Abstract
Background
Right ventricular (RV) dysfunction is associated with adverse outcomes in heart failure (HF). Mechanical unloading should be more effective than pharmacologic therapy to reduce RV afterload and improve RV function. We compared RV size and function after aggressive medical unloading therapy to that achieved in the same patients after 3 months of left ventricular assist device (LVAD) support.
Methods and Results
We studied twenty patients who underwent isolated LVAD placement (9 pulsatile and 11 axial flow). Echocardiograms were performed after inpatient optimization with diuretic and inotropic therapy and compared to studies done after 3 months of LVAD support. After medical optimization right atrial pressure was 11±5 mm Hg, mean pulmonary artery pressure 36±11 mm Hg, pulmonary capillary wedge pressure 23±9 mm Hg, and cardiac index 2.0±0.6 L/min/m2. Pre-operatively, RV dysfunction was moderate (2.6 ±0.9 on 0-4 scale), RV diameter at the base was 3.1±0.6 cm, and mid-RV was 3.5±0.6 cm. After median LVAD support of 123 days (92-170), RV size and global RV dysfunction (2.6 ±0.9) failed to improve, despite reduced RV afterload.
Conclusions
RV dysfunction seen on intensive medical therapy persisted after 3 months of LVAD unloading therapy. Selection of candidates for isolated LV support should anticipate persistence of RV dysfunction observed on inotropic therapy.
Keywords: Left ventricular assist device, echocardiogram, right ventricle, inotropic agent
Background
Left ventricular assist device (LVAD) therapy increases survival and quality of life for selected patients with advanced heart failure.1-3 Right ventricular (RV) dysfunction predicts poor outcomes and reduced exercise capacity4 in patients with heart failure,5-7 and is associated with adverse events in LVAD recipients.8-10 Forward RV output contributes to LVAD filling, while “backward” RV congestion can compromise renal and hepatic function and nutrition. RV dysfunction has shown dynamic changes related to altering loading pressures during acute pulmonary emboli and chronic pulmonary hypertension undergoing lung transplantation. In chronic dilated heart failure, mechanical circulatory support is more effective than medical unloading therapy for reducing left-sided filling pressures and pulmonary pressures to normal levels.11;12 The degree to which the RV dilation and dysfunction that persist despite intensive medical unloading therapy can reverse during LVAD support has not been established. We hypothesized that mechanical unloading with LVAD for 3 months would lead to improvement in RV function beyond that seen with medical unloading therapy.
Methods
We conducted a retrospective analysis of LVAD recipients at the Brigham and Women’s Hospital (BWH), receiving devices between January 2007 and January 2009, using echocardiograms to determine change in RV size and function after 3 months of LVAD support. LVAD recipients older than 18 years were included in the study if they received isolated LVAD support and had adequate echocardiograms for visualization of RV function both before and 3 months after LVAD implantation. Informed consent was obtained from all participants in protocols approved by the Institutional Review Board of BWH. Prior to LVAD surgery, all patients were managed on an inpatient heart failure unit by heart failure specialists, where therapy was adjusted to reduce filling pressures, with goals of right atrial pressure (RAP) ≤ 8 mm Hg and pulmonary capillary wedge pressure (PCWP) ≤ 18 mm Hg, as allowed by blood pressure and renal function. Intravenous inotropic therapy was used as needed to support blood pressure and renal function for diuresis. The need for RVAD implantation was assessed qualitatively based on patient history, pre-operative clinical status during acute unloading therapy, RV dysfunction evaluated by echocardiography, and hemodynamics.
An inotropic score was calculated on the day of LVAD implantation, before induction of general anesthesia, using a previously described formula.13;14 The score is a summation of the doses of dopamine, dobutamine, a multiple of 15 of milrinone, and a multiple of 100 of epinephrine and norepinephrine. All units are expressed in micrograms per kilogram body weight per minute. Post-operatively, clinical volume overload was treated aggressively after the first week of LVAD implantation, and echocardiogaphic assessment of LV size and aortic valve opening was used to optimize LVAD function. After stabilization, neurohormonal antagonists were used most often for treatment of hypertension and tachyarrhythmias.
Hemodynamic Measurements
Invasive hemodynamics were measured on the day of surgery before general anesthesia and at follow-up, when available, 3 weeks after surgery. Measurements included RAP, PCWP, pulmonary artery pressure (PAP) (systolic, diastolic, and mean), pulmonary vascular resistance (PVR), cardiac output (CO), and cardiac index (CI) calculated by the Fick method using an assumed O2 consumption of 125 ml O2 per min per m2.
