Abstract
To evaluate the long-term consequences of acute kidney injury (AKI) in human immunodeficiency virus (HIV)-infected persons, we studied 17,325 patients in a national HIV registry during their first hospitalization. We determined the association of AKI with risk for heart failure, cardiovascular events, end-stage renal disease (ESRD), and mortality beginning 90 days after discharge. Based on AKI Network criteria, 2453 had stage 1; 273 had stage 2 or 3; and 334 had dialysis-requiring AKI. Over a mean follow-up period of 5.7 years, 333 had heart failure, 673 had cardiovascular diseases (CVDs), 348 developed ESRD, and 8405 deaths occurred. In multivariable-adjusted analyses, AKI stage 1 was associated with death and ESRD, but not heart failure or other CVD. Dialysis-requiring AKI had much stronger and significant associations with increased risk for long-term ESRD, and death in addition to heart failure and cardiovascular events. When AKI was reclassified to account for recovery, stage 1 with recovery was still associated with death, but not ESRD. Thus, in this national sample of HIV-infected persons, we found the clinical repercussions of AKI appear to extend beyond the hospital setting contributing to excess cardiovascular risks, ESRD, and mortality. Additionally, AKI affected almost one of six patients with HIV who survived at least 90 days following discharge.
Keywords: acute kidney injury, cardiovascular disease, end-stage renal disease, HIV, mortality
Acute kidney injury (AKI), also known as acute renal failure, is commonly defined as a rapid decline in kidney function marked by an increase in serum creatinine level. AKI is increasingly recognized as an important management issue in hospitalized patients, because it is relatively common, affecting up to 7% of patients, and consistently associated with high rates of in-hospital mortality.1,2 In addition, a growing number of studies have linked AKI with long-term outcomes after discharge, such as mortality and end-stage renal disease (ESRD), even 10 years after the AKI has occurred.3,4 These reports have stimulated a debate over the mechanisms responsible for increased mortality in AKI, and indicate a need to broaden investigation to other adverse events that may be associated with AKI. For example, although the majority of deaths in chronic kidney disease (CKD) are thought to be related to cardiovascular disease (CVD), a clear association has not been established between AKI and incident heart failure or CVD.
Human immunodeficiency virus (HIV)-infected persons are a population that may experience a high burden of disease from AKI. Among hospitalized patients, AKI occurs at 2–3 times the rate observed in uninfected controls.5 Furthermore, improved survival in the HIV-infected population has shifted research priorities from effective treatment of viral replication to understanding the importance of non-AIDS-related chronic conditions and their risk factors. In the contemporary era of antiretroviral therapy, CKD and CVD have emerged as major causes of death in HIV-infected persons.6,7 We previously found that kidney function and albuminuria measured in the outpatient setting is associated with adverse cardiovascular events in HIV-infected persons.8 Although, earlier studies have linked the natural history of CKD with AKI, no studies have explored the potential relationship between AKI, CVD, and mortality.9 To enhance our understanding of the importance of AKI in HIV-infected persons, we conducted an observational cohort study in a national sample of 17,325 HIV-infected persons receiving care in the Veterans Health Administration, and examined the association between AKI experienced during their first hospitalization with the development of heart failure, atherosclerotic cardiovascular events, ESRD, and death over a period of 2 decades.
RESULTS
We identified 17,325 HIV-infected persons who survived at least 90 days after discharge from their first hospitalization over a 20-year period (Table 1). A large proportion of these patients experienced AKI (18%) during their hospital stay. Among those with AKI, stage 1 AKI (defined by a crude serum creatinine increase of 0.3 mg/dl or greater, or a relative increase between 150 and 200%) was found in 80% (n =2453). Stage 2 or 3 AKI (marked by a relative creatinine increase >200%, or a crude increase of 0.5 mg/dl in individuals with a baseline creatinine of ≥4.0 mg/dl) was much less common (9%, n =273). There were 334 patients (11%) who experienced AKI treated with dialysis. Individuals with AKI were more likely to be black, and have CKD (estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2) or risk factors for CKD such as hypertension, diabetes, low CD4 count, and elevated viral loads. Individuals who were treated in the intensive care unit were also more likely to experience AKI.
Table 1.
