Abstract
Objective
To evaluate health status and participation restrictions in childhood extremity sarcoma survivors.
Design
Members of the CCSS cohort with extremity sarcomas, who completed 1995, 2003 or 2007 questionnaires, were included.
Setting
Cohort Study of extremity sarcomas survivors.
Participants
Childhood cancer survivors diagnosed and treated between 1970–1986.
Interventions
Not applicable.
Main Outcome Measure
Prevalence rates for poor health status in six domains and five sub-optimal social participation categories were compared by tumor location and treatment exposure with generalized estimating equations adjusted for demographic/personal factors and time/age.
Results
Among 1094 survivors, median age at diagnosis 13 years (range 0–20), current age 33 years (range 10–53), 49% were male, 87.5% Caucasian, and 75% had lower extremity tumors. In adjusted models, when compared to upper extremity survivors, lower extremity survivors had increased risk of activity limitations but lower risk of not completing college. Compared to those who did not have surgery, those with limb-sparing (LS) and upper extremity amputations (UEA) were 1.6 times more likely to report functional impairment; while those with an above the knee amputation (AKA) were 1.9 times more likely to report functional impairment. Survivors treated with LS were 1.5 times more likely to report activity limitations. Survivors undergoing LS were more likely to report inactivity, incomes < $20,000, unemployment and no college degree. Those with UEA more likely reported inactivity, unmarried status and no college degree. Lastly, those with AKA more likely reported no college degree. Treatment with abdominal irradiation was associated with increased risk of poor mental health, functional impairment and activity limitation.
Conclusion
Treatment for lower extremity sarcomas is associated with a 50% increased risk for activity limitations; upper extremity survivors are at 10% higher risk for not completing college. Type of local control influences health status and participation restrictions. Both these outcomes decline with age.
Keywords: upper extremity, lower extremity, sarcoma, survivors, childhood cancer
The introduction of multi-agent chemotherapy1–3 and the use of effective local control modalities have dramatically improved outcomes for patients with pediatric sarcomas. Current series report 5 year event-free survival of 60–70% for these children.4–9 Sarcoma survivors, however, remain at high-risk for medical complications as they age, because their treatment includes high doses of chemotherapy along with aggressive surgical resection and/or high-dose radiotherapy.10 Survivors whose sarcoma was located in either the limb or the limb girdle may experience long-term neurosensory and musculoskeletal impairments that eventually interfere with overall function and health.11
Sensory impairments are particularly problematic for children whose treatment required surgical resection of peripheral nerves or extensive cutaneous tissue,12 and gonadal disorders may contribute to altered growth in children whose treatment included pelvic radiation or surgery.13 Bone mineral density deficits have been reported among survivors treated with radiation to the skeleton, and among those who were exposed to glucocorticoids or cyclophosphamide during treatment.14, 15 Skeletal dysplasia and asymmetry,16–18 limb shortening,19–21 and spinal growth abnormalities,22 such as scoliosis or kyphosis 13 are possible if the growth plate is ablated or damaged during surgery or radiation therapy. Weakened bones are susceptible to fracture;23 structural abnormalities interfere with internal organ system and limb function. Muscular hypoplasia, atrophy,21, 24 fibrosis, weakness,19, 20, 25 and limited joint range of motion19, 21 are possible outcomes. Additionally, prosthetic failure among childhood bone sarcoma survivors who undergo limb sparing surgeries often necessitates additional surgical intervention.26
Previous reports from the Childhood Cancer Survivor Study (CCSS) have shown that extremity sarcoma survivors are at increased risk of poor health status,27 and that poor health status is associated with participation restrictions, such as lower educational achievement, unemployment, and less than optimal levels of physical activity.27 Previous studies have not compared differences in health status and participation among extremity sarcoma survivors by tumor location, nor have they evaluated in detail whether or not the modalities used for local control management influence either health status or participation outcomes. This paper compares health status and participation restriction outcomes between upper and lower extremity sarcoma survivors and examines the influence of the type of local control treatment on these outcomes. We also evaluate health status and participation restriction outcomes longitudinally in this patient population to determine if the trajectory over time varies as a function of tumor location (upper or lower extremity).
Methods
Patient Population
The CCSS is a retrospective cohort study of patients diagnosed with childhood cancer before 21 years of age, who survived five or more years, and who were treated at one of 26 participating institutions between January 1, 1970 and December 31, 1986.28 The CCSS protocol was reviewed and approved by the human subjects committee at each participating institution and informed consent was obtained prior to study participation. Survivors who consented for the study were eligible to participate in a baseline questionnaire, and a subsequent series of questionnaires designed to capture major health events and other focused topics.29 Copies of the CCSS questionnaires and the treatment abstraction forms are available at: http://ccss.stjude.org/.
For the present study, we included individuals enrolled in the CCSS with either a bone or soft tissue sarcoma located in the upper (including the scapular and clavicular areas) or lower (including the sacrum and pelvis) extremity who were alive and participated in the baseline (1994–96), 2003 and/or 2007 questionnaires.
Cancer Treatment Information
Information on the initial characteristics and treatment for the cohort were obtained from the treating institution on all participants who returned a signed medical release. Information collected included initial treatment with specific chemotherapy agents, doses of these agents, surgical procedures performed following diagnosis as well as tumor site and fields and doses of radiotherapy.
Variable Definitions
Outcome
Our study evaluated health status on three separate occasions (baseline, 2003, 2007 questionnaires) using 6 different domains including: general health, mental health, functional impairment, activity limitations, pain and anxiety. Participation restrictions were also evaluated longitudinally at three different occasions (baseline, 2003, 2007 questionnaires) using educational achievement, unemployment, marital status, annual income < $ 20,000 and activity limitations.30
For general health, survivors were asked “Would you say your health is excellent, very good, good, fair or poor?” Participants who responded fair or poor were considered to have poor health. The 18-item Brief Symptom Inventory (BSI-18), a self-report measure of psychological symptoms, was used to assess mental health. Raw scores on each subscale were converted to gender specific T-scores and those who scored 63 or higher on any one of the three subscales or on the global status index31, 32 were classified as reporting poor mental health.
Poor functional status was determined based on participants’ answers to three questions that asked if any impairment or health problem resulted in: 1) needing help with personal cares; 2) needing help with household chores; or 3) difficulty attending work or school. Those who responded yes to any of these questions were classified as having poor functional status. Activity limitation was determined based on participants answers to three questions that asked if over the last two years they were limited in activity for more than three months in: 1) kinds or amounts of moderate activity (moving a table, carrying groceries); 2) walking or climbing a few flight of stairs; 3) walking one block. Those who indicated that their health limited any of these activities for three or more months over the last two years were classified as having an activity limitation.
To classify cancer related pain and anxiety, participants were asked: “Do you currently have pain as a result of your cancer or its treatment?”, and “Do you currently have anxiety/fears as a result of your cancer or its treatment?” Participants who endorsed medium, a lot, or very much pain, or anxiety/fear were classified as having cancer related pain or anxiety.
