was observed in the Rap1B morpholino LOF model [9]. Interestingly, the heart defect in CCM morphants could be rescued with exogenous expression of WT KRIT, but not a (R452E) KRIT1 mutant with decreased affinity to Rap1 and altered cellular localization [10].
In addition to its role in cardiac development, Rap1-KRIT1 interaction has been implicated in zebrafish vessel integrity. CCM1 and CCM2 LOF lead to severe and progressive dilation of major vessels in zebrafish embryos, with progressive vessel wall thinning, but structurally normal cell-cell junctions. This defect in endothelial morphogenesis is cell-autonomous, as demonstrated by transplantation studies [44]. Interestingly, at sub-effective doses, combined knockdown of CCM1 and Rap1B increases the occurrence of intracranial hemorrhage observed in Rap1B LOF morphants, suggesting Rap1 and CCM1 act in the same pathway promoting vascular integrity [9] (and M.C.-W., SL, RR, unpublished observations).
Mouse models of CCM
During early mouse development KRIT1 is ubiquitously expressed, including in the central nervous system, epithelia and endothelium [45-47]. With progressing development, endothelial expression is particularly pronounced in large vessels, a pattern different from that in human tissues [41]. Targeted mutation of CCM1 in mice failed to induce CCM pathology, however additional loss of the tumor suppressor p53, shown to increase the rate of somatic mutation, led to vascular lesions in the brains of 55% of the double-mutant animals [48] (Table). Consistent with the human phenotype, mice heterozygous for CCM2, predominantly expressed in neural parenchyma in normal brains, also develop brain lesions [47].
Complete ablation of CCM1 and CCM2 leads to early and severe vascular pathologies that result in embryo lethality. KRIT1−/− embryos develop dilatation of brain vessels and a defect in branchial arch artery formation [45]. In CCM2 total and endothelial-restricted knockout mice, vascular lumen formation and arteriogenesis are defective and the heart is malformed [49]. In comparison, mice deficient in both Rap1 isoforms in endothelium develop relatively normal vasculature, without visible defects in branchial arches or lumen but at E13.5 about 50% of them develop hemorrhage in the head, that is distinct from CCM lesions, and all embryos die before E15.5 (M.C.-W. and K.W., unpublished data). As mentioned before, most total double knockouts of Rap1A and Rap1B die before midgestation (M.C.-W., unpublished data), precluding the analysis of the vasculature.
In conclusion, CCM genes are critical for several aspects of cardio- and vasculogenesis, and functional interaction with Rap1 appears to be important for the first of these functions. Endothelial deletion of Rap1 leads to a phenotype that is distinct from CCM. However, additional cell types, such as astrocytic foot processes and pyramidal neurons in the cerebral cortex – structures integral to cerebral angiogenesis and formation of the blood-brain barrier - may contribute to the pathophysiology of CCM [41], and the role of the Rap1-KRIT1 signaling axis in these cells needs to be examined.
Molecular basis of Rap1 vascular phenotypes
Angiogenesis
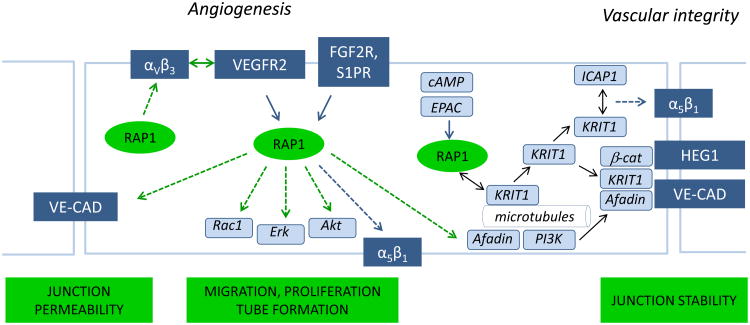
Rap1 promotes angiogenic signaling by several factors, including Fibroblast Growth Factor 2 (FGF2), VEGF and a sphingolipid, Sphingosine-1-phosphate (S1P) (Figure 1). Activated downstream from their receptors, Rap1 promotes signaling pathways leading to activation of Erk, Akt, Rac1 and Afadin, thereby regulating endothelial cell proliferation, migration, and tubule formation [29, 34]. We have recently shown both Rap1 isoforms promote responses to VEGF by an additional mechanism that implies that Rap1 impact on VEGF signaling may not be limited to selective pathways downstream from VEGFR2 [7]. Because Rap1-deficiency in ECs attenuates all VEGF-induced responses [29], we examined the ability of VEGF to activate VEGFR2 in Rap1-deficient cells. Genetic knockout of Rap1B or siRNA knockdown of either Rap1A or Rap1B leads to decreased VEGFR2 activation, indicating that Rap1 is required for full activation of that receptor. Rap1 modulation of VEGFR2 activity is mediated in part by integrin αv β3[7]. This integrin, together with integrin α5β1, plays an important role in angiogenesis [50, 51]. Upregulated in angiogenic endothelium through function-blocking studies, integrin αvβ3 has been implicated in promoting angiogenesis and, specifically in VEGF-dependent angiogenesis through cooperation with VEGFR2 [52]. The structural basis of that cooperation has recently been revealed. Integrin αvβ3 and VEGFR2 form a physical complex via their cytoplasmic domains, an interaction that is promoted by phosphorylation of the Tyr747 of the integrin β3 chain, which maintains the integrin in the activated state [53, 54]. However, under conditions when integrin αvβ3 is engaged and activated, we found that formation of the complex between VEGFR2 and the integrin is Rap1- and RIAM-independent, suggesting that Rap1 promotes integrin αvβ3-VEGFR2 cooperation via an additional signaling mechanism [7]. While both Rap1 isoforms promote integrin β1 activation and EC adhesion and migration [7, 27], Rap1 signaling to VEGFR2 is integrin β1–independent [7]. The finding that Rap1 is a positive, upstream regulator of VEGFR2 signaling implicates a broader role for Rap1 in maintaining blood vessel homeostasis. VEGF-induced EC hyperpermeability is a critical aspect of the EC response to VEGF stimulation, yet the underlying cellular mechanisms are poorly understood [55]. Tissue-specific Rap1 knockout mice may provide a valuable tool to examine the role of Rap1 in that process.