Baseline characteristics of 17,325 hospitalized HIV-infected persons, by stage of acute kidney injury (AKI)a
| No AKI (n=14,265) | AKI stage 1 (n=2453) | AKI stage 2–3 (n=273) | Dialysis-requiring AKI (n=334) | P-value | |
|---|---|---|---|---|---|
| Age | 44 (9) | 46 (10) | 47 (9) | 47 (10) | <0.001 |
| Race | <0.001 | ||||
| White | 28.0% | 20.8% | 12.8% | 12.6% | |
| Black | 39.4% | 40.0% | 61.5% | 52.4% | |
| Other | 32.6% | 39.1% | 25.6% | 35.0% | |
| Female | 2.0% | 1.4% | 1.8% | 0.9% | 0.074 |
| Comorbid conditions | |||||
| Hypertension | 18.9% | 22.2% | 31.9% | 40.7% | <0.001 |
| Diabetes | 6.8% | 10.2% | 11.0% | 18.3% | <0.001 |
| Dyslipidemia | 10.6% | 10.7% | 10.6% | 16.5% | 0.008 |
| Liver disease | 3.5% | 3.5% | 3.3% | 6.6% | 0.028 |
| Lung disease | 6.6% | 5.9% | 5.9% | 6.0% | 0.562 |
| Smoking | 17.2% | 14.7% | 15.0% | 13.5% | 0.007 |
| Cancer | 9.4% | 11.9% | 11.4% | 14.4% | <0.001 |
| Heart failure | 1.6% | 3.6% | 4.8% | 8.7% | <0.001 |
| Cardiovascular disease | 6.7% | 7.1% | 7.3% | 11.1% | 0.037 |
| Hepatitis C virus infection | 21.3% | 20.6% | 27.1% | 19.5% | 0.072 |
| Hepatitis B virus infection | 6.3% | 6.9% | 10.6% | 9.6% | 0.003 |
| Baseline eGFR | <0.001 | ||||
| ≥60 ml/min per 1.73 m2 | 93.8% | 89.4% | 66.3% | 86.8% | |
| 30–59 ml/min per 1.73 m2 | 4.8% | 8.5% | 8.8% | 2.7% | |
| <30 ml/min per 1.73 m2 | 1.4% | 2.1% | 24.9% | 10.5% | |
| Albuminuria | 29.7% | 49.9% | 67.8% | 16.5% | <0.001 |
| HIV-related characteristics | |||||
| Antiretroviral therapy | 25.7% | 24.9% | 25.6% | 21.3% | 0.261 |
| CD4 count, cells/mm3 | 267 (286) | 181 (268) | 195 (240) | 200 (248) | <0.001 |
| Viral load, log copies | 3.5 (1.5) | 3.9 (1.5) | 3.9 (1.4) | 3.5 (1.7) | <0.001 |
| Intensive care unit stay | 5.7% | 12.6% | 24.2% | 28.4% | <0.001 |
| Hospitalization after 1995 | 49.0% | 52.6% | 63.7% | 52.1% | <0.001 |
Reported as proportion with condition (%); continuous variables reported as mean (s.d.). eGFR, estimated glomerular filtration rate; albuminuria defined as ≥30 mg/dl on dipstick urinalysis.
Over 98,208 person-years of follow-up (mean 5.7, s.d. 4.8 years), 333 heart failure events, 673 CVD events, 348 ESRD events, and 8405 deaths occurred. The rates of heart failure, CVD, ESRD, and mortality increased incrementally by stage of AKI (Figure 1). Age-standardized rates of mortality were far greater than those for heart failure, CVD, and ESRD. The gradient in risk from no AKI to dialysis-requiring AKI was steeper for heart failure (3 versus 16 per 1000 person-years), than for CVD (9 versus 17 events per 1000 person-years), or death (82 versus 188 events per 1000 person-years). However, differences across AKI stages were particularly exaggerated for ESRD; ESRD was nearly 16 times as common in those with dialysis-requiring AKI compared with those without AKI (56 versus 4 events per 1000 person-years).
Figure 1. Age-standardized event rates 90 days after discharge by stage of in-hospital acute kidney injury.
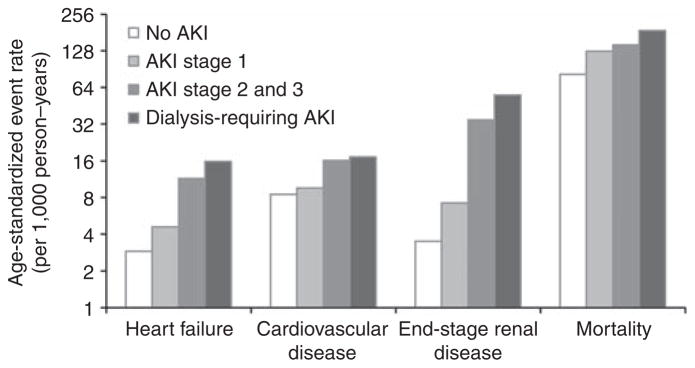
Note that y axis is on log2 scale.