Participation outcomes were dichotomized. Participation restrictions categories included 1) not graduating from college, 2) unemployment, 3) unmarried status, 4) an annual household income < $20,000, and 5) not participating in any physical activity during the last month.
The measures used to classify the outcomes in this study have been previously validated both in cancer patients,31, 32 and in childhood cancer survivors.27, 30, 33–35
Predictor Variables
Information on the cancer diagnosis was obtained from the treating institution and information on primary therapy was abstracted from medical records. Risk factors of interest included current age in ten year age groups, gender, race/ethnicity, time since diagnosis, tumor location and histologic diagnosis. Treatment-related factors of interest included tumor location, local control modality including type of surgery (none, below the knee amputation, above the knee amputation, arm amputation or limb-sparing) and/or radiotherapy (limb, abdomen and/or chest) and chemotherapy treatment (anthracyclines, alkylating agents, platinum and/or vincristine).
Statistical Analyses
The associations between prevalence of poor health status and participation restrictions with tumor location and treatment exposure were evaluated using generalized linear models with binomial distributions and log links to directly estimate risk ratios. Outcomes are reported as percentages, along with risk ratios and 95% confidence intervals. The models included host-related factors and utilized generalized estimating equations with robust variance estimates to account for within person correlations. Backward selection methods were used for model covariate selection (p < 0.10). Interactions between the age variable and tumor location/local control modality variables were evaluated to determine whether any specific factors were associated with a greater decline in either health status or participation restrictions over time. Adjusted models were used to estimate the change in predicted prevalence over time as a function of age for each outcome. Cohort mean values for other covariates were inputted into these adjusted models. SAS version 9.2a was used for all analysis.
Results
Recruitment
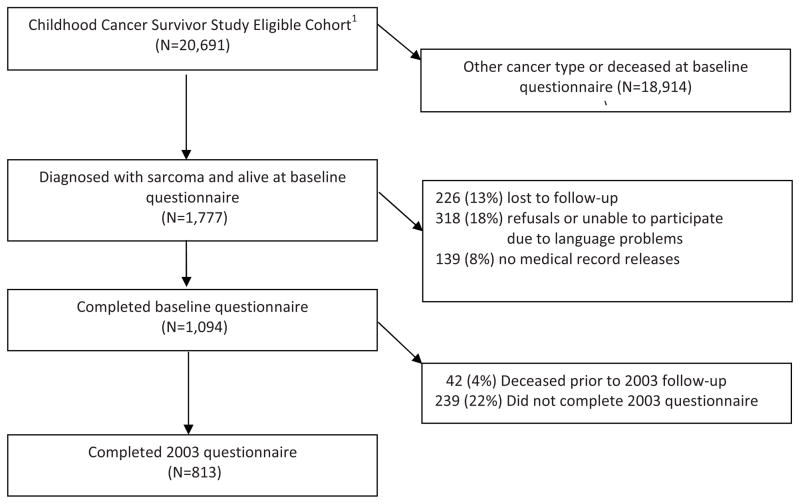
Our study population includes 1094 extremity sarcoma survivors who participated in the baseline questionnaire; 813 survivors who participated in the 2003; and 712 who participated in the 2007 questionnaire (see Figure 1 for details). Among this group of survivors, 661 (60.4%) participated in all three questionnaires. Of the 1094 persons who completed the baseline questionnaire, 42 died prior to completing the 2003 questionnaire, and 27 died prior to completing the 2007 questionnaire. Therefore, among the 1052 persons alive when the 2003 questionnaire was conducted, 77.3% participated. Among the 1025 persons alive when the 2007 questionnaire was completed, 69.5% participated. Baseline health status and participation outcomes differed by questionnaire completion status over time. Those who completed the baseline only were more likely than those who completed the first two, or all three questionnaires to report poor overall health (16.2%, 8.8%, 8.1%), poor mental health (20.0%, 14.4%, 13.5%), functional impairment (20.4%, 11.1%, 10.7%), activity limitations (27.9%, 19.1%, 17.1%), no college education (78.0%, 51.9%, 49.4%), unemployment (25.9%, 14.8%, 13.8%), and annual household incomes <$20,000 (42.3%, 30.5%, 29.0%).
Figure 1.
Flow diagram for extremity sarcoma survivors
Participant Characteristics
Table 1 illustrates the characteristics of the 1094 extremity sarcoma survivors at baseline. Their median age at diagnosis was 13 years (range, 0–20), median age at study entry 18 years (range, 5–25) and median age at questionnaire completion 33 years (range, 10–53). The vast majority of the study participants were Caucasian (87.5%); 49.3% were male and 74.9% had lower extremity tumors. Primary diagnoses were: osteosarcoma (49.0%), soft tissue sarcoma (32.0%), Ewing sarcoma (16.3%), and other bone tumors (2.7%). Chemotherapy treatment included anthracyclines in 64.4% of the population and alkylating agents in 57.1%. Local control included limb irradiation (20.6%), chest irradiation (9.3%) and above the knee amputation (35%).
Table 1.
