Abstract
Pain is the most feared symptom of cancer. New oncological cancer treatments are improving survival, but advanced cancer presents challenges that have not been seen before, often with pain that is very difficult to manage because of a recurrent tumour that is invading the central nervous system. In some of the older interventional techniques of destroying nerve pathways, expertise has diminished or has been deemed unnecessary with the development of specialist palliative care. Not all pain is managed adequately with the analgesic ladder. Knowledge of pain mechanisms, careful assessment and selection of the right technique at the right time will enhance cancer pain management. New techniques include intrathecal drug therapy, vertebroplasty, cordotomy, ultra-sound guided nerve blocks, neuromodulation and advances in drug therapies.
Introduction
Unrelieved pain is the most feared symptom of cancer and occurs in over 75% of people with advanced disease [1]. Pain relief in palliative care was founded on the oral administration of morphine regularly “by the clock”, initiated by the late Dame Cicely Saunders in the 1950s in London, and promoted by the WHO in 1986 as the third step of the analgesic ladder [2] for pain relief in cancer in developing countries around the world. The analgesic ladder approach has been outstandingly successful in alleviating pain and suffering when it is applied and used correctly, leading to a decline in the use of other interventions, such as nerve blocks. However, in the past decade, advances in the use of chemotherapy, radiotherapy and other cancer treatments have improved survival, sometimes at the expense of more complex pain from cancer that may spread to involve nerve plexuses, the spinal canal and the brain. Studies have shown that at least 20-40% of cancer pain is not adequately relieved by application of the analgesic ladder [3,4]. It is time to move on. Patients rightly want to maintain a good quality of life without the burdensome effect of sedating drugs. The long-term adverse effects of opioids on cognitive function, and on the immune and endocrine systems [5] have been largely ignored in palliative care but are significant in cancer survivors [6].
Pain mechanisms are important in the understanding of cancer pain, as is the interaction of emotions, memory, life experiences, mood, expectations, and the roller coaster of the cancer patient's journey on the pain experience. Every experience is a different one. There is always much to learn.
Advances in cancer pain management are evolving but the aim now is to manage complex and difficult pain while minimising the adverse effects of sedating drugs.
This article discusses several advances in this area:
(a) the mechanisms of cancer pain, and how knowledge of these mechanisms may lead to the development of novel analgesic agents;
(b) advances in drug therapy;
(c) intrathecal drug therapy;
(d) vertebroplasty and kyphoplasty;
(e) useful interventional therapies, such as cordotomy, coeliac plexus block, intrathecal neurolysis, and ultrasound guided techniques;
(f) the pain relieving role of chemotherapy and radiotherapy;
(g) neuromodulation;
(h) and other techniques.
Mechanisms of cancer pain
Cancer causes pain by many different routes, including direct tissue invasion, inflammation, obstruction (viscera, nerves and cerebrospinal fluid [CSF]) and can also result from cancer treatment (surgery, radiotherapy and chemotherapy). As a consequence, it has nociceptive, neuropathic and ischaemic elements. Debate is ongoing about whether cancer pain is distinct from other forms of pain or merely a composite of these separate mechanisms. Animal models have provided a greater understanding of the distinct central and peripheral mechanisms of cancer pain.
Peripheral mechanisms
Recent studies have provided more information about the molecular interactions within the cancer micro-environment [7]. These are often dependent on the tumour type. Nociceptive mediators present at high concentrations include protons [8,9], endothelin-1 [10-14], bradykinin [15,16], cytokines such as tumor necrosis factor (TNF)-α [17], nerve growth factor (NGF) and proteases such as trypsin [18]. These stimulate primary afferent sensory neuron receptors resulting in nociception, as well as peripheral and central sensitisation. As such, they are potential targets for novel selective cancer pain analgesics.
Central mechanisms
Spinal and supra-spinal mechanisms may affect the perception and perpetuation of cancer pain. In animal models of bone cancer pain, increases in descending facilitation from the rostroventral medulla mediated by serotonin (5HT3) lead to enhanced nociception from innocuous stimulation [19]. Ondansetron is an antagonist of 5HT3 though not, as yet, of known clinical benefit.
