Abstract
Cells of the gastrointestinal (GI) mucosa are subject to a constant process of renewal which, in normal adults, reflects a balance between the rates of cell production and cell loss. Detailed knowledge of these events is, therefore, essential for a better understanding of the normal aging processes as well as many GI diseases, particularly malignancy, that represent disorders of tissue growth. In general, many GI dysfunctions, including malignancy, increase with advancing age, and aging itself is associated with alterations in structural and functional integrity of the GI tract. Although the regulatory mechanisms for age-related increase in the incidence of GI-cancers are yet to be fully delineated, recent evidence suggests a role for epidermal growth factor receptors and its family members {referred to as EGFR(s)} in the development and progression of carcinogenesis during aging. The present communication discusses the involvement of EGFR(s) in regulating events of GI cancers during advancing age and summarizes the current available therapeutics targeting these receptors. The current review also describes the effectiveness of ErbB inhibitors as well as combination therapies. Additionally, the involvement of GI stem cells in the development of the age-related rise in GI cancers is emphasized.
Keywords: Gastrointestinal tract, aging and gastrointestinal cancers, tyrosine kinases, ErbB family of receptor tyrosine kinases, ErbB-targeted therapies, pan-ErbB, combination therapy, cancer stem cells
INTRODUCTION
This century has witnessed a phenomenal rate of growth of the elderly population (persons 65 years old and over) Both the developed as well as developing regions of the world are experiencing the highest increase in the population aged 60 and over [1]. In developed countries, this aging population is expected to rise from 264 million in 2009 to 416 million in 2050 [1, 2]. However, compared with the more developed world, the population of the less developed regions is aging faster. Over the next two decades, the population aged 60 and over in the developing world is projected to rise from 475 million in 2009 to 1.6 billion in 2050 [1]. Under the Census Bureau’s middle series projections, about 1 in 8 Americans were elderly in 1994, is expected to increase to 1 in 5 by the year 2030 [1]. According to the Census Bureau, the number of elderly will continue to increase and by 2050 nearly a quarter of Americans will be at least 65 years of age [1].
This increase in the aging population has led to a growing interest in achieving a better understanding of the aging processes and of diseases that are predominantly expressed during advancing age. Aging is defined as the progressive accumulation of changes over time that causes increased susceptibility to diseases and eventually to death, the final outcome. Aging is associated with marked changes in the structural and functional properties of many organ systems including the gastrointestinal (GI) tract.
AGING AND GASTROINTESTINAL CANCERS
According to the National Center for Health Statistics (NCHS), cancer remains the second leading cause of death, after heart diseases. Approximately 1.5 million new cases of cancer are expected to be diagnosed in 2009, that may result in close to 60,000 deaths [3]. Cancers of the digestive system are projected to comprise 19% of the total incidence and 24% of cancer related deaths [3]. These include the cancers of esophagus, stomach, small intestine, colon, rectum, anus, liver, gall bladder and pancreas. Colorectal cancer that comprises 53% of all GI cancers, remains a high mortality malignancy [3].
Aging is associated with increased incidence of several cancers including GI malignancies. In the US, cancer related deaths in 2006 were reported to be 23% of the total deaths among the elderly (ages 60 yrs and above), 10% of which were due to colo-rectal cancer. Despite early diagnosis, colorectal cancer remains a major concern due to metastatic recurrence of the malignancy. Colorectal cancer affects both males and females similarly, comprising 10% of total cancer cases and 9% of cancer related deaths [3].
Aging has frequently been associated with increased incidence of GI cancers of which stomach and colon show the highest proportion. Studies by Yamaji et al have reported the effect of age on the malignant potential of colorectal polyps [4,5]. They reported advancing age to be a significant factor in the incidence of non-malignant and malignant colorectal neoplasms. The polyps of older persons demonstrated higher malignant potential than younger subjects irrespective of the lesions’ size. According to Centers for Disease Control and Preventions (CDC-USA), risk of getting colorectal cancers increases with age and is greater in males compared to females [6]. In gastric cancer, intestinal metaplasia represents age-related lesions and is considered to be precancerous [7, 8]. Recently, Majumdar and Basson (2006) have extensively reviewed this topic and the readers are referred to this review [9].
Dynamics of Gastrointestinal Tract
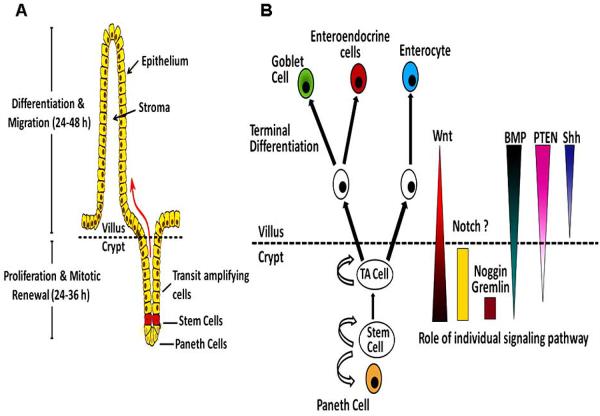
The intestinal epithelium is one of the most investigated organ system in terms of self-renewal and lineage specification. The small intestine is composed of proliferative crypts and differentiated villi, Fig. (1). The epithelial cells in the intestine migrate upwards within the crypts and villi and finally shed into the lumen. There are four principal lineages of epithelial cells in the intestine of the GI tract: 1) columnar cell termed enterocytes in small intestine and colocytes in large intestine 2) mucin secreting cells known as goblet cells in small as well large intestine, and gastric foveolar cells in gastric glands, 3) endocrine, enteroendocrine or neuroendocrine cell, 4) paneth cells in small intestine, Fig. (1). Colorectal epithelium lacks villi and paneth cells [10, 11]. Proliferative cells occupy the bottom two-thirds of the intestinal crypts, while differentiated cells constitute the upper third and the surface epithelium. The GI tract has one of the highest turn-over rates. The self-renewal of the epithelial cells of the GI tract takes 2-7 days under normal conditions, which may be altered in response to physiological stimuli.
Fig. (1).
Schematic representation of architecture and signaling in the small intestinal mucosa. A) In the epithelial lining of the normal intestinal mucosa, stem cells are located at the bottom of crypts and divide asymmetrically to give rise to different lineage of cells. The daughter cells undergoing differentiation migrate upwards to give rise to transit amplifying and terminally differentiated cells. The latter are programmed for cell death and are shed into the lumen. B) Intestinal stem cells give rise to a lineage of differentiated epithelial cells. The proliferation and differentiation of stem cells is tightly regulated by distinct signaling pathways.
The continued renewal of the GI mucosa is sustained by proliferation of stem cells in the GI tract [12, 13]. Due to lack of specific markers, the identity, location, reservoir of stem cells within each gastric gland and intestinal crypts is inconclusive. In the stomach, the differentiating cells migrate bi-directionally from the neck/isthmus region of the gland, which is considered to be the site of stem cell niche [14]. In the small intestine, they are believed to be located at the base of the crypt, just superior to the paneth cells, Fig. (1). In the large intestine, stem cells are proposed to be located in the mid-crypt of ascending colon and crypt base of the descending colon [15]. Owing to its continual self-renewal and mitosis, GI tract is one of the most frequent sites for carcinogenesis. Stem cells and the paneth cells, located at the base of crypts, evade this renewal cycle [16]. Since the lifespan of gastrointestinal epithelial cells is shorter than the time required for the induction of several “Hits”/ mutations to induce neoplastic events, intestinal stem cells are considered to be the putative targets of the oncogenic mutations. Different signaling pathways that regulate intestinal stem cells renewal include AKT/PKB, Wnt, Bone Morphogenic protein (BMP), sonic HedgeHog (HH) and Notch [17]. Dysregulation of one or more of these pathways could lead to neoplastic transformation of GI epithelium. For example, APC, a negative regulator of Wnt signaling [18-22] is frequently mutated in familial adenomatosis polyposis coli (FAP) [23-25]. The significance of aberrant Wnt signaling is gauged by the fact that almost all human adenomas and carcinomas display mutations in one of the Wnt signaling components. These are mainly APC-deletion and β-catenin activating mutations [26].
Dysregulation of Growth as a Potential Premalignant Event
Dysregulated cell proliferation and apoptosis are considered the prime factors among the different probable explanations for the initiation and progression of cancers. The mucosa of the GI tract has one of the highest turnover rates of any tissues in the body, which is maintained by a finely tuned balance between proliferation of precursor cells and ex-foliation of surface cells [13, 27]. Any dysfunction in this fine tuning may accelerate or diminish the growth rate resulting in either hyperplasia/hypertrophy or atrophy of the organ. A better understanding of normal cell proliferation kinetics and regulation of gastrointestinal mucosal growth during aging is, therefore, essential for delineating the mechanisms of pathogenesis of many GI tract diseases and disorders linked to aging, including cancer.
Recent morphological and biochemical studies from several laboratories, including our own have demonstrated that in barrier-reared Fischer-344 rats, aging is associated with increased mucosal proliferative activity in the stomach as well as in the small and large intestines [28-37]. The age-related rise in mucosal proliferative activity, at least in the colon, is also found to be accompanied by an increase in the proliferative zone, a phenomenon that has been observed in patients with precancerous conditions and in subjects with cancer of the colon and/or rectum [36]. Morphologic studies of the colonic mucosa of human volunteers have further revealed that whereas cell proliferation in young subjects is confined to the lower two-thirds of the crypt, with aging there is a major shift from the base to the middle and upper-third of the gland [38, 39]. The observations of the age-related rise in mucosal proliferative activity together with expansion of the proliferative zone in the colon, both of which are common occurrence in precancerous conditions and in cancer, have prompted us and others to postulate that aging may predispose the gastrointestinal tract to neoplasia [29, 32, 35, 40, 41].
In addition to increase in proliferation of the GI mucosa, alterations in apoptosis (programmed cell death) may also contribute to age-related rise in predisposition of the tissues to carcinogenesis [42, 43]. Decreased apoptosis in response to physiological stimuli has been documented in several cells derived from a variety of cancers [42-45]. Colorectal neoplasia, in particular demonstrated a progressive inhibition of apoptosis from normal epithelium to carcinoma [44]. Only scant information is available to associate age-related changes in apoptosis in tissues of the gastrointestinal tract with carcinogenesis. In order to understand whether changes in apoptosis could contribute to the age-related rise in the incidence of gastric and/or colorectal carcinomas, we examined the number of apoptotic cells in the gastric and colonic mucosa by TUNEL assay [37]. We have demonstrated that the number of apoptotic cells in the mucosa of the stomach and colon of 22-24 months old (aged) Fischer-344 rats are lower than in the corresponding tissues from 4 months (young) old counterparts. Additionally, we observed reduced cleavage of 115 kDa full-length poly(ADP-ribose) polymerase, a substrate for caspase-3 and probably of other caspases, in colonic mucosal lysates from 13- and 22-months old Fischer-344 rats, compared to their 4-months old counterparts [37]. These changes were accompanied by a concomitant reduction in the levels of active caspase-3, -8 and -9 [37]. Although reduction of apoptosis was more modest in the gastric mucosa, the fact that the marked increase in proliferative activity in the gastric mucosa in aged rats was not accompanied by a parallel increase in apoptosis suggests that aging may also promote accumulation of cells with secondary genetic changes in the gastric mucosa.