Characteristics of the study population
| Characteristic | All sarcoma survivors*(N=1094) | Upper Extremity (N=274) | Lower Extremity (N=820) | p-value** | |
|---|---|---|---|---|---|
| Sex | Male | 539 (49.3) | 126 (46.0) | 413 (50.4) | 0.209 |
| Female | 555 (50.7) | 148 (54.0) | 407 (49.6) | ||
|
| |||||
| Race/Ethnicity | White | 957 (87.5) | 244 (89.1) | 713 (87.0) | 0.767 |
| Black | 50 (4.6) | 10 (3.6) | 40 (4.9) | ||
| Hispanic | 49 (4.5) | 13 (4.7) | 36 (4.4) | ||
| Other | 34 (3.1) | 6 (2.2) | 28 (3.4) | ||
| Unknown | 4 (0.4) | 1 (0.4) | 3 (0.4) | ||
|
| |||||
| Age at diagnosis (years) | 0–4 | 97 (8.9) | 37 (13.5) | 60 (7.3) | <.001 |
| 5–9 | 179 (16.4) | 62 (22.6) | 117 (14.3) | ||
| 10–14 | 374 (34.2) | 87 (31.8) | 287 (35.0) | ||
| 15–20 | 444 (40.6) | 88 (32.1) | 356 (43.4) | ||
|
| |||||
| Age at baseline survey (years) | <20 | 101 (9.2) | 35 (12.8) | 66 (8.0) | 0.041 |
| 20–29 | 454 (41.5) | 119 (43.4) | 335 (40.9) | ||
| 30–39 | 497 (45.4) | 113 (41.2) | 384 (46.8) | ||
| 40–49 | 42 (3.8) | 7 (2.6) | 35 (4.3) | ||
|
| |||||
| Survival time at baseline survey (years) | 5–9 | 98 (9.0) | 22 (8.0) | 76 (9.3) | 0.682 |
| 10–14 | 362 (33.1) | 97 (35.4) | 265 (32.3) | ||
| 15–19 | 369 (33.7) | 86 (31.4) | 283 (34.5) | ||
| 20–24 | 231 (21.1) | 62 (22.6) | 169 (20.6) | ||
| 25–29 | 34 (3.1) | 7 (2.6) | 27 (3.3) | ||
|
| |||||
| Age at 2003 questionnaire (years)* | <20 | 4 (0.5) | 3 (1.5) | 1 (0.2) | 0.114 |
| 20–29 | 96 (11.8) | 25 (12.5) | 71 (11.6) | ||
| 30–39 | 420 (51.7) | 108 (54.0) | 312 (50.9) | ||
| 40–49 | 285 (35.1) | 63 (31.5) | 222 (36.2) | ||
| 50+ | 8 (1.0) | 1 (0.5) | 7 (1.1) | ||
|
| |||||
| Survival time at 2003 questionnaire (years)* | 15–19 | 172 (21.2) | 41 (20.5) | 131 (21.4) | 0.566 |
| 20–24 | 288 (35.4) | 64 (32.0) | 224 (36.5) | ||
| 25–29 | 260 (32.0) | 71 (35.5) | 189 (30.8) | ||
| 30–34 | 93 (11.4) | 24 (12.0) | 69 (11.3) | ||
|
| |||||
| Age at 2007 questionnaire (years) * | 20–29 | 25 (3.5) | 12 (6.7) | 13 (2.4) | 0.037 |
| 30–39 | 227 (31.9) | 59 (33.0) | 168 (31.5) | ||
| 40–49 | 397 (55.8) | 96 (53.6) | 301 (56.5) | ||
| 50+ | 63 (8.8) | 12 (6.7) | 51 (9.6) | ||
|
| |||||
| Survival time at 2007 questionnaire (years)* | 20–24 | 164 (23.0) | 42 (23.5) | 122 (22.9) | 0.843 |
| 25–29 | 257 (36.1) | 60 (33.5) | 197 (37.0) | ||
| 30–34 | 223 (31.3) | 60 (33.5) | 163 (30.6) | ||
| 35+ | 68 (9.6) | 17 (9.5) | 51 (9.6) | ||
|
| |||||
| Diagnosis | Ewings sarcoma | 178 (16.3) | 60 (21.9) | 118 (14.4) | <.001 |
| Osteosarcoma | 536 (49.0) | 62 (22.6) | 474 (57.8) | ||
| Soft tissue sarcoma | 350 (32.0) | 141 (51.5) | 209 (25.5) | ||
| Other bone tumors | 30 (2.7) | 11 (4.0) | 19 (2.3) | ||
|
| |||||
| Anthracyclines | None | 362 (35.6) | 125 (48.3) | 237 (31.3) | <.001 |
| Any | 655 (64.4) | 134 (51.7) | 521 (68.7) | ||
|
| |||||
| Alkylating Agents | None | 430 (42.9) | 106 (42.2) | 324 (43.1) | 0.800 |
| Any | 572 (57.1) | 145 (57.8) | 427 (56.9) | ||
|
| |||||
| Platinum | None | 865 (81.1) | 240 (88.6) | 625 (78.6) | <.001 |
| Any | 201 (18.9) | 31 (11.4) | 170 (21.4) | ||
|
| |||||
| Vincristine | None | 444 (40.6) | 105 (38.3) | 339 (41.3) | 0.378 |
| Any | 650 (59.4) | 169 (61.7) | 481 (58.7) | ||
|
| |||||
| Chest Radiation | None | 992 (90.7) | 221 (80.7) | 771 (94.0) | <.001 |
| Any | 102 (9.3) | 53 (19.3) | 49 (6.0) | ||
|
| |||||
| Abdominal Radiation | None | 1081 (98.8) | 269 (98.2) | 812 (99.0) | 0.261 |
| Any | 13 (1.2) | 5 (1.8) | 8 (1.0) | ||
|
| |||||
| Limb Radiation | None | 869 (79.4) | 214 (78.1) | 655 (79.9) | 0.529 |
| Any | 225 (20.6) | 60 (21.9) | 165 (20.1) | ||
|
| |||||
| Thoracotomy | Yes | 135 (12.3) | 21 (7.7) | 114 (13.9) | 0.007 |
| No | 959 (87.7) | 253 (92.3) | 706 (86.1) | ||
|
| |||||
| Limb Surgery | Above Knee Amputation | 381 (34.8) | 3 (1.1) | 378 (46.1) | <.001 |
| Below Knee Amputation | 44 (4.0) | 0 (0.0) | 44 (5.4) | ||
| Arm Amputation | 39 (3.6) | 39 (14.2) | 0 (0.0) | ||
| Limb sparing | 212 (19.4) | 76 (27.7) | 136 (16.6) | ||
| No surgery | 418 (38.2) | 156 (56.9) | 262 (32.0) | ||
1094 persons completed the baseline survey, 813 the 2003 questionnaire, and 712 the 2007 questionnaire
p-value based on chi-square test
Poor Health Status (Tables 2 and 3)
Table 2.
