Abstract
As women get older their oocytes become susceptible to chromosome mis-segregation. This generates aneuploid embryos, leading to increased infertility and birth defects. Here we examined the provenance of aneuploidy by tracking chromosomes and their kinetochores in oocytes from young and aged mice. Changes consistent with chromosome cohesion deterioration were found with age, including increased interkinetochore distance and loss of the centromeric protector of cohesion SGO2 in metaphase II arrested (metII) eggs, as well as a rise in the number of weakly attached bivalents in meiosis I (MI) and lagging chromosomes at anaphase I. However, there were no MI errors in congression or biorientation. Instead, premature separation of dyads in meiosis II was the major segregation defect in aged eggs and these were associated with very low levels of SGO2. These data show that although considerable cohesion loss occurs during MI, its consequences are observed during meiosis II, when centromeric cohesion is needed to maintain dyad integrity.
Keywords: Imaging, Chromosomes, Meiosis, Mouse, Oocyte
INTRODUCTION
In oocytes, chromosome mis-segregation is common and generates aneuploid embryos, so causing lowered implantation rates, pregnancy loss and birth defects (Nagaoka et al., 2012; Jones and Lane, 2013). It is generally thought that segregation errors predominantly arise during the first of the two meiotic divisions, in the hours preceding ovulation. This first meiotic division (MI) is physiologically triggered hormonally but is also induced when fully grown oocytes from Graafian follicles are released from their maturation-inhibitory ovarian environment, and so can be studied in detail through in vitro culture (Solc et al., 2010; Li and Albertini, 2013). The division at MI is unique in that it results in the segregation of homologous chromosomes (‘bivalents’); the oocyte extrudes its first polar body, undergoes a brief period of interkinesis, and assembles a second meiotic spindle before arresting at metaphase (metII) of the second meiotic division (MII) (Chiang et al., 2012; Holt et al., 2013). The fertilizing sperm triggers completion of MII, in which the chromosomes (‘dyads’ or ‘sister chromatids’) remaining in the ooplasm are segregated; an event that is the same as the separation of sister chromatids in mitosis.
Increased maternal age raises the incidence of aneuploidy, both in human and mouse oocytes (Jones, 2008; Chiang et al., 2010; Lister et al., 2010). The association of aneuploidy with maternal age has been explained mechanistically in a number of ways, but mostly in terms of losses in key proteins, deterioration in metabolic processes and gains in oxidative damage (Nagaoka et al., 2011; Selesniemi et al., 2011; Jessberger, 2012; Jones and Lane, 2012). One attractive hypothesis is that aging bivalents within germinal vesicle (GV) stage oocytes lose both the proteins that are responsible for maintaining cohesion (Tachibana-Konwalski et al., 2010; Chiang et al., 2010; Duncan et al., 2012; Merriman et al., 2012) and the proteins needed to protect centromeric cohesion, such as SGO2 (SGOL2 - Mouse Genome Informatics) (Lee et al., 2008; Lister et al., 2010). Such loss could cause aneuploidy by promoting the premature separation of bivalents into either univalents (i.e. sister chromatid pairs) or single chromatids. Indeed, single chromatids have been observed in both aged human and mouse metII eggs (Angell, 1997; Chiang et al., 2010; Lister et al., 2010; Kuliev et al., 2011; Merriman et al., 2012). The process that generates them has been called ‘premature separation of sister chromatids’ (PSSC) or ‘pre-division’; however, it remains unknown when and how they are created.
Live-cell tracking of chromosomes would be the most appropriate technique to answer the question of when PSSC occurred in aged oocytes. Previously, live-cell imaging approaches have been used to demonstrate that aging does not lead to a deterioration in the spindle assembly checkpoint (SAC) (Duncan et al., 2009; Lister et al., 2010), the pathway responsible for inhibiting the anaphase-promoting complex (APC) ahead of bivalent biorientation. Also, live imaging has shown that misalignment and anaphase defects are more common in aged oocytes (Chiang et al., 2010; Lister et al., 2010). However, with these previous experiments using only chromosomal histone labelling, it was not possible to follow any detailed dynamics of individual bivalents. The process of PSSC, in the absence of knowing kinetochore location, cannot be accurately determined.
It would be helpful if techniques were employed to track individual bivalents during the entire period of meiotic maturation. This can be accurately done by labelling the kinetochores of bivalents and tracking their movements from the time of GV breakdown until metII arrest. Recently real-time tracking of chromosomes has been performed on oocytes from young mice during maturation (Kitajima et al., 2011). This previous work showed that bivalents are error-prone in establishing correct attachment to spindle microtubules, with on average three rounds of microtubule error correction preceding stable biorientation. This puts a different emphasis on the causes of aneuploidy: that the SAC may not be responsive to small errors in microtubule-kinetochore attachment. In fact, a number of recent studies have all established that the APC becomes active, and anaphase triggered, even though chromosome alignment and attachment errors persist (Nagaoka et al., 2011; Gui and Homer, 2012; Kolano et al., 2012; Lane et al., 2012; Sebestova et al., 2012).
Here we have employed a real-time kinetochore-tracking approach to study the relative importance of cohesion and congression defects in the segregation of bivalents from aged mice. By tracking, we have managed to reduce the intensity of imaging to such an extent that we can follow the movements of individual bivalents with a temporal resolution of 2 minutes over a 12- to 15-hour time window. This has afforded us a level of detail that has not previously been used to study chromosome dynamics in aged eggs.
