SUMMARY
SETTING
There are currently no routine screening procedures for active tuberculosis (TB) or latent tuberculous infection (LTBI) in Malaysian prisons.
OBJECTIVE
To determine the prevalence and correlates of LTBI and active TB symptoms among Malaysian prisoners with and without human immunodeficiency virus (HIV) infection using the tuberculin skin test (TST) and the World Health Organization TB symptom-based screening instrument.
DESIGN
A cross-sectional survey of 266 prisoners was performed in Kelantan, Malaysia. Consenting participants underwent two-step TST and were screened for active TB symptoms. Standardized cut-offs of respectively ≥5 and ≥10 mm were used to define reactive TST among prisoners with and without HIV. Clinical and behavioral data were assessed and HIV-infected prisoners were stratified by CD4 status.
RESULTS
Overall LTBI prevalence was 87.6%, with significantly lower TST reactivity among HIV-infected than non-HIV-infected prisoners (83.6% vs. 91.5%, P < 0.05); however, TB symptoms were similar (16.9% vs. 10.1%, P = 0.105). On multivariate analysis, previous incarceration (aOR 4.61, 95%CI 1.76–12.1) was the only significant correlate of LTBI. Increasing age (aOR 1.07, 95%CI 1.01–1.13), lower body mass index (aOR 0.82, 95%CI 0.70–0.96) and TST-reactive status (aOR 3.46, 95%CI 1.20–9.97) were correlated with TB symptoms.
CONCLUSION
LTBI is highly prevalent, associated with previous incarceration, and suggests the need for routine TB screening on entry to Malaysian prisons.
Keywords: tuberculin skin test, TB, screening, prisons, HIV, substance abuse
Tuberculosis (TB) remains a global problem, with 8.7 million incident cases and 1.4 million TB-related deaths reported in 2011. TB-related mortality is disproportionately distributed among people living with human immunodeficiency virus (HIV)/acquired immunedeficiency syndrome (PLWHA), with 430 000 deaths due to TB in 2011 among PLWHA.1 HIV-related immunosuppression increases TB susceptibility and the likelihood of reactivation of latent TB. Both HIV and TB are magnified within correctional settings, where risk factors contribute to the presence and transmission of both diseases.2,3 Substance misuse, low socio-economic status, crowded housing units, malnutrition and proximity to active TB cases all increase TB risk in prisons,4–6 as does previous incarceration.7–10 Globally, HIV and TB incidence and prevalence are more concentrated in prisons than in the general population.11 Proactive steps for identifying TB in prisons, particularly among PLWHA, is important for both individual and public health.10
Malaysia, a middle-income country with intermediate TB prevalence, reported 101 active TB cases per 100 000 population in 2011.12 Although there are few data on LTBI in Malaysia, two studies have reported a latent tuberculous infection (LTBI) prevalence of 52.1% and 59.0% among Malaysian health care workers using the tuberculin skin test (TST).13,14 Malaysia’s stringent policies on illicit drug use have resulted in high incarceration rates among people who inject drugs (PWIDs), a population among whom HIV prevalence is six-fold higher than in the general population.15 Despite mandatory HIV testing and segregation of HIV-infected prisoners into separate housing units, screening for LTBI and active TB is non-existent, with reliance on ill patients presenting with advanced illness. HIV prevalence among Malaysian prisoners is estimated at 6.0%.16 Antiretroviral therapy (ART) is not available in many Malaysian prisons,17 resulting in crowded housing units full of immunocompromised prisoners.
We screened HIV-infected and non-HIV-infected prisoners in northeastern Malaysia for LTBI and active TB symptoms using the standardized recommended two-step TST and a standardized World Health Organization (WHO) symptom screening survey.18
STUDY POPULATION AND METHODS
Setting
Kelantan, a northern peninsular state in Malaysia, has one prison (n = 1400). Dedicated blocks house 40–50 inmates, including HIV-infected persons, in open-air 15 × 6 m units, consisting of three solid walls and one wall comprising a chain link fence. Sentenced and unsentenced (i.e., remand) Kelantan prisoners are housed in separate housing units; units for unsentenced prisoners often house those without previous incarcerations. Both sentenced and unsentenced prisoners were included in the study to ensure the inclusion of first-time offenders without previous incarceration.