Increased Susceptibility to Carcinogens and Mutational Events
The GI tract is frequently exposed to chemical carcinogens mainly due to ingestion of variety of substances that may include food contaminants like aflatoxin, tobacco, food preservatives such as nitrates that are known to be directly or indirectly associated with increased incidence of several GI cancers [46-54]. Among the various factors that pre-dispose the aging gut to events of carcinogenesis, increased susceptibility to carcinogens could be considered as one of the significant factors. The altered carcinogen metabolism and cumulative effects of long-term mutations has been extensively studied. Several studies, including our own, show that the incidence of aberrant crypt foci (ACF), a pre-malignant lesion increases with aging. ACF are microscopic lesions which were first observed in methylene-stained colons of azoxymethane treated mice as crypts that appeared larger and thicker [55, 56]. ACF which are considered neoplastic and precursors of adenomas and carcinomas [57, 58], show gene mutations commonly observed in adenomatous polyps [58-70]. The number of ACF formed in response to colonic carcinogen azoxymethane increases significantly in aging Sprague Dawley [71] and Fisher-344 rats [72]. We also reported that the ACF formed in aged rats showed a higher proliferative activity than their younger counterparts suggesting a higher basal proliferative activity in the colonic mucosa of aged rats [72]. Additionally, azoxymethane produced a change in distribution of proliferating cells in the colonic mucosa. Most of the proliferating cells were located at the bottom half of the crypt in young rats, whereas, in aged rats they were distributed throughout the crypt with the exception of 10-20% of the upper region. This is a significant finding, considering the fact that we have made similar observations in the colonic mucosa of patients with adenomatous polyps and ulcerative colitis [36], which are considered precancerous [27].
Carcinogenesis is a multi-step process, resulting from accumulation of mutations during progression from normal epithelium to carcinonoma [73]. For example, in colon cancer, it has been suggested that the loss or inactivation of the tumor suppressor gene in adenomatous polyposis coli (APC) initiates genomic instability that may produce the phenotypic appearance of an adenoma. Inactivation of several tumor suppressor genes, including APC, p53 and deleted in colorectal cancer (DCC), has been observed in colon as well as gastric cancer [73, 74]. However, little information is available as to whether aging is associated with increased inactivation of tumor suppressor genes. We have investigated the age-associated changes in mutations of APC, DCC, p53 and K-ras genes in the gastric mucosa of healthy subjects of varying ages (25-91 years) [75]. Specifically, we demonstrated the loss of heterozygosity (LOH) of these genes in the gastric mucosa [75]. Additional age-related events that may contribute to carcinogenesis are inactivation of estrogen receptor, a putative tumor suppressor by methylation [76] and functional inactivation of p16INK4A by hypermethylation [77].
EGFRs IN GASTEROINTESTINAL CARCINOGENESIS
The well defined stages of tumor progression [73, 78-84] has allowed gastrointestinal carcinoma to be studied extensively as an example of multi-step carcinogenesis. Though a number of mutations are associated with oncogenes and tumor susceptibility genes, there are limited studies implicating the tyrosine kinases in the processes of carcinogenesis. Despite being a minor class of cellular proteins, tyrosine kinases represent a major class of oncogenes. They play an indispensable role in regulating cell growth and apoptosis as well as other processes required for the maintenance of cellular homeostasis. Abnormally elevated tyrosine kinase activity is associated with most common human solid tumors including non-small cell lung cancer, colorectal adenocarcinoma, glioblastoma, gastric, pancreatic, breast, ovarian, cervical, and prostate cancer [85-88]. Among the tyrosine kinases studied and implicated in GI cancers are pp60 c-Src, a non-receptor protein, EGFR(s), hepatocyte growth factor receptor (c-met), vascular endothelial growth factor receptor (VEGFR) as well as focal adhesion kinases (FAK) [78, 89-94]. Since EGFR(s) are a major family of tyrosine kinases, we will be focusing on the role of EGFR(s) during aging and carcinogenesis of the gut. Furthermore, EGFR-targeted therapies will be reviewed with particular relevance to GI cancers.
EGFR and its Family Members in Gastrointestinal Cancers
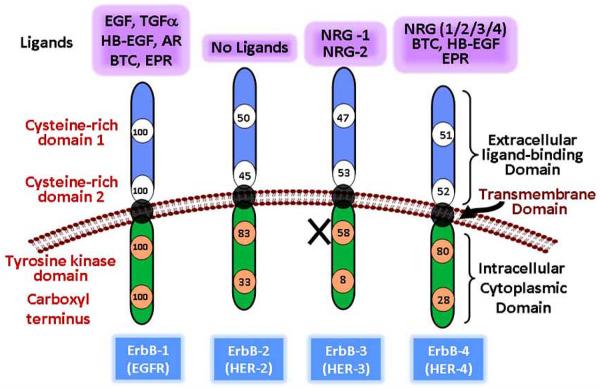
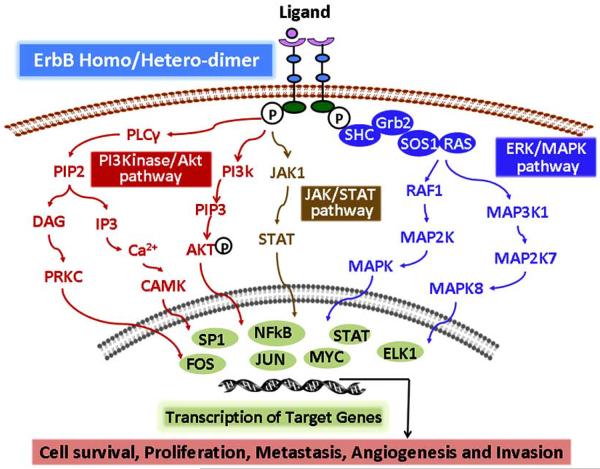
Growth factor receptors play a vital role in the normal cellular dynamics by regulating several processes that include growth, proliferation, differentiation, migration, angiogenesis, and cell death. EGFR is one of the most extensively studied growth factor receptors. EGFR family comprises of four members namely EGFR/ErbB-1/HER-1, ErbB-2/HER-2, ErbB-3/HER-3, and ErbB-4/HER-4, Fig. (2). Other than ErbB-2, each receptor has its own specific ligands which results in a highly orchestrated sequence of intracellular signal transduction, Fig. (2). The monomer receptors bind to its specific 13 polypeptide extracellular ligand and form homo-/ hetero- dimers with other members of the family. This leads to auto-/ trans activation of the intracellular tyrosine kinase domain. Owing to mutations, the intracelluar kinase domain is non-functional in ErbB-3. This necessitates the formation of hetero-dimers for ErbB-3 signaling. The EGFR signaling is pleiotropic with regards to crosstalk with other pathways as well as different signaling event that are regulated by activation of EGFR(s), Fig. (3). EGFR(s) regulate not only the processes of cellular homeostasis but also events of carcinogenesis. These include cellular proliferation, inhibition of apoptosis, migration, differentiation, invasion, angiogenesis and metastasis [95-98]. The auto/trans phosphorylation of the tyrosine residues leads to activation of EGFR signaling cascade. EGFR signaling is coupled directly or via adaptor proteins to MAPK signaling, PI3/AKT pathway, Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways [99]. EGFR (JAK/STAT) pathways [99]. EGFR signaling also modulate expression of VEGF and interleukin-8 [100].
Fig. (2).
Schematic representation of ErbB family members showing extracellular ligand binding, transmembrane and cytoplasmic domains. The numbers depict percentage homology of each domain relative to EGFR/ErbB-1. ErbB family has 10 ligands with specific affinity for each receptor. EGFR: Epidermal growth factor receptor; HER: Human Epidermal growth factor Receptor; EGF: Epidermal growth factor; TGF-α: Transformation growth factor α; HB-EGF: heparin binding-EGF; AR: amphiregulin; BTC: betacellulin; EPR: epiregulin; NRG: neuregulin; ErbB-2 has no known ligands and ErbB-3 lacks tyrosine kinase activity due to point mutation.
Fig. (3).
Schematic representation of ErbBs/EGFR(s) signaling pathways. Ligand binding to ectodomain of EGFR(s) leads to dimerization of the receptor and subsequent activation of tyrosine kinase domain. Complexity of ErbB signaling is regulated by ligand specific binding, leading to different combinations of homo/ -hetero dimerization of the receptors. Depending on the type of homo/-hetero dimer formed, a distinct intracellular pathway is activated. These pathways are involved in several processes of cellular homeostasis. PI3K: Phospholinositide-3-Kinase; Akt: phospho kinase B (PKB); JAK: Janus Kinase; STAT: Signal Transducer and Activator of Transcription; ERK: Extracellular Signal Regulated Protein Kinase; MAPK: Mitogen Activated Protein Kinase.
Overactivation of tyrosine kinase activity leading to oncogenesis may result from one of the following: 1) over expression of receptor and/ or their ligands 2) mutations leading to constitutive activation 3) inefficient mechanisms of inactivation and 4) heterodimerization leading to transactivation [97, 101, 102]. EGFR and its family members are frequently overexpressed in several solid cancers including those of the GI tract. EGFR, the first receptor tyrosine kinase to be sequenced [103], is overexpressed in gastric as well as colorectal cancers [104-111]. Similarly ErbB-2 is reported to be overexpressed in colon, esophageal, and pancreatic cancers [104, 108, 112]. Although normal colonic mucosa is negative for ErbB-2, its expression increases with progression of the cancer and is associated with poor prognosis [113]. Furthermore, even higher ErbB-2 expression is observed in colon cancer metastasis to liver or lymph nodes [114]. Although both ErbB-2 and ErbB-3 are overexpressed in gastric and colorectal cancers, ErbB-3 has been linked more strongly with gastrointestinal cancers than ErbB-2 [104, 108, 112, 115, 116]. ErbB-3 is also overexpressed in pancreatic cancer. The expression patterns of ErbB-4 with regards to different malignancies remain controversial. ErbB-4 is reported to be overexpressed in pediatric neuroblastoma [117] and GI cancers [118] whereas its loss has been implicated in the progression of breast, prostate and Head & Neck cancers [119, 120].
EGFRs as Pro-Survival and Anti-Apoptotic Factors
Age related increase in proliferation and inhibition of apoptosis is considered one of the predisposing factors for development and progression of GI cancers [28, 32, 33, 41]. Although the precise mechanisms for this regulation are not known, EGFR family of receptors plays a significant role in modulating these processes in normal, aging and malignant cells, Fig. (3). Dysregulation of EGFR signaling leads to increased activation of downstream signaling events such as Ras/Raf/MAPK kinase and PI3K/Akt signaling pathways that regulate cell proliferation and survival [9, 102, 121, 122]. Indeed, we have demonstrated increased expression and activation of EGFR and its family members ErbB-2/ErbB-3 in the GI mucosa of aging rats [72, 123, 124]. Furthermore, TGF-α, which is one of the prominent ligands of EGFR, plays a critical role in the development of colorectal neoplasia through autocrine/paracrine mechanisms. Cell lines derived from adeno-carcinomas of various tissues, including the colon, show increased expression of TGF-α and EGFR [114, 125-128].