Relative risk* (RR) and 95% Confidence Intervals (CI) of poor health status among sarcoma survivors by tumor location, age at questionnaire, sex race and tumor type
| Poor General Health | Poor Mental Health | Functional Impairment | Activity Limitation | Pain | Anxiety | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | |
| Tumor Location | ||||||||||||||||||
| Upper Extremity | 10.5 | 1.00 | 17.2 | 1.00 | 15.1 | 1.00 | 13.5 | 1.00 | 4.2 | 1.00 | 3.2 | 1.00 | ||||||
| Lower Extremity | 12.3 | 1.14 | 0.88–1.48 | 15.9 | 0.87 | 0.71–1.08 | 19.4 | 1.09 | 0.88–1.35 | 24.5 | 1.53 | 1.23–1.89 | 7.0 | 1.44 | 0.94–2.22 | 3.1 | 0.93 | 0.57–1.50 |
|
| ||||||||||||||||||
| Age at Questionnaire | ||||||||||||||||||
| <30 years | 9.0 | 1.00 | 16.4 | 1.00 | 12.5 | 1.00 | 17.9 | 1.00 | 4.0 | 1.00 | 4.0 | 1.00 | ||||||
| 30–39 years | 11.7 | 1.25 | 0.91–1.71 | 15.0 | 0.88 | 0.70–1.11 | 17.6 | 1.40 | 1.10–1.78 | 19.4 | 1.07 | 0.86–1.32 | 5.9 | 1.35 | 0.86–2.13 | 3.1 | 0.81 | 0.48–1.38 |
| 40+ years | 14.4 | 1.52 | 1.09–2.14 | 17.8 | 1.02 | 0.78–1.32 | 24.2 | 1.96 | 1.52–2.53 | 28.4 | 1.48 | 1.18–1.86 | 8.9 | 1.98 | 1.22–3.20 | 2.4 | 0.70 | 0.36–1.36 |
|
| ||||||||||||||||||
| Sex | ||||||||||||||||||
| Male | 11.1 | 1.00 | 16.6 | 1.00 | 14.0 | 1.00 | 15.6 | 1.00 | 5.4 | 1.00 | 2.2 | 1.00 | ||||||
| Female | 12.5 | 1.13 | 0.91–1.39 | 15.9 | 0.96 | 0.81–1.15 | 22.4 | 1.63 | 1.38–1.93 | 27.7 | 1.78 | 1.53–2.07 | 7.2 | 1.38 | 1.02–1.87 | 3.9 | 1.73 | 1.08–2.77 |
|
| ||||||||||||||||||
| Race | ||||||||||||||||||
| White | 11.8 | 1.00 | 16.1 | 1.00 | 17.9 | 1.00 | 21.9 | 1.00 | 6.2 | 1.00 | 3.1 | 1.00 | ||||||
| Non-white | 11.8 | 1.07 | 0.77–1.50 | 16.9 | 1.07 | 0.80–1.41 | 21.6 | 1.33 | 1.06–1.66 | 20.8 | 0.99 | 0.78–1.25 | 7.5 | 1.31 | 0.83–2.06 | 3.0 | 0.96 | 0.47–1.97 |
|
| ||||||||||||||||||
| Tumor Type | ||||||||||||||||||
| Soft tissue sarcoma | 10.8 | 1.00 | 15.0 | 1.00 | 12.5 | 1.00 | 13.4 | 1.00 | 4.8 | 1.00 | 2.4 | 1.00 | ||||||
| Ewing sarcoma | 13.4 | 1.21 | 0.88–1.67 | 17.8 | 1.21 | 0.92–1.59 | 18.5 | 1.52 | 1.16–2.00 | 21.0 | 1.65 | 1.28–2.13 | 6.5 | 1.43 | 0.88–2.31 | 4.8 | 2.08 | 1.09–3.98 |
| Osteosarcoma | 12.1 | 0.99 | 0.77–1.28 | 16.7 | 1.14 | 0.91–1.43 | 22.5 | 1.69 | 1.36–2.12 | 27.6 | 1.89 | 1.54–2.31 | 7.5 | 1.31 | 0.90–1.93 | 3.0 | 1.32 | 0.74–2.35 |
| Other bone | 8.5 | 0.69 | 0.31–1.52 | 11.8 | 0.76 | 0.38–1.49 | 8.7 | 0.60 | 0.28–1.32 | 20.0 | 1.32 | 0.80–2.16 | 1.5 | 0.25 | 0.04–1.76 | 2.9 | 1.37 | 0.32–5.93 |
Models adjusted for all variables shown and for age at diagnosis
Table 3.
Relative risk (RR) and 95% Confidence Intervals (CI) of reporting poor health status among sarcoma survivors by local control and primary cancer therapy$
| Poor General Health1 | Poor Mental Health2 | Functional Impairment1 | Activity Limitation1 | Pain3 | Anxiety4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | |
| Limb Surgery | ||||||||||||||||||
| None | 15.1 | 1.00 | 18.6 | 1.00 | ||||||||||||||
| AKA* | 23.6 | 1.56 | 1.26–1.94 | 29.8 | 1.45 | 1.21–1.73 | ||||||||||||
| BKA** | 24.1 | 1.61 | 1.09–2.36 | 18.8 | 0.95 | 0.63–1.42 | ||||||||||||
| U/E Amputation*** | 25.3 | 1.85 | 1.17–2.92 | 12.4 | 0.95 | 0.53–1.72 | ||||||||||||
| Limb sparing | 12.6 | 0.92 | 0.69–1.23 | 16.1 | 0.95 | 0.75–1.2 | ||||||||||||
|
| ||||||||||||||||||
| Alkylating Agent | ||||||||||||||||||
| None | 10.3 | 1.00 | 14.7 | 1.00 | ||||||||||||||
| Any | 12.6 | 1.23 | 0.98–1.55 | 19.9 | 1.51 | 1.26–1.81 | ||||||||||||
|
| ||||||||||||||||||
| Platinum | ||||||||||||||||||
| None | 21.0 | 1.00 | ||||||||||||||||
| Any | 24.0 | 1.10 | 0.91–1.33 | |||||||||||||||
|
| ||||||||||||||||||
| Vincristine | ||||||||||||||||||
| None | 5.4 | 1.00 | ||||||||||||||||
| Any | 7.0 | 0.70 | 0.48–0.97 | |||||||||||||||
| Abdominal Radiation | ||||||||||||||||||
| None | 16.0 | 1.00 | 18.2 | 1.00 | 21.7 | 1.00 | 6.2 | 1.00 | 3.0 | 1.00 | ||||||||
| Any | 34.8 | 2.24 | 1.25–4.02 | 26.9 | 1.83 | 1.01–3.33 | 34.6 | 2.28 | 1.5–3.45 | 20.8 | 4.63 | 1.66–12.94 | 12.5 | 4.17 | 1.42–12.26 | |||
|
| ||||||||||||||||||
| Limb Radiation | ||||||||||||||||||
| None | 11.4 | 1.00 | 2.7 | 1.00 | ||||||||||||||
| Any | 13.6 | 1.19 | 0.91–1.55 | 4.6 | 1.68 | 1.04–2.7 | ||||||||||||
|
| ||||||||||||||||||
| Thoractomy | ||||||||||||||||||
| None | 15.7 | 1.00 | ||||||||||||||||
| Any | 11.3 | 1.49 | 1.10–2.02 | |||||||||||||||
Backward selection methods used for model covariate selection and those with p<0.1 were included.
Model adjusted for all variables shown and for tumor location, age at questionnaire, gender
Model adjusted for all variables with RR shown and for tumor location, age at diagnosis
Model adjusted for all variables with RR shown and for tumor location, age at diagnosis, gender
Model adjusted for all variables with RR shown and for tumor location and gender
AKA=Above knee amputation
BKA=Below knee amputation
U/E=Upper Extremity
Compared to upper extremity survivors, lower extremity survivors more frequently reported activity limitations. Older age at questionnaire was associated with poor general health, functional impairment, activity limitations and pain; while female gender was associated with functional impairment, activity limitations, pain and anxiety. Additionally, non-Caucasian ethnicity was associated with functional impairment. When compared to those with soft tissue sarcoma, survivors with osteosarcoma and Ewing sarcoma were more likely to report functional impairments and activity limitations. Patients with a diagnosis of Ewing sarcoma had an increased risk of cancer-related anxiety.
Amputation of any type was associated with functional impairment; amputation above the knee was associated with activity limitations. Exposure to alkylating agents was also associated with functional impairment. A history of abdominal radiation was associated with poor mental health, functional impairment, activity limitations, pain and cancer related anxiety. Limb radiation was associated with poor general health and cancer related anxiety and a history of a thoracotomy was associated with reporting poor general health.
Participation Restrictions (Tables 4 and 5)
Table 4.
