Abstract
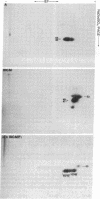
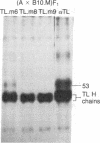
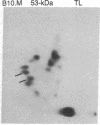
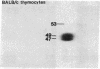
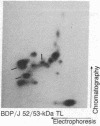
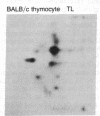
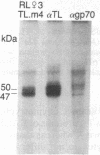
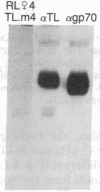
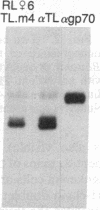
Thymocytes and leukemia cells of some mouse strains yield TL proteins, precipitable by anti-TL antiserum and by anti-TL monoclonal antibodies, that include not only the familiar heavy (H) chain of 45-50 kDa but also products of higher molecular mass. Production of a 53-kDa TL form by Tlad thymocytes was studied in detail. A cross was made between B10.M (Tlad) mice, which produce the 53-kDa TL, and mice of the A strain (Tlaa), which make only the usual H chain. Hemi-expression of apparently unaltered 53-kDa TL was observed in thymocytes of the Tlad/Tlaa heterozygous F1 progeny. Thus, there was no indication of positive or negative trans interaction with respect to production of the 53-kDa TL form associated with Tlad. We conclude that production of 53-kDa TL is governed intrachromosomally. Two-dimensional chymotryptic peptide maps of the TL H chain and the 53-kDa TL of Tlad thymocytes differed only by added features found in the map of the 53-kDa TL. With the exception of Tlaa, all Tla alleles (Tlab-f) yielded TL products of higher molecular weight than the accompanying H chain, although in the case of Tlab this was evident only in TL+ leukemia cells because Tlab thymocytes are TL-. For H-2, representing other class I genes, no products other than the familiar H chain were demonstrable under similar conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyse E. A. The biology of Tla. Cell. 1984 Aug;38(1):1–2. doi: 10.1016/0092-8674(84)90518-x. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Tucker P. W., Mushinski J. F., Blattner F. R. Mapping of heavy chain genes for mouse immunoglobulins M and D. Science. 1980 Sep 19;209(4463):1348–1353. doi: 10.1126/science.6774414. [DOI] [PubMed] [Google Scholar]
- Margulies D. H., Evans G. A., Flaherty L., Seidman J. G. H-2-like genes in the Tla region of mouse chromosome 17. Nature. 1982 Jan 14;295(5845):168–170. doi: 10.1038/295168a0. [DOI] [PubMed] [Google Scholar]
- Moriuchi T., Chang H. C., Denome R., Silver J. Thy-1 cDNA sequence suggests a novel regulatory mechanism. Nature. 1983 Jan 6;301(5895):80–82. doi: 10.1038/301080a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Nathenson S. G., Leinwand L. A. Mapping class I gene sequences in the major histocompatibility complex. Nature. 1982 Jul 22;298(5872):382–385. doi: 10.1038/298382a0. [DOI] [PubMed] [Google Scholar]
- Rosenson R. S., Flaherty L., Reinisch C. L. Induction of surface Qa2 on lymphoid cells. J Immunol. 1981 Jun;126(6):2253–2257. [PubMed] [Google Scholar]
- Rothenberg E., Boyse E. A. Synthesis and processing of molecules bearing thymus leukemia antigen. J Exp Med. 1979 Oct 1;150(4):777–791. doi: 10.1084/jem.150.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F. W., Chorney M. J., Boyse E. A. Further polymorphism of the Tla locus defined by monoclonal TL antibodies. Immunogenetics. 1982;15(6):573–578. doi: 10.1007/BF00347051. [DOI] [PubMed] [Google Scholar]
- Soloski M. J., Uhr J. W., Vitetta E. S. Primary structural studies of the Qa-2 alloantigen: implications for the evolution of the MHC. Nature. 1982 Apr 22;296(5859):759–761. doi: 10.1038/296759a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Winoto A., Steinmetz M., Hood L. Genetic mapping in the major histocompatibility complex by restriction enzyme site polymorphisms: most mouse class I genes map to the Tla complex. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3425–3429. doi: 10.1073/pnas.80.11.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Stockert E., Old L. J., Nathenson S. G. Structural comparisons of TL antigens derived from normal and leukemia cells of Tl+ and TL- strains and relationship to genetically linked H-2 major histocompatibility complex products. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7078–7082. doi: 10.1073/pnas.78.11.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]