The regulatory mechanisms for the age-related rise in mucosal proliferative activity and reduction in apoptosis are still unknown. We have demonstrated that these changes are associated with increased activation of EGFR(s) and its signaling events [124]. In Fisher-344 rats, aging has been shown to be associated with increased expression and activation of EGFR [123, 124]. A similar observation has also been made by others in preneoplastic and neoplastic lesions [36, 128-130]. This aberrant activation of EGFR signaling is in part due to increased amount of membrane associated precursor forms of TGF-α, one of the primary ligands of EGFR, bound to the receptor [124]. Thus, we suggest that in addition to increased susceptibility to carcinogens and cumulative mutations, aging may be associated with elevated levels of certain growth factors, particularly EGF-family of peptides. These factors could play a role in malignant transformation of the gastrointestinal tissues during aging.
In pursuit of a better understanding of the intracellular events regulating the EGFR signaling, we have investigated the expression of EGFR Related Protein, (ERRP), which acts as a negative regulator of EGFR(s). ERRP was isolated from rat colonic mucosa and exhibits ~ 90% homology to EGFR and ~80% to other family members [131-133]. Although the genetic identity of ERRP remains to be determined, we have observed that ERRP levels are higher in benign human colonic and gastric mucosa and in the pancreas than in the respective adenocarcinomas [40, 134]. Furthermore, we demonstrated that the age-related rise in activation of EGFR is associated with the decreased levels of ERRP [135]. ERRP expression was also decreased in dimethylhydrazine (DMH)-induced ACF in the colonic mucosa of aging rats [72]. Taken together, the results suggest that loss of ERRP resulting in increased expression and activation of EGFR in the GI mucosa during aging could in part contribute to the age-related rise in gastrointestinal cancers.
More recently, we have observed that the carcinogen-induced neoplasia in aging rats is associated with a concomitant increase in the expression of EGFR [136]. We have observed that administration of the colonic carcinogen DMH that causes a 2-fold induction in ACF formation in the colon, produces a marked 12-fold increase in EGFR expression in the colonic mucosa, when compared with the corresponding controls [136]. In humans, aging is found to be associated with increased expression of EGFR in macroscopically normal mucosa in patients with adenomatous polyps [137]. Taken together, the results suggest a potential role of EGFR in the development of colonic neoplasia during aging.
EGFRs as Modulators of Resistance to Chemotherapy and Tumor Refraction
Over-expression of EGFRs is frequently associated with a poor prognosis and the development of de novo resistance to many known therapies including chemotherapy, hormone therapy, radiotherapy in a variety of cancers, including breast, ovarian, colorectal, Head and Neck and non-small cell lung cancers (NSCLC) [138-148]. Most frequently, cancer cells are able to escape the effects of a known therapy and relapse with more aggressive phenotype. Most of the solid tumors including the colon tumor express more than one member of the EGFR family of receptors. There is evidence to suggest that EGFR/HER-2 hetero-dimers are extremely potent transforming signaling complexes [149, 150] and that co-expression of EGFR with EGFR/HER-3 receptors results in the development of enhanced drug resistance [151]. In support of this, we have recently demonstrated that EGFR expression is elevated in drug-resistant colon cancer cells [152]. 5-fluorouracil (5-FU) or 5-FU plus oxaliplatin (FOLF-OX), which remains the backbone of colorectal cancer chemotherapeutics, produces only a partial response and is limited by the recurrence of the tumor. We have demonstrated that colon cancer cells that develop de novo resistance to FOLFOX by chronic exposure to this regimen show higher expression of EGFR than the parental cells that are sensitive to FOLFOX [152]. The detailed mechanisms for this overexpression of EGFR in chemo-resistant cells remain to be investigated. The hypomethylation of EGFR promoter, in part, may be responsible for the higher expression of EGFR of the FOLFOX-resistant colon cancer cells [152].
EGFR(S) AS A PROMISING TARGET FOR GASTROINTESTINAL CANCERS
Different treatment modalities are available for GI cancers which may be employed either alone or in combination with other therapies. Due to high incidence of metastasis, surgical resection of the primary tumor is often followed by adjuvant therapy. Among the available adjuvant treatment options for GI cancers could be chemotherapy with 5-fluorouracil (5-FU) alone or in combination with other agents such as oxaliplatin, irinotecan, adriamycin, methotrexate, leucovorin. Several studies have indicated the failure of postoperative chemotherapy in providing survival benefit [153-156]. Despite the fact that chemotherapy is useful in advanced gastric cancers, the overall survival does not exceed 1 year in phase III studies. Other treatment strategy is radiotherapy, which may be utilized as pre-operative, post-operative and palliative therapy. Radiation therapy alone does not improve the survival, but is efficient in controlling the loco-regional spread of the malignancy. Since post-operative loco-regional spread is a serious concern in gastric cancers, radiation therapy appears to be an attractive therapeutic option [157, 158]. Radiotherapy is more successfully utilized in rectal cancers. Due to the anatomical location of rectum, higher doses of radiation may be delivered while sparing other sensitive organs. Several randomized and retrospective studies indicate a potential advantage of combining radiotherapy with fluorouracil-based chemotherapy with regards to loco-regional as well as survival benefit [159-161]. According to Surveillance, Epidemiology and End Results (SEER) database, the post-operative chemoradiotherapy might prove to be a superior therapeutic regimen in terms of survival [162, 163].
Targeted Therapy as a Better Strategy to Inhibit Cancer
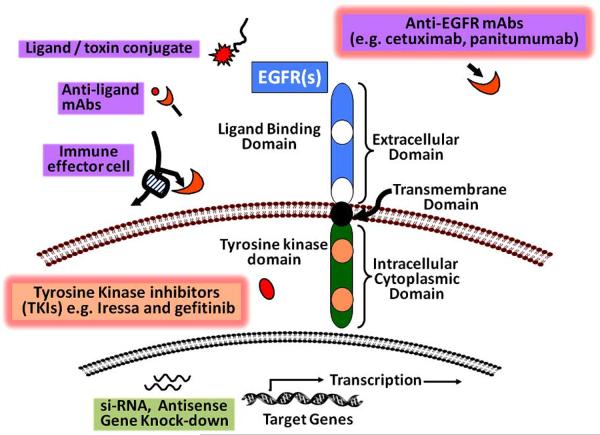
The disappointing outcome with conventional chemotherapeutics has initiated the development of other therapeutic strategies. The current cyto-toxic agents lack target specificity and does not distinguish between malignant and non-malignant cells, thereby showing undesirable toxicity profile. Though the chemoradiotherapy is shown to be beneficial, it is associated with undesirable clinically significant acute hematological as well as gastrointestinal toxicities [164]. However with the development of agents that target specific signal transduction pathway, a higher therapeutic index can be achieved. Several targeted agents are being developed for GI cancers that include perturbation of tyrosine kinases, ras/MAPK, vascular endothelial receptor (VEGF), matrix metalloproteinases, Cox-2 and c-kit signaling events [165]. Since EGFR signaling plays a significant role in the processes of GI-carcinogenesis during aging, this review will focus on EGFR(s) targeted therapies in GI cancers. EGFR-targeted interruption of signaling leads to inhibition of malignant proliferation as well as survival [166-168]. EGFR targeting strategies involve the inhibition of growth factor receptor signaling by targeting either the 1) ligand binding ecto-domain, 2) intracellular tyrosine kinase domain 3) or by manipulating the transcription of several target genes. Fig. (4) demonstrates some of the strategies being employed to achieve EGFR inhibition. Most of the agents developed based upon these strategies have shown success in vitro and with xenograft models [169-186]. However, after completion of several clinical and pre-clinical studies, only two classes of anti-EGFR agents have demonstrated clinical activity and achieved regulatory approval for treatment of cancers. These are 1) monoclonal antibodies that target and inhibit the extracellular ligand binding domain of EGFRs and 2) small molecule inhibitors of intracellular tyrosine kinase activity of the receptor. Some of these agents in different stages of their development are listed in Table 1.
Fig. (4).
Schematic representation of different strategies to inhibit EGFR(s). Inhibition of EGFRs can be achieved by targeting extracellular ligand binding domain and/or intracellular tyrosine kinase domain of the receptor. Among the available strategies, mAbs that target the ligand binding domain and TKIs that target intracellular tyrosine kinase activity have been shown to be more successful. mAbs: monoclonal antibodies; siRNA: small interfering ribonucleic acid.
Table 1.
ErbB Targeted Therapies in Gl-Cancers
| Agent | Type and Target | Developer | Development Phase |
|---|---|---|---|
|
Monoclonal Antibodies
Cetuximab (Erbitux / C225) Panitumamab (vectibix / ABX-EGF) Nimotuzumab (Thera CIM / hR3) Zalutumumab (MDX-447 / HuMax™-EGFR) Trastuzumab (Heceptin) Pertuzumab (Omnitarg / 2C4) Matuzumab (EMD-72000) mAb-806 |
Human-mouse chimeric / EGFR Fully Human / EGFR Humanized / EGFR Fully Human / EGFR Humanized / ErbB-2 Humanized / ErbB-2 Humanized / EGFR Human-mouse chimeric / EGFRvIII |
ImClone/Merck KGaA Amgen YM BioSciences Medarex Genentech / Roche Genentech Merck KGaA Ludwig Institute |
Approved in 2004 Approved in 2006 Phase II / III Phase I / II Phase II Phase II Phase II Preclinical |
|
Tyrosine Kinase Inhibitors
Gefitinib (ZD-1839 / Iressa) Erlotinib (OSI-774 / Tarceva) BMS-599626 Lapatinib (GW-572016) PK1166 PD153035 EKB-569 Canertinib (Cl-1033) Cervene (TP-38) |
Reversible TKI / EGFR Reversible TKI / EGFR Reversible TKI / Multiple ErbB Reversible TKI / Dual EGFR / ErbB-2 Reversible TKI / Dual EGFR / ErbB-2 Reversible TKI / Dual EGFR / ErbB-2 Irreversible TKI / Multiple ErbB Irreversible TKI / Multiple ErbB Chimeric toxin made of human TGFα / Pseudomonas endotoxin |
AstraZeneca Genentech / OSI / Roche Bristol-Myers Squibb GlaxoSmithKline Novartis Tocris Cookson Wyeth-Ayerst Pfizer IVAX |
Phase II / III Phase I-III Phase I Phase II / III Phase I Preclinical Phase II Phase I-III Phase I |
EGFR = Epidermal Growth Factor Receptor; mAb = Monoclonal Antibody; TKI = Tyrosine Kinase Inhibitor
Since cancer cells develop resistance to therapies by overexpressing one or more members of the EGFR family, a better therapeutic effect is expected by targeting more than one EGFR(s) simultaneously. To meet this end, either a combination of therapies targeting EGFR(s) or a therapy targeting more than one EGFR can be employed. Combination of EGFR and ErbB-2 targeting therapies has demonstrated some success in solid tumors like ovarian and breast [187-189]. Currently different clinical trials are ongoing to investigate the efficacy of combination of EGFR targeted therapies in GI cancers [190]. While pan-ErbB inhibitors have not met with success in clinical trials [191], they remain in various stages of development [192]. One such agent, ERRP, was developed and characterized in our laboratory. ERRP shows anti-tumor properties in variety of cancer cells lines as well as tumor xenografts [131, 132]. ERRP inhibits growth and/or stimulates apoptosis of different colon, gastric, breast, pancreatic and prostate cancer cells [193-196]. ERRP attenuates constitutive and ligand-induced activation of EGFR in a wide variety of epithelial cancer cell lines, including colon cancer cells [193-196]. Down-stream signaling events of EGFR, specifically activation of Akt and NF-κB in several epithelial cancer cell lines are also attenuated by ERRP [193, 197]. Comparison of ERRP with cetuximab (MoAb to EGFR) or trastuzumab (MoAb to HER-2) revealed that ERRP inhibits growth and stimulates apoptosis and suppresses ligand-induced activation of EGFR and HER-2 in different colon and breast cancer cells that express varying levels EGFR(s) [197]. In contrast, cetuximab is found to be effective only in cells that express high levels of EGFR, whereas trastuzumab exerts its growth inhibitory effect only on HER-2 overexpressing cells [197]. Since rat origin of ERRP may be a concern for its therapeutic application, we have further generated human chimera of ERRP. Our recent unpublished data show similar in vitro as well as in vivo efficacy of the humanized version of ERRP, referred as ErbB inhibitory protein (EBIP) in different solid tumors.