N-methyl-D-aspartate (NMDA) receptor activation in the cord can result in long term potentiation and central sensitisation. Increase in the ratio of wide dynamic range to nociceptive only neurones in the dorsal horn can lead to augmented responses to non-noxious stimuli.
Ketamine and dextromethorphan have been used as NMDA antagonists and, although evidence for effectiveness in cancer pain as an adjunct to opioids is weak [20], they have a role in opioid-induced hyperalgesia, caused by high dose opioid use, and can “reset” opioid receptors to regain responsiveness.
Specific combinations of spinal cord changes are seen in animal models of cancer pain [21]. Increased glial fibrillary acidic protein (GFAP) is seen in the spinal cord in bone cancer models. This is associated with astrocyte activation. Activation of glia, which is also seen in other pain states, can perpetuate pain by secreting inflammatory cytokines such as interleukin (IL)-1 & 6, TNF-α and prostaglandins, leading to a positive feedback loop. Glial inhibitors, such as anti-TNF-α drugs, are a potential target for analgesics. Increased dynorphin levels are also seen, which at high concentration leads to NMDA receptor activation and pro-nociception. Again, this may be reversed by ketamine.
Advances in drug therapy
New preparations of fentanyl
Buccal, sublingual and intranasal preparations of fentanyl (a highly potent opioid analgesic with a short duration of action) for breakthrough pain, are used widely in palliative care. Analgesia is of rapid onset. Within 15 minutes, the drug is absorbed through the oral mucosa, is usually well tolerated and is effective in about 75% of patients for breakthrough cancer pain. These preparations can be difficult to withdraw in cancer survivors and should not be used outside their licensed indication.
Agents for neuropathic pain
Tricyclic antidepressants and gabapentinoids are often used as adjuncts to opioids for neuropathic pain. In neuropathic cancer pain they are rarely used alone, prior to opioids: a recent study showed that all antineuropathic agents are effective and there is a morphine-sparing effect of pregabalin [22]. Combinations are more effective than one drug alone, and only 15-30% of patients attain the gold standard of 50% pain relief. Preliminary studies show that duloxetine, a serotonin and noradrenaline re-uptake inhibitor originally developed as an antidepressant, reduces chemotherapy-induced peripheral neuropathic pain in some patients [23].
Tapentadol
Tapentadol is a novel centrally acting analgesic that is both a noradrenaline uptake inhibitor and a mu agonist (acts on opioid receptors). A study by Mercadante et al. concluded that it is effective for cancer pain [24]. This 50 patient 4-week open label prospective study of opioid naïve patients with moderate to severe cancer pain showed significantly improved pain scores and quality of life.
Capsaicin
Capsaicin, the active ingredient of the hot red chilli pepper, activates the transient receptor potential vanilloid 1 (TRPV) receptor expressed in nociceptive sensory nerves and defunctionalizes nociceptor activity. A recent Cochrane review suggests that low dose topical capsaicin is no better than placebo cream for peripheral neuropathic pain but high concentrations can be effective [25]. It is available as an 8% skin patch (Qutenza), which is applied to a hypersensitive area after local anaesthetic as a “one off” treatment. If effective it can be repeated after 3 months. Qutenza is not licensed for cancer- or chemotherapy-related neuropathic pain and its role in cancer treatment has not yet been evaluated.
Lidocaine
Topical lidocaine 5% medicated plasters are licensed for post herpetic neuralgia but are recommended in a recent review as first-line treatment for localized neuropathic pain from a variety of causes [26]. The plasters are cooling, liked by patients, and are remarkably safe. There are no studies of their use in cancer pain.
Intrathecal drug therapy
Neuraxial (epidural and intrathecal) infusions of local anaesthetic with or without opioid make a most important contribution to the management of severe cancer pain. Indeed, it has been suggested that intrathecal morphine should be the top rung of the analgesic ladder and that intraspinal therapy should be used when the usual pharmacological therapies have failed [27]. Sadly, the provision of intrathecal drug therapy is all too rare in the UK, although the expertise is available [28].
