Abstract
Papillary thyroid cancer (PTC) rates continue to increase in the United States and Europe, and while most patients do well, some recur and die of their disease. Patients with PTC harboring the BRAFV600E mutation appear to display a more aggressive clinical behavior but little is known about the role of this mutation in crucial processes in the tumor microenvironment such as tumor adhesion, migration, invasion, and metastasis. The extracellular matrix (ECM) microenvironment is not merely a structural scaffold for the cellular elements of the epithelial and stromal microenvironment, but it elicits also a profound influence on cell behavior affecting viability, proliferation, adhesion, motility. The effects of BRAFV600E on cell surface receptors (i.e. integrins) and ECM non-cellular components (i.e. TSP-1, FN) appear to trigger different pathological biological effects in a cell context-dependent manner. This review will focus on the recent progress in understanding the role of BRAFV600E in the regulation of some ECM non-cellular components and trans-membrane receptors of the microenvironment in PTC in order to design novel targeted therapies directed at the BRAFV600E multifaceted signaling cascades. Some of these targeted therapeutics such as ATP-competitive BRAFV600E inhibitors (i.e. orally bioavailable PLX4720 and PLX4032 compounds) are already under investigation.
Keywords: BRAFV600E, thrombospondin-1, integrins, extracellular matrix, fibronectin, papillary thyroid cancer, metastasis
Introduction
The incidence of thyroid cancer is increasing more rapidly than other cancers in both the US (1) and other countries (2). Papillary thyroid carcinoma (PTC) originates in the follicular cells of the thyroid and represents the most frequent endocrine malignancies. Well-differentiated papillary thyroid cancers (PTC) typically have a favorable prognosis with thyroidectomy followed by thyroid hormone suppressive therapy and radioactive iodine (RAI) ablation of normal thyroid tissue and any residual tumor in some (3). However, for the group of patients who fails to respond to this treatment paradigm or presents initially with aggressive and refractory thyroid carcinomas, rates of neck recurrence and distance metastases are high and survival rates are very low and rationale targeted therapies are being investigated (3).
The BRAFV600E mutation, thyroid cancer and tumor microenvironment
Molecular targets for recurrent PTC are mostly centered on the RAS/BRAF/MAPK (mitogen-activated extracellular signal regulated kinase, i.e. ERK1/2) kinase signaling pathway given the prominence of this pathway as an oncogenic event in PTC progression (4). The BRAF gene is located on human chromosome 7q24 and encodes a cytosolic serine–threonine protein kinase that is expressed in many human cells, including thyroid follicular cells (5). The wild type (wt) BRAF is activated at the plasma membrane through a complex process that involves RAS activity, phosphorylation events and protein/lipid interactions. BRAF kinase exhibits a characteristic bilobar structure similar to all protein kinases. The inactive conformation of BRAF involves the simultaneous binding of 14-3-3 to phosphorylated sites S365 and S729 (6). The activated wt BRAF is phosphorylated at site S446, leading to a maximally negatively charged amino-region. Extracellular signals (i.e. mitogens, hormones, and neurotransmitters) induce a tyrosine kinase receptor, act on RAS-GTP, and activates wt BRAF (7) (6). Two conserved sites (T598 and S601) of wt BRAF are oncogenic RAS-dependent phosphorylation sites. This event not only renders BRAF constitutively active, but also induces ERK1/2 activation, causing cell transformation (7) (6).
Constitutive activation of the RAS-ERK signaling pathway is common to numerous cancers. Approximately 15% of human cancers have activating RAS mutations (8). Over 30 mutations of the BRAF gene associated with human cancers have been identified, the majority of which are located within the kinase domain (8). In 2002, Davies et al. (1) identified an oncogene widespread amongst human cancers, mutant BRAFV600E. BRAFV600E is expressed in different human cancer cell lines including melanoma, colorectal cancer, and thyroid cancer (9) (4). An activating mutation located on exon 15 of the B isoform of the RAF kinase gene results in a valine-to-glutamic acid substitution at amino acid 600 (BRAFV600E). The V600E mutation strongly enhances BRAF kinase activity by inserting a negatively charged residue adjacent to the phosphorylation site at T598 and mimicking phosphorylation at Thr598 and Ser601 residues (10) (7) with increased ERK1/2 phosphorylation (11) (8). These molecular features render BRAFV600E as a unique kinase able to elicit a strong phosphorylation activity on ERK1/2, 480-fold higher than wt BRAF or other mutants BRAF (8). This mutation is very prevalent in PTC and is clearly seen much more frequently in the tumors with larger size, lymphovascular invasion or metastases, and mortality, and may play a role in the progression of PTC to anaplastic thyroid cancer (ATC) (4) (9) (12) (13).