KEY AREAS FOR FUTURE RESEARCH
The dawn of era of targeted therapies has met with some exciting success in the clinic. However, many intriguing questions regarding the sustained remission of the cancer remain to be answered. For example developing predictors of response and therapeutics that not only target primary tumor but also inhibits the refractory tumor are highly desirable. The current scenario warrants the investigation of following research areas in more details enabling development of better therapeutic strategies.
Gastrointestinal Cancer Stem Cells
More than a decade ago, existence of cancer stem cells was first detected in hematopoietic malignancies by John Dick’s group [198]. Since then, numerous studies have indicated the presence of cancer stem cells (CSCs) which are known to be the driving force for initiation, progression and refraction of solid tumors as well as leukemia [199-208]. The establishment of technique of sorting cells on the basis of cell surface marker expression and dilutional tumor initiating assays have allowed identification of the cancer stem cells. This technique was utilized for the first time in solid tumors by Al-Hajj [199]. There is a fairly good amount of evidence for the presence of CSCs in GI cancers as well [201, 205, 207]. It is widely accepted that CSCs constitute a very small proportion of the tumor that are presumed to be the driving force for the tumor growth due to their ability to self-renew and give rise to heterogeneous lineages of cells that form the bulk of the tumor. Colon CSCs are capable of forming tumors in immune-suppressed mice in numbers as low as few hundred [201].
Recently we have demonstrated that aging as well as carcinogen exposure lead to increase in CSC population in the rat colonic crypts [136]. We have also observed that the macroscopically normal mucosa of patients with adenomatous polyps show an increase in CSCs and this increases with aging [137]. To examine a potential association between CSCs and growth factor receptors, specifically EGFR and its family members in the development of resistance and refraction of tumors, we investigated the levels of EGFR in normal tissue as well as adenomatous polyps from patients and carcinogen induced rat colonic crypts. Expression of EGFR rises with aging in macroscopically normal mucosa of patients with polyps [137] as well in carcinogen-induced rat colonic crypts [137]. We have also reported that the FOLFOX surviving colon cancer cells that show increased expression of several CSC markers, including CD133, CD44, CD166 and ALDH-1 is associated with a parallel rise in EGFR [152]. FOLFOX-surviving colon cancer cells were also found to form anchorage dependent colonies as well as colonospheres [152]. However, the role of EGFR and its family members in regulating CSCs in drug-resistant colon cancer cells as well in the aging GI tract remains to be determined. A better understanding of the role of different signaling pathways with particular reference to aging will enable us to develop novel therapeutics that target CSCs unlike the current anti-tumor therapies that are primarily directed towards the bulk of the tumor.
Combinatorial Approach for Development of Therapeutics
It is becoming increasingly clear that employing one therapeutic agent may not be as efficient as combination of several compounds directed at different processes of tumor initiation and progression. There is evidence that tumor cells are able to evade the effects of a therapy by over-expressing more than one regulatory pathways. For example, EGFR is co-overexpressed with c-Src in several tumors including the colon suggesting a synergy between the two tyrosine kinases [209-214]. Similarly, VEGF expression has been shown to be augmented in response to activation of EGFR in variety of tumor cell lines [215-217]. Therefore EGFR(s) targeted therapies may prove to be a better strategy when combined with inducers of differentiation, apoptosis or inhibitors of other kinases. Several studies were conducted where inhibition of one type of growth factor receptor was combined with another agent(s) that inhibit other receptor and/ or effector molecules [187, 218, 219]. We have also reported that a combined therapy of ERRP and curcumin, a dietary agent, was more effective in inhibiting the growth and inducing apoptosis in colon cancer HCT-116 and HT-29 cells [220]. Curcumin [diferuloylmethane; I,7-bis-(4-hydroxy-3-methoxyphenyl)-1 ,6-heptadiene-3,5-dione], the major pigment in turmeric powder, possesses anti-inflammatory and anti-oxidant properties [221]. The combined therapy was more effective in inhibiting the activation of EGFR(s) as well as IGF-1R than monotherapy [220]. Furthermore, unpublished data from our lab revealed that EBIP, the humanized version of ERRP, synergizes with dasatinib, a specific inhibitor of Src Family kinase (SFK), in inhibiting the growth of different breast cancer cells expressing varying levels of EGFR(s). This synergy was also observed in inhibiting the growth of breast cancer derived xenografts. Similar investigations need to be conducted for colon cancer. Utilizing dasatinib, we demonstrated that the combination of dasatinib and curcumin is more effective in inhibiting growth and aggressive behavior of variety of colon cancer cells than either agent alone. Although the two agents inhibit colony and tubule formation and cell invasion differentially, the combined therapy produced a significantly marked inhibition of the processes of transformation. Further, we observed that the combination therapy was highly effective in regressing the adenomas formed in APCMin+/− mice (unpublished data), which carry a mutation in the APC gene predisposing them to the development of spontaneous intestinal adenomas. This superior effect of combined therapy is in part due to increased apoptosis and decreased proliferation in the tumor remnants.
Cure vs. Prevention
Since aging predisposes the GI tract to processes of carcinogenesis, one can speculate that consumption of chemo-preventive agent(s) could be beneficial in reducing the incidence of age-related GI-carcinogenesis. With regard to this several compounds are being investigated for their chemo-preventive efficacy. One such class of compounds is phytochemicals that are derived from fruits, vegetables, and grains. Many phytochemicals are shown to possess anti-cancer properties and therefore, represent a promising therapeutic approach for prevention and treatment of many cancers [222, 223]. The ability of phytochemicals to inhibit tumor formation in experimental animals is well documented [224, 225]. Many of these compounds possess anti-oxidant, antiproliferative and pro-apoptotic effects on a variety of cancers, including colon tumors that contain CSCs [199, 205, 224, 226, 227]. The benefit of many of these phytochemicals is that they are well tolerated and are found in food products that can be added to the diet [228]. Furthermore, phytochemicals could be taken on a long-term basis to either prevent primary tumor formation or tumor recurrence. Phytochemicals target several molecules that are involved in processes of carcinogenesis [229, 230]. For instance, curcumin, obtained from Curcuma longa, used in many South Asian cuisines, has been shown to inhibit chemically induced carcinogenesis in the skin, forestomach and colon when administered during initiation and/or post-initiation phases [225, 231-234]. Development of azoxymethane-induced preneoplastic and neoplastic lesions of the colon is also inhibited in experimental animals fed a diet containing curcumin [235, 236]. In addition, curcumin has been reported to prevent adenoma development in the intestinal tract of APCMin+/− mice, a model of human familial adenomatous polyposis [237]. In a Phase I clinical trial, curcumin was shown to be effective in inhibiting tumor growth [238]. Taken together, the data generated from many of the chemo-preventive studies suggest that some of these natural products can not only be chemo-preventive but could also exert therapeutic benefit alone or in combination with conventional therapeutics. We can certainly say that increase in age-related rise in GI cancers warrants life style changes which may include consumption of natural chemopreventive agents.
ACKNOWLEDGEMENTS
A part of the work presented in this communication has been supported by grants to Dr Majumdar by NIH/NIA (AG014343) and the Department of Veterans Affairs (VA Merit Review).
REFERENCES
- 1.United Nations Population Division 2009 Sep; http://www.un.org/esa/population/publications/wpp2008/pressrelease.pdf. accessed.
- 2.U.S. Census Bureau Population Profile of the United States 2009 Sep; http://www.census.gov/population/www/pop-profile/elderpop.html. accessed.
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics. CA Cancer J. Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto M, Shiratori Y, Yamaji Y, Kato J, Ikenoue T, Togo G, Yoshida H, Kawabe T, Omata M. Relationship between age and site of colorectal cancer based on colonoscopy findings. Gastrointest. Endosc. 2002;55(4):548–51. doi: 10.1067/mge.2002.122335. [DOI] [PubMed] [Google Scholar]
- 5.Yamaji Y, Mitsushima T, Yoshida H, Watabe H, Okamoto M, Wada R, Ikuma H, Kawabe T, Omata M. The malignant potential of freshly developed colorectal polyps according to age. Cancer Epidemiol. Biomarkers Prev. 2006;15(12):2418–21. doi: 10.1158/1055-9965.EPI-06-0136. [DOI] [PubMed] [Google Scholar]
- 6.Centers For Disease Control and Prevention 2009 Sep; http://www.cdc.gov/cancer/colorectal/statistics/age.htm. accessed.