Epidural infusions of local anaesthetic with opioid, targeting the appropriate nerve roots, are effective but require large infusion volumes (2-10 mls/hour) delivered by an external pump. This can be achieved in a hospice or hospital setting but is not practicable for use at home. Intrathecal infusions (into cerebrospinal fluid) are of low volume and can be delivered via a fully implanted pump, which allows the patient complete independence in between pump reservoir refills, at intervals between 3 weeks and 3 months.
The main indications for use of intrathecal drug delivery are:
(a) uncontrolled pain despite high doses of opioids and adjunct drugs;
(b) unacceptable side effects from analgesics;
(c) cancer involving a nerve plexus, most commonly the lumbosacral plexus;
(d) and widespread bony metastases.
Battery powered fully programmable pumps (e.g. those made by Medtronic Ltd; Fig 1) contain a drug reservoir of 20 or 40 ml. The intrathecal catheter and pump are inserted under general anaesthesia by a surgeon and are programmed via a computer and telemetric head held over the abdominal skin where the pump is sited. Drugs that can be used are: preservative-free morphine, hydromorphone, clonidine, baclofen, bupivacaine and ziconotide. Doses can be altered as often as necessary and the patient can deliver pre-set boluses via a hand held device called a PTM (Personal Therapy Manager; Fig 2).
Figure 1. Intrathecal pump (Medtronic Ltd.).

Battery powered fully programmable intrathecal pump.
Figure 2. Personal Therapy Manager (PTM).

Wal is pictured in his garden with his PTM remote control (with permission).
A randomised controlled trial showed improved quality of life, sustained pain control and significantly less drug-related toxicity with intrathecal drug delivery (ITDD) compared to comprehensive medical management [29]. Survival was significantly longer in the ITDD arm of the trial. There have been other case series and a recent review article supportive of the technique [30-32].
Personal experience consisting of a case series of 24 patients with pain from advanced cancer (where other therapies have failed to provide pain relief) supports the findings of sustained pain control, lack of sedation, no serious complications and improved quality of life.
Here is a recent quote from the widow of a patient: “…without the benefit of the pump I cannot imagine how much additional medication he would have needed and what effect that would have had on the quality of his life”.
Vertebroplasty and kyphoplasty
Metastatic spread of cancer to the vertebral bodies in the spine occurs in one third of patients with carcinoma and half of those with distant metastases [33], most commonly in the thoracic spine. Pain is a common symptom, presenting as back pain if the metastasis is isolated to the body of the vertebra, or with radiating pain in the distribution of compressed nerve roots if the tumour starts to invade the spinal canal. Vertebroplasty is a technique of injecting acrylic cement percutaneously into the affected vertebral body or bodies under fluoroscopic imaging. Kyphoplasty is a newer modification of vertebroplasty where a specialised balloon is inflated in the vertebral body prior to injection of cement. This minimises leakage of cement and reduces kyphotic deformity. A systematic review of safety and efficacy of percutaneous vertebroplasty in malignancy in 987 cases indicated pain reduction ranging between 47 and 87%, but a 2% risk of serious complications including 5 deaths, 4 cement pulmonary emboli, and frequent cement leaks, 12 of which caused neuropathy requiring emergency decompression [34]. The authors comment on a lack of robust data. The same authors published a more optimistic prospective study of percutaneous vertebroplasy in 125 patients with myeloma and spinal metastases and reported reduction in pain and improvement in mobility without serious complications [35].
Figure 3. Vertebroplasty to a thoracic vertebral osteolytic lesion.
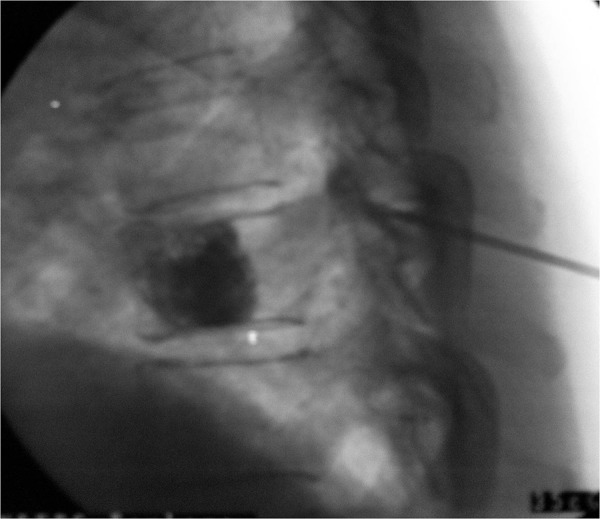
Credit, Dr Arun Bhaskar, with permission.