RESULTS
Balanced pre-division in the eggs of aged mice generates single chromatids
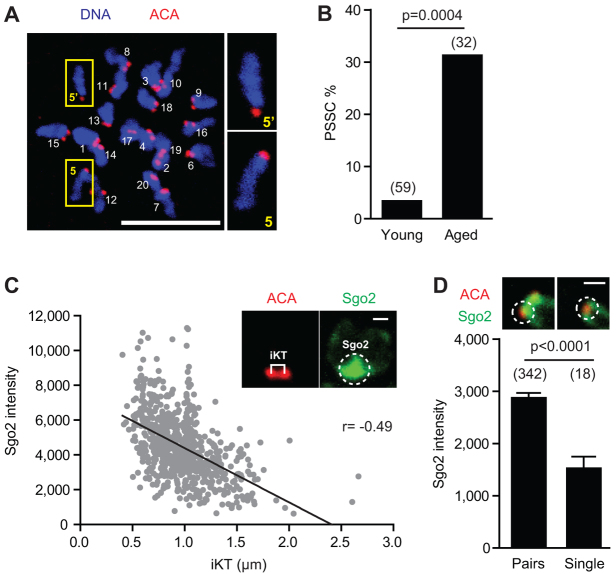
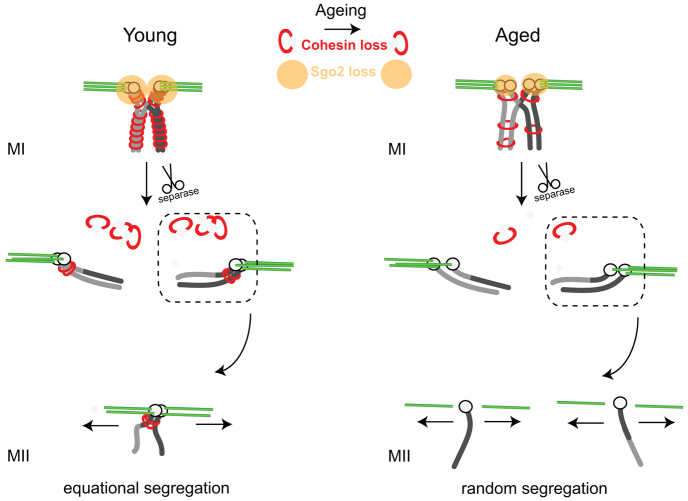
To explore the association of aneuploidy in oocytes with maternal age, we compared 1-month-old (young) and >12-month-old (aged) mice. GV-stage mouse oocytes were in vitro matured for 13-14 hours, by which time they had extruded their first polar body, and the resulting mature eggs arrested at metII. No procedures were performed on these oocytes following collection and during subsequent maturation. The dyads in these eggs were in situ spread with monastrol, to observe their morphology with Hoechst dye and their kinetochores with anti-centromeric antibodies (ACA). We counted a low, 3% incidence of single chromatids in eggs from young mice, which increased to 31% with maternal age (Fig. 1A,B; supplementary material Fig. S1A).
Fig. 1.
Loss of chromosome cohesion in aged eggs correlates with less SGO2. (A) Nineteen sister chromatid pairs (dyads) and two single chromatids (boxed, 5 and 5′; inset, zoomed) in an aged metII mouse egg. Numbering done to aid counting. (B) PSSC rate in young versus aged fixed eggs (Fisher’s exact test). (C) Scatter plot demonstrating association of reduced centromeric SGO2 intensity with increasing iKT distance (measured as shown in inset, Spearman correlation). (D) Centromeric SGO2 intensity (mean and s.e.m.) at one kinetochore of a sister chromatid pair or single chromatid, with representative images shown on top (Mann-Whitney U). Scale bars: 1 μm in C,D; 10 μm in A. ACA, anti-centromeric antibodies.
PSSC can result in either balanced pre-division, in which the two sisters of a pair remain in the egg, or unbalanced, in which only a single chromatid can be observed. If PSSC is after cytokinesis at completion of MI, then only balanced pre-division is possible, because the single chromatids so generated cannot be partitioned between the oocyte and already-extruded polar body. However, if PSSC is before cytokinesis, then either balanced or unbalanced pre-division can occur, dependent on the direction of the segregation, into either the polar body or the oocyte (supplementary material Fig. S1B). In human eggs, the ratio of balanced to unbalanced pre-division is 5:1 (Angell, 1991; Angell et al., 1991; Angell, 1997). Consistent with this here, for the ∼30% of aged mouse metII eggs showing PSSC, all pre-division gave even numbers of chromatids. As such eggs had either two or four single chromatids, and contained a total of 40 kinetochores (supplementary material Fig. S1C). The lack of any unbalanced pre-division is suggestive of an error after cytokinesis; however, the use of only metII eggs precludes the accurate timing of such an event.
Loss of chromosome cohesion in aged eggs correlates with less SGO2
The in situ spreads of aged metII eggs additionally demonstrated both a reduction in levels of SGO2 and also an increase in the distance between sister kinetochores [interkinetochore (iKT) distance], a measurement that would be expected to increase when dyads lose their cohesion (supplementary material Fig. S2A-C) (Lister et al., 2010; Duncan et al., 2012; Merriman et al., 2012). The iKT measurement can be taken as an inverse measure of the cohesive ties holding sister chromatids together, with larger measurements equating to less cohesion. Indeed, here we observed an inverse correlation between SGO2 levels and iKT distance (Fig. 1C). As such, those dyads with the largest iKT distance tended to have reduced SGO2. It was also found that SGO2 intensity on kinetochores of single chromatids in metII eggs was about half that on single kinetochores from dyads (Fig. 1D), suggesting that chromosomes that had developed a segregation error possessed less SGO2. In summary, the measurements show a strong association between an age-related loss in sister chromatid cohesion and reduced SGO2 in metII eggs.
Weakly attached bivalents are common in aged oocytes
In order to understand the influence of maternal age on meiotic chromosomes more fully, we moved to examine maturing oocytes by fixing them during meiosis I. In metaphase I (metI) oocytes from aged mice, bivalents appearing to be weakly held together, as assessed by the lack of Hoechst staining between the two sister chromatid pairs, were commonly observed (Fig. 2A; supplementary material Fig. S3A-C). Such bivalents were found in just 15% (n=13) of young, but in all (n=6) aged oocytes (Fig. 2B). Additionally, young oocytes contained a maximum of one weakly attached bivalent, whereas aged oocytes had a mean of 2.7 (Fig. 2C). Furthermore, these were associated with an increased kinetochore separation, defined as the distance separating the two sister kinetochore pairs within a bivalent (supplementary material Fig. S3A,D). One possibility is that these bivalents could be prone to undergo premature separation into univalents in MI. However, we had no direct observation of the separation, and therefore refer to them as weakly attached bivalents. This was a more accurate description, because we noted that these affected bivalents were congressed and under tension, rather than randomly distributed on the meiotic spindle, which would have been anticipated if association was lacking. Therefore, we conclude that some cohesive ties were maintained, albeit at a weak level. However, it was possible that ties could eventually be lost at later time points in MI, so generating univalents.
Fig. 2.
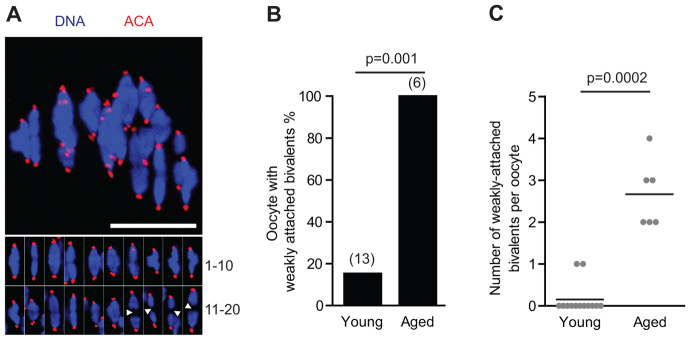
Weakly attached bivalents in aged oocytes. (A) Four weakly attached bivalents in an aged oocyte, marked by arrowheads (inset). (B,C) Percentage of oocytes with weakly attached bivalents (B) and the number present per oocyte (C; the horizontal line indicates the mean). Scale bar: 10 μm. ACA, anti-centromeric antibodies. (B) Fisher’s exact test; (C) Mann-Whitney U.