Relative risk* (RR) and 95% Confidence Intervals of reporting participation restrictions among sarcoma survivors (limited to those 25+ years old)
| Did not graduate from college | Unemployed | Not married or living as married | Personal income <$20,000/year | No physical activity in the past month | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | ||||
| Tumor Location | ||||||||||||||||||
| Upper Extremity | 45.6 | 1.00 | 13.1 | 1.00 | 34.5 | 1.00 | 27.4 | 1.00 | 27.1 | 1.00 | ||||||||
| Lower Extremity | 41.8 | 0.87 | 0.77–0.97 | 12.5 | 0.81 | 0.62–1.06 | 32.2 | 0.95 | 0.83–1.10 | 31.6 | 1.09 | 0.93–1.28 | 32.3 | 1.07 | 0.91–1.26 | |||
|
| ||||||||||||||||||
| Age at Questionnaire | ||||||||||||||||||
| <30 years | 47.1 | 1.00 | 11.7 | 1.00 | 48.2 | 1.00 | 30.4 | 1.00 | 32.8 | 1.00 | ||||||||
| 30–39 years | 40.6 | 0.85 | 0.74–0.97 | 11.4 | 0.96 | 0.69–1.33 | 31.9 | 0.68 | 0.59–0.78 | 27.2 | 0.96 | 0.80–1.16 | 32.2 | 0.97 | 0.81–1.15 | |||
| 40+ years | 43.9 | 0.92 | 0.80–1.07 | 14.9 | 1.25 | 0.88–1.78 | 26.9 | 0.59 | 0.50–0.70 | 35.9 | 1.29 | 1.06–1.57 | 28.6 | 0.82 | 0.67–1.00 | |||
|
| ||||||||||||||||||
| Sex | ||||||||||||||||||
| Male | 42.4 | 1.00 | 10.2 | 1.00 | 35.0 | 1.00 | 20.8 | 1.00 | 29.4 | 1.00 | ||||||||
| Female | 43.1 | 1.01 | 0.92–1.11 | 14.9 | 1.44 | 1.16–1.80 | 30.6 | 0.86 | 0.76–0.96 | 40.2 | 1.90 | 1.66–2.18 | 32.6 | 1.13 | 1.00–1.27 | |||
|
| ||||||||||||||||||
| Race | ||||||||||||||||||
| White | 41.7 | 1.00 | 12.2 | 1.00 | 31.5 | 1.00 | 30.4 | 1.00 | 30.5 | 1.00 | ||||||||
| Non-white | 51.8 | 1.23 | 1.07–1.41 | 16.7 | 1.42 | 1.04–1.93 | 43.6 | 1.32 | 1.13–1.53 | 32.6 | 1.04 | 0.85–1.27 | 35.6 | 1.12 | 0.93–1.34 | |||
|
| ||||||||||||||||||
| Tumor Type | ||||||||||||||||||
| Soft tissue sarcoma | 43.7 | 1.00 | 9.1 | 1.00 | 32.5 | 1.00 | 26.3 | 1.00 | 27.1 | 1.00 | ||||||||
| Ewings sarcoma | 36.1 | 0.84 | 0.71–0.99 | 12.4 | 1.38 | 0.96–2.00 | 34.6 | 1.06 | 0.88–1.26 | 26.4 | 1.05 | 0.85–1.29 | 27.6 | 1.02 | 0.82–1.26 | |||
| Osteosarcoma | 44.9 | 1.07 | 0.95–1.20 | 14.7 | 1.64 | 1.23–2.20 | 32.7 | 1.07 | 0.93–1.24 | 34.4 | 1.30 | 1.11–1.52 | 35.1 | 1.24 | 1.06–1.45 | |||
| Other bone | 31.1 | 0.73 | 0.50–1.06 | 14.3 | 1.44 | 0.74–2.80 | 26.6 | 0.96 | 0.64–1.42 | 30.0 | 1.05 | 0.70–1.59 | 16.9 | 0.60 | 0.35–1.05 | |||
Models adjusted for all variables shown and for age at diagnosis
Table 5.
Relative risk (RR) and 95% Confidence Intervals (CI) of reporting participation restrictions among sarcoma survivors by local control and primary cancer therapy$
| Did not graduate from college1 | Unemployed2 | Not married or living as married3 | Income <$20,000K/year4 | No physical activity in past month5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | % | RR | 95% CI | |
| Limb Surgery | |||||||||||||||
| None | 37.0 | 1.00 | 10.2 | 1.00 | 31.7 | 1.00 | 27.0 | 1.00 | 28.1 | 1.00 | |||||
| AKA* | 45.8 | 1.36 | 1.18–1.56 | 16.3 | 1.88 | 1.38–2.55 | 33.3 | 1.15 | 0.99–1.34 | 36.8 | 1.69 | 1.31–2.19 | 36.3 | 1.34 | 1.14–1.56 |
| BKA** | 47.4 | 1.46 | 1.15–1.86 | 14.1 | 1.78 | 1.0–3.17 | 38.6 | 1.31 | 1.01–1.71 | 30.9 | 1.34 | 0.81–2.22 | 38.8 | 1.39 | 1.06–1.83 |
| U/E Amputation*** | 62.3 | 1.8 | 1.48–2.18 | 20.3 | 1.65 | 0.97–2.80 | 39.7 | 1.06 | 0.77–1.47 | 35.2 | 1.73 | 0.97–3.09 | 21.8 | 0.76 | 0.48–1.19 |
| Limb sparing | 43.3 | 1.11 | 0.95–1.30 | 8.8 | 0.84 | 0.58–1.24 | 31.1 | 0.96 | 0.81–1.14 | 25.4 | 0.94 | 0.70–1.26 | 26.4 | 0.94 | 0.78–1.14 |
| Alkylating Agent | |||||||||||||||
| None | 40.9 | 1.00 | 11.1 | 1.00 | 32.6 | 1.00 | 28.5 | 1.00 | |||||||
| Any | 43.8 | 1.21 | 1.07–1.37 | 13.6 | 1.44 | 1.11–1.86 | 34 | 0.95 | 0.83–1.09 | 31.6 | 1.50 | 1.18–1.91 | |||
| Anthracycline | |||||||||||||||
| None | 44.5 | 1.00 | 11.4 | ||||||||||||
| Any | 41.2 | 0.81 | 0.71–0.91 | 12.9 | |||||||||||
| Vincristine | |||||||||||||||
| None | 13.1 | 1.00 | 31.9 | 1.00 | 31.3 | 1.00 | |||||||||
| Any | 12.3 | 1.33 | 1.03–1.71 | 33.4 | 0.90 | 0.79–1.03 | 30.1 | 1.27 | 1.00–1.62 | ||||||
| Chest Radiation | |||||||||||||||
| None | 33.4 | 1.00 | 31.4 | 1.00 | |||||||||||
| Any | 25.7 | 0.80 | 0.62–1.03 | 22.7 | 0.62 | 0.41–0.92 | |||||||||
Backward selection methods used for model covariate selection and those with p<0.1 were included.