Figure 1.
In endothelium, Rap1 regulates angiogenesis and vascular integrity. Rap1 promotes angiogenic responses to VEGF by promoting VEGF Receptor 2 (VEGFR2) activation, in part via integrin αvβ3. Rap1 also acts downstream from VEGF, FGF2 and S1P receptors in signaling pathways leading to EC migration, proliferation, tube formation, and regulation of vascular permeability. Activated by cAMP-dependent GEF, Epac, Rap1 promotes junctional stability by translocating KRIT1, a molecule implicated in cavernous cerebral malformations, from the microtubules to the plasma membrane and cell-cell junctions, where KRIT1 binds Heart-of-Glass (HEG1) receptor and other junctional proteins, including b-catenin (β-cat) and a Rap1GEF, Afadin, promoting junctional stability. KRIT1 at the plasma membrane may also interact with ICAP-1, promoting integrin α5β1 activation. VE-CAD: VE-cadherin.
Vascular integrity
Rap1 GEFs
A number of Rap1 GEFs and several Rap1 effectors regulate cell-cell junction formation in epithelial and ECs, and the mechanisms involved have been discussed in several comprehensive reviews [12, 13]. Of the Rap1 GEFs involved in this process, the function of Epac is probably best known. Activation of Rap1 in endothelium by cAMP-activated Epac promotes cell-cell junction formation and enhances endothelial barrier. First described in cultured cells [56-58], significance of Epac-Rap1 signaling pathway in barrier formation in vivo has been demonstrated in PAF-induced microvascular hyperpermeability of rat mesentery [59] and in protection of lung endothelium in a ventilator-induced lung injury [60]. Recently, targeting the Epac-Rap1 pathway has been validated as a potential therapeutic for endothelial hyperpermeability in vivo [61].
Rap1 effectors
Afadin
Afadin is known for regulating epithelial cell-cell junctions [32], but also acts as a Rap1 effector in promoting endothelial cell-cell junction formation and facilitates angiogenesis in vivo. Studies in model cell systems suggest that Afadin is required for the tubulogenesis step of angiogenesis. In HUVEC and HEK293 cells, Rap1 activated downstream from VEGFR2 and S-1-P receptors, promotes association of Afadin with PI3K and their translocation to AJs. Knockdown of Afadin blocks accumulation of AJ and tight junction (TJ) proteins in cell-cell contact sites and tubule formation. Once localized to junctions, Afadin further promotes Rap1 activation, providing a positive feedback [34].
In addition to its function during vasculogenesis, Afadin mediates barrier-protective effects in the murine model of ventilator induced lung injury. Cyclopenthenone-containing products resulting from oxidation of a natural phospholipid, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC), have protective effects in lung EC barrier dysfunction models and act by promoting Rac1-and cdc42-mediated peripheral actin assembly and accumulation of AJ and TJ proteins, a process dependent on Rap1 activity [62]. Recent in vivo studies demonstrate that Afadin mediates that effect as its depletion in vivo with Afadin-specific siRNA abolishes protective effects by OxPAPC [63].
KRIT1
Studies in animal models demonstrate a critical role for CCM proteins in endothelium in the regulation of blood vessel development. While it is likely that CCM proteins in other tissue compartments also contribute to the pathophysiology of CCM, studies in cultured ECs and analysis of human samples implicate Rap-KRIT1 functional interactions in the regulation of endothelial cell barrier.
Molecular interactions
CCM1/KRIT1 is a multidomain protein containing C-terminal band 4.1/ezrin/radixin/moesin (FERM) which mediates the linkage between cortical actin and integral membrane proteins, four ankyrin repeats involved in protein-protein interactions, and three NPXY motifs, the first two involved in binding CCM2 [64] and the most N-terminus proximal in binding integrin β1-binding protein ICAP-1 [65]. The ICAP-1 binding site on KRIT1 is similar to the I-CAP1 binding site on integrin β1 and therefore it was proposed that KRIT1 and integrin compete for binding to ICAP-1 [66]. Recent studies defined the structural basis of this interaction and revealed the role of ICAP-1 in modulation of integrin activation [67]. ICAP-1 bound to integrin β1 acts as an antagonist of talin-mediated integrin β1 activation; KRIT1 binding of ICAP-1 releases integrin β1 from this inhibitory association, allowing binding of talin and subsequent steps of integrin activation.