Real-time 4D chromosome imaging by confocal tracking algorithm
We employed a technique for labelling and tracking both bivalents and kinetochores by 4D confocal microscopy, using histone 2B-mCherry and EGFP-CENPC, respectively (Fig. 3A). This follows the same approach used recently to explore bivalent dynamics in young oocytes (Kitajima et al., 2011). By this technique, which tracked the movements of chromosomes frame by frame, we managed to limit our x-y imaging to a 30×30 μm square - the area occupied by all the bivalents in a meiotic spindle. In the absence of such tracking, when the x-y area was increased to 80×80 μm, to accommodate the oocyte circumference, most oocytes arrested in MI and so did not complete meiotic maturation (not shown). Therefore, tracking was essential in order for oocytes to be imaged continuously over a period of 12 hours.
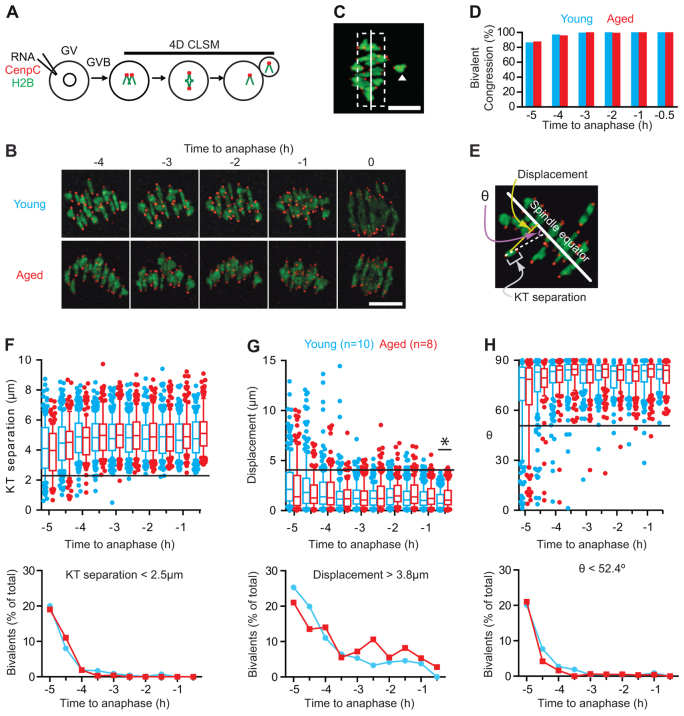
Fig. 3.
Congression and establishment of tension for bivalents are not affected by age. (A,B) Method used for chromatin and kinetochore labelling in live oocytes (A), and the resulting bivalent images at selected time points in young and aged oocytes (B). (C) A meiotic spindle, with one bivalent (arrowhead), classified as nonaligned because it is >4 μm (dashed line) from the spindle equator (solid line). (D) Percentage of congressed bivalents in young (n=10) and aged (n=8) oocytes at the times indicated. (E-H) Three parameters used on bivalents and their kinetochores (E); the separation distance between the two pairs of sister kinetochores (F, top), the displacement of the midpoint of the bivalent from the equator (G, top), and the bivalent axis of orientation (θ) relative to the spindle equator (H, top). Horizontal lines indicate minimum (F,H) or maximum (G) measurements made on young oocytes at the -0.5 hour time point; bivalents outside the lines were considered ‘at risk’ of mis-segregation. The percentage of bivalents found to be ‘at risk’ for each time point (F-H, bottom). Box plots, whiskers at 10-90 centiles; the asterisk indicates P<0.05 (Kruskal-Wallis). Scale bar: 10 μm in B,C.
Initially, the effects of this 4D imaging protocol were examined on oocytes with respect to their ability to complete MI and the chromosome complement at metII. Bivalents underwent normal segregation during MI when imaged every 2 minutes over several hours (supplementary material Movie 1); and no significant differences were observed in either MI completion (70-90% for all conditions), or premature separation of sister chromatids (supplementary material Fig. S1D). As such it is concluded that the imaging protocol had no significant impact on the faithful segregation of chromosomes.
Real-time tracking of bivalents in aged oocytes would be informative for three reasons: (1) to determine whether the process of bivalent congression and establishment of tension necessary for faithful segregation is affected by age; (2) to examine the fate of the bivalents classified as weakly attached in fixed metI oocytes; (3) to determine the origin of the balanced pre-division/PSSC also observed on fixed cells, such balanced pre-division possibly being derived from those weakly attached bivalents in MI. The first reason, relating to the measurement of congression and tension establishment, is especially relevant to oocytes given recent reports showing that the SAC becomes satisfied, and the APC activated, when the initial interactions of microtubules with kinetochores are established, rather than when they are correct and bivalents are under tension (Nagaoka et al., 2011; Gui and Homer, 2012; Kolano et al., 2012; Lane et al., 2012). With age, there may be a reduction in the ability of bivalents to establish correct attachments to both spindle poles, and given APC activation would be unaffected by such events, chromosome segregation would still occur but with greater errors. This idea can be pursued by tracking kinetochores and measuring the tension developing across each bivalent - here tension gives a readout of amphitelic attachment. It is important to note that tension can also result from incorrect, merotelic attachment (Cimini et al., 2003); however, these would be observed at anaphase onset as lagging chromosomes (Thompson and Compton, 2011).
Bivalent congression is not affected by maternal age
Bivalent congression was measured in histone 2B-mCherry and EGFP-CENPC expressing oocytes, by tracking chromosome dynamics over several hours leading up to anaphase in both young and aged oocytes (Fig. 3B). Previously we established that bivalent nonalignment was most accurately defined when displacement was >4 μm from the spindle equator (Fig. 3C) (Lane et al., 2012). As such here, congression of all bivalents was achieved at least 3 hours ahead of anaphase independent of age (Fig. 3D). This suggests there was no gross malfunctioning of bivalent congression in aged oocytes.
To examine the dynamics of bivalents in more detail, with the possibility that subtler differences may be found, three measurements were performed on each bivalent over a 5-hour window, up to 30 minutes before anaphase onset (Fig. 3E). Firstly, we measured the distance between the two sister kinetochore pairs within a bivalent, defined here as ‘kinetochore separation’. This increases as tension develops across the bivalent owing to microtubule pulling forces from opposing spindle poles. The second measurement is the distance that separates the bivalent from the spindle equator, defined as ‘bivalent displacement’, which reduces to a minimum level once congression is achieved. The third measurement is the angle of orientation that the axis of the bivalent makes in relation to the spindle equator. In our calculation, this angle (θ) tends towards 90°, as bivalents align perpendicular to the spindle equator. Kinetochore separation, bivalent displacement and θ could be measured for each bivalent in all oocytes and at every time point.