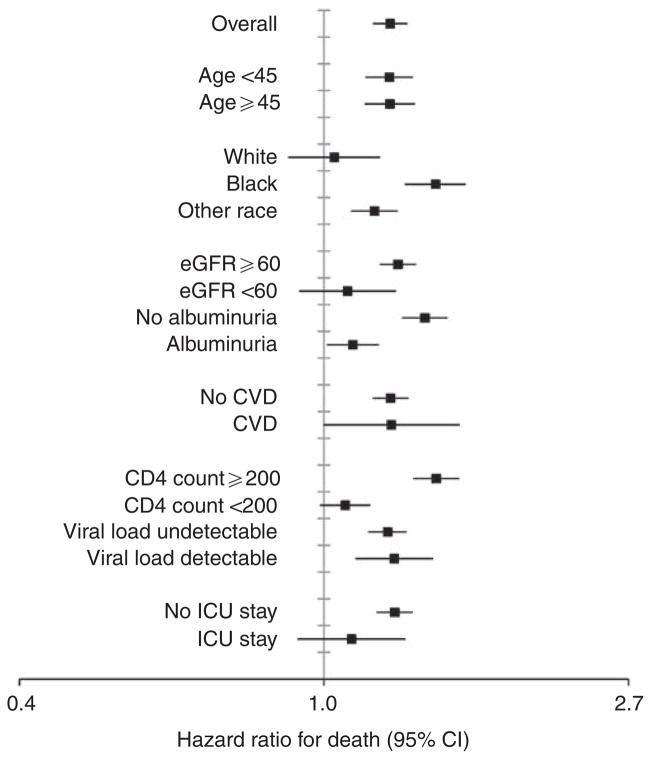
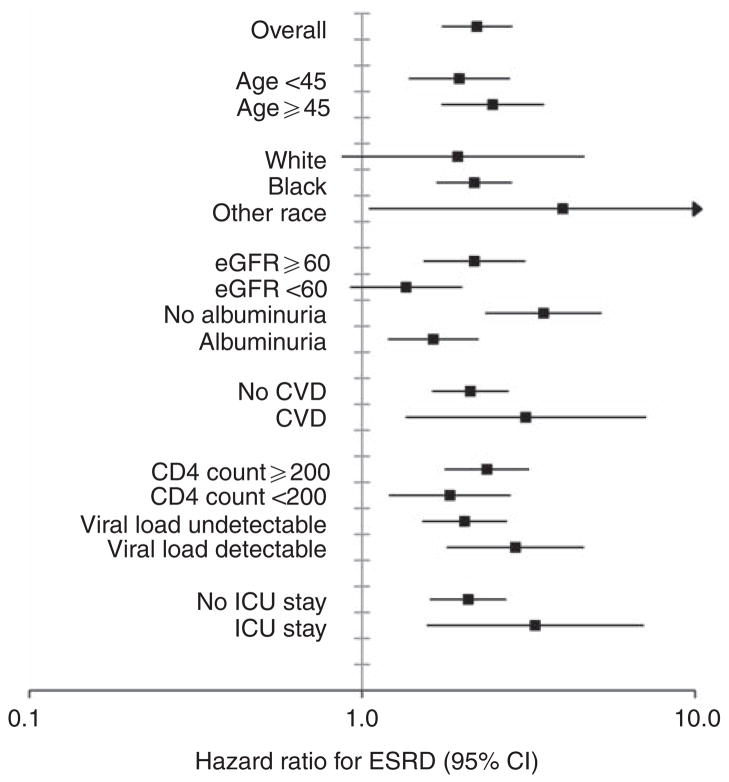
In Cox proportional hazards regression models (Table 2) adjusted for characteristics in Table 1, adjusted hazard ratios for stage of AKI increased incrementally. Associations were strongest for ESRD, where a 37% increased risk for long-term chronic dialysis therapy was observed in those with the mildest form of AKI. Despite multivariable adjustment for demographic factors, baseline kidney function and albuminuria, HIV-related factors, and comorbid conditions, all stages of AKI remained associated with an increased risk for mortality. The association between AKI and the outcomes of heart failure and CVD changed minimally when results were stratified by those with or without a history of these conditions. We also did not detect significant interactions between AKI and calendar year (i.e., antiretroviral treatment era). In subgroup analyses for mortality (Figure 2), AKI was consistently associated with harm; however, hazard ratios were smaller in magnitude among those with greater severity of disease at baseline marked by lower CD4 count, detectable viral load, prevalent kidney disease, or hospitalization in the intensive care unit. Also, to better characterize the effect of baseline eGFR<60 ml/min per 1.73 m2 and albuminuria, we divided the cohort into four mutually exclusive categories defined by (1) the absence of these conditions, (2) albuminuria only, (3) eGFR<60 ml/min per 1.73 m2 only, and (4) both conditions. Results were consistent with the primary subgroup analysis (Figure 2), with the adjusted risk of mortality associated with AKI: 1.41 (1.31–1.52), 1.16 (1.06–1.28), 1.02 (0.73–1.43), and 1.06 (0.86–1.31) in respective categories. Similar patterns were observed in the subgroup analysis for ESRD (Figure 3); however, the magnitude of associations between AKI and ESRD were stronger than for death.
Table 2.