Decades of research have demonstrated that tumorigenesis is strongly affected by non-malignant cells (i.e. stromal cells) that comprise the tumor microenvironment (14). Interestingly, a large number of genes abnormally expressed in human cancer encode secreted proteins and receptors, with paracrine and autocrine effects on other components of the tumor such as stromal cells (including fibroblasts, macrophages, endothelial cells, smooth muscle cells, T-lymphocytes, monocytes, etc.), and extracellular matrix (ECM) non-cellular components (15) (16). Dynamic and reciprocal interactions involving cell adhesion molecules (e.g. integrins, CD44), ECM non-cellular components (i.e. thrombospondin-1 (TSP-1), Fibronectin (FN)), and soluble cytokines occur between tumor epithelial cells and tumor microenvironment stroma cells (17). The degree of these interactions may represent the basis of triggering of intracellular signaling pathways that confer tissue-specific characteristics to the epithelium (17). The ECM is therefore a fundamental component of cell microenvironment and has been substantially expanded during the evolution of vertebrates. It provides more than mechanical support and is a locus for cell adhesion, with potential roles in basement membranes and tumors. All epithelial cells are in association with basement membranes during their lives and include the ECM. ECM composition and organization undergo radical alterations in human cancers and could affect cell survival, proliferation, adhesion, migration, and other properties of both tumor and stromal cells.
Importantly, the BRAFV600E mutation has been associated with aggressive clinical behavior in some patients with PTC (4). Little data shed light on how the oncogene BRAFV600E can affect the tumor microenvironment in thyroid cancer, including interactions between neoplastic thyroid follicular cells and ECM components. Deregulated pathways downstream BRAFV600E in human cancers harboring this mutation include: tumor suppressor genes (i.e. TIMP-3), deregulation of micro-RNAs, and positive regulation of Skp-2 and NF-kB signaling (4). It has also been recently shown that BRAFV600E expression correlates significantly with VEGF protein expression in PTCs with extra-thyroidal invasion, perhaps via BRAFV600E modulation of hypoxia-inducible factors (HIF) (4). Mesa et al. have shown that BRAFV600E-activated normal rat thyroid cells express genes such as metallo-proteases (MMPs) (i.e. MMP-3, MMP-9, MMP-13) (18). Traditionally, these enzymes may promote tumor invasion by breaking down various non-cellular components of the ECM. It has been observed that PTC harboring BRAFV600E show a more aggressive clinic-pathologic behavior (e.g. extra-thyroid extension) and a significant increase in MMP-2 and MMP-9 protein levels, thus suggesting that these proteins may play a role in PTC progression (12).
The BRAFV600E mutation and extracellular matrix non-cellular components
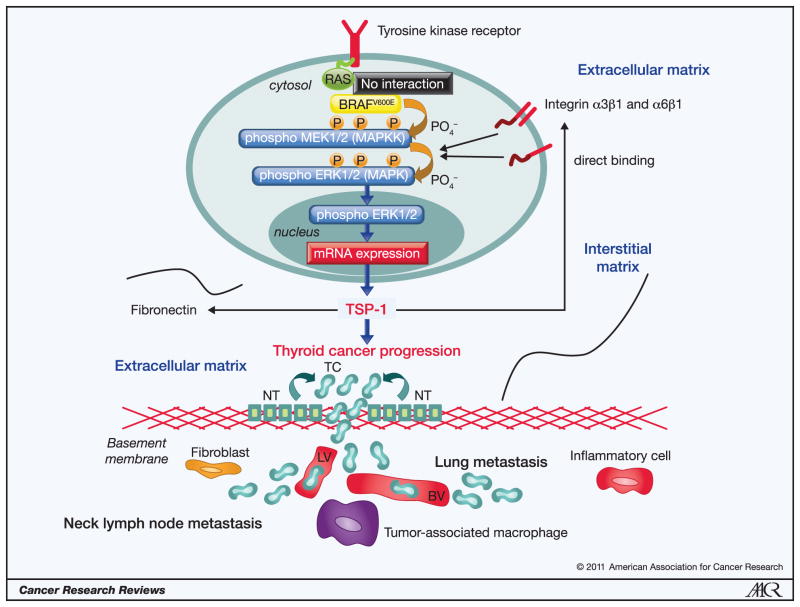
We have recently investigated the role of this single mutation in the gene expression patterns of PTC and ATC cells and in the tumor microenvironment, leading to a better understanding in order to design future targeted therapies directed at BRAFV600E signaling cascades (19). Our results suggest that BRAFV600E pathway plays an important role in PTC progression through proteins crucial for the ECM remodeling processes including tumor cell adhesion, migration, invasion, and metastasis (19). Using both in vitro and in vivo (i.e. orthotopic mouse models) of human thyroid