- 7.Katz LA, Spiro HM. Gastrointestinal manifestations of diabetes. N. Engl. J. Med. 1966;275(24):1350–61. doi: 10.1056/NEJM196612152752406. [DOI] [PubMed] [Google Scholar]
- 8.Morson BC, Sobin LH, Grundmann E, Johansen A, Nagayo T, Serck-Hanssen A. Precancerous conditions and epithelial dysplasia in the stomach. J. Clin. Pathol. 1980;33(8):711–21. doi: 10.1136/jcp.33.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majumdar APN, Basson MD. Effect of Aging on the Gastrointestinal Tract. Academic Press; New York: 2006. pp. 405–433. [Google Scholar]
- 10.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134(3):849–64. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 11.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J. Pathol. 2009;217(2):307–17. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 12.Leblond CP, Messier B. Renewal of chief cells and goblet cells in the small intestine as shown by radioautography after injection of thymidine-H3 into mice. Anat. Rec. 1958;132(3):247–59. doi: 10.1002/ar.1091320303. [DOI] [PubMed] [Google Scholar]
- 13.Leblond CP, Stevens CE. The constant renewal of the intestinal epithelium in the albino rat. Anat. Rec. 1948;100(3):357–77. doi: 10.1002/ar.1091000306. [DOI] [PubMed] [Google Scholar]
- 14.Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–98. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- 15.Wright NA. Epithelial stem cell repertoire in the gut: clues to the origin of cell lineages, proliferative units and cancer. Int. J. Exp. Pathol. 2000;81(2):117–43. doi: 10.1046/j.1365-2613.2000.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1998;353(1370):821–30. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307(5717):1904–9. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 18.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 19.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86(3):391–9. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 20.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli(APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. USA. 1995;92(7):3046–50. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272(5264):1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 22.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262(5140):1731–4. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 23.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomarkers Prev. 2005;14(1):120–5. [PubMed] [Google Scholar]
- 24.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, Mcpherson J, Wasmuth J, LePaslier D, Abderrahim H, Cohen D, Leppert M, White R. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 25.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Matrkam A, Groffen J, Boguski MS, Altschul SF, Horii A, Ando H, Miyoshi Y, Miki Y, Nishisho I, Nakamura Y. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253(5020):661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 26.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–51. [PubMed] [Google Scholar]
- 27.Lipkin M. Proliferation and Differentiation of Normal and Diseased Gastrointyestinal Cells. Raven Press; New York: 1987. [Google Scholar]
- 28.Atillasoy E, Holt PR. Gastrointestinal proliferation and aging. J. Gerontol. 1993;48(2):B43–9. doi: 10.1093/geronj/48.2.b43. [DOI] [PubMed] [Google Scholar]
- 29.Holt PR, Yeh KY. Colonic proliferation is increased in senescent rats. Gastroenterology. 1988;95(6):1556–63. doi: 10.1016/s0016-5085(88)80077-5. [DOI] [PubMed] [Google Scholar]
- 30.Holt PR, Yeh KY. Small intestinal crypt cell proliferation rates are increased in senescent rats. J. Gerontol. 1989;44(1):B9–14. doi: 10.1093/geronj/44.1.b9. [DOI] [PubMed] [Google Scholar]
- 31.Holt PR, Yeh KY, Kotler DP. Altered controls of proliferation in proximal small intestine of the senescent rat. Proc. Natl. Acad. Sci. USA. 1988;85(8):2771–5. doi: 10.1073/pnas.85.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaszewski R, Ehrinpreis MN, Majumdar AP. Aging and cancer of the stomach and colon. Front. Biosci. 1999;4:D322–8. doi: 10.2741/jaszewsk. [DOI] [PubMed] [Google Scholar]
- 33.Majumdar AP, Edgerton EA, Arlow FL. Gastric mucosal tyrosine kinase activity during aging and its relationship to cell proliferation in rats. Biochim. Biophys. Acta. 1988;965(2-3):97–105. doi: 10.1016/0304-4165(88)90043-8. [DOI] [PubMed] [Google Scholar]
- 34.Majumdar AP, Jasti S, Hatfield JS, Tureaud J, Fligiel SE. Morphological and biochemical changes in gastric mucosa of aging rats. Dig. Dis. Sci. 1990;35(11):1364–70. doi: 10.1007/BF01536742. [DOI] [PubMed] [Google Scholar]
- 35.Majumdar AP, Jaszewski R, Dubick MA. Effect of aging on the gastrointestinal tract and the pancreas. Proc. Soc. Exp. Biol. Med. 1997;215(2):134–44. doi: 10.3181/00379727-215-44120. [DOI] [PubMed] [Google Scholar]
- 36.Malecka-Panas E, Kordek R, Biernat W, Tureaud J, Liberski PP, Majumdar AP. Differential activation of total and EGF receptor(EGF-R) tyrosine kinase(tyr-k) in the rectal mucosa in patients with adenomatous polyps, ulcerative colitis and colon cancer. Hepatogastroenterology. 1997;44(14):435–40. [PubMed] [Google Scholar]
- 37.Xiao ZQ, Moragoda L, Jaszewski R, Hatfield JA, Fligiel SE, Majumdar AP. Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mech. Ageing Dev. 2001;122(15):1849–64. doi: 10.1016/s0047-6374(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 38.Roncucci L, Ponz de Leon M, Scalmati A, Malagoli G, Pratissoli S, Perini M, Chahin NJ. The influence of age on colonic epithelial cell proliferation. Cancer. 1988;62(11):2373–7. doi: 10.1002/1097-0142(19881201)62:11<2373::aid-cncr2820621120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 39.Stadler J, Stern HS, Yeung KS, McGuire V, Furrer R, Marcon N, Bruce WR. Effect of high fat consumption on cell proliferation activity of colorectal mucosa and on soluble faecal bile acids. Gut. 1988;29(10):1326–31. doi: 10.1136/gut.29.10.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majumdar AP. Regulation of gastrointestinal mucosal growth during aging. J. Physiol. Pharmacol. 2003;54(4):143–54. [PubMed] [Google Scholar]
- 41.Majumdar APN, Jaszewski R. Aging of the Esophagus and Stomach. Krager; New York: 2003. pp. 40–56. [Google Scholar]
- 42.Carson DA, Ribeiro JM. Apoptosis and disease. Lancet. 1993;341(8855):1251–4. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- 43.Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr. Rev. 1993;14(2):133–51. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- 44.Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, Hamilton SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55(9):1811–6. [PubMed] [Google Scholar]
- 45.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 46.Bartsch H, Ohshima H, Shuker DE, Pignatelli B, Calmels S. Exposure of humans to endogenous N-nitroso compounds: implications in cancer etiology. Mutat. Res. 1990;238(3):255–67. doi: 10.1016/0165-1110(90)90017-6. [DOI] [PubMed] [Google Scholar]
- 47.Enzinger PC, Mayer RJ. Esophageal cancer. N. Engl. J. Med. 2003;349(23):2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 48.Groopman JD, Cain LG, Kensler TW. Aflatoxin exposure in human populations: measurements and relationship to cancer. Crit. Rev. Toxicol. 1988;19(2):113–45. doi: 10.3109/10408448809014902. [DOI] [PubMed] [Google Scholar]
- 49.Kamiyama S, Ohshima H, Shimada A, Saito N, Bourgade MC, Ziegler P, Bartsch H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants in high- and low-risk areas for stomach cancer in northern Japan. IARC. Sci. Publ. 1987;87:497–502. [PubMed] [Google Scholar]
- 50.Lu SH, Ohshima H, Fu HM, Tian Y, Li FM, Blettner M, Wahrendorf J, Bartsch H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for esophageal cancer in Northern China: endogenous formation of nitrosoproline and its inhibition by vitamin C. Cancer Res. 1986;46(3):1485–91. [PubMed] [Google Scholar]
- 51.Scholl P, Musser SM, Kensler TW, Groopman JD. Molecular biomarkers for aflatoxins and their application to human liver cancer. Pharmacogenetics. 1995;5:S171–6. doi: 10.1097/00008571-199512001-00022. [DOI] [PubMed] [Google Scholar]
- 52.Sugimura T. Food and cancer. Toxicology. 181-182. 2002:17–21. doi: 10.1016/s0300-483x(02)00250-0. [DOI] [PubMed] [Google Scholar]
- 53.Wogan GN. Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res. 1992;52(7):2114s–2118s. [PubMed] [Google Scholar]
- 54.Zatonski W, Ohshima H, Przewozniak K, Drosik K, Mierzwinska J, Krygier M, Chmielarczyk W, Bartsch H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for stomach cancer in Poland. Int. J. Cancer. 1989;44(5):823–7. doi: 10.1002/ijc.2910440513. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee S, Hussain M, Wang Z, Saliganan A, Che M, Bonfil D, Cher M, Sarkar FH. in vivo and In vivo molecular evidence for better therapeutic efficacy of ABT-627 and taxotere combination in prostate cancer. Cancer Res. 2007;67(8):3818–26. doi: 10.1158/0008-5472.CAN-06-3879. [DOI] [PubMed] [Google Scholar]
- 56.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37(2):147–51. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 57.McLellan EA, Bird RP. Specificity study to evaluate induction of aberrant crypts in murine colons. Cancer Res. 1988;48(21):6183–6. [PubMed] [Google Scholar]
- 58.Takayama T, Katsuki S, Takahashi Y, Ohi M, Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H, Niitsu Y. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N. Engl. J. Med. 1998;339(18):1277–84. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 59.Pretlow TP, Brasitus TA, Fulton NC, Cheyer C, Kaplan EL. K-ras mutations in putative preneoplastic lesions in human colon. J. Natl. Cancer Inst. 1993;85(24):2004–7. doi: 10.1093/jnci/85.24.2004. [DOI] [PubMed] [Google Scholar]
- 60.Pretlow TP, O’Riordan MA, Pretlow TG, Stellato TA. Aberrant crypts in human colonic mucosa: putative preneoplastic lesions. J. Cell. Biochem. Suppl. 16G. 1992:55–62. doi: 10.1002/jcb.240501111. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita N, Minamoto T, Ochiai A, Onda M, Esumi H. Frequent and characteristic K-ras activation in aberrant crypt foci of colon. Is there preference among K-ras mutants for malignant progression? Cancer. 1995;(6);75:1527–33. doi: 10.1002/1097-0142(19950315)75:6+<1527::aid-cncr2820751524>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita N, Minamoto T, Ochiai A, Onda M, Esumi H. Frequent and characteristic K-ras activation and absence of p53 protein accumulation in aberrant crypt foci of the colon. Gastroenterology. 1995;108(2):434–40. doi: 10.1016/0016-5085(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 63.Heinen CD, Shivapurkar N, Tang Z, Groden J, Alabaster O. Microsatellite instability in aberrant crypt foci from human colons. Cancer Res. 1996;56(23):5339–41. [PubMed] [Google Scholar]
- 64.Nucci MR, Robinson CR, Longo P, Campbell P, Hamilton SR. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum. Pathol. 1997;28(12):1396–407. doi: 10.1016/s0046-8177(97)90230-6. [DOI] [PubMed] [Google Scholar]
- 65.Pedroni M, Sala E, Scarselli A, Borghi F, Menigatti M, Benatti P, Percesepe A, Rossi G, Foroni M, Losi L, Di Gregorio C, De Pol A, Nascimbeni R, Di Betta E, Salerni B, de Leon MP, Roncucci L. Microsatellite instability and mismatch-repair protein expression in hereditary and sporadic colorectal carcinogenesis. Cancer Res. 2001;61(3):896–9. [PubMed] [Google Scholar]