Echocardiographic Data
Transthoracic echocardiograms were performed within 5 days prior to LVAD implantation (baseline), and repeated 3 months after surgery. Studies were interpreted by a cardiologist blinded to the other studies obtained in the same patient, as well as the clinical data and LVAD settings. We measured left ventricular end-diastolic dimension (LVEDD) in the parasternal long axis view. Left ventricular ejection fraction (LVEF) was assessed semi-quantitatively using the wall motion score and, whenever feasible, the modified Simpson’s rule. Mitral regurgitation (MR) was graded using semi-quantitative evaluation, pulmonary vein flow blunting or reversal, and when available proximal isovelocity surface area calculation. Basal and mid-RV diameters were measured in the 4-chamber view. Severity of MR, tricuspid regurgitation (TR), and RV dysfunction were graded semi-quantitatively using a scale from 1 to 4 (1 = normal; 2 = mild, 3 = moderate, and 4 = severe). Inferior vena cava (IVC) size was measured in the subcostal view. Systolic pulmonary artery pressure was estimated using the summation of TR gradient and RAP estimate. TR gradient was derived from the highest TR jet velocity recorded, using simplified Bernoulli’s principle. RAP was estimated using IVC size and respiratory variation. All measurements were performed according to American Society of Echocardiography guidelines.15
Laboratory Measurements
Estimated glomerular filtration rate (GFR) by the Cockcroft-Gault formula, serum creatinine, and total bilirubin were recorded on the day of surgery, at 48 hours, and at 3 months follow-up. Serum albumin and pre-albumin levels were assessed at baseline and at the 3-month follow-up visit.
Statistical Analyses
Continuous data were expressed as mean ± standard deviation or median with interquartile range (IQR) when the data were not normally distributed. Follow-up parameters were compared to baseline using paired Student’s t-test and Wilcoxon signed-rank test for continuous and categorical variables respectively. A p value less than 0.05 was considered statistically significant. A Kaplan-Meier curve for the freedom from death and heart transplant after LVAD support was calculated starting at the 3 month visit because patients that had an event before 3 months of follow-up were excluded. SAS software version 9.1 (Cary, N.C.) was used to perform the analyses.
Results
Of 46 ventricular assist device recipients, 17 were excluded due to concomitant RVAD implantation, and 9 were excluded for transplant or death prior to 3 months, leaving 20 patients for comparison of RV function on intensive medical therapy versus mechanical unloading. (Figure 1). The mean age was 52 years, 75% were male, and 75% had non-ischemic cardiomyopathy (Table 1). Five patients received LVAD with the initial intent of permanent mechanical support. Concomitant tricuspid valve repair (TVR) was performed in 8 patients, and 2 patients had a prior history of TVR.
Figure 1. Flow Diagram for Study Population.
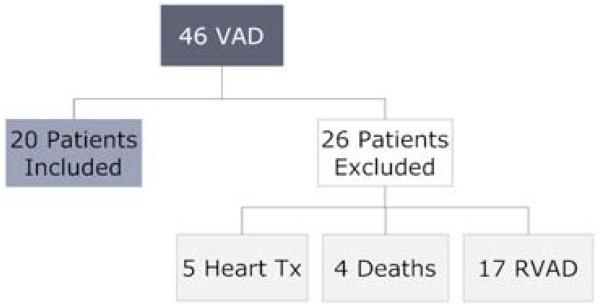
Shown are the total number of ventricular assist devices implanted at BWH between 01/2007 and 01/2009, and the 26 patients excluded from this analysis. VAD, ventricular assist device; Tx, transplant; RVAD, right ventricular assist device.
Table 1. Baseline Characteristics.
| Parameter | Mean or Number of Patients (%) |
|---|---|
| Age (years) | 52 ± 15 |
| Male | 15 (75) |
| Non ischemic cardiomyopathy | 15 (75) |
| LVEF (%) | 18 ± 7 |
| History of diabetes | 5 (25) |
| Prior cardiac surgery | 4 (20) |
| eGFR (ml/min) | 58 ± 26 |
| Total bilirubin (mg/dl) | 1.4 ± 1.0 |
| Inotrope-dependent | 19 (95) |
| Inotrope score (median) | 7.5 (IQR: 3.0;9.5) |
| IABP | 5(25) |
| Axial flow pump (HeartMate II) | 11 (55) |
| Pulsatile device | 9 (45) |
| TVR | 10 (50) |
eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump; IQR, interquartile range; LVEF, left ventricular ejection fraction; TVR, tricuspid valve repair.