First shown in a yeast two-hybrid system [40], direct interaction between KRIT1 and Rap1 was controversial for a time [66], likely because binding of KRIT1 RA domain to purified Rap1B is rather weak with a micromolar affinity, and requires neighboring ankyrin repeats and FERM domain [68].Studies from Faurobert and Ginsberg labs shed more light on this interaction. [69] In BHK cells, exogenously expressed WT and, in particular, DA Rap1A interacts with KRIT1 and promotes KRIT1 interaction with PIP2 in liposomes. Rap1 interacts with the FERM domain of KRIT1 in vitro [70] and a point mutation in KRIT1 that significantly decreases KRIT1's affinity for Rap1 (R452E) alters KRIT1's cellular localization [10] (as described below). Recently, a co-crystal structure of KRIT1 in complex with Rap1 has been solved [71]. Rap1-KRIT1 interaction encompasses an extended surface, including Rap1 Switch I and II and KRIT1 FERM F1 and F2 subdomains.
Cellular localization and function
The FERM domain of KRIT1, like that of other FERM-containing proteins, exists in two conformations: open and closed, mediated by intramolecular interactions between the N- and C-terminus [69, 72]. Exogenously expressed KRIT1 localizes to the nucleus or the cytoplasm and the shuttling between the two compartments was proposed to depend on KRIT1's FERM domain open or closed conformation and interaction with CCM2 and ICAP-1, respectively [72]. In ECs [70, 73], KRIT1 localizes to microtubules [69, 73], with increased localization to microtubule plus ends during mitosis [73]. Overexpression of DA mutant of Rap1 induces release of KRIT1 from microtubules and its translocation to the membrane and, specifically, to cell-cell junctions [69]. In cell-cell junctions, KRIT1 binds Heart-of-glass (HEG1) receptor [74] and associates with junctional proteins, including β-catenin and Afadin [70]. Rap1 activity is required for KRIT1's interaction with junctional molecules, but not localization to junctions [71]. Interestingly, (R452E) KRIT1 mutant with decreased affinity for Rap1 does not localize to junctions (as it is sequestered by microtubules) and fails to support endothelial cell-cell junctions [71].
Knockdown of endogenous KRIT1 decreases β-catenin localization to junctions, increasing vascular permeability to thrombin in BAEC monolayers, and prevents Epac1- and Rap1-mediated stabilization of junctions [70]. Further, physical interaction of KRIT1 and CCM2 is required for endothelial cell-cell junctional localization, and lack of either protein destabilizes endothelial barrier by promoting RhoA-mediated contractility. Therefore, the KRIT1-CCM2 interaction promotes endothelial barrier by suppressing Rho/ROCK signaling. Inhibition of ROCK corrects the vascular leak in KRIT1+/− and CCM2+/− mice [75].
A model for regulation of endothelial barrier
Based on the above-described cellular studies, a couple of mechanisms have been proposed to explain the role of Rap1 in regulating KRIT1 function in cell-cell junctions (Figure 1). One proposes that KRIT1 is transported along microtubules by an unknown motor protein to the plasma membrane, where it encounters active Rap1, which releases it from microtubules. Next, KRIT1 sequesters ICAP-1, releasing it from integrin β1 and thus facilitating integrin activation. It has been proposed that loss of KRIT1 may reduce integrin β1 activation, contributing to increased vascular permeability. However, integrin expression pattern indicates that integrins αvβ and αvβ5 rather than αvβ1 are expressed more strongly in CCM endothelium, and therefore regulation of these integrins may be more significant in CCM pathology [76]. In an alternative model Rap1 plays an active role in releasing KRIT1 from microtubules allowing KRIT1 binding to HEG1 [74] and localization to cell-cell junctions, where it associates with junctional proteins, including β-catenin and VE-cadherin [10].
Additional function
Most KRIT1 LOF mutations described in patients occur within the FERM domain, leading to a truncated KRIT1 protein [77]. It has been suggested that the open conformation assumed by such mutants, blocks their ability to shuttle to the nucleus and that the loss of that function, mediated by interaction with ICAP-1, may contribute to CMM pathogenesis [72]. In the nucleus KRIT1 may modulate the expression levels of the antioxidant protein SOD2, contributing to the maintenance of intracellular Reactive Oxygen Species homeostasis [78].
A study in HUVECs overexpressing KRIT1 demonstrated its anti-angiogenic functions, mediated by DLL4/Notch signaling, and suggested that during early stages of CCM development, loss of functional KRIT1 promotes endothelial proliferation and capillary sprouting by dysregulated Notch signaling [79]. Consistent with this, increased endothelial proliferation has been observed in KRIT1−/− embryos [45], a phenotype distinct from Rap1B−/− mice [26].
Lastly, studies using human and mouse ECs cultured in 3D implicate Rap1 as a downstream target of VE-cadherin and CCM1, as a component of a signaling complex in AJs that is required for establishment of endothelial polarity and formation of vascular lumen [80]. The role of CCM proteins in lumen formation has been demonstrated in a mouse knockout model [49].
Rap1 functions in other cells
Outside of endothelium, there is little information about Rap1 functions in pericytes and other cellular components contributing to vasculogenesis. Emerging reports, however, demonstrate that in cardiomyocytes and smooth muscle cells the Epac-Rap1 signaling axis, often in cooperation with PKA signaling, regulates several basic functions, including permeability and cellular contractility, disruption of which would be expected to lead to vascular dysfunction.