cancer, we found that TSP-1, a multifunctional molecule known to have important effects on tumor stroma and endothelium, serves as a mediator of invasiveness and aggressive tumor behavior when the BRAFV600E mutation is present. Using a novel technique based on genome-wide expression profiling and designed to look at alterations in gene sets (Gene Set Enrichment Analysis or GSEA) we identified seventeen up-regulated gene sets that were significantly associated with PTC with BRAFV600E when compared to PTCs without the mutation or in normal thyroid tissue. Many of these altered gene sets are involved in the composition and remodeling of ECM such as TSP-1, TGF-β1, integrin-α3, -α6, -β1, FN, CD44, cathepsin-B (CTS-B) and cathepsin-B (CTS-S). These genes appear to be either targeted or affected by the BRAFV600E mutation in PTCs (19). They might act in concert and elicit important biological cross-talk during tumor cell adhesion, migration, and invasion processes involving tumor microenvironment, and ultimately trigger thyroid cancer progression (Figure 1). Furthermore, our data showed that decreasing mutant BRAF with knockdown or using a drug (PLX4720) designed to selectively deactivate BRAFV600E in those thyroid cancer cells with at least one copy of mutant BRAF results in reversal of tumor migration and invasion, and metastasis, which is translatable to decreased tumor volume in mice with orthotopic thyroid cancers at 5 weeks post tumor implantation (19).
Figure 1. The BRAFV600E oncoprotein activates phospho-ERK1/2 signaling pathway and regulates the expression of extracellular matrix non-cellular components.
The BRAFV600E oncoprotein elicits dramatically an increased kinase activity and activates phospho-ERK1/2. This oncoprotein is constitutively active and does not require RAS signaling. The BRAFV600E -activated phospho-ERK1/2 pathway is able to transform normal thyroid cells (NT) to thyroid cancer cells (TC). It up-regulates some extracellular matrix (ECM) non-cellular components (i.e. Thrombospondin-1 (TSP-1), Fibronectin) and cellular trans-membrane receptors (e.g. integrins), which together may increase the levels of phospho-ERK1/2 through a positive feedback driven by TSP-1. These pathological processes trigger ECM remodeling and determines tumor cell invasion into the lymphatic (LV) and/or blood vessels (BV) through basement membrane causing thyroid cancer progression.
For those familiar with the broad range of Thrombospondins (TSP) and their important role in development, angiogenesis and tumor stroma it is not surprising to find them implicated in BRAF mediated tumor progression. TSPs are a family of 5 secreted proteins that play distinct roles in development and physiology with TSP-1 and TSP-2 playing potential roles in tumors. TSP-1 is not only the most abundant protein in α-granules of platelets but is also expressed in the stroma of tumors (20). TSP-1 binds to a wide variety of integrins and non-integrin (i.e. proteoglycans) cell surface receptors, matrix proteins (i.e. FN), cytokines (i.e. TGF-β1), pro-angiogenic factors (e.g. VEGF), and matrix proteases (i.e. MMP-9), indicating its importance in cross-talk between surface receptors, and (iii) serves as a key-regulator of tumor cell adhesion and migration, metastasis, and angiogenesis, and may direct clustering of receptors to specialized domains for these biological processes. TSP-1 influences VEGF activity by inhibiting the activation of MMP-9 and suppressing the release of VEGF from the ECM and is also a major activator of TGFβ1 (20).