Medical Therapy
Medical therapy was intensified over a median duration of 15 days (IQR: 12;20) prior to LVAD implantation. Aiming for a goal of PCWP ≤ 18 mm Hg and RAP ≤ 8 mm Hg as possible, the median daily dose of intravenous furosemide was 340 mg (IQR: 115;480), and 19 patients (95%) required positive inotropic agents, with a median inotropic score of 7.5 (IQR: 3.0;9.5). Use of multiple vasopressors or an intra-aortic balloon pump was necessary in 2 and 5 patients, respectively. Only 1 patient was able to tolerate an angiotensin blocker (ACEI/ARB), and 1 patient was still on a beta-blocker at the time of surgery. Diuresis was associated with pre-operative weight reduction of 5.4 ± 3.7 kg.
After surgery, inotropic agents were weaned over a median period of 12 days (IQR: 9;18). ACEI/ARB and beta-blocker, for arrhythmias and hypertension unless otherwise contraindicated, were introduced prior to discharge in 5 and 12 patients, respectively. Median post-operative stay was 34 days (IQR: 26;41), and all patients were discharged home or to a rehabilitation facility. Before the 3-month visit, no new medications were started, and all patients initially treated with ACEI/ARB or beta-blocker remained on them. No patients required the addition of chronic nitrates or sildenafil to allow adequate VAD function during weaning of inotropic therapy.
Hemodynamic Improvement on LV Support
Baseline hemodynamics were available for 17 patients of whom 10 patients had repeat hemodynamics measured when stable, 28 ± 13 days after LVAD implantation. Inotropic agents had been stopped in all patients prior to follow-up measurements. The subgroup of patients with follow-up measurements was representative of the original cohort at baseline (Table 2). Compared to the hemodynamic status on inotropic agents prior to LVAD implantation, PCWP was markedly reduced, and CI increased after 3 weeks of LV assist. Concordantly, repeat echocardiography demonstrated significant reduction in LVEDD and MR severity after a median LVAD support time of 123 days (IQR 93;67 days) (Figure 2). When measured, systolic PAP was reduced from 49 to 33 mm Hg (p= 0.016), with a trend toward reduction in PVR, although PVR was not markedly elevated (2.2 Wood units) on the intensive medical therapy prior to LVAD. TR gradient was reduced from 41 ± 15 mm Hg at baseline to 21 ± 7 mmHg at 3 months follow-up (p = 0.001), consistent with a substantial reduction of pulmonary artery systolic pressure.
Table 2. Pre-LVAD Hemodynamic Data and Changes after 3 Weeks of Mechanical Support in a Subgroup of Patients.
| Parameter | Baseline (17 patients) |
Baseline Subgroup (10 patients) |
Follow-up Subgroup (10 patients) |
P value* |
|---|---|---|---|---|
| RAP (mm Hg) | 11 ± 5 | 10 ± 5 | 7 ± 4 | 0.192 |
| Systolic PAP (mm Hg) | 50 ± 13 | 49 ± 15 | 33 ± 13 | 0.016 |
| Mean PAP (mm Hg) | 36 ± 11 | 35 ± 11 | 21 ± 8 | 0.01 |
| PVR (Wood units) | 2.5 (IQR 2.1; 4.5) n=15 | 2.2 (IQR 1.6;3.2) | 1.9 (IQR 1.6; 2.4) | 0.30 |
| PCWP (mm Hg) | 23 ± 9 | 22 ± 9 | 9 ± 5 | 0.002 |
| CI (L/min/m2) | 2.0 ± 0.6 | 2.0 ± 0.5 | 2.5 ± 0.5 | 0.044 |
Follow-up vs. baseline in subgroup of patients (n=10) with available hemodynamic measurements at 3 weeks.
CI, cardiac index; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance;
RAP, right atrial pressure.
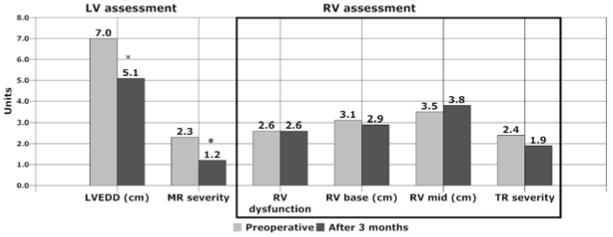
Figure 2. Echocardiographic Changes after 3 Months of LVAD Unloading Therapy.