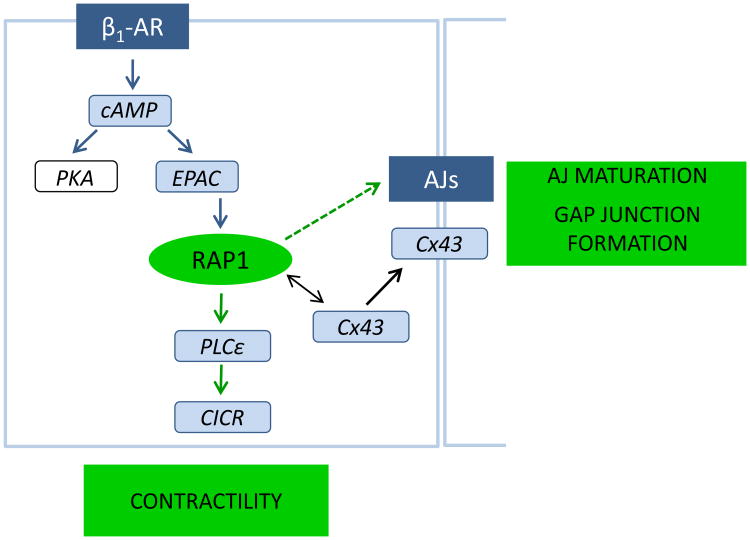
In cardiomyocytes, Epac regulates the activity of various cellular compartments and influences calcium handling and excitation-contraction coupling [81]. In cultured rat neonatal cardiomyocytes, PKA and Epac/Rap1 cooperate in cAMP-induced gap junction formation, with PKA signaling promoting gating and Epac/Rap1 increasing recruitment of connexin43 at cell–cell contacts and promoting maturation of AJs [82] (Figure 2). Epac is activated by β-adrenergic receptor (β-AR), a G-protein-coupled receptor that enhances cardiomyocyte contractility in response to acute stress and leads to cardiac hypertrophy and ventricular dysfunction in chronic stress. Following acute β-AR stimulation, Epac-mediated and Rap1-dependent activation of PLC epsilon is critical for Ca2+ -induced Ca2+ release (CICR) [83]. Epac expression correlates with pathological cardiac hypertrophy [84] and silencing Epac expression blocks the hypertrophic response [85]; however, Epac targets in this case are other small GTPases Rap2B, Rac1 and H-Ras but not Rap1 [86]. Therefore, Rap1 mediates a subset of Epac functions in the heart: regulation of cell-cell junctions and contractility, but there is no evidence for Rap1's direct involvement in promoting hypertrophy.
Figure 2.
In cardiomyocytes, Epac-Rap1 axis transmits signals from β1-adrenergic receptor (β1-AR) promoting adherens junction (AJs) maturation and gap junction formation by inducing recruitment of connexin 43 (Cx43). Acute stimulation of β1-AR leads to activation of PLCs, critical for Ca2+ -induced Ca release (CICR) and cardiomyocyte contractility, in a Rap1-dependent manner.
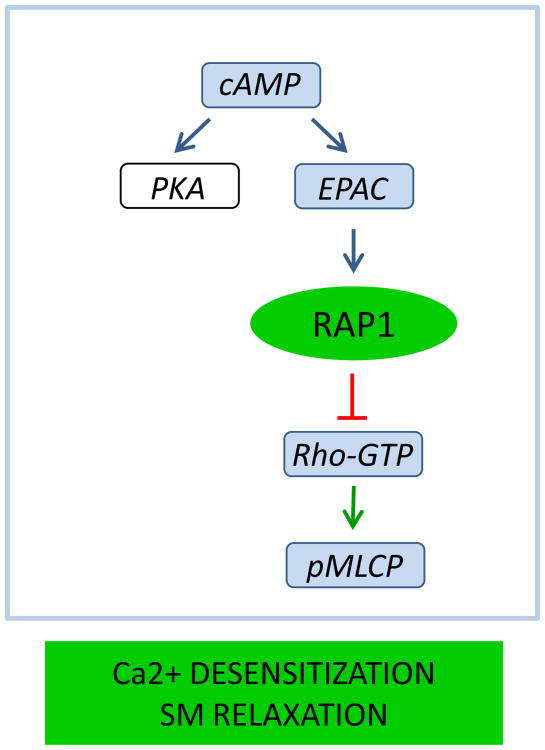
Rap1 also regulates smooth muscle contractility, albeit by a different mechanism. At a given intracellular concentration of Ca2+, contractility of smooth muscle and fibroblasts is regulated by myosin regulatory light chain (RLC20) phosphorylation, which promotes actomyosin contractility. Agonist-induced activation of small GTPase Rho and its effector Rho kinase (ROCK) leads to increased inhibitory phosphorylation of myosin phosphatase (MLCP) increasing RLC20 phosphorylation (“Ca2+-sensitization”) [87-89]. In contrast, elevation of intracellular second messenger cAMP or cGMP reduces Ca2+-sensitivity and induces SM relaxation (“Ca2+ -desensitization”) [90, 91]. We demonstrated that Epac-Rap1 signaling axis contributes to Ca -desensitization of SM and fibroblast contractility [92]. We found that Epac-specific analog reduces agonist-induced contractility, RLC20, and myosin light chain phosphatase phosphorylation in isolated vessels independently of PKA. Further, agonist-induced elevation of cAMP led to activation of Rap1, which was independent of PKA, but inhibited by silencing Epac. Lastly, RhoA activity was reduced by activating Epac in WT but not Rap1B null fibroblasts, consistent with Epac signaling through Rap1B inhibition of RhoA [92] (Figure 3). These findings demonstrated a novel cAMP-mediated signaling mechanism in which Epac-activated Rap1 leads to desensitization of SM contractility by inhibiting activation of Rho. Additional in vivo studies are needed to address the relevance of this mechanism in regulation of vascular tone.
Figure 3.