Whereas the role of TSP-1 as an anti-angiogenic factor is well documented (20), its biological action in tumor progression and metastasis is still controversial. Yee et al. have recently demonstrated that TSP-1 can promote metastasis to lungs in a transgenic mouse model of breast cancer (21). We have shown that TSP-1 knockdown in aggressive human thyroid cancer cells harboring BRAFV600E prevent the progression of this cancer by resulting in a decreased phospho-ERK1/2 protein levels and reduced cell proliferation, adhesion, migration, and invasion, as well as metastasis in vivo (19). In addition, the G1 arrest of these cells demonstrates that TSP-1 promotes cell cycle progression (19). Overall, these results suggest that TSP-1 can be considered a regulator of thyroid cancer microenvironment, eliciting pro-migratory and pro-invasive roles in thyroid cancer cells harboring BRAFV600E (19). By contrast, (i) mutated RAS is able to repress TSP-1 expression via the c-myc oncogene or activate by RAF/ERK pathway in human breast cancer models (22); and (ii) loss of p53 function has been shown to correlate with reduction in TSP-1 expression and switch to a pro-angiogenic phenotype in fibroblasts derived from a patient with Li-Fraumeni syndrome (20). Overall, varied TSP-1 expression is regulated differently depending on the genetic context of the cells.
Our results also point to the importance of certain key integrins (i.e. α3β1 and α6β1) that showed significantly higher mRNA levels in BRAFV600E positive PTC compared with wt BRAF PTC or normal thyroid tissue and may mediate thyroid tumor cells migration and invasion (19). Some integrins mediate tumor cell-ECM adhesion and provide both the connection to the adhesive substrate and cellular signaling (known as “outside-in” signaling or extracellular to intracellular) crucial for cell proliferation, migration, and invasion (17). Some integrins (e.g. α2β1 in breast cancer cells) are decreased as tumors progress, thus suggesting that α2β1 could function as a tumor suppressor; whereas elevated expression of integrin β3 appears to be closely associated with melanoma progression (23). Interestingly, integrins α5β1 may become activated upon p53 mutation and reflect enhanced FN-binding integrin (24). We also found a potential link between FN and BRAFV600E in our analysis (19); BRAFV600E positive PTCs showed significantly higher FN mRNA levels compared with wt BRAF PTC or normal thyroid tissue. Our data support the hypothesis that FN overexpression may be involved in cancer progression harboring BRAFV600E and may influence the control of metastasis by mediating integrin-associated signaling pathways. Human cells mediate FN matrix assembly through integrins binding to the RGD cell-binding domain. Integrin α5β1 is the primary receptor for FN matrix assembly, which binds to the RGD sequence (Arg-Gly-Asp) (17). Importantly, melanoma cells over express FN, which controls many fundamental pathobiological processes. There is strong evidence that over expression of FN is tightly correlated with the acquisition of invasive and metastatic behavior of melanoma cells by constitutive BRAFV600E/ERK kinase signaling (25). FN binding to integrin induces receptor clustering, which brings together cytoplasmic molecules such as focal adhesion kinase (FAK), Src kinase, paxillin, and others to form protein rich focal complexes that activate polymerization of actin filaments and intracellular signaling through kinase cascades (26). FAK activation by integrin–ligand interactions promotes PI3K signaling, which is essential to promote cancer invasion. In addition, it has been recently demonstrated from Shibue and Weinberg that integrin β1 is also fundamental to activate FAK signaling axis in controlling the initial proliferation of micrometastatic mouse breast cancer cells disseminated in the lungs (27).
In addition to FN-Integrins interactions, TSP-1-Integrins interaction also contributes to initiate “outside-in” signal transduction events that modulate gene expression, cell proliferation, migration, and invasion (17). TSP-1 has many important functional interactions through its various domains, some of which (3TSR domain or termed as type 1 repeats) lay an important role in activation of TGF-β1 in vivo (20). Our data showed that the N-terminal of TSP-1 appears to be the critical element involved in BRAF mediated invasion in thyroid cancer cells (19). Chandrasekaran et al. (28) also showed a critical role for the TSP-1 N-terminal domain in breast cancer cell invasion via putative binding site(s) to integrin α3β1, which have an important role for tumor cell migration and invasion. Sumimoto et al. (29) have shown that BRAFV600E knockdown down-regulated phospho-ERK1/2 protein levels and inhibited matrigel invasion of melanoma cells accompanied with a decrease of matrix metalloproteinase activity and integrin β1 expression. These results clarify that the mutated BRAFV600E is essentially involved in malignant phenotype of melanoma cells by regulating genes involved in the ECM remodeling through the ERK1/2 activation and would thus serve as an attractive molecular target for melanoma treatment.
TSP-1 also binds FN directly (30) or indirectly through TSG6 (also termed as tumor necrosis factor-stimulated gene 6, secreted protein that is produced during inflammation processes) (31). The binding by FN to TSP-1 induces conformational changes in TSP-1 that enhance the ability of TSP-1 to be recognized by integrin α3β1 (30); such interactions appear to enhance FN matrix assembly and increase adhesive properties of TSP-1 to integrins. In addition, Decker et al. have shown that FN can form a complex with integrin α4β1 and TSP-1; the complex α4β1/FN/TSP1 increased adhesion of osteosarcoma cells (32).