This graphic depicts LV and RV changes after 3 months of LVAD support, compared to baseline assessment performed after medical unloading therapy. LVEDD reference range is 3.9 to 5.3 cm for women and 4.2 to 5.9 cm for men. RV diameter reference ranges are 2.0 to 2.8 cm at the base and 2.7 to 3.3 cm at mid-RV. LV, left ventricle; LVEDD, left ventricular end diastolic dimension; MR, mitral regurgitation; RV, right ventricle; TR, tricuspid regurgitation. *p<0.0001
Right Ventricular Function
RV size remained globally dilated without reduction in RV dimensions after 3 months of LVAD support (Table 3). There was a trend toward increasing mid-RV diameter from mild to moderate at 3 months, although this was not statistically significant. RV dysfunction (score ≥ 2) was present in 17 of 20 patients (85%) at baseline; and in the group as a whole, moderate RV dysfunction persisted 3 months after LVAD implantation (Figure 2). Although subgroups were too small for statistical comparison, there was no apparent difference in RV function changes in patients supported with axial versus pulsatile flow devices. For the 5 patients who were transplanted before the 3-month visit and excluded from the paired analysis, echocardiograms showed no improvement in RV function prior to transplantation (data not shown).
Table 3. Echocardiographic Changes after 3 Months of Mechanical Unloading Therapy.
| Parameter | Pre-LVAD (n = 20) |
Follow-up (n = 20) |
P value |
|---|---|---|---|
| LVEDD (cm) | 7.0 ± 0.8 | 5.1 ± 0.8 | <0.0001 |
| MR severity | 2.3 ± 1.1 | 1.2 ± 0.5 | <0.0001 |
| RV base (cm) | 3.1 ± 0.6 | 2.9 ± 0.7 | 0.226 |
| Mid-RV (cm) | 3.5 ± 0.6 | 3.8 ± 0.7 | 0.075 |
| RV dilation | 2.0 ± 1.0 | 2.4 ± 0.9 | 0.126 |
| RV function | 2.6 ± 0.9 | 2.6 ± 0.9 | 0.961 |
| TR severity | 2.4 ± 0.8 | 1.9 ± 0.8 | 0.095 |
LVEDD reference range: 3.9-5.3 cm for women, and 4.2-5.9 cm for men; RV base diameter reference range: 2.0-2.8 cm; mid-RV diameter reference range: 2.7-3.3 cm.
LVEDD, left ventricular end diastolic dimension; MR, mitral regurgitation; RV, right ventricle; TR, tricuspid regurgitation.
Clinical Outcomes
Improved end-organ perfusion and nutrition reflected increased systemic perfusion during LVAD support (Table 4). Serum creatinine was reduced from 1.4 ± 0.6 mg/dl at baseline to 1.2 ± 0.3 mg/dl at 3 months. Nutritional status, as reflected by serum albumin and pre-albumin, also improved substantially during LVAD support. All patients except 2 were discharged before the 3-month follow-up visit. One patient was readmitted before 3 months for fluid overload. At 3 months, 18 patients stated that they were physically active. Jugular venous pressures (JVP) were within the normal range at the 3-month visit (mean JVP 6.5 ± 1.5 cm H2O). Oral diuretics were still prescribed in 18 patients, but at a median dose of 40 mg furosemide (IQR: 35;150), which was significantly reduced from baseline (−120 mg with IQR of −450;40).
Table 4. Laboratory Changes 48 hours and 3 Months after LVAD Placement.
| Parameter | Baseline (n = 20) |
48 hours (n = 20) |
P value* | Follow-up (n=20) |
P value† |
|---|---|---|---|---|---|
| eGFR (ml/min) | 58 ± 26 | 65 ± 44 | 0.36 | 66 ± 21 | 0.08 |
| Creatinine (mg/dl) | 1.4 ± 0.6 | 1.4 ± 0.6 | 0.93 | 1.2 ± 0.3 | 0.003 |
| Total bilirubin (mg/dl) | 1.4 ± 1.0 | 4.8 ± 4.1 | <0.0001 | 0.6 ± 0.2 | 0.0002 |
| (median 2.9) | |||||
| Albumin (g/dl) | 3.6 ± 0.6 | ----- | ----- | 4.0 ± 0.4 | <0.0001 |
| Pre-albumin (mg/dl) | 20.3 ± 5.5 | ----- | ----- | 25.1 ± 5.0 | <0.0001 |
48 hours vs. baseline;
follow-up vs. baseline.
eGFR, estimated glomerular filtration rate.
After the 3-month visit, 1 death, 9 transplants, and 4 device malfunctions (2 requiring LVAD replacement) occurred. Median duration of LVAD support was 358 days (IQR: 246;498). Among those transplanted, 3 patients were listed as Status 1A due to LVAD dysfunction. In the 5 patients with LVAD dysfunction, this occurred after a mean support duration of 406 ± 95 days. Survival free from death or heart transplant was 84% at 6 months and 71% at 1 year (Figure 3).