Useful interventional techniques
Since the palliative care movement gathered pace in the 1970s and 1980s, requests for “permanent” nerve blocks have become fewer, partly because of perceived lack of effectiveness or harm from the blocks and partly because anaesthetists (who perform the blocks) became more remote and less experienced. There are a few interventional techniques that still have an important role in complex cancer pain management.
Percutaneous cervical cordotomy
This is a radiofrequency lesion to the spinothalamic tract at C1-2 level in the cervical spinal cord for pain on the opposite side of the body, usually from mesothelioma or breast carcinoma causing severe unilateral pain. It is performed at four centres only in the UK. Case series show effectiveness in 85-90% cases, and a reduction in drug-related toxicity, with minimal adverse effects [36].
Coeliac plexus block
This is a neurolytic block of the coeliac ganglia, situated anterior to the body of L1 vertebra, which innervate the organs of the upper abdomen through the sympathetic nervous system. It is a technique that was widely performed via a posterior approach until the mid 1990s when a retrospective postal survey showed an incidence of paraplegia following the block of 0.15% [37]. However, evidence points to effective pain relief and reduction in opioid use following a successful block [38-40]. There are many approaches to the coeliac ganglia described in the literature: none is better than another, but use of CT scanning, ultrasonography-guided blocks via the anterior route, or the video-thorascopic approach adopted in recent years may improve safety [41].
Intrathecal neurolysis
This is the injection of phenol or alcohol onto the dorsal roots of selected nerves via a spinal needle sited within the dural sac. Safety depends on correct positioning of the patient and skill of the operator. The technique is performed in a few centres only in the UK but selective dorsal thoracic root blocks are helpful for chest wall pain and saddle blocks for pelvi-sacral pain [42].
Ultrasound guided nerve blocks
Improvements in ultrasound technology have greatly improved accuracy of placement of needles and catheters adjacent to nerves, and there is increasing use by anaesthetists for regional anaesthesia. A literature review by Tran et al. concluded that for axillary blocks the use of ultrasound increases the likelihood of the block being successful [43]. Portable machines make bedside use in hospices and other settings a realistic goal. Brachial plexus, lumbar plexus, femoral, intercostal, sciatic and other nerve blocks are now readily achievable and may become more commonplace again.
The pain-relieving role of radiotherapy and chemotherapy
Palliative radiotherapy is used to relieve pain from brain and bony metastases. A Cochrane review shows a complete response in 25% at one month, with a further 40% of patients with painful bony metastases getting 50% pain relief [44-46]. The radionuclides strontium-89 and radium-223 have been used successfully to treat pain from prostate cancer bone secondaries [47].
Palliative chemotherapy is most successful in lymphoma, germ cell tumours, small cell lung tumours and breast carcinoma. Chemotherapy can also induce pain, especially neuropathic pain.
Bisphosphonates reduce cancer bone pain in 50% of recipients regardless of the primary tumour [48] and should be considered where analgesics and/or radiotherapy are inadequate for the management of painful bony metastases [49]. Serious adverse effects include osteonecrosis of the jaw.
Hormone therapy improves pain in breast and prostate cancer. Dual oncogenic and nociceptive signalling antagonists are being developed [50].
Neuromodulation
Neuromodulation is the technique of altering nerve activity by the application of electric currents. The most common technique is spinal cord stimulation with wires/paddles adjacent to the dorsal columns, but other modalities include peripheral stimulation, motor cortex and deep brain stimulation. Spinal cord stimulation has been shown to be particularly effective in neuropathic pain (15-40% of cancer pain), vascular pain and recently high-frequency stimulation has been shown to be effective in controlling nociceptive pain [51]. Despite its success in chronic pain, there has been little research into neuromodulation for cancer pain.