Recruitment and sample
Information sessions consisted of study description, elaboration of risks and benefits, and an explanation of the voluntary nature of participation. All HIV-infected sentenced and unsentenced prisoners and a convenience sample of non-HIV-infected prisoners participated in the information sessions. A G*Power (Institute for Digital Research and Education, UCLA, Los Angeles, CA, USA) power analysis determined the sample size necessary to detect the difference in LTBI prevalence between groups. A minimum sample of 134 with a moderate effect size of 0.3, α of 0.05 and a power of 0.95 was required. Based on the number of HIV-infected participants who provided consent, a similar number of non-HIV-infected inmates were recruited by housing block until the number of non-HIV-infected participants matched the number of HIV-infected participants. Interested prisoners provided signed informed consent, and were interviewed and underwent TST in a private room. HIV-infected individuals underwent CD4 testing. Reasons for not completing the study protocol included transfer or release. Inmates were not rewarded or punished based on their decision to participate.
Survey administration
A structured questionnaire, administered by a trained research assistant in Bahasa Malaysia, was used to collect information on demographics, HIV risk behavior, TB history, active TB contacts, incarceration history and presence of active TB symptoms. Active TB symptoms were assessed using the standardized WHO TB symptom screening assessment survey.18 We defined ‘high risk’ for active TB as a cough lasting >2 weeks plus the presence of one or more of the following symptoms: night sweats, weight loss, fever or hemoptysis. Chronic cough is the most specific symptom, and inclusion of another symptom increases sensitivity.18 The English-language version was translated into Bahasa Malaysia and validated by back-translation. Patients with symptoms suggestive of TB were referred to the prison’s medical staff for further evaluation. Research personnel did not have jurisdiction over the quality or the content of the prison-based assessment after referral, and were therefore not allowed further access to assessment information. After the survey, height and weight was measured and body mass index (BMI) was calculated. Interviews were conducted in private counseling rooms with no correctional officer present to ensure privacy and reduce coercion.
Tuberculin skin testing
All participants underwent TST unless one of the following three conditions was met: 1) self-reported previous positive TST, 2) documented current receipt of anti-tuberculosis treatment (confirmed by prison medical records), or 3) self-reported previous TB diagnosis and treatment. To avoid variations in implantation and reading, a single trained investigator (BM) intradermally injected two units of purified protein derivative (PPD-RT23) using the Mantoux method; induration was measured in the transverse direction 48–72 h later. Cut-off values of respectively 5 and 10 mm were used for HIV-infected and non-HIV-infected prisoners. The two-step TST as recommended by the Centers for Disease Control and Prevention (CDC) was performed to identify participants with remote TB exposure, immunosuppression or who were malnourished who may falsely test negative, but exhibit the ‘booster phenomenon’ and mount an immune response to a second TST placed 1–3 weeks after an initial negative TST. Initially negative TST participants underwent a repeat TST after 1–3 weeks. The TST result is reported as positive only if the repeat test was positive. Participants who reported a previous positive TST or reported previous active TB diagnosis and uncompleted treatment were considered TST-positive for analysis. When calculating induration size for repeat testers, the larger of the induration diameters was recorded.
Analysis of CD4 lymphocyte count
CD4 testing was performed at a local AIDS Center after phlebotomy by a trained medical assistant. Twenty-seven (19.7%) HIV-infected participants refused testing because they had recently been tested or expected to be released before obtaining the results. Reports were provided to patients and clinicians to guide treatment.
Data analysis
Analyses were conducted using IBM SPSS Statistics 19 (IBM Corporation, Somers, NY, USA). χ2 testing was used to determine significant differences between HIV-infected and non-HIV-infected participants. Bivariate logistic regression was used to estimate odds ratios (ORs) for each independent variable; those with P < 0.20 were included in the final multivariate logistic regression model. Akaike Information Criterion (AIC) was used to assess the goodness of fit of the model.
Ethical approval
The Institutional Review Boards (IRBs) at Yale University and the University of Malaya approved the study. Participants were advised that they could refuse participation during any part of the study or withdraw at any time. After data collection, all data were de-identified and personal identifiers were destroyed.
RESULTS
Population characteristics
Figure 1 describes the sampling for the study, and Table 1 compares HIV-infected and non-HIV-infected subjects. Nearly all (n = 266, 92%) of the 288 participants initially recruited completed the study survey: LTBI was identified in 227 (87.6%) of the 259 participants with complete TST results. The surveyed population had a mean age of 33.4 years (standard deviation 7.2); 96.2% were male, and 97.0% were of Malay ethnicity.
Figure 1.