Figure 3. Conditional Survival Free of Death or Transplant after 3-month Follow-up Visit.
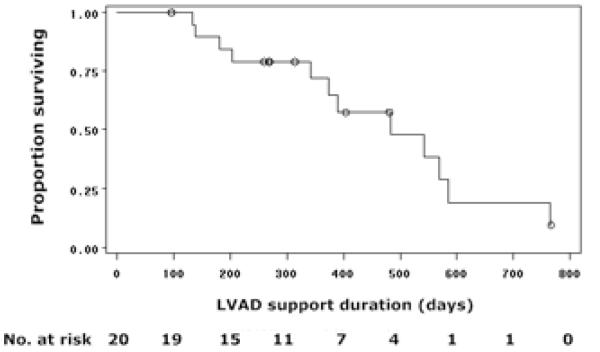
This Kaplan-Meier curve shows survival free of outcomes (death or transplant) in 20 patients following their 3-month visit. LVAD, left ventricular assist device.
Discussion
This study of patients receiving isolated LVAD support demonstrated that RV dilation and dysfunction seen on inotropic support after intensive diuresis failed to improve after 3 months of mechanical unloading with an LVAD. Factors contributing to persistent RV dysfunction may include irreversible chronic RV remodeling, intra-operative injury, incomplete RV unloading with current VAD management, and unfavorable RV effects of ventricular interdependence.
Importance of Preoperative RV Dysfunction
Underlying RV dysfunction may have limited the benefit that could be achieved by afterload reduction, either pharmacologic or mechanical. It is not known if there is a tipping point beyond which RV dysfunction becomes irreversible regardless of loading conditions. Perhaps at this point RV function worsens when challenged by the increased venous return following LVAD placement. Short-term LVAD support was not shown to alter RV function16;17 in normal hearts, but in animal models of significant RV dysfunction due to right coronary occlusion18 or tachycardia-induced cardiomyopathy,19;20 RV stroke volume and CO diminished after LVAD implantation. To our knowledge, only the recent study from Australia in patients with end-stage cardiomyopathy supported with an axial flow device (VentraAssist) demonstrated persistent moderate RV dysfunction after a median support time of 140 days21 Our experience showed similar results with pharmacologic and mechanical support, and with pulsatile and non-pulsatile support devices.
Intra-operative ischemia and activation of the inflammatory and complement cascades during surgery may add further damage limiting potential improvement of RV function.22 Even in patients with normal LV function, RV ejection fraction and RV stroke volume index diminish transiently in the post-operative period.23;24
Impact of LV Unloading on RV function
The LV assisted circulation translocates blood volume from the pulmonary venous circulation to the systemic circulation, thereby restoring forward flow and reducing LV filling pressures, but increasing RV preload. PAP, RV systolic pressure, and wall stress (afterload) are usually reduced with LVAD support.25 We observed the expected decrease in left-heart filling pressure and pulmonary artery pressures when hemodynamics were measured invasively at 3 weeks. Reduction of LV dimension, MR severity and RA-RV pressure gradient (assessed from TR velocity) provide additional non-invasive evidence of effective LV unloading and reduction of filling pressures.
There is an inverse relationship between RV function and PAP. 26 In fact, in humans with normal or mildly-reduced LV function, studies using an LV assistance model at the time of cardiac surgery to mimic LVAD physiology demonstrated an acute increase in RV fractional area change (42 to 63%) in response to a 19 mm Hg reduction in PAP.27;28 However, in the advanced chronic heart failure population, we did not observe improvement in RV function after at least 3 months of LV assistance, when compared to RV function seen on inotropic support pre-operatively. It is possible that some post-operative improvement might have been seen if patients had gone directly to LVAD without aggressive unloading therapy and inotropic support pre-operatively. In addition, we cannot exclude the possibility that RV function would have improved with a greater reduction in PVR.
Contribution of Septal Contraction to RV Function
Anatomic ventricular interactions are accentuated in heart failure and LVAD recipients, reducing RV septal contractile forces.29 In the setting of RV dysfunction, the septum generates 25 to 35% of RV stroke work.30 However, after LVAD placement, as the septum shifts toward the LV, the LV septal contribution to RV stoke work is further reduced.18 Thus, the effect of exaggerated leftward septal movement, particularly in our patients with pre-existing RV dysfunction, may also have contributed to the lack of improvement seen in this study.