The association between in-hospital acute kidney injury (AKI) with long-term adverse eventsa
| No AKI (n=14,265) | AKI stage 1 (n=2453) | AKI stage 2–3 (n=273) | Dialysis-requiring AKI (n=334) | P-value for trend | |
|---|---|---|---|---|---|
| Heart failure | |||||
| Number of events | 250 | 53 | 14 | 16 | |
| Demographic adjusted | 1.0 | 1.39 (0.98–1.95) | 3.28 (1.73–6.22) | 4.57 (2.53–8.28) | <0.001 |
| Multivariable adjusted | 1.0 | 1.17 (0.82–1.67) | 2.11 (1.07–4.16) | 4.20 (2.24–7.88) | <0.001 |
| CVD | |||||
| Number of events | 559 | 85 | 14 | 15 | |
| Demographic adjusted | 1.0 | 1.01 (0.80–1.27) | 1.71 (1.00–2.91) | 1.85 (1.10–3.10) | 0.120 |
| Multivariable adjusted | 1.0 | 0.98 (0.77–1.24) | 1.34 (0.77–2.35) | 1.96 (1.14–3.38) | 0.051 |
| End-stage renal disease | |||||
| Number of events | 230 | 63 | 23 | 32 | |
| Demographic adjusted | 1.0 | 2.03 (1.54–2.69) | 9.09 (5.89–14.02) | 23.54 (16.09–34.44) | <0.001 |
| Multivariable adjusted | 1.0 | 1.37 (1.02–1.84) | 3.80 (2.32–6.23) | 20.36 (12.65–32.78) | <0.001 |
| Death | |||||
| Number of events | 6681 | 1377 | 150 | 197 | |
| Demographic adjusted | 1.0 | 1.31 (1.23–1.38) | 1.53 (1.30–1.80) | 1.69 (1.46–1.94) | <0.001 |
| Multivariable adjusted | 1.0 | 1.20 (1.13–1.28) | 1.18 (1.00–1.41) | 1.73 (1.49–2.01) | <0.001 |
Adjusted estimates reported as hazard ratio (95% confidence interval). Demographic adjusted models control for age, sex, and race. All factors in Table 1 were candidate variables for fully adjusted models.
Multivariable models for cardiovascular disease and heart failure include: age, sex, race, baseline estimated glomerular filtration rate, albuminuria, CD4 count, hypertension, diabetes, history of heart failure, history of cardiovascular disease (CVD), hospitalization in the intensive care unit, and calendar year.
Multivariable models for end-stage renal disease include: age and age square, sex, race, baseline estimated glomerular filtration rate, albuminuria, CD4 count, hypertension, diabetes, hospitalization in the intensive care unit, and calendar year.
Multivariable adjusted models for death include: age, sex, race, baseline estimated glomerular filtration rate, albuminuria, viral load, CD4 count, hypertension, diabetes, lung disease, smoking, cancer, history of CVD, hospitalization in the intensive care unit, and calendar year.
Figure 2. Association of acute kidney injury with mortality within subgroups defined by baseline characteristics.
Stages of acute kidney injury combined into a dichotomous variable (no AKI versus AKI) to allow adequate statistical power to analyze subgroups. CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ICU, intensive care unit. Units for variables: age (years), eGFR (ml/min per 1.73 m2), CD4 count (cells/ mm3). All estimates adjusted for factors as described in Table 2.
Figure 3. Association of acute kidney injury with ESRD within subgroups defined by baseline characteristics.
Stages of acute kidney injury combined into a dichotomous variable (no AKI versus AKI) to allow adequate statistical power to analyze subgroups. CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ICU, intensive care unit. Units for variables: age (years), eGFR (ml/min per 1.73 m2), CD4 count (cells/ mm3). All estimates adjusted for factors as described in Table 2.
For the primary analysis, creatinine changes were defined by increases from the most recent outpatient creatinine measure before hospitalization. However, in 13% of the study sample, in whom pre-hospitalization outpatient creatinine measurements were not available, the first in-patient creatinine level was used as the baseline measure of kidney function. In sensitivity analyses, we tested alternative definitions of AKI using the nadir in-patient creatinine to define baseline kidney function and also by applying a 48-h time criterion for creatinine increase. For both of these analyses, results changed minimally from the primary findings (data not shown). In addition, when using the CKD-EPI equation to define eGFR instead of the Modification of Diet in Renal Disease formula, we found that results did not change.
Next, we examined the risk for each outcome after refining the classification for AKI to account for recovery status at the time of discharge from the hospital (Table 3). In contrast to patients without recovery from AKI stage 1, those who recovered had no increased risk for ESRD. Nonetheless, patients with stage 1 AKI with recovery had a 20% higher risk of mortality, compared with those who did not experience AKI. The risk for ESRD was altered most dramatically when AKI was reclassified by recovery status. Those with AKI stages 2–3 with recovery had a twofold risk for ESRD, in contrast to the 10-fold increase in risk observed among those with AKI stages 2–3 without recovery.
Table 3.