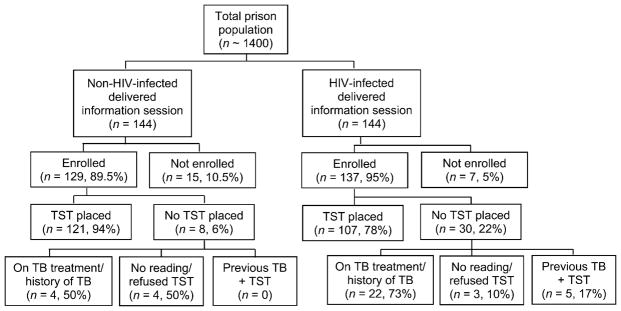
Study sampling and participant disposition. HIV = human immunodeficiency virus; TST = tuberculin skin test; TB = tuberculosis.
Table 1.
Study population characteristics
| Total population (n = 266) n (%) |
Non-HIV-infected (n = 129, 48.4%) n (%) |
HIV-infected (n = 137, 51.5%) n (%) |
P value* | |
|---|---|---|---|---|
| Demographic | ||||
| Age, years, mean ± SD | 33.4 ± 7.2 | 31.3 ± 7.8 | 35.5 ± 6.0 | <0.001 |
| Sex | 0.923 | |||
| Male | 256 (96.2) | 124 (96.1) | 132 (96.4) | |
| Female | 10 (3.8) | 5 (3.9) | 5 (3.6) | |
| Ethnicity | 0.216 | |||
| Malay | 258 (97.0) | 122 (94.6) | 136 (99.3) | |
| Chinese | 4 (1.5) | 3 (2.3) | 1 (0.7) | |
| Indian | 2 (0.8) | 2 (1.6) | 0 | |
| Asli (native) | 1 (0.4) | 1 (0.8) | 0 | |
| Other | 1 (0.4) | 1 (0.8) | 0 | |
| History of incarceration | ||||
| Previously incarcerated | 179 (67.3) | 66 (51.2) | 113 (82.5) | <0.001 |
| Cumulative time incarcerated, months | <0.001 | |||
| None | 87 (32.7) | 63 (48.8) | 24 (17.5) | |
| 1–6 | 29 (10.9) | 17 (13.2) | 12 (8.8) | |
| >6 | 150 (56.4) | 49 (38.0) | 101 (73.7) | |
| Health history | ||||
| History of smoking | 261 (98.1) | 124 (96.1) | 137 (100) | 0.020 |
| History of alcohol use | 232 (87.2) | 106 (82.2) | 126 (92.0) | 0.017 |
| History of BCG vaccination | 260 (97.7) | 127 (98.4) | 133 (97.1) | 0.452 |
| History of needle sharing | 148 (55.6) | 17 (13.2) | 131 (95.6) | <0.001 |
| TB history and work-up | ||||
| History of previous active TB | 28 (10.5) | 4 (3.1) | 24 (17.5) | <0.001 |
| Contact with active TB cases† | 151 (56.8) | 56 (43.4) | 95 (69.3) | <0.001 |
| Family contact | 26 (9.8) | 16 (12.4) | 10 (7.3) | 0.161 |
| Cell contact | 132 (49.6) | 44 (34.1) | 88 (64.2) | <0.001 |
| Friend contact | 9 (3.4) | 5 (3.9) | 4 (2.9) | 0.532 |
| TST results available (n = 259) | 0.040 | |||
| Positive | 227 (87.6) | 115 (91.5) | 112 (83.6) | |
| Negative | 32 (12.4) | 10 (8.0) | 22 (16.4) | |
| Induration size, mm, mean ± SD | 13.8 ± 6.1 | 14.9 ± 5.2 | 12.4 ± 8.5 | 0.007 |
| Body mass index, kg/m2, mean ± SD | 22.4 ± 3.4 | 23.2 ± 3.7 | 21.6 ± 2.8 | <0.001 |
| TB symptom screen | ||||
| Positive screen | 36 (13.6) | 13 (10.1) | 23 (16.9) | 0.105 |
From χ2 analyses.
Includes anyone with reported contact with active TB cases. As some persons reported multiple exposures, totals do not equal 100%.
HIV = human immunodeficiency virus; SD = standard deviation; BCG = bacille Calmette-Guérin; TB = tuberculosis; TST = tuberculin skin test.