A 2013 Cochrane review on spinal cord stimulation in cancer-related pain found no randomised controlled trials, 4 longitudinal case series and 14 case reports [52]. The most significant case series was performed by Shimoji et al. [53]. This was a retrospective analysis of 454 patients with spinal cord stimulators, of which 52 had cancer pain. A total of 87% of these had significant pain relief. Notably, this was a much higher percentage response to spinal cord stimulation than for their overall response (56%). Other studies have shown efficacy of spinal cord stimulation in post thoracotomy pain for lung carcinoma [54], back pain related to carcinoma [55], leg pain [56,57] and groin/testicular pain [58,59]. Whilst more studies are needed, spinal cord stimulation appears to be a promising therapy for intractable cancer pain. Evidence for other forms of neuromodulation in cancer pain is very limited.
Other techniques used for cancer pain
Complementary therapies
Complementary and alternative therapies are widely used for the management of cancer pain and associated distress. Despite this, the evidence base is relatively poor. A systematic review performed by Bardia et al. found 18 randomised controlled trials on this subject, of which only 7 were of high quality [60]. Improvements in pain control were seen with acupuncture, support groups, hypnosis and relaxation/imaging therapies. Many studies were of poor quality with small numbers and no blinding. Further, higher quality studies are needed to confirm the efficacy of these treatments.
Educational interventions
Although opioids are effective drugs at reducing cancer pain, there are significant psychological barriers that prevent physicians from prescribing them and patients from accepting them. These are illustrated in the recent National Institute for Health and Clinical Excellence (NICE) guidelines on opioids in palliative care [61]. Several interview-based studies have highlighted patients' concerns about opioids [62-64]. They include concerns over addiction, tolerance, side effects and issues such as acceptance of palliation rather than treatment, and the feeling that starting opioids is a landmark for end-of-life care.
Educational interventions aim to reduce these barriers by providing information, thus increasing patient satisfaction and adherence to medication, leading to improved pain control. There have been many studies on the efficacy of educational interventions. The meta-analysis performed by Bennett et al. in 2009 demonstrated that educational interventions, compared to usual care, led to improvements in patients knowledge and attitudes and an improvement in average pain score of around 1 point out of 10 [65]. Interestingly, they did not find any improvement in the consequence of pain on patient function or adherence to medication. A systematic review by Ling et al. in 2012 again found a significant improvement in pain control with educational interventions but no improvement in quality of life [66].
In summary
Cancer pain is complex and multifactorial in origin, which makes management of pain difficult in at least a quarter of patients. Careful assessment of the types and sources of pain must be made and then treated holistically, adapting treatment to the needs of the individual. Recent advances in drug therapies, interventional techniques and the multiplicity of approaches that may be necessary are outlined in this short article; there are few rigorous trials in cancer patients and there remain many unknowns. The specialities of oncology, palliative care and pain medicine must work together to achieve the best possible pain management for patients.
Acknowledgments
The authors would like to thank Dr Mark Linch, Consultant Medical Oncologist, Sarcoma Unit, Royal Marsden Hospital, Fulham Road, London SW3 6JJ for advice about the pain relieving role of radiotherapy and chemotherapy.