The association between in-hospital acute kidney injury (AKI), recovery status, and adverse events 90 days after dischargea
| No AKI (n=14,265) | AKI stage 1 with recovery (n=1487) | AKI stage 1 without recovery (n=966) | AKI stage 2–3 with recovery (n=201) | AKI stage 2–3 without recovery (n=72) | P-value for trend | |
|---|---|---|---|---|---|---|
| Heart failure | ||||||
| Number of events | 250 | 28 | 25 | 7 | 7 | |
| Demographic adjusted | 1.0 | 1.22 (0.78–1.92) | 1.63 (1.02–2.62) | 2.18 (0.90–5.31) | 6.65 (2.73–16.23) | <0.001 |
| Multivariable adjusted | 1.0 | 1.00 (0.63–1.59) | 1.45 (0.90–2.34) | 1.90 (0.76–4.71) | 2.38 (0.88–6.42) | <0.001 |
| Cardiovascular disease | ||||||
| Number of events | 559 | 54 | 31 | 9 | 5 | |
| Demographic adjusted | 1.0 | 1.05 (0.79–1.39) | 0.94 (0.65–1.35) | 1.43 (0.74–2.76) | 2.66 (1.10–6.42) | 0.001 |
| Multivariable adjusted | 1.0 | 1.01 (0.76–1.35) | 0.93 (0.64–1.34) | 1.22 (0.62–2.41) | 1.67 (0.65–4.29) | 0.013 |
| End-stage renal disease | ||||||
| Number of events | 230 | 25 | 38 | 12 | 11 | |
| Demographic adjusted | 1.0 | 1.36 (0.90–2.05) | 3.05 (2.16–4.30) | 5.39 (3.00–9.65) | 37.33 (20.22–68.94) | <0.001 |
| Multivariable adjusted | 1.0 | 0.89 (0.58–1.35) | 2.18 (1.52–3.13) | 2.51 (1.34–4.70) | 10.62 (4.99–22.60) | <0.001 |
| Death | ||||||
| Number of events | 6681 | 826 | 551 | 103 | 47 | |
| Demographic adjusted | 1.0 | 1.40 (1.30–1.51) | 1.41 (1.30–1.54) | 1.36 (1.12–1.65) | 2.02 (1.52–2.69) | <0.001 |
| Multivariable adjusted | 1.0 | 1.19 (1.10–1.28) | 1.23 (1.12–1.34) | 1.13 (0.92–1.38) | 1.33 (0.98–1.80) | <0.001 |
Non-recovery was only for those who were defined as AKI stages 1–3, and defined as not being able to become AKI stage 0 (for previous stage I) or not being able to become AKI stage I (for previous stages 2 or 3). Demographic and multivariable adjustment was performed as described in Table 2.
DISCUSSION
In this large cohort of 17,325 hospitalized HIV-infected veterans receiving care in a relatively uniform health system, AKI was a common occurrence, affecting one of six patients who survived at least 90 days after discharge. We observed graded, independent associations between severity of AKI with heart failure, CVD, ESRD, and death. Our study is the first to report an association between AKI and the incidence of heart failure and CVD, while confirming previously established associations with ESRD and death. Furthermore, we refined the prognostic value of these associations by examining the effect of recovery from AKI. We found that AKI stage I was not associated with heart failure, CVD, or ESRD in those who recovered kidney function, yet surprisingly maintained an association with death. In subgroup analyses, the association between AKI with mortality and ESRD appeared to be stronger in those without prevalent CKD, and reduced GFR in particular. Finally, our study extends the epidemiology of AKI to an understudied population, HIV-infected persons, in whom the long-term consequences of AKI have never been reported.
Previous reports of AKI in HIV-infected persons
Earlier studies have found that AKI is associated with an increased risk of mortality in HIV-infected persons in the era of antiretroviral therapy. A study of adult hospitalized patients in New York State found AKI, ascertained by diagnostic codes, occurred in 6% of HIV-infected individuals, and was associated with an approximate fivefold increase in in-hospital mortality.5 Lopes et al.10 used the Risk, Injury, Failure, Loss, and End Stage Kidney Disease (RIFLE) criteria to classify AKI in 97 critically ill HIV-infected patients in Portugal. These authors found that 47% of the patients studied had AKI, and there was a graded risk of 60-day mortality based on RIFLE staging.10 No studies have evaluated the association of AKI with long-term survival or other adverse events in HIV-infected persons.
Earlier studies of cardiovascular events after AKI
In the general population without HIV, a number of recent studies have examined long-term clinical outcomes.3,4,9,11,12 Although the majority of studies have identified associations between AKI with ESRD and death, few have investigated intermediary outcomes such as cardiovascular events. All of these studies have been conducted in persons either receiving percutaneous coronary intervention or admitted for myocardial infarction.12–14 Goldberg et al.14 also addressed the issue of recovery from AKI in a study of 1957 patients who survived acute myocardial infarction; they also found that recovery from AKI mitigated the risk for heart failure and CVD associated with AKI. We confirm these associations in HIV-infected patients unselected for prevalent CVD, and demonstrate a dose-dependent relationship between AKI and risk of adverse cardiovascular events.