BiVAD versus LVAD
Almost as many patients received BiVAD as received isolated LVAD during this time period. The choice of patients for BiVAD was based, as it often is, on clinical judgment taking into account numerous clinical and echocardiographic features. It is not known whether some patients would have been better served with the alternate approach. LVAD unloading induces LV structural and functional reverse remodeling, and can restore the neurohormonal31 and cytokine32 milieu. However, LV assist does not directly unload the RV, and its benefits on RV remodeling have not been clearly established. Some molecular remodeling in both ventricles has been demonstrated in patients with end-stage heart failure, with normalization of N-terminus dystrophin levels in both the LV and RV after 1 and 3 months of continuous and pulsatile LVAD support.33;34 On the other hand, complete LVAD unloading failed to improve RV myocyte function.35;36 At the present time, only LVAD with RVAD support (56 ± 27 days) tested in one small study induced RV structural remodeling.36;37 Perhaps RV filling pressures need to be lowered to very low levels in order to see reverse remodeling in this ventricle. It may be that biventricular mechanical support is the only method to protect the RV sufficiently to allow biventricular recovery in the presence of significant pre-existing RV dysfunction.
Another surgical approach to RV protection is correction of severe tricuspid regurgitation. Notably, 50% of our patients underwent TVR prior to or at the time of LVAD implant. We would have predicted that correction of underlying tricuspid regurgitation would have contributed to improvement in RV function, which we did not observe.
Clinical Impact of Persistent RV Dysfunction
RV dysfunction has been considered an adverse prognostic marker and a crucial factor limiting exercise capacity during medical therapy for heart failure.4 As well, right heart failure is increasingly implicated in the cardiorenal syndrome. Although the persistence of RV dysfunction after mid-term LVAD support is concerning, the favorable clinical outcomes in our study population are reassuring. At least for this short-term follow-up, RV function appeared adequate to prevent clinical signs and symptoms of right-sided failure. The majority of our patients were ambulatory through rehabilitation, and were not re-hospitalized for hemodynamic reasons. Furthermore, end-organ perfusion, as measured by renal and liver function, as well as nutritional status, was markedly improved. The need for diuretics was decreased but not eliminated. The majority of patients in this study underwent heart transplantation after 3 months of follow-up and do not contribute information about the later consequences of impaired RV function in recipients of isolated LVAD.
Limitations
For this retrospective study, we did not have adequate images for full quantitative assessment of RV function, such as RV fractional area change, tissue Doppler imaging, RV myocardial performance index, or tricuspid annular plane systolic excursion. We also did not assess ventricular septal motion during systole. Moreover, the severity of illness did not permit the weaning of inotropic agents before surgery in order to assess unsupported RV function. Patients with more clinically-evident RV dysfunction received biventricular support, so it is not known how and whether those decisions were made correctly. The current patients represent the majority of our overall population. The number of patients in this study with normal pre-implantation RV function (n=3) was too small to allow any meaningful comparison of echocardiographic changes in RV size and function between patients with or without pre-existing RV dysfunction. In addition, the relative contributions of the potential mechanisms of persistent RV dysfunction cannot be determined until more focused physiologic studies are performed before and after LVAD implantation.
Although baseline and follow-up echocardiograms were reviewed separately, the interpreter could not be blinded to the presence of the LVAD. The absence of follow-up right heart catheterization data at 3 months precluded confirmation that LV filling pressures remained optimized. Finally, these results are limited to a single center, but raise important considerations regarding expansion of the field of potential recipients for long-term support.
Conclusions
RV function did not improve after 3 months of isolated mechanical LV support in this consecutive experience, in which patients demonstrated major pre-operative RV dysfunction despite intensive pharmacologic therapy, including positive inotropic support. It is not known if RV function might be reversible in patients with a shorter duration of heart failure or longer periods of support. Clinical outcomes were acceptable during intermediate follow-up, but it is not known whether persistent RV dysfunction would limit the degree and duration of benefit of LVAD during prolonged support. The potential compromise of peak exercise and cardiorenal interactions with RV dysfunction is particularly relevant when recommending LVAD to less sick patients with higher expectations of excellent function as well as survival. Until reverse RV remodeling can be demonstrated with longer time or newer strategies, only those patients in whom pre-operative RV function is adequate for rehabilitation should be selected for life-time mechanical circulatory support.