- 66.Shivapurkar N, Huang L, Ruggeri B, Swalsky PA, Bakker A, Finkelstein S, Frost A, Silverberg S. K-ras and p53 mutations in aberrant crypt foci and colonic tumors from colon cancer patients. Cancer Lett. 1997;115(1):39–46. doi: 10.1016/s0304-3835(97)04709-5. [DOI] [PubMed] [Google Scholar]
- 67.Shpitz B, Bomstein Y, Shalev M, Liverant S, Kaufman Z, Klein E, Mekori Y, Bernheim J. Oncoprotein coexpression in human aberrant crypt foci and minute polypoid lesions of the large bowel. Anticancer Res. 1999;19(4B):3361–6. [PubMed] [Google Scholar]
- 68.Smith AJ, Stern HS, Penner M, Hay K, Mitri A, Bapat BV, Gallinger S. Somatic APC and K-ras codon 12 mutations in aberrant crypt foci from human colons. Cancer Res. 1994;54(21):5527–30. [PubMed] [Google Scholar]
- 69.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 2004;36(4):417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 70.Takayama T, Ohi M, Hayashi T, Miyanishi K, Nobuoka A, Nakajima T, Satoh T, Takimoto R, Kato J, Sakamaki S, Niitsu Y. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121(3):599–611. doi: 10.1053/gast.2001.27203. [DOI] [PubMed] [Google Scholar]
- 71.Magnuson BA, South EH, Exon JH, Dashwood RH, Xu M, Hendrix K, Hubele S. Increased susceptibility of adult rats to azoxymethane-induced aberrant crypt foci. Cancer Lett. 2000;161(2):185–93. doi: 10.1016/s0304-3835(00)00609-1. [DOI] [PubMed] [Google Scholar]
- 72.Schmelz EM, Levi E, Du J, Xu H, Majumdar AP. Age-related loss of EGF-receptor related protein(ERRP) in the aging colon is a potential risk factor for colon cancer. Mech. Ageing Dev. 2004;125(12):917–22. doi: 10.1016/j.mad.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 74.Yokozaki H, Yasui W, Tahara E. Genetic and epigenetic changes in stomach cancer. Int. Rev. Cytol. 2001;204:49–95. doi: 10.1016/s0074-7696(01)04003-7. [DOI] [PubMed] [Google Scholar]
- 75.Moragoda L, Jaszewski R, Kulkarni P, Majumdar AP. Age-associated loss of heterozygosity of tumor suppressor genes in the gastric mucosa of humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282(6):G932–6. doi: 10.1152/ajpgi.00312.2001. [DOI] [PubMed] [Google Scholar]
- 76.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat. Genet. 1994;7(4):536–40. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 77.Sherr CJ. Cancer cell cycles. Science. 1996;274(5293):1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 78.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988;319(9):525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 79.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol. 2001;25(5):579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Kloppel G, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 2004;28(8):977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 81.Lin J, Beerm DG. Molecular biology of upper gastrointestinal malignancies. Semin. Oncol. 2004;31(4):476–86. doi: 10.1053/j.seminoncol.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 82.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 83.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10(8):789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 84.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat. Rev. Cancer. 2003;3(8):592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]
- 85.Glenney JR., Jr. Tyrosine-phosphorylated proteins: mediators of signal transduction from the tyrosine kinases. Biochim. Biophys. Acta. 1992;1134(2):113–27. doi: 10.1016/0167-4889(92)90034-9. [DOI] [PubMed] [Google Scholar]
- 86.Hunter T, Cooper JA. Protein-tyrosine kinases. Annu. Rev. Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- 87.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N. Engl. J. Med. 2001;344(14):1052–6. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 88.Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu. Rev. Biochem. 1988;57:443–78. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- 89.Brunton VG, Ozanne BW, Paraskeva C, Frame MC. A role for epidermal growth factor receptor, c-Src and focal adhesion kinase in an in vivo model for the progression of colon cancer. Oncogene. 1997;14(3):283–93. doi: 10.1038/sj.onc.1200827. [DOI] [PubMed] [Google Scholar]
- 90.Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W. pp60c-src activation in human colon carcinoma. J. Clin. Invest. 1989;83(6):2025–33. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cartwright CA, Coad CA, Egbert BM. Elevated c-Src tyrosine kinase activity in premalignant epithelia of ulcerative colitis. J. Clin. Invest. 1994;93(2):509–15. doi: 10.1172/JCI117000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S, Varnum B, Liu ET, Cance WG. Receptor tyrosine kinases expressed in metastatic colon cancer. Int. J. Cancer. 1995;60(6):791–7. doi: 10.1002/ijc.2910600611. [DOI] [PubMed] [Google Scholar]
- 93.Pena SV, Melhem MF, Meisler AI, Cartwright CA. Elevated c-yes tyrosine kinase activity in premalignant lesions of the colon. Gastroenterology. 1995;108(1):117–24. doi: 10.1016/0016-5085(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55(18):3964–8. [PubMed] [Google Scholar]
- 95.Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7(90004):2–8. doi: 10.1634/theoncologist.7-suppl_4-2. [DOI] [PubMed] [Google Scholar]
- 96.Bundred NJ, Chan K, Anderson NG. Studies of epidermal growth factor receptor inhibition in breast cancer. Endocr. Relat. Cancer. 2001;8(3):183–9. doi: 10.1677/erc.0.0080183. [DOI] [PubMed] [Google Scholar]
- 97.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr. Relat. Cancer. 2001;8(1):11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 98.Wells A. EGF receptor. Int. J. Biochem. Cell Biol. 1999;31(6):637–43. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 99.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 100.Rak J, Yu JL, Klement G, Kerbel RS. Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J. Investig. Dermatol. Symp. Proc. 2000;5(1):24–33. doi: 10.1046/j.1087-0024.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 101.Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr. Opin. Cell Biol. 1999;11(2):184–9. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 102.Moghal N, Sternberg PW. Multiple positive and negative regulators of signaling by the EGF-receptor. Curr. Opin. Cell. Biol. 1999;11(2):190–6. doi: 10.1016/s0955-0674(99)80025-8. [DOI] [PubMed] [Google Scholar]
- 103.Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, Mayes ELV, Whittle N, Waterfield MD, Seeburg PH. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309(5967):418–25. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 104.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 105.Itakura Y, Sasano H, Shiga C, Furukawa Y, Shiga K, Mori S, Nagura H. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer. 1994;74(3):795–804. doi: 10.1002/1097-0142(19940801)74:3<795::aid-cncr2820740303>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 106.Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37(3):285–9. doi: 10.1080/028418698429595. [DOI] [PubMed] [Google Scholar]
- 107.Raymond E, Faivre S, Armand JP. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60(1):15–23. doi: 10.2165/00003495-200060001-00002. discussion 41-2. [DOI] [PubMed] [Google Scholar]
- 108.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995;19(3):183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 109.Slichenmyer WJ, Fry DW. Anticancer therapy targeting the erbB family of receptor tyrosine kinases. Semin. Oncol. 2001;28(16):67–79. doi: 10.1016/s0093-7754(01)90284-2. [DOI] [PubMed] [Google Scholar]
- 110.Yarden Y. The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur. J. Cancer. 2001;37(4):3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 111.Yasui W, Sumiyoshi H, Hata J, Kameda T, Ochiai A, Ito H, Tahara E. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 1988;48(1):137–41. [PubMed] [Google Scholar]
- 112.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 113.Kapitanovic S, Radosevic S, Kapitanovic M, Andelinovic S, Ferencic Z, Tavassoli M, Primorac D, Sonicki Z, Spaventi S, Pavelic K, Spaventi R. The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology. 1997;112(4):1103–13. doi: 10.1016/s0016-5085(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 114.Saeki T, Salomon DS, Johnson GR, Gullick WJ, Mandai K, Yamagami K, Moriwaki S, Tanada M, Takashima S, Tahara E. Association of epidermal growth factor-related peptides and type I receptor tyrosine kinase receptors with prognosis of human colorectal carcinomas. Jpn. J. Clin. Oncol. 1995;25(6):240–9. [PubMed] [Google Scholar]
- 115.Lemoine NR, Lobresco M, Leung H, Barton C, Hughes CM, Prigent SA, Gullick WJ, Kloppel G. The erbB-3 gene in human pancreatic cancer. J. Pathol. 1992;168(3):269–73. doi: 10.1002/path.1711680305. [DOI] [PubMed] [Google Scholar]
- 116.Prigent SA, Lemoine NR, Hughes CM, Plowman GD, Selden C, Gullick WJ. Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene. 1992;7(7):1273–8. [PubMed] [Google Scholar]
- 117.Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57(15):3272–80. [PubMed] [Google Scholar]
- 118.Kataoka H, Joh T, Kasugai K, Okayama N, Moriyama A, Asai K, Kato T. Expression of mRNA for heregulin and its receptor, ErbB-3 and ErbB-4, in human upper gastrointestinal mucosa. Life Sci. 1998;63(7):553–64. doi: 10.1016/s0024-3205(98)00306-3. [DOI] [PubMed] [Google Scholar]
- 119.Lyne JC, Melhem MF, Finley GG, Wen D, Liu N, Deng DH, Salup R. Tissue expression of neu differentiation factor/heregulin and its receptor complex in prostate cancer and its biologic effects on prostate cancer cells in vivo. Cancer J. Sci. Am. 1997;3(1):21–30. [PubMed] [Google Scholar]
- 120.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J. Pathol. 1998;185(3):236–45. doi: 10.1002/(SICI)1096-9896(199807)185:3<236::AID-PATH118>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 121.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO. J. 1997;16(10):2783–93. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Majumdar AP, Du J. Phosphatidylinositol 3-kinase/Akt signaling stimulates colonic mucosal cell survival during aging. Am. J. Physiol. Gastrointest. Liver. Physiol. 2006;290(1):G49–55. doi: 10.1152/ajpgi.00106.2005. [DOI] [PubMed] [Google Scholar]
- 123.Tureaud J, Sarkar FH, Fligiel SE, Kulkarni S, Jaszewski R, Reddy K, Yu Y, Majumdar AP. Increased expression of EGFR in gastric mucosa of aged rats. Am. J. Physiol. 1997;273(2 Pt 1):G389–98. doi: 10.1152/ajpgi.1997.273.2.G389. [DOI] [PubMed] [Google Scholar]
- 124.Xiao ZQ, Majumdar AP. Increased in vivo activation of EGFR by membrane-bound TGF-alpha from gastric and colonic mucosa of aged rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281(1):G111–6. doi: 10.1152/ajpgi.2001.281.1.G111. [DOI] [PubMed] [Google Scholar]
- 125.Anzano MA, Rieman D, Prichett W, Bowen-Pope DF, Greig R. Growth factor production by human colon carcinoma cell lines. Cancer Res. 1989;49(11):2898–904. [PubMed] [Google Scholar]
- 126.Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108(2):564–80. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 127.Coffey RJ, Jr., Shipley GD, Moses HL. Production of transforming growth factors by human colon cancer lines. Cancer Res. 1986;46(3):1164–9. [PubMed] [Google Scholar]
- 128.Podolsky D. Raven Press; New York: 1994. Peptide growth factors in the gastrointestinal tract; pp. 129–167. [Google Scholar]