Targeting BRAFV600E positive human cancers with orally available selective inhibitors
Recent advances in understanding the molecular changes that take place in human tumorigenesis have led to the development of novel therapeutic strategies that are based on various molecular targets. Pharmacologic targeting BRAFV600E may provide selective and rationale advantages for treatment of patients with PTC harboring this mutation. Two Plexxikon compounds- PLX4720 and PLX4032 are novel orally available selective small molecules inhibitors of BRAFV600E that have been specifically designed to insert into the ATP-binding site and trap oncogenic BRAFV600E in an inactive conformation (33) (34). Consistent with their high degree of selectivity for the mutant BRAFV600E, these compounds inhibit BRAFV600E kinase activity both in melanoma and colorectal cancer cells, leading to the inhibition of ERK1/2 phosphorylation and G1-phase cell cycle arrest (33) (34). PLX compounds demonstrate efficacy against either homozygous or heterozygous BRAFV600E mutated cell lines and animals with tumor implantation (i.e. melanoma, colorectal cancer, or anaplastic thyroid cancer) (33) (34) (19).
It has been shown that these ATP-competitive RAF inhibitors could have unexpected effects in some cell and genotype contexts (e.g. presence of wt BRAF along with mutated RAS) due to ERK1/2 hyper-phosphorylation by dimerization between wt BRAF with another RAF isoform, CRAF (35) (36) (37). The results from these aforementioned studies highlight the importance of individualized genomic profiling to guide patient selection for inclusion in targeted therapy trials.
Recently, phase I-II clinical trials in patients with BRAFV600E positive melanomas have shown a partial or complete response to PLX4032, even though duration of the response to this inhibitor is yet unknown (38). PLX4032 induced complete or partial tumor regression in 81% of patients who had melanoma with the BRAFV600E mutation. Responses were observed at all sites of disease, including the bone, liver, and small bowel (38). Most side effects related to PLX4032 appeared to be proportional to the dose and exposure to the drug. Cutaneous side effects, fatigue, and arthralgia were the main clinical problems in the treated patients. Thirty-one percent of these patients treated with PLX4032 developed skin lesions described as cutaneous squamous cell carcinomas and keratoacanthoma. These skin lesions generally appear within a few months of treatment initiation in sun exposed areas of skin, suggesting that pre-existing oncogenic mutations may potentiate the RAF inhibitor effects (34). This drug-induced effect is of particular interest, because other RAF inhibitors such as sorafenib (used in clinical trials, either alone or in combination with chemotherapy that has not had significant anti-melanoma effects) also caused these skin lesions in a subset of treated patients (38) (39). The ability of PLX4032 to cause tumor regression in a large proportion of patients with BRAFV600E, advanced-stage, metastatic melanoma provides strong support for the hypothesis that the BRAFV600E protein is a dominant driver of tumor growth and maintenance. However, in some patients with BRAFV600E mutation positive melanoma, the tumors showed resistance without evidence of an early response, and the mechanism of this primary resistance (refractory state) is still unknown. Importantly, results from an advanced human thyroid cancer preclinical model (40) using PLX4720 (19), suggest that these inhibitors might be an effective therapy in clinical trials for the treatment of patients with BRAFV600E positive thyroid cancers that are refractory to conventional therapy.
Conclusions and Perspectives
In summary, the BRAFV600E mutation may affect the expression of tumor ECM non-cellular components and alters microenvironment in papillary thyroid cancer. The molecular action of this oncogene appears to affect both the migratory and invasive properties of the thyroid cancer cell itself as well as components of tumor ECM microenvironment. Knowledge about these new downstream targets of BRAF may help identify biomarkers (i.e. secreted factors) and/or targets for innovative therapeutic strategies in BRAFV600E positive human cancers. Therapeutic strategies aimed at modulating the host microenvironment (i.e. ECM cellular and non-cellular components) may offer a complementary perspective for the treatment of patients with these types of cancers. It will be of considerable interest therefore to reveal the spectrum of molecular mechanisms underlying the signaling cross-talks of tumor microenvironment and to determine the extent to which they participate in the aberrant behavior of BRAFV600E positive human cancer cells.