Model adjusted for all variables shown and for tumor location, age at questionnaire, and race
Model adjusted for all variables shown and for tumor location, gender, and race
Model adjusted for all variables shown and for tumor location, age at questionnaire, gender, and race
Model adjusted for all variables shown and for tumor location, age at questionnaire, age at diagnosis, and gender
Model adjusted for all variables shown and for tumor location, age at questionnaire and age at diagnosis
AKA=Above knee amputation
BKA=Below knee amputation
U/E=Upper Extremity
Tumor location was associated only with educational attainment. Those who had an upper extremity tumor were more likely than those who had a lower extremity tumor to report no college education (45.6% vs. 41.8%). Survivors aged 30–39 years were less likely than those younger than 30 years of age to report no college education and be unmarried; while females were more likely than males to be unemployed, have annual household incomes < $20,000 and report no activity during the past month. Non-Caucasian ethnicity was associated with no college education, unemployment, and unmarried status. Osteosarcoma survivors were more likely than soft tissue sarcoma survivors to report no activity in the past month.
Amputations were associated with not having a college education, and when in the lower extremity, with unemployment, unmarried status and no physical activity in the past month. Above the knee amputation was also associated with annual household incomes < $20,000. Exposure to alkylating agents was associated with no college education, unemployment and incomes < $20,000.
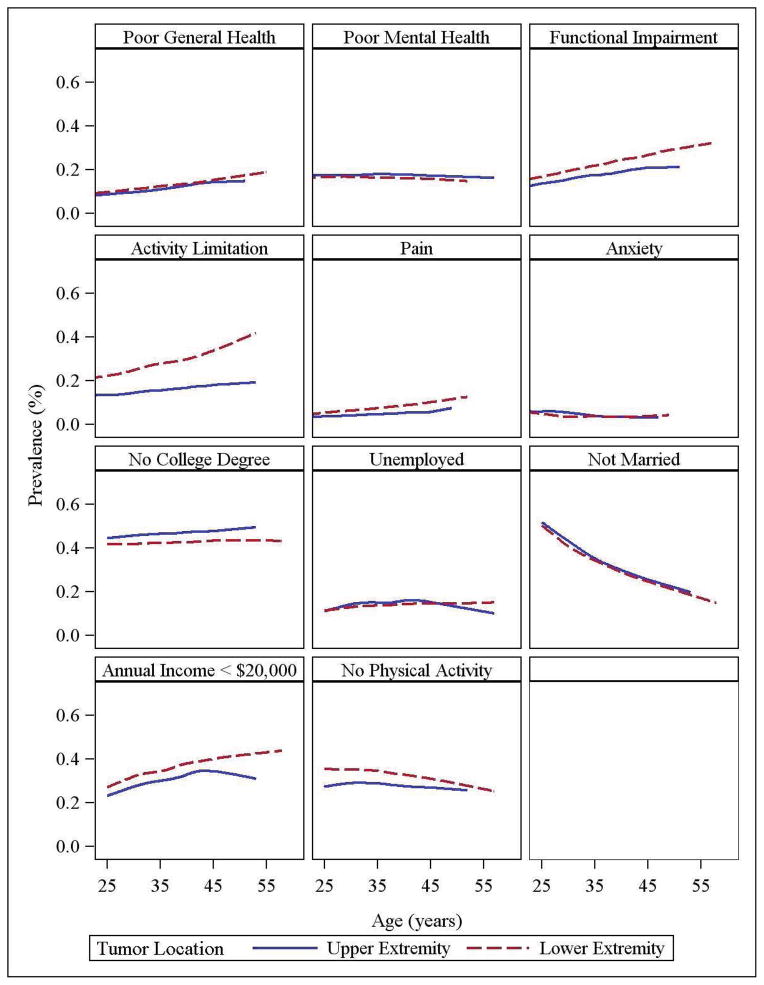
Longitudinal Evaluation of Health Status and Participation Restrictions (Figure 2)
Figure 2.
Longitudinal Evaluation of Health Status and Participation Restrictions
Based on models including tumor location, time, age at diagnosis, sex, race, and diagnosis, the adjusted proportion of extremity tumor survivors who report poor general health, functional impairment, activity limitations and cancer related pain increases with age. The number of unmarried survivors decreases as the cohort ages. Older survivors appear at increased risk of having an income <$20,000. These age-related changes do not differ as a function of tumor location (all p values for interaction > 0.05).
Discussion
This study evaluated health status and participation restrictions in upper and lower extremity sarcoma survivors treated for cancer during childhood. We report that survivors of lower extremity sarcomas were at 50% higher risk for activity limitations when compared to those with upper extremity sarcomas, and that upper extremity survivors were at 10% higher risk than lower extremity survivors of not completing college. It is likely these outcomes are not only a result of local control, i.e. surgery or radiation, but also related to the impact of chemotherapy on normal tissue (e.g. vincristine induced peripheral neuropathy). As suspected, local control methods, particularly amputation, and advancing age, influenced these outcomes. The results of our study expand 27, 30, 33 on previous findings from the CCSS by reporting the impact of tumor location, local control methods, and aging on both health status and participation restrictions among a group of survivors now decades from their original therapy.
This study adds to the literature by comparing large groups of upper extremity tumor survivors to lower extremity tumor survivors, and including tumor type and local control mechanisms in adjusted models. Previous work comparing survivors by tumor location were constrained by the small numbers of upper extremity survivors, limiting power for multiple variable assessments.36, 37 New information in this manuscript includes the finding that arm amputation is associated with functional limitations, and an increased likelihood of not graduating from college, when compared to persons who did not have surgery. Additionally, our results show that abdominal irradiation, likely applied because of tumor metastases and an indicator of more severe initial disease is a more important predictor of poor health status than is radiotherapy used locally to control the tumor. Abdominal radiation is associated with gastrointestinal motility problems, nausea, hematological abnormalities and fatigue.
Although a number of previous studies have evaluated outcomes in extremity sarcoma survivors,36–44 most studies were small, used different outcome measures and did not include survivors who were decades from their original diagnosis. Many studies have focused on the disability differences between survivors with limb sarcoma who were treated with limb sparing surgery compared to those who had amputation.38, 40, 45 In general, these studies indicate there are little differences between amputees and patients undergoing limb-salvage surgery in terms of disability. One particular study suggests that outcomes are better in survivors with more functional lower limbs regardless of surgical procedure performed.36 Another study suggests that though limb sparing surgery is associated with a greater need for re-operation, functional outcomes are better.44 Clearly, the results and conclusions regarding outcome for patients receiving surgery are dependent on the outcome measures used. Furthermore, our results suggest the possibility that, over time, there may be a difference in functional outcomes and ability to participate in life roles by mechanism of surgical control. We found individuals treated with amputation were at the greatest risk for functional impairments and activity limitations; amputations of the lower limb were associated with unemployment, unmarried status and low levels of physical activity; and amputations above the knee were associated with annual incomes <$20,000 per year.
Our study is the first that we know of to evaluate the longitudinal trajectory of health status and participation in a large aging cohort of extremity sarcoma survivors most of whom were enrolled on study during adulthood. Our study indicates that functional impairment, activity limitations, general health, cancer-related pain, and a low income all worsen over time as survivors’ age. Although the worsening health is not surprising given that aging in the general population is also associated with worsening health status, the increased percentage of survivors whose annual household income falls below $20,000 per year when they are in their fifth decade of life is very concerning. It is possible that physical disability and or declining health eventually limit these survivors’ abilities to be employed and earn an income adequate for self-support.