- 129.Relan NK, Saeed A, Ponduri K, Fligiel SE, Dutta S, Majumdar AP. Identification and evaluation of the role of endogenous tyrosine kinases in azoxymethane induction of proliferative processes in the colonic mucosa of rats. Biochim. Biophys. Acta. 1995;1244(2-3):368–76. doi: 10.1016/0304-4165(95)00024-6. [DOI] [PubMed] [Google Scholar]
- 130.Yoshida K, Kyo E, Tsuda T, Tsujino T, Ito M, Niimoto M, Tahara E. EGF and TGF-alpha, the ligands of hyperproduced EGFR in human esophageal carcinoma cells, act as autocrine growth factors. Int. J. Cancer. 1990;45(1):131–5. doi: 10.1002/ijc.2910450124. [DOI] [PubMed] [Google Scholar]
- 131.Majumdar AP. Therapeutic potential of EGFR-related protein, a universal EGFR family antagonist. Future Oncol. 2005;1(2):235–45. doi: 10.1517/14796694.1.2.235. [DOI] [PubMed] [Google Scholar]
- 132.Nautiyal J, Rishi AK, Majumdar AP. Emerging therapies in gastrointestinal cancers. World J. Gastroenterol. 2006;12(46):7440–50. doi: 10.3748/wjg.v12.i46.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu Y, Rishi AK, Turner JR, Liu D, Black ED, Moshier JA, Majumdar AP. Cloning of a novel EGFR-related peptide: a putative negative regulator of EGFR. Am. J. Physiol. Cell Physiol. 2001;280(5):C1083–9. doi: 10.1152/ajpcell.2001.280.5.C1083. [DOI] [PubMed] [Google Scholar]
- 134.Feng J, Adsay NV, Kruger M, Ellis KL, Nagothu K, Majumdar AP, Sarkar FH. Expression of ERRP in normal and neoplastic pancreata and its relationship to clinicopathologic parameters in pancreatic adenocarcinoma. Pancreas. 2002;25(4):342–9. doi: 10.1097/00006676-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 135.Majumdar AP, Du J, Hatfield JS, Levi E, Adsay V, Schmelz EM, Nagothu KK, Jaszewski R, Kucuk O, Sarkar FH. Expression of EGF-receptor related protein(ERRP) decreases in gastric mucosa during aging and carcinogenesis. Dig. Dis. Sci. 2003;48(5):856–64. doi: 10.1023/a:1023078924686. [DOI] [PubMed] [Google Scholar]
- 136.Levi E, Misra S, Du J, Patel BB, Majumdar AP. Combination of aging and dimethylhydrazine treatment causes an increase in cancer-stem cell population of rat colonic crypts. Biochem. Biophys. Res. Commun. 2009;385(3):430–3. doi: 10.1016/j.bbrc.2009.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel BB, Yu Y, Du J, Levi E, Phillip PA, Majumdar AP. Age-related increase in colorectal cancer stem cells in macroscopically normal mucosa of patients with adenomas: a risk factor for colon cancer. Biochem. Biophys. Res. Commun. 2009;378(3):344–347. doi: 10.1016/j.bbrc.2008.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ang KK, Andratschke NH, Milas L. Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):959–65. doi: 10.1016/j.ijrobp.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 139.Dassonville O, Formento JL, Francoual M, Ramaioli A, Santini J, Schneider M, Demard F, Milano G. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J. Clin. Oncol. 1993;11(10):1873–8. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 140.Dickstein B, Valverius EM, Wosikowski K, Saceda M, Pearson JW, Martin MB, Bates SE. Increased epidermal growth factor receptor in an estrogen-responsive, adriamycin-resistant MCF-7 cell line. J. Cell Physiol. 1993;157(1):110–8. doi: 10.1002/jcp.1041570115. [DOI] [PubMed] [Google Scholar]
- 141.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032–44. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 142.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71(8):2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 143.Montgomery RB, Guzman J, O’Rourke DM, Stahl WL. Expression of oncogenic epidermal growth factor receptor family kinases induces paclitaxel resistance and alters beta-tubulin isotype expression. J. Biol. Chem. 2000;275(23):17358–63. doi: 10.1074/jbc.M000966200. [DOI] [PubMed] [Google Scholar]
- 144.Scambia G, Benedetti-Panici P, Ferrandina G, Distefano M, Salerno G, Romanini ME, Fagotti A, Mancuso S. Epidermal growth factor, oestrogen and progesterone receptor expression in primary ovarian cancer: correlation with clinical outcome and response to chemotherapy. Br. J. Cancer. 1995;72(2):361–6. doi: 10.1038/bjc.1995.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsai CM, Chang KT, Li L, Perng RP, Yang LY. Interrelationships between cellular nucleotide excision repair, cisplatin cytotoxicity, HER-2/neu gene expression, and epidermal growth factor receptor level in non-small cell lung cancer cells. Jpn. J. Cancer Res. 2000;91(2):213–22. doi: 10.1111/j.1349-7006.2000.tb00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Dam PA, Vergote IB, Lowe DG, Watson JV, van Damme P, van der Auwera JC, Shepherd JH. Expression of c-erbB-2, c-myc, and c-ras oncoproteins, insulin-like growth factor receptor I, and epidermal growth factor receptor in ovarian carcinoma. J. Clin. Pathol. 1994;47(10):914–9. doi: 10.1136/jcp.47.10.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wosikowski K, Silverman JA, Bishop P, Mendelsohn J, Bates SE. Reduced growth rate accompanied by aberrant epidermal growth factor signaling in drug resistant human breast cancer cells. Biochim. Biophys. Acta. 2000;1497(2):215–26. doi: 10.1016/s0167-4889(00)00062-8. [DOI] [PubMed] [Google Scholar]
- 148.Yu D, Liu B, Jing T, Sun D, Price JE, Singletary SE, Ibrahim N, Hortobagyi GN, Hung MC. Overexpression of both p185c-erbB2 and p170mdr-1 renders breast cancer cells highly resistant to taxol. Oncogene. 1998;16(16):2087–94. doi: 10.1038/sj.onc.1201729. [DOI] [PubMed] [Google Scholar]
- 149.Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, Di Fiore PP, Kraus MH. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10(9):1813–21. [PubMed] [Google Scholar]
- 150.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO. J. 1996;15(10):2452–67. [PMC free article] [PubMed] [Google Scholar]
- 151.Chen X, Yeung TK, Wang Z. Enhanced drug resistance in cells coexpressing ErbB2 with EGF receptor or ErbB3. Biochem. Biophys. Res. Commun. 2000;277(3):757–763. doi: 10.1006/bbrc.2000.3731. [DOI] [PubMed] [Google Scholar]
- 152.Yu Y, Kanwar S, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl. Oncol. 2009;2(4):321–8. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur. J. Cancer. 1999;35(7):1059–1064. doi: 10.1016/s0959-8049(99)00076-3. [DOI] [PubMed] [Google Scholar]
- 154.Gianni L, Panzini I, Tassinari D, Mianulli AM, Desiderio F, Ravaioli A. Meta-analyses of randomized trials of adjuvant chemotherapy in gastric cancer. Ann. Oncol. 2001;12(8):1178–80. [PubMed] [Google Scholar]
- 155.Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J. Clin. Oncol. 1993;11(8):1441–7. doi: 10.1200/JCO.1993.11.8.1441. [DOI] [PubMed] [Google Scholar]
- 156.Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: A meta-analysis of published randomised trials: A study of the GISCAD(Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente) Ann. Oncol. 2000;11(7):837–835. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 157.Jansen EPM, Boot H, Verheij M, van de Velde CJH. Optimal locoregional treatment in gastric cancer. J. Clin. Oncol. 2005;23(20):4509–4517. doi: 10.1200/JCO.2005.21.196. [DOI] [PubMed] [Google Scholar]
- 158.Smalley SR, Gunderson L, Tepper J, Martenson JA, Minsky B, Willett C, Rich T. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int. J. Radiat. Oncol. Biol. Phys. 2002;52(2):283–293. doi: 10.1016/s0360-3016(01)02646-3. [DOI] [PubMed] [Google Scholar]
- 159.Moertel CG, Childs DS, O’Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J. Clin. Oncol. 1984:1771–77. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 160.Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J. Clin. Oncol. 1985;3(3):373–8. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 161.Moertel CG, Childs DS, O’Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J. Clin. Oncol. 1984;2(11):1249–54. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 162.Coburn NG, Guller U, Baxter NN, Kiss A, Ringash J, Swallow CJ, Law CHL. Adjuvant therapy for resected gastric cancer--rapid, yet incomplete adoption following results of intergroup 0116 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(4):1073–1080. doi: 10.1016/j.ijrobp.2007.07.2378. [DOI] [PubMed] [Google Scholar]
- 163.Kozak KR, Moody JS. The Survival Impact of the Intergroup 0116 Trial on Patients With Gastric Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(2):517–521. doi: 10.1016/j.ijrobp.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 164.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001;345(10):725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 165.Gill S, Thomas RR, Goldberg RM. New targeted therapies in gastrointestinal cancers. Curr. Treat. Opt. Oncol. 2003;4(5):393–403. doi: 10.1007/s11864-003-0040-9. [DOI] [PubMed] [Google Scholar]
- 166.Berlin J. New directions in the treatment of advanced colorectal cancer. Oncology(Williston Park) 2001;15(3 Suppl 5):27–30. [PubMed] [Google Scholar]
- 167.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94(5):1593–611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- 168.Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr. Relat. Cancer. 2001;8(1):3–9. doi: 10.1677/erc.0.0080003. [DOI] [PubMed] [Google Scholar]
- 169.Azemar M, Schmidt M, Arlt F, Kennel P, Brandt B, Papadimitriou A, Groner B, Wels W. Recombinant antibody toxins specific for ErbB2 and EGF receptor inhibit the in vivo growth of human head and neck cancer cells and cause rapid tumor regression in vivo. Int. J. Cancer. 2000;86(2):269–75. doi: 10.1002/(sici)1097-0215(20000415)86:2<269::aid-ijc18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 170.Baselga J, Averbuch SD. ZD1839(’Iressa’) as an anticancer agent. Drugs. 2000;60(1):33–40. doi: 10.2165/00003495-200060001-00004. discussion 41-2. [DOI] [PubMed] [Google Scholar]
- 171.Chandler LA, Sosnowski BA, McDonald JR, Price JE, Aukerman SL, Baird A, Pierce GF, Houston LL. Targeting tumor cells via EGF receptors: selective toxicity of an HBEGF-toxin fusion protein. Int. J. Cancer. 1998;78(1):106–11. doi: 10.1002/(sici)1097-0215(19980925)78:1<106::aid-ijc17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 172.Ciardiello F, Caputo R, Troiani T, Borriello G, Kandimalla ER, Agrawal S, Mendelsohn J, Bianco AR, Tortora G. Antisense oligonucleotides targeting the epidermal growth factor receptor inhibit proliferation, induce apoptosis, and cooperate with cytotoxic drugs in human cancer cell lines. Int. J. Cancer. 2001;93(2):172–8. doi: 10.1002/ijc.1335. [DOI] [PubMed] [Google Scholar]
- 173.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1995;1(11):1311–8. [PubMed] [Google Scholar]
- 174.Hambek M, Solbach C, Schnuerch HG, Roller M, Stegmueller M, Sterner-Kock A, Kiefer J, Knecht R. Tumor necrosis factor alpha sensitizes low epidermal growth factor receptor(EGFR)-expressing carcinomas for anti-EGFR therapy. Cancer Res. 2001;61(3):1045–9. [PubMed] [Google Scholar]
- 175.He Y, Zeng Q, Drenning SD, Melhem MF, Tweardy DJ, Huang L, Grandis JR. Inhibition of human squamous cell carcinoma growth in vivo by epidermal growth factor receptor antisense RNA transcribed from the U6 promoter. J. Natl. Cancer Inst. 1998;90(14):1080–7. doi: 10.1093/jnci/90.14.1080. [DOI] [PubMed] [Google Scholar]
- 176.Hirota N, Ueda M, Ozawa S, Abe O, Shimizu N. Suppression of an epidermal growth factor receptor-hyperproducing tumor by an immunotoxin conjugate of gelonin and a monoclonal anti-epidermal growth factor receptor antibody. Cancer Res. 1989;49(24 Pt 1):7106–9. [PubMed] [Google Scholar]