Acknowledgments
Dr. Palardy’s research was supported by a fellowship grant from the Heart Failure Society of America
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Stevenson LW, Miller LW, svigne-Nickens P, Ascheim DD, Parides MK, Renlund DG, et al. Left ventricular assist device as destination for patients undergoing intravenous inotropic therapy: a subset analysis from REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure) Circulation. 2004;110:975–81. doi: 10.1161/01.CIR.0000139862.48167.23. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N.Engl.J.Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Bank AJ, Mir SH, Nguyen DQ, Bolman RM, III, Shumway SJ, Miller LW, et al. Effects of left ventricular assist devices on outcomes in patients undergoing heart transplantation. Ann.Thorac.Surg. 2000;69:1369–74. doi: 10.1016/s0003-4975(00)01083-3. [DOI] [PubMed] [Google Scholar]
- 4.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J.Am.Coll.Cardiol. 1995;25:1143–53. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 5.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am.Coll.Cardiol. 1998;32:948–54. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 6.Dini FL, Conti U, Fontanive P, Andreini D, Banti S, Braccini L, et al. Right ventricular dysfunction is a major predictor of outcome in patients with moderate to severe mitral regurgitation and left ventricular dysfunction. Am.Heart J. 2007;154:172–79. doi: 10.1016/j.ahj.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Field ME, Solomon SD, Lewis EF, Kramer DB, Baughman KL, Stevenson LW, et al. Right ventricular dysfunction and adverse outcome in patients with advanced heart failure. J Card Fail. 2006;12:616–20. doi: 10.1016/j.cardfail.2006.06.472. [DOI] [PubMed] [Google Scholar]
- 8.Kormos RL, Gasior TA, Kawai A, Pham SM, Murali S, Hattler BG, et al. Transplant candidate’s clinical status rather than right ventricular function defines need for univentricular versus biventricular support. J Thorac.Cardiovasc.Surg. 1996;111:773–82. doi: 10.1016/s0022-5223(96)70337-9. [DOI] [PubMed] [Google Scholar]
- 9.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–72. doi: 10.1016/j.jacc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002;106:I198–I202. [PubMed] [Google Scholar]
- 11.Zimpfer D, Zrunek P, Roethy W, Czerny M, Schima H, Huber L, et al. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J.Thorac.Cardiovasc.Surg. 2007;133:689–95. doi: 10.1016/j.jtcvs.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 12.Salzberg SP, Lachat ML, von HK, Zund G, Turina MI. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur.J.Cardiothorac.Surg. 2005;27:222–25. doi: 10.1016/j.ejcts.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Kormos RL, Gasior TA, Kawai A, Pham SM, Murali S, Hattler BG, et al. Transplant candidate’s clinical status rather than right ventricular function defines need for univentricular versus biventricular support. J Thorac.Cardiovasc.Surg. 1996;111:773–82. doi: 10.1016/s0022-5223(96)70337-9. [DOI] [PubMed] [Google Scholar]
- 14.Potapov EV, Hennig F, Wagner FD, Volk HD, Sodian R, Hausmann H, et al. Natriuretic peptides and E-selectin as predictors of acute deterioration in patients with inotrope-dependent heart failure. Eur.J Cardiothorac.Surg. 2005;27:899–905. doi: 10.1016/j.ejcts.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J.Am.Soc.Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Farrar DJ, Compton PG, Dajee H, Fonger JD, Hill JD. Right heart function during left heart assist and the effects of volume loading in a canine preparation. Circulation. 1984;70:708–16. doi: 10.1161/01.cir.70.4.708. [DOI] [PubMed] [Google Scholar]
- 17.Farrar DJ, Compton PG, Hershon JJ, Foner JD, Hill JD. Right ventricular pressure-dimension relationship during left ventricular assistance in dogs. Trans.Am.Soc.Artif.Intern.Organs. 1984;30:121–23. [PubMed] [Google Scholar]
- 18.Farrar DJ, Chow E, Compton PG, Foppiano L, Woodard J, Hill JD. Effects of acute right ventricular ischemia on ventricular interactions during prosthetic left ventricular support. J.Thorac.Cardiovasc.Surg. 1991;102:588–95. [PubMed] [Google Scholar]
- 19.Chow E, Farrar DJ. Right heart function during prosthetic left ventricular assistance in a porcine model of congestive heart failure. J.Thorac.Cardiovasc.Surg. 1992;104:569–78. [PubMed] [Google Scholar]