Rap1 promotes smooth muscle relaxation by mediating Ca2+ -desensitization of contraction. Following elevation of cAMP, activated Rap1 inhibits activation of RhoA and downstream signaling leading to myosin light chain phosphatase (pMLCP), resulting in decreased phosphorylation of myosin regulatory light chains (RLC20) and decreased contractility.
Genetic models have revealed important roles of Rap1 signaling in promoting angiogenesis and maintenance of vascular stability. Studies in cells have provided some understanding of the underlying mechanisms, in particular, with new insights into regulation of angiogenic responses, where Rap1 acts upstream from VEGFR2; and into the role of Rap1-KRIT1 interactions in meditating endothelial barrier. However, these studies do not fully explain vascular defects observed in the in vivo models of Rap1 deficiency or Rap1's role in the pathophysiology of CCM. Further, emerging studies in cardiac and smooth muscle cells demonstrate that Rap1 is an important regulator of several basic functions, including contractility. The role of Rap1 in smooth muscle cells in regulation of vasculogenesis and vascular tone needs to be addressed in vivo. Additional models that allow temporal and tissue-specific modulation of Rap1 signaling, in particular in pericytes, should help address these outstanding questions.
Acknowledgments
We thank Dr. Sribalaji Lakshmikanthan for assistance with preparing figures and Tina Heil and Portlynne Joseph for proofreading the manuscript. This work was supported by NHLBI grant HL111582.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frische EW, Zwartkruis FJT. Rap1, a mercenary among the Ras-like GTPases. Developmental Biology. 2010;340:1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin (alpha)v(beta)3. Blood. 2011;118:2015–2026. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC. Rap1 b is required for normal platelet function and hemostasis in mice. Journal of Clinical Investigation. 2005;115:2296–2296. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Yan J, De P, Chang HC, Yamauchi A, Christopherson KW, II, Paranavitana NC, Peng X, Kim C, Munugulavadla V, Kapur R, Chen H, Shou W, Stone JC, Kaplan MH, Dinauer MC, Durden DL, Quilliam LA. Rap1a Null Mice Have Altered Myeloid Cell Functions Suggesting Distinct Roles for the Closely Related Rap1a and lb Proteins. J Immunol. 2007;179:8322–8331. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJT. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Molecular and Cellular Biology. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Molecular and Cellular Biology. 2008;28:5803–5810. doi: 10.1128/MCB.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin (alpha)v(beta)3. Blood. 2011;118:2015–2026. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical Role of the TIE2 Endothelial Cell Receptor in the Development of Definitive Hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- 9.Gore AV, Lampugnani MG, Dye L, Dejana E, Weinstein BM. Combinatorial interaction between CCM pathway genes precipitates hemorrhagic stroke. Dis Model Mech. 2008;1:275–281. doi: 10.1242/dmm.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu JJ, Stockton RA, Gingras AR, Ablooglu AJ, Han J, Bobkov AA, Ginsberg MH. A mechanism of Rap1-induced stabilization of endothelial cell-cell junctions. Molecular Biology of the Cell. 2011;22:2509–2519. doi: 10.1091/mbc.E11-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gore AV, Lampugnani MG, Dye L, Dejana E, Weinstein BM. Combinatorial interaction between CCM pathway genes precipitates hemorrhagic stroke. DMM Disease Models and Mechanisms. 2008;1:275–281. doi: 10.1242/dmm.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannekoek WJ, Kooistra MRH, Zwartkruis FJT, Bos JL. Cell-cell junction formation: The role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochimica Et Biophysica Acta-Biomembranes. 2009;1788:790–796. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Boettner B, Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Current Opinion in Cell Biology. 2009;21:684–693. doi: 10.1016/j.ceb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Kariya Ki, Hu CD, Shibatohge M, Goshima M, Okada T, Watari Y, Gao X, Jin TG, Yamawaki-Kataoka Y, Kataoka T. RA-GEF, a Novel Rap1A Guanine Nucleotide Exchange Factor Containing a Ras/Rap1A-associating Domain, Is Conserved between Nematode and Humans. J Biol Chem. 1999;274:37815–37820. doi: 10.1074/jbc.274.53.37815. [DOI] [PubMed] [Google Scholar]
- 15.Liao Y, Satoh T, Gao X, Jin TG, Hu CD, Kataoka T. RA-GEF-1, a Guanine Nucleotide Exchange Factor for Rap1, is Activated by Translocation Induced by Association with Rap1-GTP and Enhances Rap1-dependent B-Raf Activation. Journal of Biological Chemistry. 2001;276:28478–28483. doi: 10.1074/jbc.M101737200. [DOI] [PubMed] [Google Scholar]
- 16.Wei P, Satoh T, Edamatsu H, Aiba A, Setsu T, Terashima T, Kitazawa S, Nakao K, Yoshikawa Y, Tamada M, Kataoka T. Defective vascular morphogenesis and mid-gestation in mice lacking RA-GEF-1. Biochemical and Biophysical Research Communications. 2007;363:106–112. doi: 10.1016/j.bbrc.2007.08.149. [DOI] [PubMed] [Google Scholar]
- 17.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by [alpha] 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 18.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 19.Kanemura H, Satoh T, Bilasy SE, Ueda S, Hirashima M, Kataoka T. Impaired vascular development in the yolk sac and allantois in mice lacking RA-GEF-1. Biochemical and Biophysical Research Communications. 2009;387:754–759. doi: 10.1016/j.bbrc.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Takahashi S, Haramoto Y, Onuma Y, Nagamine K, Okabayashi K, Hashizume K, Iwanaka T, Asashima M. XRASGRP2 is essential for blood vessel formation during Xenopus development. International Journal of Developmental Biology. 2010;54:609–615. doi: 10.1387/ijdb.092929ks. [DOI] [PubMed] [Google Scholar]