Mechanisms
Our study results indicate that the clinical repercussions of AKI appear to extend beyond the hospital setting, and may contribute to adverse outcomes years after the AKI has occurred. These findings raise the possibility that AKI may be an inciting event that triggers a cascade of perturbations that never completely resolve. In animal models of AKI, permanent alterations in tubular, glomerular, and vascular structure such as atrophy and dilatation and loss of function are evident 40 weeks after injury.15–18 Importantly, AKI also triggers endothelial dysfunction, hypercoagulability, and upregulation of cytokines, such as interleukin-1, interleukin-6, tumor necrosis factor-α, which lead to distant organ injury and dysfunction in the heart, lungs, and brain.19–23 In these organs, it is possible that the long-term effects may be due to a loss of functional reserve that is only manifest in later years by a reduced ability to withstand an acute physiologic stressor event.
Similar processes have been described as a result of HIV infection. HIV triggers a systemic inflammatory response that leads to chronic immune activation and a prothrombotic state, which also features increases in interleukin-1, interleukin-6, and tumor necrosis factor-α.24–26 These abnormalities have been implicated in the pathogenesis of atherosclerosis, progression to AIDS, and mortality in HIV-infected persons;25–28 we speculate that AKI may magnify these derangements through these common mechanisms. Understanding the precise role of AKI in persistent immune activation and inflammation may also provide insights into both the pathogenesis of HIV and CKD.
Strengths and limitations
The strengths of this study include the broad scope of long-term clinical outcomes examined, including heart failure, CVD, ESRD, and mortality over a 20-year period, the application of consensus recommendations to define AKI, and the study population, which was a national sample of patients, all of whom had accessed care in a relatively uniform, low-cost health system. We also improve on the limitations of previous studies by using outpatient creatinine measurements as the baseline level of kidney function and defining AKI, and its severity, based on actual creatinine changes instead of diagnostic codes (which lack sensitivity and may be prone to secular trends in physician reporting). In addition, the associations between AKI with adverse events were robust, despite adjustment for a large number of clinical measures, including comorbid conditions, medications, and laboratory measurements. Finally, our understanding of AKI is further enhanced by our analysis of recovery from AKI and its impact on the prognostic implications for each outcome.
Our study also has a number of limitations. First, our study was retrospective in nature and several pieces of key information were not obtainable. For example, we could not conduct the study with measurement of creatinine at regular time points, we did not have urine output information to supplement our definition of AKI, and other biomarkers of kidney damage may be more sensitive in detecting AKI and preferred for prospective analysis. These factors may have led to misclassification of AKI in some patients. In addition, we were unable to perform medical record review to determine causes of AKI or death in the cohort. Despite these limitations, serum creatinine is the only marker of kidney function available for widespread use in the clinical setting, and thus, our findings may be directly applicable to the care of hospitalized HIV-infected persons with AKI. Second, although we accounted for a large number of potential confounders in our analysis, AKI may still be a marker of severity of underlying systemic illness, rather than an independent pathway for adverse events. Third, outpatient baseline creatinine information was not available for 13% of patients. Although we tested multiple definitions for baseline creatinine, AKI may have been misclassified in this subgroup. Fourth, other studies of long-term outcomes after AKI have been conducted in the Veterans Affairs (VA) system.12,29 However, our study differs significantly from these reports which (1) only examined mortality; (2) did not account for important confounders such as albuminuria; and (3) defined AKI within an arbitrary time period and thus, did not include individuals at the time of their first hospitalization. Fifth, we used the AKI Network criteria to classify changes in creatinine levels; however, other guidelines define AKI differently, and none has been validated against a gold standard of directly measured GFR. Finally, we focused our analysis on HIV-infected persons receiving care in the VA; study results may not be applicable to other populations or health-care systems and women in particular.
Conclusions
AKI is common in HIV-infected persons, affecting 18% of hospitalized patients. The clinical repercussions of AKI appear to extend beyond the hospital setting, and may contribute to excess cardiovascular risk, ESRD, and mortality. Furthermore, the prognostic associations of AKI with long-term adverse events may be refined by accounting for recovery status. These results may provide the foundation for research aimed at identifying the specific derangements responsible for adverse event years after AKI and help guide follow-up care after discharge from the hospital.