Study Limitations
Several limitations including the possibility of bias need to be considered when interpreting the results. It appears that, particularly at later time points, our cohort included healthier survivors. That is, survivors who only answered the first questionnaire were sicker than those who responded to more than one questionnaire. This suggests that our estimates of prevalence of poor health and participation restrictions may be conservative. Another limitation is the self-reported nature of the outcomes. Additionally, since the study population includes cancer survivors treated between 1970 and 1986, the health outcomes reported here may not apply to patients treated more recently. This is particularly true for patients undergoing limb sparing surgeries more recently since modern implants appear to have better function.46 However, we believe the results reported here are important since they provide baseline information regarding health outcomes in extremity sarcoma survivors, which represent a small but important group of cancer survivors. Chemotherapy for sarcoma survivors has evolved to consistently include the use of ifosfamide, another alkylating agent. Though the incorporation of this agent is likely to improve long term survival, it is associated with an increased risk of sterility, which could lead to further psychological problems. The use of modern local control methods will increase the number of patients who have limb function sparing surgery as the sole method of local control. The procedures are designed to decrease some of the complications experienced by the survivors in this cohort. Research with more recently treated cohorts of survivors should include longitudinal evaluation of functional outcomes, not only to document likely improved function in the modern surgical era, but also to identify the timing of and the need for rehabilitation interventions in this patient population.
Conclusions
In conclusion, childhood sarcoma survivors are a population at high risk of late sequelae including activity limitations, cancer related pain, unemployment, and low income. Some of these deficits appear to worsen over time as the cohort ages. Although this may be intuitive, this is the first study to evaluate changes in health status longitudinally, and as such, provides an important contribution to the literature. Since the CCSS is assembling an expanded cohort that includes childhood cancer survivors treated more recently, our study serves as a baseline against which to measure further evaluation of health over time for childhood cancer survivors. It will be both interesting and important to evaluate the expansion cohort to determine if the therapeutic advances have improved outcome and decreased late sequelae or whether they have improved initial outcome but worsen the functional outcomes for a group of survivors with a high risk of complications. An important goal for pediatric oncologists is to develop treatment strategies that diminish the negative health impact of therapy and to provide resources to address the probability of decline in social role functions over time. It is clear from our study that survivors of extremity sarcoma may benefit from physical therapy or rehabilitation services to allow them to optimize their functional and participation outcomes over time. Although these patients are referred to physical therapy during treatment, the intensity of chemotherapy often precludes early intense intervention for immediate musculoskeletal complications. Patients with sarcoma likely would benefit from post-chemotherapy rehabilitation interventions in the first year after therapeutic cancer treatment ends to address their musculoskeletal issues. Additionally, organ system dysfunction or joint deterioration over time may require additional bouts of rehabilitation to help sarcoma survivors maintain their functional abilities as they age.
Acknowledgments
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
Abbreviations
- CCSS
childhood cancer survivor study
- LS
limb-sparing
- UEA
upper extremity amputation
- AKA
above the knee amputation
- BKA
below the knee amputation
- BSI-18
18-item Brief Symptom Inventory
Footnotes
SAS version 9.2 (SAS Institute, Inc., 100 SAS Campus Drive Cary, NC 27513-2414)
Presented at the International Society of Pediatric Oncology (SIOP) meeting October 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. The New England journal of medicine. 1986;314(25):1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 2.Raney RB, Maurer HM, Anderson JR, Andrassy RJ, Donaldson SS, Qualman SJ, et al. The Intergroup Rhabdomyosarcoma Study Group (IRSG): Major Lessons From the IRS-I Through IRS-IV Studies as Background for the Current IRS-V Treatment Protocols. Sarcoma. 2001;5(1):9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesbit ME, Jr, Gehan EA, Burgert EO, Jr, Vietti TJ, Cangir A, Tefft M, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol. 1990;8(10):1664–74. doi: 10.1200/JCO.1990.8.10.1664. [DOI] [PubMed] [Google Scholar]
- 4.Arndt CA, Stoner JA, Hawkins DS, Rodeberg DA, Hayes-Jordan AA, Paidas CN, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children’s oncology group study D9803. J Clin Oncol. 2009;27(31):5182–8. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 6.Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children’s Oncology Group Study. J Clin Oncol. 2009;27(15):2536–41. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 8.Jurgens H, Exner U, Gadner H, Harms D, Michaelis J, Sauer R, et al. Multidisciplinary treatment of primary Ewing’s sarcoma of bone. A 6-year experience of a European Cooperative Trial. Cancer. 1988;61(1):23–32. doi: 10.1002/1097-0142(19880101)61:1<23::aid-cncr2820610106>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Marina N. Long-term survivors of childhood cancer. The medical consequences of cure. Pediatr Clin North Am. 1997;44(4):1021–42. doi: 10.1016/s0031-3955(05)70543-5. [DOI] [PubMed] [Google Scholar]
- 11.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of Risk- Based Guidelines for Pediatric Cancer Survivors: The Children’s Oncology Group Long-Term Follow-Up Guidelines From the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg JP, Goodman P, Leisenring W, Ness KK, Meyers PA, Wolden SL, et al. Long-term survivors of childhood Ewing sarcoma: report from the childhood cancer survivor study. Journal of the National Cancer Institute. 2010;102(16):1272–83. doi: 10.1093/jnci/djq278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spunt SL, Sweeney TA, Hudson MM, Billups CA, Krasin MJ, Hester AL. Late effects of pelvic rhabdomyosarcoma and its treatment in female survivors. J Clin Oncol. 2005;23(28):7143–51. doi: 10.1200/JCO.2005.12.096. [DOI] [PubMed] [Google Scholar]
- 14.Kaste SC, Ahn H, Liu T, Liu W, Krasin MJ, Hudson MM, et al. Bone mineral density deficits in pediatric patients treated for sarcoma. Pediatr Blood Cancer. 2008;50(5):1032–8. doi: 10.1002/pbc.21281. [DOI] [PubMed] [Google Scholar]