- 21.Takino J, Nagamine K, Hori T. Ras guanyl nucleotide releasing protein 2 affects cell viability and cell-matrix adhesion in ECV304 endothelial cells. Cell Adhes Migr. 2013;7 doi: 10.4161/cam.24082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotoh T, Hattori S, Nakamura S, Kitayama H, Noda M, Takai Y, Kaibuchi K, Matsui H, Hatase O, Takahashi H. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol Cell Biol. 1995;15:6746–6753. doi: 10.1128/mcb.15.12.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, Kurokawa K, Mayer BJ, Maki K, Miyazaki JI, Matsuda M. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO Journal. 2001;20:3333–3341. doi: 10.1093/emboj/20.13.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voss AK, Gruss P, Thomas T. The guanine nucleotide exchange factor C3G is necessary for the formation of focal adhesions and vascular maturation. Development. 2003;130:355–367. doi: 10.1242/dev.00217. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 26.Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficientmice. Blood. 2008;111:2647–2656. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmona G, Göttig S, Orlandi A, Scheele J, Bäuerle T, Jugold M, Kiessling F, Henschler R, Zeiher AM, Dimmeler S, Chavakis E. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood. 2009;113:488–497. doi: 10.1182/blood-2008-02-138438. [DOI] [PubMed] [Google Scholar]
- 28.Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chrzanowska-Wodnicka M. Regulation of angiogenesis by a small GTPase Rap1. Vascular Pharmacology. 2010;53:1–10. doi: 10.1016/j.vph.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Chen YH, Yu M, Podd A, Wen R, Chrzanowska-Wodnicka M, White GC, Wang D. A critical role of Rap1b in B-cell trafficking and marginal zone B-cell development. Blood. 2008;111:4627–4636. doi: 10.1182/blood-2007-12-128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu H, Awasthi A, White GC, II, Chrzanowska-Wodnicka M, Malarkannan S. Rap1b Regulates B Cell Development, Homing, and T Cell-Dependent Humoral Immunity. J Immunol. 2008;181:3373–3383. doi: 10.4049/jimmunol.181.5.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 33.Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. PNAS. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tawa H, Rikitake Y, Takahashi M, Amano H, Miyata M, Satomi-Kobayashi S, Kinugasa M, Nagamatsu Y, Majima T, Ogita H, Miyoshi J, Hirata Ki, Takai Y. Role of Afadin in Vascular Endothelial Growth Factor- and Sphingosine 1-Phosphate-Induced Angiogenesis. Circ Res. 2010;106:1731–1742. doi: 10.1161/CIRCRESAHA.110.216747. [DOI] [PubMed] [Google Scholar]
- 35.Revencu N, Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. Journal of Medical Genetics. 2006;43:716–721. doi: 10.1136/jmg.2006.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laberge-le Couteulx S, Jung HH, Labauge P, Houtteville JP, Lescoat C, Cecillon M, Marechal E, Joutel A, Bach JF, Tournier-Lasserve E. Truncating mutations in CCM1 encoding KRIT1, cause hereditary cavernous angiomas. Nature Genetics. 1999;23:189–193. doi: 10.1038/13815. [DOI] [PubMed] [Google Scholar]
- 37.Sahoo T, Johnson EW, Thomas JW, Kuehl PM, Jones TL, Dokken CG, Touchman JW, Gallione CJ, Lee-Lin SQ, Kosofsky B, Kurth JH, Louis DN, Mettler G, Morrison L, Gil-Nagel A, Rich SS, Zabramski JM, Boguski MS, Green ED, Marchuk DA. Mutations in the gene encoding KRIT1, a Krev-1/Rap1a binding protein, cause cerebral cavernous malformations (CCM1) Hum Mol Genet. 1999;8:2325–2333. doi: 10.1093/hmg/8.12.2325. [DOI] [PubMed] [Google Scholar]
- 38.Liquori CL. Mutations in a gene encoding a novel protein containing a phosphotyrosine binding domain cause type 2 cerebral cavernous malformations. Am J Hum Genet. 2003;73:1459–1464. doi: 10.1086/380314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergametti F. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. 2005;76:42–51. doi: 10.1086/426952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serebriiskii I, Estojak J, Sonoda G, Testa JR, Golemis EA. Association of Krev-1/Rap1a with Kritl, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21-22. Oncogene. 1997;15:1043–1049. doi: 10.1038/sj.onc.1201268. [DOI] [PubMed] [Google Scholar]
- 41.Guzeloglu-Kayish O, Amankulor NM, Voorhees J, Luleci G, Lifton RP, Gunel M. KRIT1/cerebral cavernous malformation 1 protein localizes to vascular endothelium, astrocytes and pyramidal cells of the adult human cerebral cortex. Neurosurgery. 2004;54:943–949. doi: 10.1227/01.neu.0000114512.59624.a5. [DOI] [PubMed] [Google Scholar]
- 42.Uhlik MT, Abell AN, Johnson NL, Sun WY, Cuevas BD, Lobel-Rice KE, Home EA, Dell'Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nature Cell Biology. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 43.Mably JD, Chuang LP, Serluca FC, Mohideen MAPK, Chen JN, Fishman MC. Santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development. 2006;133:3139–3146. doi: 10.1242/dev.02469. [DOI] [PubMed] [Google Scholar]
- 44.Hogan BM, Bussmann J, Wolburg H, Schulte-Merker S. Ccml cell autonomously regulates endothelial cellular morphogenesis and vascular tubulogenesis in zebrafish. Hum Mol Genet. 2008;17:2424–2432. doi: 10.1093/hmg/ddn142. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccml is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131:1437–1448. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- 46.Denier C, Gasc JM, Chapon F, Domenga V, Lescoat C, Joutel A, Tournier-Lasserve E. Krit1/cerebral cavernous malformation 1 mRNA is preferentially expressed in neurons and epithelial cells in embryo and adult. Mechanisms of Development. 2002;117:363–367. doi: 10.1016/s0925-4773(02)00209-5. [DOI] [PubMed] [Google Scholar]