During the period of observation, bivalents were under tension, with a mean kinetochore separation of ∼5 μm (Fig. 3F); they were aligned on the spindle equator, having a mean displacement of <2 μm (Fig. 3G); and were essentially parallel with the spindle poles, subtending a >80° angle with respect to the spindle equator (Fig. 3H). No significant differences were observed between the two age groups at any time point within 5 hours of anaphase, with the single exception of greater bivalent displacement in aged oocytes at 30 minutes before anaphase (Fig. 3G). The box plots made for each measurement help identity outlying bivalents with ‘at risk’ characteristics for mis-segregation (KT separation <2.5 μm; displacement >3.8 μm; and θ <52.4°). The proportion of such bivalents has its maximal value at ∼20-25% 5 hours before anaphase, but decreases with time owing to establishment of congression and biorientation (Fig. 3F-H). Using these ‘at-risk’ measures, no differences were observed between young and aged oocytes. Therefore, in response to the first question we conclude that the process of congression and establishment of tension across the bivalent is not affected by age.
Weakly attached bivalents retained integrity during MI
Weakly attached bivalents, with no apparent H2B-mCherry signal between the two sister chromatid pairs, were more frequent in live aged oocytes (Fig. 4), confirming earlier observations on fixed cells. Such bivalents did not, however, undergo premature separation, but instead maintained the appearance of biorientation (Fig. 4). Although premature separation of a bivalent into two univalents was not observed, 2 from 26 aged oocytes contained univalents in the 5-hour assessment period (supplementary material Fig. S4A). By contrast, univalents were never found in young oocytes (n=39). Of the two aged oocytes, kinetochore tracking was only possible in one. Univalents have previously been reported to undergo biorientation in MI, and so divide equationally (Kouznetsova et al., 2007), an event that would have accounted for the balanced PSSC observed in the metII eggs. However, in this oocyte we found that neither univalent was biorientated. Specifically, one univalent that was positioned near the spindle pole separated into two single chromatids at anaphase, both of which remained in the oocyte. The other univalent also separated into two single chromatids, both of which partitioned into the polar body after lagging for several minutes at the spindle equator (supplementary material Fig. S4B).
Fig. 4.
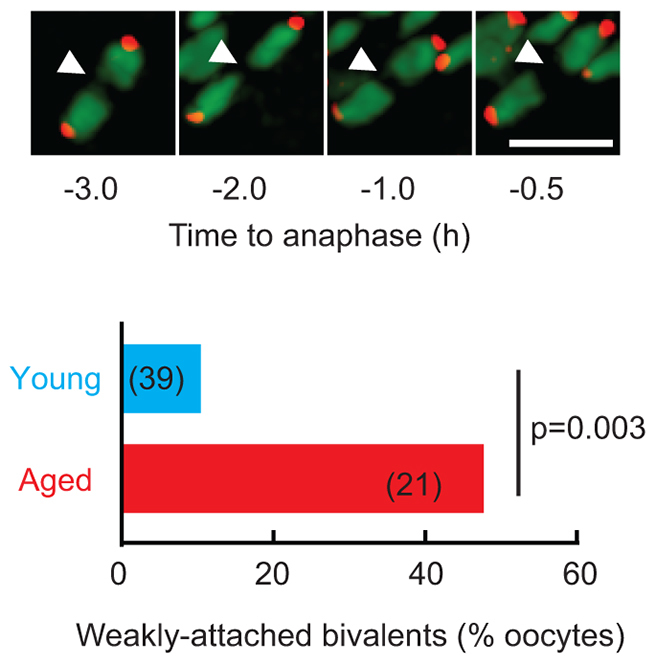
Weakly attached bivalents in aged oocytes maintain association in MI. The two sister chromatid pair halves of a weakly attached bivalent maintained their association during MI (arrowheads) and were more prevalent in aged oocytes (Fisher’s exact test). Scale bar: 5 μm.
Synchrony of bivalent separation at the metaphase-anaphase transition is independent of age
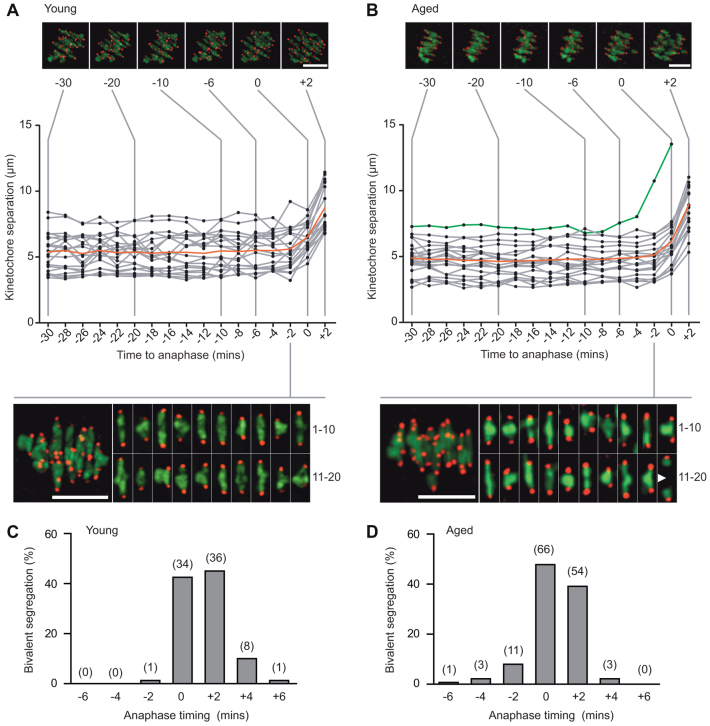
The presence of weakly attached bivalents and even univalents in aged MI oocytes is consistent with a loss of cohesion, most likely during prophase arrest. To understand the provenance of single chromatids in metII eggs, and to better study the fate of weakly attached bivalents, we went on to study the separation of bivalents at the metaphase-anaphase transition in more detail. Excepting those two aged oocytes in which univalents were already present, 20 bivalents were observed at every time point before anaphase onset, and all were biorientated on the metaphase plate (Fig. 5A,B). The precise timing of separation of each bivalent (relative to when the mean kinetochore separation first increases, i.e. Fig. 5A,B, time 0) revealed that most weakly attached bivalents separated 2-4 minutes earlier (Fig. 5B, green line; supplementary material Fig. S3E,F). However, this did not lead to mis-segregation of the two sister chromatid pairs (supplementary material Fig. S3F). In conclusion, early separation of bivalents that appeared either normally or weakly attached was a feature of aged oocytes, possibly indicative of reduced cohesion, but was not itself responsible for age-related PSSC. Indeed, for all bivalents segregation was a highly synchronous event, lasting 8-10 minutes, with >80% separating in a 4-minute window regardless of oocyte age (Fig. 5C,D).
Fig. 5.