Abbreviations
- ITDD
intrathecal drug delivery
- NMDA
N-methyl-D-aspartate
- PTM
personal therapy manager
- TNF
tumor necrosis factor
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/6/10
References
- 1.Bonica J VV, Twycross RG. The Management of Pain. 2. Philadelphia: Lea and Febiger; 1990. Cancer pain; pp. 400–401. [Google Scholar]
- 2.WHO Cancer Pain Relief. Geneva. 1986 [Google Scholar]
- 3.Twycross R, Harcourt J, Bergl S. A survey of pain in patients with advanced cancer. J Pain Symptom Manage. 1996;12:273–82. doi: 10.1016/S0885-3924(96)00149-2. [DOI] [PubMed] [Google Scholar]
- 4.Weiss SC, Emanuel LL, Fairclough DL, Emanuel EJ. Understanding the experience of pain in terminally ill patients. Lancet. 2001;357:1311–5. doi: 10.1016/S0140-6736(00)04515-3. [DOI] [PubMed] [Google Scholar]
- 5.Seyfried O, Hester J. Opioids and Endocrine Dysfunction. British Journal of Pain. 2012;6(1):17–24. doi: 10.1177/2049463712438299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100:851–8. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL. Mechanism of cancer pain. Mol Interv. 2010;10:164–78. doi: 10.1124/mi.10.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–31. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242815
- 9.Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007;25:99–104. doi: 10.1007/s00774-006-0734-8. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242816
- 10.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–6. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 11.Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci. 2001;21:9367–76. doi: 10.1523/JNEUROSCI.21-23-09367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242818
- 12.Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–66. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242819
- 13.Quang PN, Schmidt BL. Peripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in mice. Pain. 2010;149:254–62. doi: 10.1016/j.pain.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quang P, Schmidt B. Endothelin receptor-mediated attenuation of carcinoma-induced nociception is opioid-dependent in mice. The Journal of Pain. 2009;104(4, suppl):S22. doi: 10.1016/j.jpain.2009.01.096. [DOI] [Google Scholar]
- 15.Sevcik MA, Ghilardi JR, Halvorson KG, Lindsay TH, Kubota K, Mantyh PW. Analgesic efficacy of bradykinin B1 antagonists in a murine bone cancer pain model. J Pain. 2005;6:771–5. doi: 10.1016/j.jpain.2005.06.010. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242820
- 16.Fujita M, Andoh T, Ohashi K, Akira A, Saiki I, Kuraishi Y. Roles of kinin B1 and B2 receptors in skin cancer pain produced by orthotopic melanoma inoculation in mice. Eur J Pain. 2010;14:588–94. doi: 10.1016/j.ejpain.2009.10.010. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242821
- 17.Constantin CE, Mair N, Sailer CA, Andratsch M, Xu Z, Blumer MJF, Scherbakov N, Davis JB, Bluethmann H, Ji R, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28:5072–81. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242822
- 18.Lam DK, Schmidt BL. Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway. Pain. 2010;149:263–72. doi: 10.1016/j.pain.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242823
- 19.Gordon-Williams RM, Dickenson AH. Central neuronal mechanisms in cancer-induced bone pain. Curr Opin Support Palliat Care. 2007;1:6–10. doi: 10.1097/SPC.0b013e328133f5e9. [DOI] [PubMed] [Google Scholar]
- 20.Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2012;11:CD003351. doi: 10.1002/14651858.CD003351.pub2. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717964702
- 21.Currie GL, Delaney A, Bennett MI, Dickenson AH, Egan KJ, Vesterinen HM, Sena ES, Macleod MR, Colvin LA, Fallon MT. Animal models of bone cancer pain: systematic review and meta-analyses. Pain. 2013;154:917–26. doi: 10.1016/j.pain.2013.02.033. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242824
- 22.Mishra S, Bhatnagar S, Goyal GN, Rana SPS, Upadhya SP. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. Am J Hosp Palliat Care. 2012;29:177–82. doi: 10.1177/1049909111412539. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242825
- 23.Smith EML, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–67. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718000622
- 24.Mercadante S, Porzio G, Ferrera P, Aielli F, Adile C, Ficorella C, Giarratano A, Casuccio A. Tapentadol in cancer pain management: a prospective open-label study. Curr Med Res Opin. 2012;28:1775–9. doi: 10.1185/03007995.2012.739151. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242826
- 25.Derry S, Lloyd R, Moore RA, McQuay HJ. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2009:CD007393. doi: 10.1002/14651858.CD007393.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242827