METHODS
Data sources
The primary data source was the Department of Veterans Affairs HIV Clinical Case Registry (CCR).30 The VA HIV CCR contains demographic, clinical, laboratory, pharmacy, utilization, and death information from the VA electronic medical record system. To supplement demographic, clinical, and vital status data, and to capture health-care utilization outside of the VA system, we further linked this data source to the VA National Patient Care Database, Medicare claims, and the VA Beneficiary Identification and Records Locator Subsystem (BIRLS) Death File.31 We also used the United States Renal Data System to ascertain prevalent ESRD cases at the time of cohort entry and new ESRD incidence during follow-up.32
Study population
We identified a national sample of 21,439 HIV-infected US veterans at the time of their first hospitalization between the years 1986–2006; hospitalizations for non-medical conditions were not included in this group. We excluded 1615 patients who died during their hospitalization, and 1581 who died before the 90th day after discharge from the hospital. An additional 918 patients who were already receiving chronic dialysis treatment at the time of admission were also eliminated from the cohort. The remaining 17,325 individuals were included and entered the study 90 days after their discharge date.
Definition of AKI
We characterized AKI on the basis of the changes in serum creatinine levels according to AKI Network (AKIN) criteria.33 AKI stage 1 was categorized as a crude serum creatinine increase of 0.3 mg/dl or greater, or a relative increase between 150 and 200%; stage 2 was categorized as a relative increase between 200 and 300%; and stage 3 as either a relative increase greater than 300%, or in individuals with a serum creatinine level of 4.0 mg/dl or greater, a crude increase of 0.5 mg/dl from the baseline. Stages 2 and 3 AKI were combined in the analysis because of the small number of individuals in these groups. We also separately categorized individuals who experienced AKI-requiring dialysis therapy during their hospitalization, identified using validated diagnostic and procedural codes.34 The serum creatinine change was defined as the difference between the peak serum creatinine level during hospitalization and the most recent outpatient creatinine measure before hospitalization. In 13% of the study sample, pre-hospitalization outpatient creatinine measurements were not available; in these patients, the first in-patient creatinine level was used as the baseline measure of kidney function. Among those who experienced AKI, ‘recovery’ was defined as a decrease in serum creatinine below the threshold level for stage 1 AKI.
Outcomes
The outcomes were time from study entry to: (1) heart failure; (2) CVD, defined as coronary, cerebrovascular, or peripheral arterial disease; (3) ESRD, defined as receipt of chronic dialysis therapy ascertained by the United States Renal Data System; and (4) death. Cardiovascular outcomes, including heart failure, were defined by hospitalization for these conditions based on primary discharge diagnoses and procedural codes in VA and non-VA data sources using validated algorithms described previously.8,35
Covariates
Comorbid conditions were identified using a combination of physician problem lists, ambulatory and hospitalization discharge diagnoses, procedures, laboratory results, and medication prescriptions in the 2 years period before hospitalization. These included diabetes, hypertension, dyslipidemia, CVD, lung disease, smoking, liver disease, and cancer.35–38 We accounted for HIV-related characteristics such as hepatitis B or C virus co-infection, antiretroviral therapy, CD4 count, and HIV viral load.39–41 We also estimated severity of illness based on whether an individual was hospitalized in the intensive care unit. eGFR at the time of admission was calculated using the abbreviated Modification of Diet in Renal Disease formula based on age, sex, race, and serum creatinine; eGFR was then categorized as ≥60, 30–59, and <30 ml/min per 1.73 m2.42 Albuminuria was defined as urine dipstick measurements greater than 30 mg/dl. We included a missing indicator category to retain observations in the analysis. Overall, only 5% of the data was missing; the majority of missing information was due to HIV viral load and CD4 count in patients who entered the cohort before when these tests became widely clinically available. Albuminuria was also missing in 19% of patients.
Statistical analysis
We compared age-standardized rates of heart failure, CVD, ESRD, and death by stage of AKI. Rates were calculated using Poisson regression models, and standardized to the age distribution of the source population. We used Cox proportional hazards regression models to determine the adjusted association between AKI with each outcome independent of demographic characteristics (age, sex, and race), eGFR category, albuminuria, comorbid conditions, calendar year, and intensive care unit stay during the first hospitalization. We built parsimonious models using a backward stepwise procedure with P<0.05, used as the criterion for inclusion; age, sex, race, eGFR category, and albuminuria were forced into all models. For the outcomes of heart failure, CVD, and ESRD, individuals with events that occurred within the 90 days period after discharge were excluded from the analysis. Between the date of discharge and study entry, 550 patients experienced a heart failure or CVD event and 1616 had ESRD.
The final models for all outcomes included age, sex, race, eGFR category, albuminuria, CD4 count, hypertension, diabetes, hospitalization in the intensive care unit, and calendar year. Models for heart failure and CVD additionally included history of CVD and history of heart failure. For mortality, models also included viral load, lung disease, smoking, and history of CVD. The same models for each outcome were used for both the initial AKI analyses and the subsequent models of recovery from AKI. For the outcomes of CVD and heart failure, respectively, we also stratified by the presence of CVD, heart failure, and CKD at baseline to determine whether AKI is associated with de novo cardiovascular events in individuals without prevalent CKD. We also checked for interactions with calendar year (antiretroviral treatment era). For the outcomes of mortality and ESRD, we performed subgroup analyses by age, race, CKD, CVD, CD4 count, viral load, and intensive care unit stay; in these analyses, stages of AKI were combined into a dichotomous variable (AKI versus no AKI) to allow adequate statistical power. We also further explored the potential effects of prevalent albuminuria and reduced kidney function on the association between AKI with mortality by dividing the cohort into four mutually exclusive categories defined by (1) eGFR>60 ml/min per 1.73 m2 and no albuminuria, (2) albuminuria only, (3) eGFR<60 ml/min per 1.73 m2 only, and (4) both reduced GFR and albuminuria.