In aged oocytes anaphase onset is synchronous. (A,B) Kinetochore separation in a representative young (A) or aged (B) oocyte plotted against time to anaphase onset. Separation is measured as the distance between the two sister kinetochore pairs. Above, selected time points showing a z-projection of bivalents. Below, bivalents in the last frame before anaphase onset. Red lines show the mean value; grey lines show individual values. The green line in B shows tracking of a weakly attached bivalent performing ‘premature but successful separation’ at -4 minutes, marked by the arrowhead in the inset. (C,D) Timing of individual bivalent segregation in young (C) or aged (D) oocytes, relative to the time of the initial increase in mean kinetochore separation (n=12, 4 from young and 8 from aged; 91% counting efficiency of bivalents). In parenthesis, bivalent number. Scale bars: 10 μm.
Lagging chromosomes at anaphase onset in aged oocytes
Despite the synchrony in the timing of bivalent segregation, we nonetheless observed lagging chromosomes. These were defined as persisting for a period of at least 30 minutes following anaphase onset, and they were significantly more common in aged than young oocytes (>40%, n=23 versus <5%, n=29; Fig. 6A,B), and 64% (n=11) of these produced aneuploid metII eggs. However, lagging chromosomes were not derived from weakly attached bivalents, because 82% (n=11) of oocytes containing lagging chromosomes previously had no weakly attached bivalents, and conversely 86% (n=14) of oocytes that had weakly attached bivalents had no lagging chromosomes at anaphase.
Fig. 6.
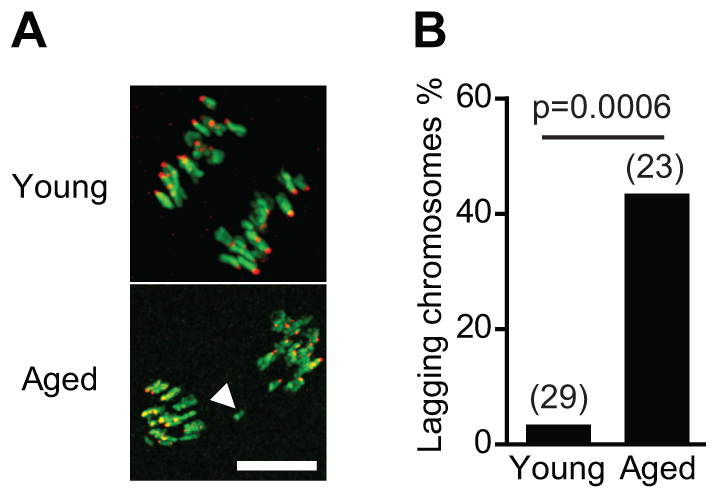
Lagging chromosomes in aged oocytes. (A) Representative images of anaphase in a young and an aged oocyte; the arrowhead shows lagging chromosome. (B) Higher incidence of oocytes with lagging chromosomes at anaphase in aged mice (Fisher’s exact test). The number of oocytes is indicated in parentheses. Scale bar: 10 μm.
Single chromatids are generated in aged MII eggs following interkinesis
Finally, we addressed the third of our questions asked of live cell tracking: when following anaphase I were single chromatids generated? They must arise either simultaneously with the MI division, such that their segregation is at the same time as sister chromatid pairs, or alternatively be produced when the metII spindle is forming.
Following polar body extrusion a period of interkinesis separates MI from MII, and this lasts between 1.5 and 2 hours, ending with the formation of the metII spindle (Madgwick et al., 2006). Decondensation of chromatin, probably caused by a loss of CDK1 activity (Kubiak et al., 1992), begins within several minutes of anaphase onset and disrupts both CENPC and H2B fluorescent protein signals, making it impossible to resolve individual kinetochores (Fig. 7A; supplementary material Movie 2).
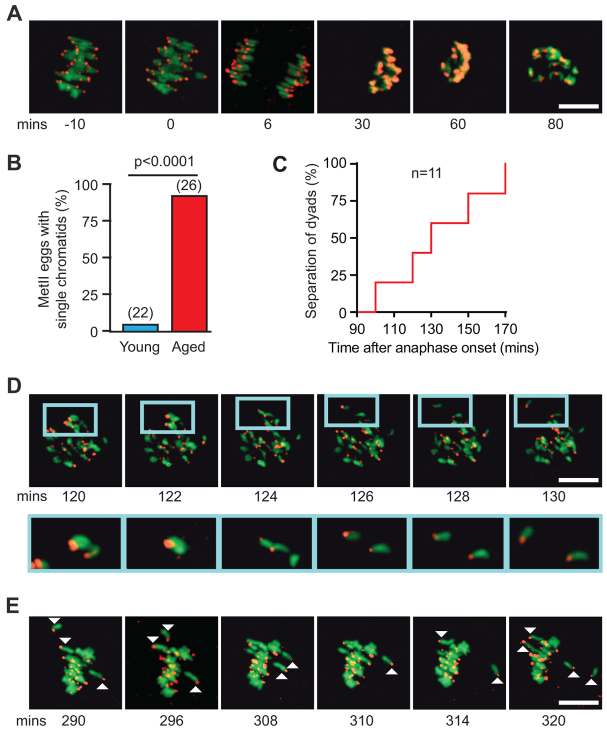
Fig. 7.
Generation of single chromatids in aged eggs during MII. (A) Loss and then gain of the H2B chromatin and CENPC kinetochore signals associated with MI exit, interkinesis and establishment of a metII spindle. (B) Percentage of metII eggs with single chromatids is higher in aged oocytes (Fisher’s exact test). (C) Timing of separation of a dyad into single chromatids in oocytes from aged mice. (D) Premature separation of a sister chromatid pair (dyad) following recondensation, boxed and expanded in inset. (E) Oscillatory behaviour of single chromatids during metII arrest. Representative of 26 aged oocytes examined. All times are relative to anaphase onset. Scale bars: 10 μm.
In the period following anaphase but preceding decondensation, separation of sister chromatids in aged oocytes was not observed to occur, with the single exception of the one containing two univalents (supplementary material Fig. S4B). However, in aged oocytes during the subsequent metII arrest, 92% (n=24/26) had single chromatids, as opposed to a rate in young mice of 4.5% (n=1/22; Fig. 7B). Despite the lost resolution during interkinesis, we could observe the premature separation of a sister chromatid pair in 46% of those aged eggs, at 133±24 minutes after anaphase onset (n=11/24; Fig. 7C,D). This period was shortly after the recondensation of CENPC and H2B fluorescent protein signals, and during the assembly of the metII spindle (Madgwick et al., 2006).
In the remaining 13 oocytes, the exact timing of the sister chromatid separation could not be determined, and so may have occurred at any point from anaphase I onwards. It remains possible that sister chromatids that have lost cohesion nonetheless travel together to the same pole and cannot be distinguished in the brief period of poleward movement that precedes decondensation. However, for those where sister separation was observed in MII, the newly formed single chromatids oscillated about the spindle equator, presumably because they have only a single kinetochore that fails to establish simultaneous attachment to both spindle poles (Fig. 7E; supplementary material Movie 2). Such movement is consistent with the interaction of the kinetochore with microtubules from one pole continually being destabilized, only to be replaced by attachment to the other pole.