- 15.Kelly J, Damron T, Grant W, Anker C, Holdridge S, Shaw S, et al. Cross-sectional study of bone mineral density in adult survivors of solid pediatric cancers. J Pediatr Hematol Oncol. 2005;27(5):248–53. doi: 10.1097/01.mph.0000162526.77400.78. [DOI] [PubMed] [Google Scholar]
- 16.Fromm M, Littman P, Raney RB, Nelson L, Handler S, Diamond G, et al. Late effects after treatment of twenty children with soft tissue sarcomas of the head and neck. Experience at a single institution with a review of the literature. Cancer. 1986;57(10):2070–6. doi: 10.1002/1097-0142(19860515)57:10<2070::aid-cncr2820571032>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Fiorillo A, Migliorati R, Vassallo P, Canale G, Tranfa F, Fariello I, et al. Radiation late effects in children treated for orbital rhabdomyosarcoma. Radiother Oncol. 1999;53(2):143–8. doi: 10.1016/s0167-8140(99)00137-1. [DOI] [PubMed] [Google Scholar]
- 18.Raney RB, Anderson JR, Kollath J, Vassilopoulou-Sellin R, Klein MJ, Heyn R, et al. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: Report from the Intergroup Rhabdomyosarcoma Study (IRS)-III, 1984–1991. Med Pediatr Oncol. 2000;34(6):413–20. doi: 10.1002/(sici)1096-911x(200006)34:6<413::aid-mpo6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004;60(1):265–74. doi: 10.1016/j.ijrobp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Paulino AC, Nguyen TX, Mai WY, Teh BS, Wen BC. Dose response and local control using radiotherapy in non-metastatic Ewing sarcoma. Pediatr Blood Cancer. 2007;49(2):145–8. doi: 10.1002/pbc.20904. [DOI] [PubMed] [Google Scholar]
- 21.Craft AW, Cotterill SJ, Bullimore JA, Pearson D. Long-term results from the first UKCCSG Ewing’s Tumour Study (ET-1) United Kingdom Children’s Cancer Study Group (UKCCSG) and the Medical Research Council Bone Sarcoma Working Party. Eur J Cancer. 1997;33(7):1061–9. doi: 10.1016/s0959-8049(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 22.Hughes LL, Baruzzi MJ, Ribeiro RC, Ayers GD, Rao B, Parham DM, et al. Paratesticular rhabdomyosarcoma: delayed effects of multimodality therapy and implications for current management. Cancer. 1994;73(2):476–82. doi: 10.1002/1097-0142(19940115)73:2<476::aid-cncr2820730237>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Wall JE, Kaste SC, Greenwald CA, Jenkins JJ, Douglass EC, Pratt CB. Fractures in children treated with radiotherapy for soft tissue sarcoma. Orthopedics. 1996;19(8):657–64. doi: 10.3928/0147-7447-19960801-09. [DOI] [PubMed] [Google Scholar]
- 24.Raney RB, Asmar L, Vassilopoulou-Sellin R, Klein MJ, Donaldson SS, Green J, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and - III. IRS Group of the Children’s Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33(4):362–71. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Tsauo JY, Li WC, Yang RS. Functional outcomes after endoprosthetic knee reconstruction following resection of osteosarcoma near the knee. Disabil Rehabil. 2006;28(1):61–6. doi: 10.1080/09638280500164008. [DOI] [PubMed] [Google Scholar]
- 26.Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70(2):109–15. doi: 10.1002/(sici)1096-9098(199902)70:2<109::aid-jso9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Ness KK, Hudson MM, Ginsberg JP, Nagarajan R, Kaste SC, Marina N, et al. Physical Performance Limitations in the Childhood Cancer Survivor Study Cohort. Journal of Clinical Oncology. 2009;27(14):2382–9. doi: 10.1200/JCO.2008.21.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Medical and Pediatric Oncology. 2002;38(4):229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 29.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–92. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 31.Derogatis L. Brief Symptom Inventory (BSI) 18: Administration, Scoring, and Procedures MAnual. Minneapolis, Minn: National Computer Systems; 2000. [Google Scholar]
- 32.Zabora J, BrintzenhofeSzoc K, Jacobsen P, Curbow B, Piantadosi S, Hooker C, et al. A new psychosocial screening instrument for use with cancer patients. Psychosomatics. 2001;42(3):241–6. doi: 10.1176/appi.psy.42.3.241. [DOI] [PubMed] [Google Scholar]
- 33.Ness KK, Mertens AC, Hudson MM, Wall MM, Leisenring WM, Oeffinger KC, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143(9):639–47. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 34.Nagarajan R, Kamruzzaman A, Ness KK, Marchese VG, Sklar C, Mertens A, et al. Twenty years of follow-up of survivors of childhood osteosarcoma. Cancer. 2011;117(3):625–34. doi: 10.1002/cncr.25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(14):2390–5. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert RS, Ottaviani G, Huh WW, Palla S, Jaffe N. Psychosocial and functional outcomes in long-term survivors of osteosarcoma: A comparison of limb-salvage surgery and amputation. Pediatric Blood & Cancer. 2010:n/a–n/a. doi: 10.1002/pbc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertucio CS, Wara WM, Matthay KK, Ablin AR, Johnston JO, O’Donnell RJ, et al. Functional and clinical outcomes of limb-sparing therapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2001;49(3):763–9. doi: 10.1016/s0360-3016(00)01415-2. [DOI] [PubMed] [Google Scholar]
- 38.Davis AM, Devlin M, Griffin AM, Wunder JS, Bell RS. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case-control study. Arch Phys Med Rehabil. 1999;80(6):615–8. doi: 10.1016/s0003-9993(99)90161-2. [DOI] [PubMed] [Google Scholar]
- 39.Parsons JA, Davis AM. Rehabilitation and quality-of-life issues in patients with extremity soft tissue sarcoma. Curr Treat Options Oncol. 2004;5(6):477–88. doi: 10.1007/s11864-004-0036-0. [DOI] [PubMed] [Google Scholar]
- 40.Nagarajan R, Neglia JP, Clohisy DR, Yasui Y, Greenberg M, Hudson M, et al. Education, employment, insurance, and marital status among 694 survivors of pediatric lower extremity bone tumors. Cancer. 2003;97(10):2554–64. doi: 10.1002/cncr.11363. [DOI] [PubMed] [Google Scholar]
- 41.Gerber L, Hoffman K, Chaudhry U, Augustine E, Parks R, Bernad M, et al. Functional Outcomes and Life Satisfaction in Long-Term Survivors of Pediatric Sarcomas. Archives of Physical Medicine and Rehabilitation. 2006;87(12):1611–7. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]
- 42.Weddington WW, Jr, Segraves KB, Simon MA. Psychological outcome of extremity sarcoma survivors undergoing amputation or limb salvage. J Clin Oncol. 1985;3(10):1393–9. doi: 10.1200/JCO.1985.3.10.1393. [DOI] [PubMed] [Google Scholar]
- 43.Lane JM, Christ GH, Khan SN, Backus SI. Rehabilitation for limb salvage patients: kinesiological parameters and psychological assessment. Cancer. 2001;92(4 Suppl):1013–9. doi: 10.1002/1097-0142(20010815)92:4+<1013::aid-cncr1414>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 44.Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. The Journal of bone and joint surgery. 1994;76(5):649–56. doi: 10.2106/00004623-199405000-00004. American volume. [DOI] [PubMed] [Google Scholar]
- 45.Zahlten-Hinguranage A, Bernd L, Ewerbeck V, Sabo D. Equal quality of life after limb-sparing or ablative surgery for lower extremity sarcomas. British Journal of Cancer. 2004 doi: 10.1038/sj.bjc.6602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clinical orthopaedics and related research. 2010;468(8):2198–210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]