- 47.Plummer NW, Squire TL, Srinivasan S, Huang E, Zawistowski JS, Matsunami H, Hale LP, Marchuk DA. Neuronal expression of the Ccm2 gene in a new mouse model of cerebral cavernous malformations. Mammalian Genome. 2006;17:119–128. doi: 10.1007/s00335-005-0098-8. [DOI] [PubMed] [Google Scholar]
- 48.Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN, Marchuk DA. Loss of p53 Sensitizes Mice with a Mutation in Ccml (KRIT1) to Development of Cerebral Vascular Malformations. Am J Pathol. 2004;165:1509–1518. doi: 10.1016/S0002-9440(10)63409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunningham K, Uchida Y, O'Donnell E, Claudio E, Li WL, Soneji K, Wang HS, Mukouyama YS, Siebenlist U. Conditional deletion of Ccm2 causes hemorrhage in the adult brain: a mouse model of human cerebral cavernous malformations. Human Molecular Genetics. 2011;20:3198–3206. doi: 10.1093/hmg/ddr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hynes RO. Cell-matrix adhesion in vascular development. Journal of Thrombosis and Haemostasis. 2007;5:32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 51.Robinson SD, Hodivala-Dilke KM. The role of (β3-integrins in tumor angiogenesis: Context is everything. Current Opinion in Cell Biology. 2011;23:630–637. doi: 10.1016/j.ceb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alpha(V)beta(3) and VEGFR2 in angiogenesis. Angiogenesis. 2009;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.West XZ, Meller N, Malinin NL, Deshmukh L, Meller J, Mahabeleshwar GH, Weber ME, Kerr BA, Vinogradova O, Byzova TV. Integrin (β 3 crosstalk with VEGFR accommodating tyrosine phosphorylation as a regulatory switch. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0031071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deshmukh L, Meller N, Alder N, Byzova T, Vinogradova O. Tyrosine phosphorylation as a conformational switch: A case study of integrin (β 3 cytoplasmic tail. Journal of Biological Chemistry. 2011;286:40943–40953. doi: 10.1074/jbc.M111.231951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagy JA, Benjamin L, Zeng HY, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuhara S. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 58.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase Inhibits Leukocyte Transmigration by Promoting Endothelial Barrier Function. J Biol Chem. 2005;280:11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 59.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol. 2008;294:H1188–1196. doi: 10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- 60.Birukova AA, Fu P, Xing J, Birukov KG. Rap1 mediates protective effects of iloprost against ventilator-induced lung injury. Journal of Applied Physiology. 2009;107:1900–1910. doi: 10.1152/japplphysiol.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bubik MF, Wilier EA, Bihari P, Jürgenliemk G, Ammer H, Krombach F, Zahler S, Vollmar AM, Fiirst R. A novel approach to prevent endothelial hyperpermeability: The Crataegus extract WS® 1442 targets the cAMP/Rap1 pathway. Journal of Molecular and Cellular Cardiology. 2012;52:196–205. doi: 10.1016/j.yjmcc.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 62.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. Journal of Cellular Physiology. 2011;226:2052–2062. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birukova AA, Tian Y, Dubrovskyi O, Zebda N, Sarich N, Tian X, Wang Y, Birukov KG. VE-cadherin trans-interactions modulate Rac activation and enhancement of lung endothelial barrier by iloprost. Journal of Cellular Physiology. 2012;227:3405–3416. doi: 10.1002/jcp.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zawistowski JS. CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum Mol Genet. 2005;14:2521–2531. doi: 10.1093/hmg/ddi256. [DOI] [PubMed] [Google Scholar]
- 65.Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA. KRIT1 association with the integrin-binding protein ICAP-1: a new direction in the elucidation of cerebral cavernous malformations (CCM1) pathogenesis. Human Molecular Genetics. 2002;11:389–396. doi: 10.1093/hmg/11.4.389. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Clatterbuck RE, Rigamonti D, Chang DD, Dietz HC. Interaction between krit1 and icap1 alpha infers perturbation of integrin beta 1-mediated angiogenesis in the pathogenesis of cerebral cavernous malformation. Human Molecular Genetics. 2001;10:2953–2960. doi: 10.1093/hmg/10.25.2953. [DOI] [PubMed] [Google Scholar]
- 67.Liu W, Draheim KM, Zhang R, Calderwood DA, Boggon TJ. Mechanism for KRIT1 Release of ICAP1-Mediated Suppression of Integrin Activation. Molecular Cell. 2013;49:719–729. doi: 10.1016/j.molcel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wohlgemuth S, Kiel C, Kramer A, Serrano L, Wittinghofer F, Herrmann C. Recognizing and Defining True Ras Binding Domains I: Biochemical Analysis. Journal of Molecular Biology. 2005;348:741–758. doi: 10.1016/j.jmb.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 69.Béraud-Dufour S, Gautier R, Albiges-Rizo C, Chardin P, Faurobert E. Krit 1 interactions with microtubules and membranes are regulated by Rap1 and integrin cytoplasmic domain associated protein-1. Febs J. 2007;274:5518–5532. doi: 10.1111/j.1742-4658.2007.