DISCUSSION
Here we employed a chromosome-tracking approach to examine bivalent dynamics in oocytes from aged mice. An algorithm allowed the imaging area to be reduced to that containing only the meiotic spindle, and real-time tracking allowed imaging to follow the movement of the spindle from the centre to the oocyte cortex during MI. This protocol allowed us to perform 4D spindle imaging with a temporal resolution of 2 minutes continuously for a period of greater than 10 hours, without any noticeable loss in rates of meiotic maturation. By contrast, with conventional imaging of the entire oocyte, completion of MI was severely compromised. This is the first imaging-based study on oocytes from aged mice to be able to track chromosomes throughout the period of MI, with high temporal resolution, from the time of meiotic resumption until metII arrest. Such long-term confocal imaging with kinetochore tracking has only been applied previously to oocytes from young mice (Kitajima et al., 2011), whereas in aged mice, imaging has been limited to the period of anaphase (Chiang et al., 2010) or based on epi-fluorescence analysis, which lacks resolution in the z-axis and cannot reliably track individual bivalents or their kinetochores (Lister et al., 2010). In so doing we have been able to catalogue the movements of bivalents and kinetochores in a way not previously performed, and by tracking establish the effects of maternal aging on chromosome dynamics in MI through to metII arrest.
We observed no defects in bivalent congression with age, and conclude that deterioration in the dynamics of spindle assembly is probably not a cause of maternal age-related aneuploidy. However, it remains significant for the aetiology of aneuploidy that during assembly, the initial attachments of microtubules to bivalents are often incorrect and yet, despite this, satisfy the SAC and lead to APC activity (Kitajima et al., 2011; Nagaoka et al., 2011; Gui and Homer, 2012; Kolano et al., 2012; Lane et al., 2012). Such a system would be prone to segregation errors if these attachments failed to get corrected.
It is likely that with age, microtubule-kinetochore attachment errors increase. This is inferred by the raised numbers of lagging chromosomes during anaphase in aged oocytes. Their presence failed to alter segregation dynamics, implying that they have no influence on the activity of the SAC. Lagging chromosomes are most likely generated by increased incorrect microtubule attachment, which would have been promoted by reduced cohesion, a conclusion supported by the finding of univalents, weakly attached bivalents, raised interkinetochore distance, and reduced SGO2 on kinetochores in aged oocytes seen in this study. It may be that lowered chromosome cohesion, measured here and in other aging mouse and human oocyte studies (Chiang et al., 2010; Lister et al., 2010; Duncan et al., 2012; Merriman et al., 2012), allows greater flexibility within a sister kinetochore pair, so promoting incorrect attachment. Indeed, the importance of rigidity in bivalent structure for their faithful segregation at MI, in this case caused through recombination, has previously been established in yeasts (Lacefield and Murray, 2007; Sakuno et al., 2011). The lagging chromosomes, if analogous to mitosis, are likely to be generated from merotelic kinetochore-microtubule attachment, in which microtubules from both spindle poles attach to one of the sister kinetochore pairs. Merotelic bivalents would, if the same as sister chromatids, align normally on the metaphase plate (Cimini et al., 2003; Cimini et al., 2004; Thompson and Compton, 2011), and as such have gone undetected in our previous analysis of bivalent congression and tension. Indeed, increased kinetochore stretch, although often assumed to equate to amphitelic attachment, is appreciated to be consistent with such attachment but does not guarantee it.
Live imaging has helped resolve the fate of the weakly attached bivalents. The two sister chromatid pairs that constitute such a bivalent typically separated 2-4 minutes earlier; however, their segregation was always faithful. They were also not responsible for generating the lagging chromosomes at anaphase. Although not appearing detrimental in this age of mouse, it is likely, however, that they are a manifestation of a weakening of chromosome cohesion. This would only worsen with further maternal aging, and probably predispose the egg to aneuploidy by prematurely generating univalents or single chromatids. In this regard it would be interesting for future studies to determine how univalents behave in oocytes from aged mice, and such a study is possible using models with obligatory univalents (Nagaoka et al., 2011). It may be that the fate of the division of the univalent in MI is affected by maternal age.
Despite the above observations in MI, the main defect with age was the premature separation of dyads during metII arrest, revealed from the live-cell imaging. In about half of oocytes the event of sister chromatid separation was captured during imaging, and occurred 2 hours after anaphase I, as the metII spindle was assembling. Such visible separation was probably as a result of newly established tension across the dyad as the spindle microtubules assemble and attach to kinetochores. In the remaining half the event could not be captured, but likely times when this occurred are during the recondensation event itself, which may also be associated with microtubule attachment, and when resolution of the separation not possible, or alternatively during the anaphase I poleward movement of sister chromatids. In all instances the molecular reason for the premature separation is likely to be the same: cohesion loss associated with MI occurs at the centromere to a sufficient degree that sisters are no longer tethered together (Fig. 8).
Fig. 8.
Model for the generation of single chromatids following maternal aging. The effect of maternal aging is depicted on a bivalent (dark and light grey) during meiosis I (MI) and entry into meiosis II (MII). Aging is associated with a loss of cohesin and SGO2, which results in centromeric cohesion loss at anaphase. However, the two single chromatids co-segregate normally because they are attached to the same pole. When attachment of microtubules (green) is established following exit from interkinesis, the two single chromatids no longer are able to maintain their cohesion, and attach independently to microtubules. They are therefore subject to random segregation in MII, as opposed to the normal equational segregation, and consequently are liable to generate aneuploid embryos.
The increase of pre-division from 31% observed on fixed eggs to 92% on live cells are probably from the exogenous proteins or/and illumination, although the same procedure had no obvious impact on the faithful chromosome segregation tested on the young oocytes. These findings highlight the selective nature of the present study into maternal aging: in assessing one mouse type (CD1), at one time point (12 months) using one type of procedure (in vitro maturation performed without supporting cumulus cells and often following imaging). Further aging studies are needed in order to determine the relative importance sometimes attributed to hormone concentrations, metabolic status and environmental factors (Jones and Lane, 2012) - all of which may preferentially affect oocytes from aged mice. Our observations suggest that aged oocytes are more sensitive to extrinsic factors, which would lend support to a multifactorial mechanism of age-related aneuploidy. The resulting single chromatids contained the least SGO2, supporting a model in which the primary cause of increased aneuploidy with maternal age is a loss in centromeric chromosome cohesion (Chiang et al., 2010; Lister et al., 2010; Tachibana-Konwalski et al., 2010; Chiang et al., 2011; Jessberger, 2012). Indeed, it has been demonstrated directly in mouse oocytes that centromeric cohesion is more vulnerable to the actions of separase (Espl1 - Mouse Genome Informatics) than arm cohesion (Chiang et al., 2011), and because of an age-related loss in SGO2 this vulnerability would only be increased.