Acknowledgments
Carmelo Nucera (M.D., Ph.D.) was a recipient of a Ph.D. fellowship in Experimental Endocrinology and Metabolic Diseases (MIUR, Italy). Sareh Parangi (M.D.) was funded through the American Thyroid Association and NIH Grant. Jack Lawler (Ph.D.) was funded through NIH Grant CA130895. We thank those authors who we have neglected to cite due to limitation on the number of references.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295(18):2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14(12):1056–60. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 3.Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8(2):148–56. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- 4.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T, Nakazawa T, Murata S, et al. Enhanced B-Raf protein expression is independent of V600E mutant status in thyroid carcinomas. Human pathology. 2007;38(12):1810–8. doi: 10.1016/j.humpath.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon AS, Kolch W. Oncogenic B-Raf mutations: crystal clear at last. Cancer cell. 2004;5(4):303–4. doi: 10.1016/s1535-6108(04)00087-x. [DOI] [PubMed] [Google Scholar]
- 7.Ikenoue T, Hikiba Y, Kanai F, et al. Functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer research. 2003;63(23):8132–7. [PubMed] [Google Scholar]
- 8.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 9.Nucera C, Goldfarb M, Hodin R, Parangi S. Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf(V600E) Biochimica et biophysica acta. 2009;1795(2):152–61. doi: 10.1016/j.bbcan.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene. 2008;27(7):877–95. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- 11.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 12.Frasca F, Nucera C, Pellegriti G, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocrine-related cancer. 2008;15(1):191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 13.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. The Journal of clinical endocrinology and metabolism. 2008;93(10):3943–9. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 14.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25(1):30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 16.McAllister SS, Weinberg RA. Tumor-Host Interactions: A Far-Reaching Relationship. J Clin Oncol. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- 17.Hynes RO. The extracellular matrix: not just pretty fibrils. Science (New York, NY. 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesa C, Jr, Mirza M, Mitsutake N, et al. Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer research. 2006;66(13):6521–9. doi: 10.1158/0008-5472.CAN-06-0739. [DOI] [PubMed] [Google Scholar]
- 19.Nucera C, Porrello A, Antonello ZA, et al. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(23):10649–54. doi: 10.1073/pnas.1004934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65(5):700–12. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee KO, Connolly CM, Duquette M, Kazerounian S, Washington R, Lawler J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res Treat. 2009;114(1):85–96. doi: 10.1007/s10549-008-9992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watnick RS, Cheng YN, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer cell. 2003;3(3):219–31. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 23.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature reviews. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller PA, Caswell PT, Doyle B, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139(7):1327–41. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Gaggioli C, Robert G, Bertolotto C, et al. Tumor-derived fibronectin is involved in melanoma cell invasion and regulated by V600E B-Raf signaling pathway. The Journal of investigative dermatology. 2007;127(2):400–10. doi: 10.1038/sj.jid.5700524. [DOI] [PubMed] [Google Scholar]
- 26.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2(11):793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 27.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10290–5. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin-like growth factor-1 and CD98. The Journal of biological chemistry. 1999;274(16):11408–16. doi: 10.1074/jbc.274.16.11408. [DOI] [PubMed] [Google Scholar]
- 29.Sumimoto H, Miyagishi M, Miyoshi H, et al. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene. 2004;23(36):6031–9. doi: 10.1038/sj.onc.1207812. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues RG, Guo N, Zhou L, et al. Conformational regulation of the fibronectin binding and alpha 3beta 1 integrin-mediated adhesive activities of thrombospondin-1. The Journal of biological chemistry. 2001;276(30):27913–22. doi: 10.1074/jbc.M009518200. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsova SA, Mahoney DJ, Martin-Manso G, et al. TSG-6 binds via its CUB_C domain to the cell-binding domain of fibronectin and increases fibronectin matrix assembly. Matrix Biol. 2008;27(3):201–10. doi: 10.1016/j.matbio.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker S, van Valen F, Vischer P. Adhesion of osteosarcoma cells to the 70-kDa core region of thrombospondin-1 is mediated by the alpha 4 beta 1 integrin. Biochemical and biophysical research communications. 2002;293(1):86–92. doi: 10.1016/S0006-291X(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 33.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464(7287):431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 36.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell. 2010;140(2):209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010 doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. The New England journal of medicine. 2010;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27(23):e59–61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 40.Nucera C, Nehs MA, Mekel M, et al. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid. 2009;19(10):1077–84. doi: 10.1089/thy.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]