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X, Zhang R, Draheim KM, Liu W, Calderwood DA, Boggon TJ. Structural basis for small G protein effector interaction of ras-related protein 1 (Rap1) and adaptor protein krev interaction trapped 1 (KRIT1) Journal of Biological Chemistry. 2012;287:22317–22327. doi: 10.1074/jbc.M112.361295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Francalanci F, Avolio M, De Luca E, Longo D, Menchise V, Guazzi P, Sgro F, Marino M, Goitre L, Balzac F, Trabalzini L, Retta SF. Structural and functional differences between KRIT1A and KRIT1B isoforms: A framework for understanding CCM pathogenesis. Experimental Cell Research. 2009;315:285–303. doi: 10.1016/j.yexcr.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Gunel M, Laurans MSH, Shin D, DiLuna ML, Voorhees J, Choate K, Nelson-Williams C, Lifton RP. KRIT1, a gene mutated in cerebral cavernous malformation, encodes a microtubule-associated protein. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10677–10682. doi: 10.1073/pnas.122354499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, Chen M, Guo L, Lu Mm, Zhou D, Kitajewski J, Affolter M, Ginsberg MH, Kahn ML. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–176. doi: 10.1038/nm.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. Journal of Experimental Medicine. 2010;207:881–896. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seker A. Expression of integrins in cerebral arteriovenous and cavernous malformations. Neurosurgery. 2006;58:159–168. doi: 10.1227/01.neu.0000192174.55131.09. [DOI] [PubMed] [Google Scholar]
- 77.Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. The Lancet Neurology. 2007;6:237–244. doi: 10.1016/S1474-4422(07)70053-4. [DOI] [PubMed] [Google Scholar]
- 78.Guazzi P, Goitre L, Ferro E, Cutano V, Martino C, Trabalzini L, Retta SF. Identification of the Kelch Family Protein Nd1-L as a Novel Molecular Interactor of KRIT1. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0044705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wüstehube J, Bartol A, Liebler SS, Brütsch R, Zhu Y, Felbor U, Sure U, Augustin HG, Fischer A. Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12640–12645. doi: 10.1073/pnas.1000132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. Journal of Cell Science. 2010;123:1073–1080. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 81.Laurent AC, Breckler M, Berthouze M, Lezoualc'h F. Role of Epac in brain and heart. Biochemical Society Transactions. 2012;40:51–57. doi: 10.1042/BST20110642. [DOI] [PubMed] [Google Scholar]
- 82.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circulation Research. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- 83.Oestreich EA, Wang HA, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C epsilon plays a critical role in 6-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. Journal of Biological Chemistry. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- 84.Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Gamier A, Lompre AM, Vandecasteele G, Lezoualc'h F. cAMP-Binding Protein Epac Induces Cardiomyocyte Hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- 85.Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc'h F. Epac Mediates {beta}-Adrenergic Receptor-Induced Cardiomyocyte Hypertrophy. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.107.164947. CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 86.Metrich M, Laurent AC, Breckler M, Duquesnes N, Hmitou I, Courillau D, Blondeau JP, Crozatier B, Lezoualc'h F, Morel E. Epac activation induces histone deacetylase nuclear export via a Ras-dependent signalling pathway. Cellular Signalling. 2010;22:1459–1468. doi: 10.1016/j.cellsig.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 87.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 88.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 89.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. The Journal of Cell Biology. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Somlyo AP, Somlyo AV. Ca 2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiological Reviews. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 91.Puetz S, Lubomirov LT, Pfitzer G. Regulation of Smooth Muscle Contraction by Small GTPases. Physiology. 2009;24:342–356. doi: 10.1152/physiol.00023.2009. [DOI] [PubMed] [Google Scholar]
- 92.Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Chrzanowska-Wodnicka M, Somlyo AV. The cAMP-responsive Rap1 Guanine Nucleotide Exchange Factor, Epac, Induces Smooth Muscle Relaxation by Down-regulation of RhoA Activity. Journal of Biological Chemistry. 2011;286:16681–16692. doi: 10.1074/jbc.M110.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa Si, Takai Y. Afadin: A Key Molecule Essential for Structural Organization of Cell-Cell Junctions of Polarized Epithelia during Embryogenesis. The Journal of Cell Biology. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Marchuk DA, Davis GE, Li DY. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nature Medicine. 2009;15:177–184. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boulday G, Blécon A, Petit N, Chareyre F, Garcia LA, Niwa-Kawakita M, Giovannini M, Tournier-Lasserve E. Tissue-specific conditional CCM2 knockout mice establish the essential role of endothelial CCM2 in angiogenesis: Implications for human cerebral cavernous malformations. DMM Disease Models and Mechanisms. 2009;2:168–177. doi: 10.1242/dmm.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boulday G, Rudini N, Maddaluno L, Blécon A, Arnould M, Gaudric A, Chapon F, Adams RH, Dejana E, Tournier-Lasserve E. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. Journal of Experimental Medicine. 2011;208:1835–1847. doi: 10.1084/jem.20110571. [DOI] [PMC free article] [PubMed] [Google Scholar]