Our observations are consistent with human studies that have shown a prevalence of pre-division in eggs from older women (Angell, 1997; Kuliev et al., 2011), but suggest that they result from errors that manifest themselves in MII, despite having origins in MI. This conclusion agrees with recent work on human oocytes, which has extensively screened both polar bodies and suggested that a large component of the maternal age effect is due to segregation errors arising in MII (Fragouli et al., 2011). The present work highlights that biopsy of the first polar body alone, which would have been normal in most aged oocytes here, may not be an effective screening method for aneuploidy. Therefore, one important clinically relevant consequence of this study is that maternal aging can result in oocytes that have a minimally defective first meiotic division, have a normal chromosomal complement to their first polar body, and yet contain a latent chromosome defect of separated sister chromatids at metII that could generate aneuploid embryos following fertilization.
MATERIALS AND METHODS
Materials
All chemicals were from Sigma-Aldrich (Australia), unless stated otherwise.
Oocyte collection and culture
Mice were used in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, and were approved by the University of Newcastle Animal Care and Ethics Committee. For the aging study, 1-month- or >12-month-old Swiss CD-1 outbred females were used (Animal Resources Centre, Perth, Australia and Charles River Breeding Laboratories, Kingston, NY, USA). These mice were housed in pathogen-free facilities. All mice were hormonally primed with intraperitoneal injection of 10 IU pregnant mares’ serum gonadotrophin (Intervet, Australia). After 44-48 hours, GV-stage oocytes were collected from the ovary and mechanically stripped of surrounding cumulus cells. Only oocytes with an integral cumulus were used. A range of 10-30 oocytes for young, and 1-5 oocytes for old, were collected per mouse. Denuded oocytes were handled in M2 media containing 2.5 μM milrinone at 37°C for a period between 1 to 3 hours. Meiotic maturation was stimulated by milrinone washout, and all further culture was under mineral oil at 37°C.
Preparation of RNA and microinjection
H2B-mCherry, subcloned into a pMDL vector, which is optimized for expression in mammalian oocytes (Madgwick et al., 2006) and EGFP-CENPC, contained within a pGEM vector, were linearized via digestion with KpnI and SwaI respectively. 5′-capped cRNAs were synthesized using either T3 (H2B) or T7 (CENPC) mMessage mMachine (Ambion) according to the manufacturer’s instructions. cRNA (1500 ng/μl) was dissolved in nuclease-free water and stored at -80°C. Microinjection was performed as previously described using fabricated micropipettes that were inserted into the oocyte using the negative capacitance facility of an electrophysiological amplifier (Madgwick et al., 2006). cRNA was injected at a pipette concentration of 500 ng/μl for EGFP-CENPC and 200 ng/μl for H2B-mCherry. A measured 0.1-0.3% of oocyte volume injection was given using a timed pressure injection facility of a Pneumatic PicoPump (World Precision Instruments, Stevenage, UK).
Immunofluorescence
Oocytes were fixed and permeabilized in 2% formaldehyde in PHEM buffer (60 mM PIPES, 25 mM HEPES, 25 mM EGTA, 4 mM MgSO4) with 0.5% Triton X-100 and 1 μM taxol. Blocking was performed in 7% normal goat serum in phosphate-buffered saline (PBS) with 0.1% Tween 20. Primary antibodies were diluted in PBS with 3% bovine serum albumin (BSA)/0.1% Tween 20 with overnight incubation at 4°C: SGO2, gift of Y. Watanabe (Lee et al., 2008), or anti-ACA (CREST; 90C1058, Cortex Biochem, CA, USA). Secondary antibodies were Alexa-633 (SGO2) or Alexa-555 (ACA) conjugated (Invitrogen). Oocytes were counterstained with Hoechst (20 μg/ml) before mounting in Citifluor (Citifluor, UK).
Confocal time-lapse imaging
4D imaging was performed using an Olympus FV1000 confocal laser scanning microscope (CLSM) equipped with a 60×/1.2 NA UPLSAPO oil immersion objective lens. The CLSM was housed in a 37°C temperature-controlled environment. Chromosomes were imaged using 15-18 z-sections, with z-resolution of 2.0 μm. The x-y area was set to 320×320 pixels (′30×30 μm) and tracking was performed in real time using ImageJ (NIH) macros that were written to maintain chromosomes within the centre of field of view. The minimum time between consecutive frames was 2 minutes. The imaging period was between 0 to 15 hours after GV breakdown.
In situ chromosome analysis
MetII eggs were incubated in M2 media containing 200 μM monastrol for 1 hour, a process that spreads chromosomes sufficiently for analysis (Lane et al., 2010). Following incubation, eggs were assessed for aneuploidy by kinetochore and chromatin imaging using either EGFP-CENPC and H2B-mCherry; or anti-ACA antibodies and Hoechst.
Image processing and analysis
To improve the kinetochore signals in live oocytes, the images were processed using ImageJ software, by the subtraction of a ten-pixel Gaussian blur from a two-pixel Gaussian blur, as suggested (Kitajima et al., 2011). The positions of the kinetochores for each bivalent and at every time point were logged using semi-automated ImageJ macros. Algorithms then trialled 1,000,000 spindle orientations to establish the best fit with the bivalent kinetochore positions in 3D. A second-best-fit algorithm then fixed the position of the spindle equator. Finally, for each bivalent the angle of intersect with the equator was determined, as well as its distance from the equator and the separation of the two kinetochore pairs. SGO2 fluorescence calculation was performed as previously described (Lane et al., 2012) with modification. Specifically, SGO2 intensity between two sister kinetochores with a pair in metII eggs was made by measuring mean fluorescence within a 2 μm diameter circle centred on the midpoint joining the two kinetochores. For single chromatids in metII eggs, only a 1 μm diameter circle was required, and when comparison with sister chromatid pairs, measurement was against a 1 μm diameter circle centred on one of the kinetochores of the pair.
Statistical analysis and replicate numbers
Dichotomous data were analysed using Fisher’s exact test. For multiple comparisons we employed Kruskal-Wallis ANOVA, with Dunn’s post-test. All of other means analysis was performed using Mann-Whitney test. Data were processed using GraphPad Prism 5, with P<0.05 set for significance. At least three separate aged mice were tested from at least two independent experiments. All data are pooled.
Supplementary Material
Acknowledgments
We thank Y. Watanabe for the CENPC construct and the SGO2 antibody.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
The experiments were performed and figures prepared by Y.Y. S.I.R.L. wrote the software developed for live chromosome tracking and kinetochore analysis. Y.Y. and S.I.R.L. performed all data analysis. K.T.J. instigated the study, supervised the work and was the lead author in writing of the manuscript, on which all authors contributed.