- 177.Modjtahedi H, Eccles S, Sandle J, Box G, Titley J, Dean C. Differentiation or immune destruction: two pathways for therapy of squamous cell carcinomas with antibodies to the epidermal growth factor receptor. Cancer Res. 1994;54(7):1695–701. [PubMed] [Google Scholar]
- 178.Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP, Neveu M, Pollack VA, Pustilnik LR, Reynolds MM, Sloan D, Theleman A, Miller P. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57(21):4838–4848. [PubMed] [Google Scholar]
- 179.Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA, Vaidya MP, Arnold LD, Doty JL, Iwata KK, Morin MJ. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J. Pharmacol. Exp. Ther. 1999;291(2):739–48. [PubMed] [Google Scholar]
- 180.Prewett M, Rockwell P, Rockwell RF, Giorgio NA, Mendelsohn J, Scher HI, Goldstein NI. The biologic effects of C225, a chimeric monoclonal antibody to the EGFR, on human prostate carcinoma. J. Immunother. Emphasis. Tumor Immunol. 1996;19(6):419–27. doi: 10.1097/00002371-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 181.Rubin Grandis J, Chakraborty A, Melhem MF, Zeng Q, Tweardy DJ. Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene. 1997;15(4):409–16. doi: 10.1038/sj.onc.1201188. [DOI] [PubMed] [Google Scholar]
- 182.Siegall CB, Xu YH, Chaudhary VK, Adhya S, Fitzgerald D, Pastan I. Cytotoxic activities of a fusion protein comprised of TGF alpha and Pseudomonas exotoxin. FASEB J. 1989;3(14):2647–52. doi: 10.1096/fasebj.3.14.2556314. [DOI] [PubMed] [Google Scholar]
- 183.Uckun FM, Narla RK, Zeren T, Yanishevski Y, Myers DE, Waurzyniak B, Ek O, Schneider E, Messinger Y, Chelstrom LM, Gunther R, Evans W. In vivo toxicity, pharmacokinetics, and anticancer activity of Genistein linked to recombinant human epidermal growth factor. Clin. Cancer Res. 1998;4(5):1125–34. [PubMed] [Google Scholar]
- 184.Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo : a role for altered tumor angiogenesis. Cancer Res. 2001;61(13):5090–101. [PubMed] [Google Scholar]
- 185.Vollmar AM, Banker DE, Mendelsohn J, Herschman HR. Toxicity of ligand and antibody-directed ricin A-chain conjugates recognizing the epidermal growth factor receptor. J. Cell Physiol. 1987;131(3):418–25. doi: 10.1002/jcp.1041310314. [DOI] [PubMed] [Google Scholar]
- 186.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG, Jakobovits A. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res. 1999;59(6):1236–43. [PubMed] [Google Scholar]
- 187.Normanno N, Campiglio M, De Luca A, Somenzi G, Maiello M, Ciardiello F, Gianni L, Salomon DS, Menard S. Cooperative inhibitory effect of ZD1839(Iressa) in combination with trastuzumab(Herceptin) on human breast cancer cell growth. Ann. Oncol. 2002;13(1):65–72. doi: 10.1093/annonc/mdf020. [DOI] [PubMed] [Google Scholar]
- 188.Schmidt M, Wels W. Targeted inhibition of tumour cell growth by a bispecific single-chain toxin containing an antibody domain and TGF alpha. Br. J. Cancer. 1996;74(6):853–62. doi: 10.1038/bjc.1996.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18(3):731–8. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- 190.Sep, 2009. http://www.clinicaltrials.gov/ct2/results?term=combination+of++ EGFR+inhibitors+for+Gastrointestinal+cancers. accessed.
- 191.Rixe O, Franco SX, Yardley DA, Johnston SR, Martin M, Arun BK, Letrent SP, Rugo HS. A randomized, phase II, dose-finding study of the pan-ErbB receptor tyrosine-kinase inhibitor CI-1033 in patients with pretreated metastatic breast cancer. Cancer Chemother. Pharmacol. 2009;64(6):1139–48. doi: 10.1007/s00280-009-0975-z. [DOI] [PubMed] [Google Scholar]
- 192.Zinner RG, Nemunaitis J, Eiseman I, Shin HJC, Olson SC, Christensen J, Huang X, Lenehan PF, Donato NJ, Shin DM. Phase I clinical and pharmacodynamic evaluation of oral CI-1033 in patients with refractory cancer. Clin. Cancer Res. 2007;13(10):3006–3014. doi: 10.1158/1078-0432.CCR-06-1958. [DOI] [PubMed] [Google Scholar]
- 193.Marciniak DJ, Rishi AK, Sarkar FH, Majumdar AP. Epidermal growth factor receptor-related peptide inhibits growth of PC-3 prostate cancer cells. Mol. Cancer Ther. 2004;3(12):1615–21. [PubMed] [Google Scholar]
- 194.Moon WS, Chang KJ, Majumdar AP, Tarnawski AS. Reduced expression of epidermal growth factor receptor-related protein in hepatocellular carcinoma: implications for cancer growth. Digestion. 2004;69(4):219–24. doi: 10.1159/000079151. [DOI] [PubMed] [Google Scholar]
- 195.Wang Z, Sengupta R, Banerjee S, Li Y, Zhang Y, Rahman KM, Aboukameel A, Mohammad R, Majumdar AP, Abbruzzese JL, Sarkar FH. Epidermal growth factor receptor-related protein inhibits cell growth and invasion in pancreatic cancer. Cancer Res. 2006;66(15):7653–60. doi: 10.1158/0008-5472.CAN-06-1019. [DOI] [PubMed] [Google Scholar]
- 196.Zhang Y, Banerjee S, Wang Z, Xu H, Zhang L, Mohammad R, Aboukameel A, Adsay NV, Che M, Abbruzzese JL, Majumdar AP, Sarkar FH. Antitumor activity of epidermal growth factor receptor-related protein is mediated by inactivation of ErbB receptors and nuclear factor-kappaB in pancreatic cancer. Cancer Res. 2006;66(2):1025–32. doi: 10.1158/0008-5472.CAN-05-2968. [DOI] [PubMed] [Google Scholar]
- 197.Xu H, Yu Y, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar AP. Epidermal growth factor receptor(EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol. Cancer. Ther. 2005;4(3):435–42. doi: 10.1158/1535-7163.MCT-04-0280. [DOI] [PubMed] [Google Scholar]
- 198.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 199.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 201.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA. 2007;104(24):10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 203.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 204.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–56. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 205.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 206.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA. 2007;104(3):973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 208.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8. [PubMed] [Google Scholar]
- 209.Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20(12):1465–75. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- 210.Biscardi JS, Belsches AP, Parsons SJ. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol. Carcinog. 1998;21(4):261–72. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 211.Luttrell DK, Lee A, Lansing TJ, Crosby RM, Jung KD, Willard D, Luther M, Rodriguez M, Berman J, Gilmer TM. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc. Natl. Acad. Sci. USA. 1994;91(1):83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc. Natl. Acad. Sci. USA. 1995;92(15):6981–5. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Muthuswamy SK, Muller WJ. Direct and specific interaction of c-Src with Neu is involved in signaling by the epidermal growth factor receptor. Oncogene. 1995;11(2):271–9. [PubMed] [Google Scholar]
- 214.Ottenhoff-Kalff AE, Rijksen G, van Beurden EACM, Hennipman A, Michels AA, Staal GEJ. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 1992;52(17):4773–4778. [PubMed] [Google Scholar]
- 215.Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol. Biol. Cell. 1993;4(1):121–33. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.O-charoenrat P, Rhys-Evans P, Modjtahedi H, Eccles SA. Vascular endothelial growth factor family members are differentially regulated by c-erbB signaling in head and neck squamous carcinoma cells. Clin. Exp. Metastasis. 2000;18(2):155–61. doi: 10.1023/a:1006764100867. [DOI] [PubMed] [Google Scholar]
- 217.Ravindranath N, Wion D, Brachet P, Djakiew D. Epidermal growth factor modulates the expression of vascular endothelial growth factor in the human prostate. J. Androl. 2001;22(3):432–43. [PubMed] [Google Scholar]
- 218.Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J. Gastroenterol. 2008;43(4):256–64. doi: 10.1007/s00535-008-2177-6. [DOI] [PubMed] [Google Scholar]
- 219.Rivera F, Vega-Villegas ME, Lopez-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat. Rev. 2007;33(4):315–24. doi: 10.1016/j.ctrv.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 220.Reddy S, Rishi AK, Xu H, Levi E, Sarkar FH, Majumdar AP. Mechanisms of curcumin- and EGF-receptor related protein(ERRP)-dependent growth inhibition of colon cancer cells. Nutr. Cancer. 2006;55(2):185–94. doi: 10.1207/s15327914nc5502_10. [DOI] [PubMed] [Google Scholar]
- 221.Aggarwal BB, Ichikawa H, Garodia P, Weerasinghe P, Sethi G, Bhatt ID, Pandey MK, Shishodia S, Nair MG. From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin. Ther. Targets. 2006;10(1):87–118. doi: 10.1517/14728222.10.1.87. [DOI] [PubMed] [Google Scholar]
- 222.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol. Biomarkers Prevent. 2007;16(11):2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 223.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxidants Amp. Redox Signal. 2008;10(3) doi: 10.1089/ars.2007.1740. 475(36) [DOI] [PubMed] [Google Scholar]
- 224.Ichikawa H, Nakamura Y, Kashiwada Y, Aggarwal BB. Anticancer drugs designed by mother nature: ancient drugs but modern targets. Curr. Pharm. Des. 2007;13(33):3400–16. [PubMed] [Google Scholar]
- 225.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255(2):170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 226.Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, Aderka D, Ben-Yosef R. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest. 2007;25(6):411–8. doi: 10.1080/07357900701359577. [DOI] [PubMed] [Google Scholar]
- 227.Majumdar APN, Banerjee S, Nautiyal J, Patel BB, Patel V, Du J, Yu Y, Elliott AA, Levi E, Sarkar FH. Curcumin Synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer. 2009;61(4):544, 553. doi: 10.1080/01635580902752262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J. Cell Biochem. 2007;102(3):580–92. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- 229.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71(10):1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 230.Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell. Signal. 2009;21(11):1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54(22):5841–7. [PubMed] [Google Scholar]
- 232.Huang MT, Ma W, Yen P, Xie JG, Han J, Frenkel K, Grunberger D, Conney AH. Inhibitory effects of topical application of low doses of curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion and oxidized DNA bases in mouse epidermis. Carcinogenesis. 1997;18(1):83–8. doi: 10.1093/carcin/18.1.83. [DOI] [PubMed] [Google Scholar]
- 233.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48(21):5941–6. [PubMed] [Google Scholar]
- 234.Huang MT, Wang ZY, Georgiadis CA, Laskin JD, Conney AH. Inhibitory effects of curcumin on tumor initiation by benzoapyrene and 7,12-dimethylbenzaanthracene. Carcinogenesis. 1992;13(11):2183–6. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 235.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55(2):259–66. [PubMed] [Google Scholar]
- 236.Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993;14(11):2219–25. doi: 10.1093/carcin/14.11.2219. [DOI] [PubMed] [Google Scholar]
- 237.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomarkers Prev. 2002;11(6):535–40. [PubMed] [Google Scholar]
- 238.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10(20):6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]