- 26.Mick G, Correa-Illanes G. Topical pain management with the 5% lidocaine medicated plaster--a review. Curr Med Res Opin. 2012;28:937–51. doi: 10.1185/03007995.2012.690339. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242828
- 27.de Leon-Casasola OA, Medicis ED. My patient with rectal cancer does not have pain control despite high doses of opioids and optimal doses of gabapentin and desipramine. Now what?-Advanced strategies for cancer pain management. Techniques in Regional Anaesthesia and Pain Management. 2000;4:167–173. doi: 10.1053/trap.2000.20598. [DOI] [Google Scholar]
- 28.Williams JE, Grady K. Pain News: Intrathecal drug delivery for the management of pain and spasticity in adults; a national audit. BPS National Intrathecal Drug Audit group. 2008 [ http://www.britishpainsociety.org/bps_nl_winter_2008.pdf] [Google Scholar]
- 29.Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, Buchser E, Català E, Bryce DA, Coyne PJ, Pool GE. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040–9. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242830
- 30.Burton AW, Rajagopal A, Shah HN, Mendoza T, Cleeland C, Hassenbusch SJ, Arens JF. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239–47. doi: 10.1111/j.1526-4637.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 31.Deer TR, Smith HS, Burton AW, Pope JE, Doleys DM, Levy RM, Staats PS, Wallace MS, Webster LR, Rauck RL, Cousins M. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician. 2011;14:E283–312. [PubMed] [Google Scholar]
- 32.Upadhyay SP, Mallick PN. Intrathecal drug delivery system (IDDS) for cancer pain management: a review and updates. Am J Hosp Palliat Care. 2012;29:388–98. doi: 10.1177/1049909111426134. [DOI] [PubMed] [Google Scholar]
- 33.Drury AB, Palmer PH, Highman WJ. Carcinomatous metastasis to the vertebral bodies. J Clin Pathol. 1964;17:448–57. doi: 10.1136/jcp.17.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chew C, Craig L, Edwards R, Moss J, O'Dwyer PJ. Safety and efficacy of percutaneous vertebroplasty in malignancy: a systematic review. Clin Radiol. 2011;66:63–72. doi: 10.1016/j.crad.2010.09.011. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242831
- 35.Chew C, Ritchie M, O'Dwyer PJ, Edwards R. A prospective study of percutaneous vertebroplasty in patients with myeloma and spinal metastases. Clin Radiol. 2011;66:1193–6. doi: 10.1016/j.crad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Jackson MB, Pounder D, Price C, Matthews AW, Neville E. Percutaneous cervical cordotomy for the control of pain in patients with pleural mesothelioma. Thorax. 1999;54:238–41. doi: 10.1136/thx.54.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242832
- 37.Davies DD. Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993;86:264–6. doi: 10.1177/014107689308600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, Mantilla CB, Warner DO. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092–9. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 39.Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg. 1998;85:199–201. doi: 10.1046/j.1365-2168.1998.00563.x. [DOI] [PubMed] [Google Scholar]
- 40.Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefaniak T, Basinski A, Vingerhoets A, Makarewicz W, Connor S, Kaska L, Stanek A, Kwiecinska B, Lachinski AJ, Sledzinski Z. A comparison of two invasive techniques in the management of intractable pain due to inoperable pancreatic cancer: neurolytic celiac plexus block and videothoracoscopic splanchnicectomy. Eur J Surg Oncol. 2005;31:768–73. doi: 10.1016/j.ejso.2005.03.012. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242833
- 42.Slatkin NE, Rhiner M. Phenol saddle blocks for intractable pain at end of life: report of four cases and literature review. Am J Hosp Palliat Care. 2003;20:62–6. doi: 10.1177/104990910302000114. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242834
- 43.Tran DQH, Clemente A, Tran DQ, Finlayson RJ. A comparison between ultrasound-guided infraclavicular block using the “double bubble” sign and neurostimulation-guided axillary block. Anesth Analg. 2008;107:1075–8. doi: 10.1213/ane.0b013e31817ef259. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242835
- 44.McQuay HJ, Collins SL, Carroll D, Moore RA. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev. 2000:CD001793. doi: 10.1002/14651858.CD001793. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242836