Funding
The work was supported by an Australian Research Council grant [DP110100418 and DP1201000946] to K.T.J.; Y.Y. is the recipient of a China Scholarship Council PhD scholarship; S.I.R.L. was the recipient of a Northcote Scholarship Award scholarship awarded by the Menzies Trust and administered through King’s College London. Deposited in PMC for immediate release.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.100206/-/DC1
References
- Angell R. R. (1991). Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum. Genet. 86, 383–387 [DOI] [PubMed] [Google Scholar]
- Angell R. (1997). First-meiotic-division nondisjunction in human oocytes. Am. J. Hum. Genet. 61, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell R. R., Ledger W., Yong E. L., Harkness L., Baird D. T. (1991). Cytogenetic analysis of unfertilized human oocytes. Hum. Reprod. 6, 568–573 [DOI] [PubMed] [Google Scholar]
- Chiang T., Duncan F. E., Schindler K., Schultz R. M., Lampson M. A. (2010). Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 20, 1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T., Schultz R. M., Lampson M. A. (2011). Age-dependent susceptibility of chromosome cohesion to premature separase activation in mouse oocytes. Biol. Reprod. 85, 1279–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T., Schultz R. M., Lampson M. A. (2012). Meiotic origins of maternal age-related aneuploidy. Biol. Reprod. 86, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D., Moree B., Canman J. C., Salmon E. D. (2003). Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116, 4213–4225 [DOI] [PubMed] [Google Scholar]
- Cimini D., Cameron L. A., Salmon E. D. (2004). Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr. Biol. 14, 2149–2155 [DOI] [PubMed] [Google Scholar]
- Duncan F. E., Chiang T., Schultz R. M., Lampson M. A. (2009). Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol. Reprod. 81, 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan F. E., Hornick J. E., Lampson M. A., Schultz R. M., Shea L. D., Woodruff T. K. (2012). Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell 11, 1121–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragouli E., Alfarawati S., Goodall N. N., Sánchez-García J. F., Colls P., Wells D. (2011). The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. Mol. Hum. Reprod. 17, 286–295 [DOI] [PubMed] [Google Scholar]
- Gui L., Homer H. (2012). Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development 139, 1941–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. E., Lane S. I., Jones K. T. (2013). The control of meiotic maturation in mammalian oocytes. Curr. Top. Dev. Biol. 102, 207–226 [DOI] [PubMed] [Google Scholar]
- Jessberger R. (2012). Age-related aneuploidy through cohesion exhaustion. EMBO Rep. 13, 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. T. (2008). Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum. Reprod. Update 14, 143–158 [DOI] [PubMed] [Google Scholar]
- Jones K. T., Lane S. I. (2012). Chromosomal, metabolic, environmental, and hormonal origins of aneuploidy in mammalian oocytes. Exp. Cell Res. 318, 1394–1399 [DOI] [PubMed] [Google Scholar]
- Jones K. T., Lane S. I. (2013). Molecular causes of aneuploidy in mammalian eggs. Development 140, 3719–3730 [DOI] [PubMed] [Google Scholar]
- Kitajima T. S., Ohsugi M., Ellenberg J. (2011). Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 146, 568–581 [DOI] [PubMed] [Google Scholar]
- Kolano A., Brunet S., Silk A. D., Cleveland D. W., Verlhac M. H. (2012). Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc. Natl. Acad. Sci. USA 109, E1858–E1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsova A., Lister L., Nordenskjöld M., Herbert M., Höög C. (2007). Bi-orientation of achiasmatic chromosomes in meiosis I oocytes contributes to aneuploidy in mice. Nat. Genet. 39, 966–968 [DOI] [PubMed] [Google Scholar]
- Kubiak J. Z., Weber M., Géraud G., Maro B. (1992). Cell cycle modification during the transitions between meiotic M-phases in mouse oocytes. J. Cell Sci. 102, 457–467 [DOI] [PubMed] [Google Scholar]
- Kuliev A., Zlatopolsky Z., Kirillova I., Spivakova J., Cieslak Janzen J. (2011). Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod. Biomed. Online 22, 2–8 [DOI] [PubMed] [Google Scholar]
- Lacefield S., Murray A. W. (2007). The spindle checkpoint rescues the meiotic segregation of chromosomes whose crossovers are far from the centromere. Nat. Genet. 39, 1273–1277 [DOI] [PubMed] [Google Scholar]
- Lane S. I., Chang H. Y., Jennings P. C., Jones K. T. (2010). The Aurora kinase inhibitor ZM447439 accelerates first meiosis in mouse oocytes by overriding the spindle assembly checkpoint. Reproduction 140, 521–530 [DOI] [PubMed] [Google Scholar]
- Lane S. I., Yun Y., Jones K. T. (2012). Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development 139, 1947–1955 [DOI] [PubMed] [Google Scholar]
- Lee J., Kitajima T. S., Tanno Y., Yoshida K., Morita T., Miyano T., Miyake M., Watanabe Y. (2008). Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol. 10, 42–52 [DOI] [PubMed] [Google Scholar]
- Li R., Albertini D. F. (2013). The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 14, 141–152 [DOI] [PubMed] [Google Scholar]
- Lister L. M., Kouznetsova A., Hyslop L. A., Kalleas D., Pace S. L., Barel J. C., Nathan A., Floros V., Adelfalk C., Watanabe Y., et al. (2010). Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 20, 1511–1521 [DOI] [PubMed] [Google Scholar]
- Madgwick S., Hansen D. V., Levasseur M., Jackson P. K., Jones K. T. (2006). Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J. Cell Biol. 174, 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman J. A., Jennings P. C., McLaughlin E. A., Jones K. T. (2012). Effect of aging on superovulation efficiency, aneuploidy rates, and sister chromatid cohesion in mice aged up to 15 months. Biol. Reprod. 86, 49 [DOI] [PubMed] [Google Scholar]
- Nagaoka S. I., Hodges C. A., Albertini D. F., Hunt P. A. (2011). Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr. Biol. 21, 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S. I., Hassold T. J., Hunt P. A. (2012). Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuno T., Tanaka K., Hauf S., Watanabe Y. (2011). Repositioning of aurora B promoted by chiasmata ensures sister chromatid mono-orientation in meiosis I. Dev. Cell 21, 534–545 [DOI] [PubMed] [Google Scholar]
- Sebestova J., Danylevska A., Novakova L., Kubelka M., Anger M. (2012). Lack of response to unaligned chromosomes in mammalian female gametes. Cell Cycle 11, 3011–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K., Lee H. J., Muhlhauser A., Tilly J. L. (2011). Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. USA 108, 12319–12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc P., Schultz R. M., Motlik J. (2010). Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol. Hum. Reprod. 16, 654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana-Konwalski K., Godwin J., van der Weyden L., Champion L., Kudo N. R., Adams D. J., Nasmyth K. (2010). Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 24, 2505–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. L., Compton D. A. (2011). Chromosome missegregation in human cells arises through specific types of kinetochore-microtubule attachment errors. Proc. Natl. Acad. Sci. USA 108, 17974–17978 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.