We conducted a number of sensitivity analyses using alternate definitions of AKI. When outpatient creatinines were not available, we explored reclassification of AKI using the nadir in-patient creatinine to define the baseline measurement. In addition, we performed a supplementary analysis applying the AKIN 48-h time criterion for the definition of AKI. An alternative analysis was also performed using the CKD-EPI equation, instead of the Modification of Diet in Renal Disease formula, to calculate eGFR.43 We checked the proportional hazard assumption of the Cox regression models by comparing plots of log(−log(survival)) versus log of survival time and the Schoenfeld test. All analyses were conducted using Stata version 11.0 (Stata Corp, College Station, TX, USA).
Acknowledgments
This study was supported by the National Institutes of Health (K23DK080645-01A1, K23AI65244, R01 DK066488-01), the National Center for Research Resources (KL2 RR024130), the American Heart Association Early Investigator Award, and the VA Public Health Strategic Health Care Group. These funding sources had no involvement in the design or execution of this study.
Footnotes
DISCLOSURE
PAV serves on a data safety and monitoring board for Merck and TaiMed, an end points adjudication committee for Schering, and scientific advisory boards for Pfizer, BMS, Gilead, and Schering.
References
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 3.Newsome BB, Warnock DG, McClellan WM, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 4.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt CM, Arons RR, Klotman PE, et al. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006;20:561–565. doi: 10.1097/01.aids.0000210610.52836.07. [DOI] [PubMed] [Google Scholar]
- 6.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 7.Selik RM, Byers RH, Jr, Dworkin MS. Trends in diseases reported on U.S. death certificates that mentioned HIV infection, 1987–1999. J Acquir Immune Defic Syndr. 2002;29:378–387. doi: 10.1097/00126334-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Choi AI, Li Y, Deeks SG, et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes JA, Fernandes J, Jorge S, et al. An assessment of the RIFLE criteria for acute renal failure in critically ill HIV-infected patients. Crit Care. 2007;11:401. doi: 10.1186/cc5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amdur RL, Chawla LS, Amodeo S, et al. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay J, Apple S, Pinnow EE, et al. Percutaneous coronary intervention-associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv. 2003;59:338–343. doi: 10.1002/ccd.10534. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg A, Kogan E, Hammerman H, et al. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int. 2009;76:900–906. doi: 10.1038/ki.2009.295. [DOI] [PubMed] [Google Scholar]
- 15.Basile DP, Donohoe D, Roethe K, et al. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 16.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 17.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Basile DP, Fredrich K, Weihrauch D, et al. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol. 2004;286:F893–F902. doi: 10.1152/ajprenal.00328.2003. [DOI] [PubMed] [Google Scholar]
- 19.Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. Kidney Int. 2008;74:849–851. doi: 10.1038/ki.2008.390. [DOI] [PubMed] [Google Scholar]
- 20.Klein CL, Hoke TS, Fang WF, et al. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–909. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 21.Grigoryev DN, Liu M, Hassoun HT, et al. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 25.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. Aids. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 29.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backus L, Mole L, Chang S, et al. The immunology case registry. J Clin Epidemiol. 2001;54(Suppl 1):S12–S15. doi: 10.1016/s0895-4356(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 31.Maynard C, Chapko MK. Data resources in the Department of Veterans Affairs. Diabetes Care. 2004;27(Suppl 2):B22–B26. doi: 10.2337/diacare.27.suppl_2.b22. [DOI] [PubMed] [Google Scholar]
- 32.United States Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 33.Molitoris BA, Levin A, Warnock DG, et al. Improving outcomes from acute kidney injury. J Am Soc Nephrol. 2007;18:1992–1994. doi: 10.1681/ASN.2007050567. [DOI] [PubMed] [Google Scholar]
- 34.Waikar SS, Wald R, Chertow GM, et al. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 35.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 36.Goulet JL, Fultz SL, McGinnis KA, et al. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. Aids. 2005;19(Suppl 3):S99–S105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 37.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–B21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 38.Jonk YC, Sherman SE, Fu SS, et al. National trends in the provision of smoking cessation aids within the Veterans Health Administration. Am J Manag Care. 2005;11:77–85. [PubMed] [Google Scholar]
- 39.Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 40.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44:S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 41.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 42.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 43.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]