- 45.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–36. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 46.Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S, Sahgal A, Silverman L, Gunten C von, Mendel E, Vassil A, Bruner DW, Hartsell W. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–76. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal M, Pigott K, O'Bryan-Tear CG, Thuresson M, Bolstad B, Bruland ØS. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11:20–6. doi: 10.1016/j.clgc.2012.07.002. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242837
- 48.Coleman RE, Guise TA, Lipton A, Roodman GD, Berenson JR, Body J, Boyce BF, Calvi LM, Hadji P, McCloskey EV, Saad F, Smith MR, Suva LJ, Taichman RS, Vessella RL, Weilbaecher KN. Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clin Cancer Res. 2008;14:6387–95. doi: 10.1158/1078-0432.CCR-08-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong R, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database Syst Rev. 2002:CD002068. doi: 10.1002/14651858.CD002068. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242838
- 50.Linch M. Cancer treatments for Pain Relief. Royal Marsden Hospital. 2013 [Google Scholar]
- 51.van Buyten J, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16:59–65. doi: 10.1111/ner.12006. discussion 65-6. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242839
- 52.Lihua P, Su M, Zejun Z, Ke W, Bennett MI. Spinal cord stimulation for cancer-related pain in adults. Cochrane Database Syst Rev. 2013;2:CD009389. doi: 10.1002/14651858.CD009389.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Shimoji K, Hokari T, Kano T, Tomita M, Kimura R, Watanabe S, Endoh H, Fukuda S, Fujiwara N, Aida S. Management of intractable pain with percutaneous epidural spinal cord stimulation: differences in pain-relieving effects among diseases and sites of pain. Anesth Analg. 1993;77:110–6. doi: 10.1213/00000539-199307000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Yakovlev AE, Resch BE, Karasev SA. Treatment of cancer-related chest wall pain using spinal cord stimulation. Am J Hosp Palliat Care. 2010;27:552–6. doi: 10.1177/1049909110373240. [DOI] [PubMed] [Google Scholar]
- 55.Yakovlev AE, Resch BE. Spinal cord stimulation for cancer-related low back pain. Am J Hosp Palliat Care. 2012;29:93–7. doi: 10.1177/1049909111410414. [DOI] [PubMed] [Google Scholar]
- 56.Cata JP, Cordella JV, Burton AW, Hassenbusch SJ, Weng H, Dougherty PM. Spinal cord stimulation relieves chemotherapy-induced pain: a clinical case report. J Pain Symptom Manage. 2004;27:72–8. doi: 10.1016/j.jpainsymman.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Tsubota S, Higaki N, Nagaro T. [A case of neuropathic cancer pain in the lower extremities successfully treated with spinal cord stimulation] Masui. 2009;58:1460–1. [PubMed] [Google Scholar]
- 58.Yakovlev AE, Ellias Y. Spinal cord stimulation as a treatment option for intractable neuropathic cancer pain. Clin Med Res. 2008;6:103–6. doi: 10.3121/cmr.2008.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nouri KH, Brish EL. Spinal cord stimulation for testicular pain. Pain Med. 2011;12:1435–8. doi: 10.1111/j.1526-4637.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 60.Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol. 2006;24:5457–64. doi: 10.1200/JCO.2006.08.3725. [DOI] [PubMed] [Google Scholar]
- 61.NICE clinical guideline 140 Opioids in palliative care: safe and effective prescribing of strong opioids for pain in palliative care in adults. 2012 National Institute for Health and Clinical Excellence. [ http://www.nice.org.uk/nicemedia/live/13745/59285/59285.pdf] [PubMed] [Google Scholar]
- 62.Blanchard H, Batten B. Designing and producing a patient leaflet on morphine. Eu J Pall Care. 1996;3:106–8. [Google Scholar]
- 63.Bender JL, Hohenadel J, Wong J, Katz J, Ferris LE, Shobbrook C, Warr D, Jadad AR. What patients with cancer want to know about pain: a qualitative study. J Pain Symptom Manage. 2008;35:177–87. doi: 10.1016/j.jpainsymman.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Reid CM, Gooberman-Hill R, Hanks GW. Opioid analgesics for cancer pain: symptom control for the living or comfort for the dying? A qualitative study to investigate the factors influencing the decision to accept morphine for pain caused by cancer. Ann Oncol. 2008;19:44–8. doi: 10.1093/annonc/mdm462. [DOI] [PubMed] [Google Scholar]
- 65.Bennett MI, Bagnall A, José Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain. 2009;143:192–9. doi: 10.1016/j.pain.2009.01.016. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242840
- 66.Ling C, Lui LYY, So WKW. Do educational interventions improve cancer patients' quality of life and reduce pain intensity? Quantitative systematic review. J Adv Nurs. 2012;68:511–20. doi: 10.1111/j.1365-2648.2011.05841.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242841