- 20.Santamore WP, Gray LA., Jr. Left ventricular contributions to right ventricular systolic function during LVAD support. Ann.Thorac.Surg. 1996;61:350–56. doi: 10.1016/0003-4975(95)01056-4. [DOI] [PubMed] [Google Scholar]
- 21.Maeder MT, Leet A, Ross A, Esmore D, Kaye DM. Changes in right ventricular function during continuous-low left ventricular assist device support. J.Heart Lung Transplant. 2009;28:360–66. doi: 10.1016/j.healun.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Edmunds LH., Jr. Inflammatory response to cardiopulmonary bypass. Ann.Thorac.Surg. 1998;66:S12–S16. doi: 10.1016/s0003-4975(98)00967-9. [DOI] [PubMed] [Google Scholar]
- 23.Pegg TJ, Selvanayagam JB, Karamitsos TD, Arnold RJ, Francis JM, Neubauer S, et al. Effects of off-pump versus on-pump coronary artery bypass grafting on early and late right ventricular function. Circulation. 2008;117:2202–10. doi: 10.1161/CIRCULATIONAHA.107.735621. [DOI] [PubMed] [Google Scholar]
- 24.Stein KL, Breisblatt W, Wolfe C, Gasior T, Hardesty R. Depression and recovery of right ventricular function after cardiopulmonary bypass. Crit Care Med. 1990;18:1197–200. doi: 10.1097/00003246-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy PM, Savage RM, Fraser CD, Vargo R, James KB, Goormastic M, et al. Hemodynamic and physiologic changes during support with an implantable left ventricular assist device. J Thorac.Cardiovasc.Surg. 1995;109:409–17. doi: 10.1016/S0022-5223(95)70271-7. [DOI] [PubMed] [Google Scholar]
- 26.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J.Am.Coll.Cardiol. 2001;37:183–88. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 27.Hershon JJ, Farrar DJ, Compton PG, Hill JD. Right ventricular dimensions with transesophageal echocardiography during an operating room model of left heart assist. Trans.Am.Soc.Artif.Intern.Organs. 1984;30:129–32. [PubMed] [Google Scholar]
- 28.Farrar DJ, Compton PG, Hershon JJ, Hill JD. Right ventricular function in an operating room model of mechanical left ventricular assistance and its effects in patients with depressed left ventricular function. Circulation. 1985;72:1279–85. doi: 10.1161/01.cir.72.6.1279. [DOI] [PubMed] [Google Scholar]
- 29.Farrar DJ, Compton PG, Hershon JJ, Fonger JD, Hill JD. Right heart interaction with the mechanically assisted left heart. World J.Surg. 1985;9:89–102. doi: 10.1007/BF01656260. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman D, Sisto D, Frater RW, Nikolic SD. Left-to-right ventricular interaction with a noncontracting right ventricle. J.Thorac.Cardiovasc.Surg. 1994;107:1496–502. [PubMed] [Google Scholar]
- 31.James KB, McCarthy PM, Thomas JD, Vargo R, Hobbs RE, Sapp S, et al. Effect of the implantable left ventricular assist device on neuroendocrine activation in heart failure. Circulation. 1995;92:II191–II195. doi: 10.1161/01.cir.92.9.191. [DOI] [PubMed] [Google Scholar]
- 32.Torre-Amione G, Stetson SJ, Youker KA, Durand JB, Radovancevic B, Delgado RM, et al. Decreased expression of tumor necrosis factor-alpha in failing human myocardium after mechanical circulatory support : A potential mechanism for cardiac recovery. Circulation. 1999;100:1189–93. doi: 10.1161/01.cir.100.11.1189. [DOI] [PubMed] [Google Scholar]
- 33.Vatta M, Stetson SJ, Perez-Verdia A, Entman ML, Noon GP, Torre-Amione G, et al. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–41. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- 34.Vatta M, Stetson SJ, Jimenez S, Entman ML, Noon GP, Bowles NE, et al. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J.Am.Coll.Cardiol. 2004;43:811–17. doi: 10.1016/j.jacc.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 35.Barbone A, Holmes JW, Heerdt PM, The’ AH, Naka Y, Joshi N, et al. Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure: a primary role of mechanical unloading underlying reverse remodeling. Circulation. 2001;104:670–75. doi: 10.1161/hc3101.093903. [DOI] [PubMed] [Google Scholar]
- 36.Klotz S, Naka Y, Oz MC, Burkhoff D. Biventricular assist device-induced right ventricular reverse structural and functional remodeling. J.Heart Lung Transplant. 2005;24:1195–201. doi: 10.1016/j.healun.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Klotz S, Jan Danser AH, Burkhoff D. Impact of left ventricular assist device (LVAD) support on the cardiac reverse remodeling process. Prog.Biophys.Mol.Biol. 2008;97:479–96. doi: 10.1016/j.pbiomolbio.2008.02.002. [DOI] [PubMed] [Google Scholar]