Compared to non-HIV-infected participants, HIV-infected subjects were significantly older (35.5 vs. 31.3 years), more likely to have been previously incarcerated (82.5% vs. 51.2%) and more likely to report pre-incarceration alcohol use (92.0% vs. 82.2%) and needle sharing (95.6% vs. 13.2%). HIV-infected participants were also significantly more likely to report previous active TB (17.5% vs. 3.1%) and contact with active TB cases (69.3% vs. 43.4%) than non-HIV-infected participants. Nearly all TB contacts were cellmates with active TB. None of the HIV-infected participants in our survey were receiving ART.
Prevalence of latent tuberculous infection
LTBI prevalence in the entire sample was 87.6% (Table 1) and was higher among non-HIV-infected prisoners (91.5% vs. 83.6%, P < 0.05). High overall TST reactivity resulted in only 41 participants undergoing booster testing: 6 of 24 HIV-infected and 7 of 17 non-HIV-infected participants transitioned to TST-positive results. On bivariate analysis, HIV-infected subjects, particularly those at the lowest CD4 strata (<200 cells/ml), had the lowest likelihood of being TST-positive compared to non-HIV-infected participants (OR 0.24, 95% confidence interval [CI] 0.08–0.076, P < 0.05). Low CD4 strata did not, however, remain significant in the multivariate model. After controlling for other potentially confounding variables in the final model, inmates with previous incarceration history were nearly five times more likely to be TST-positive (adjusted OR [aOR] 4.61, 95%CI 1.76–12.1, P < 0.05) (Table 2). TST reactivity between sentenced and unsentenced prisoners (P = 0.65) did not differ (data not shown).
Table 2.
Bivariate and multivariate correlates of positive TST results (n = 259, AIC = 41.4)
| Characteristic | Total (n = 259) n (%) |
TST-negative (n = 32, 12.4%) n (%) |
TST-positive (n = 227, 87.6%) n (%) |
Unadjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value |
|---|---|---|---|---|---|---|---|
| Age, years, mean ± SD | 33.4 ± 7.2 | 31.5 ± 6.2 | 33.7 ± 7.3 | 1.05 (0.99–1.11) | 0.120 | ||
| Sex | |||||||
| Female | 10 (3.9) | 3 (9.4) | 7 (3.1) | 0.31 (0.08–1.26) | 0.100 | 0.32 (0.07–1.41) | 0.131 |
| Male | 249 (96.1) | 29 (90.6) | 220 (96.9) | Referent | — | Referent | — |
| BMI, kg/m2 | 22.4 (3.4) | 22.3 (3.0) | 22.5 (3.4) | 1.02 (0.91–1.15) | 0.712 | . | |
| BMI ≤18.5 | 22 (8.5) | 3 (9.4) | 19 (8.4) | 0.88 | 0.849 | ||
| BMI >18.5 | 237 (91.5) | 29 (90.6) | 208 (91.6) | Referent | — | ||
| Previously incarcerated | 175 (67.6) | 15 (46.9) | 160 (70.5) | 2.71 (1.28–5.73) | 0.009 | 4.61 (1.76–12.1) | 0.002 |
| Health history | |||||||
| History of needle sharing | 144 (55.6) | 22 (68.8) | 122 (53.7) | 0.53 (0.24–1.17) | 0.114 | 0.61 (0.10–3.61) | 0.584 |
| HIV status and CD4 count, cells/μl (n = 235)* | |||||||
| HIV-infected, CD4 ≥200 | 86 (36.6) | 13 (44.8) | 73 (35.4) | 0.48 (0.20–1.16) | 0.104 | 0.37 (0.06–2.09) | 0.259 |
| HIV-infected, CD4 <200 | 23 (9.8) | 6 (20.7) | 17 (8.3) | 0.24 (0.08–0.76) | 0.015 | 0.25 (0.04–1.61) | 0.143 |
| Non-HIV-infected | 126 (53.6) | 10 (34.5) | 116 (56.3) | Referent | — | — | — |
| HIV status† | |||||||
| Non-infected | 125 (48.3) | 10 (20.8) | 115 (54.5) | 2.26 (1.02– 4.99) | 0.044 | ||
| Infected | 134 (51.7) | 22 (68.8) | 112 (49.5) | Referent | — | ||
Excludes HIV-infected participants who did not complete CD4 testing, but underwent TST.
Not included in multivariate analysis as HIV status stratified by CD4 count.
TST = tuberculin skin test; AIC = Akaike Information Criterion; OR = odds ratio; CI = confidence interval; SD = standard deviation; BMI = body mass index; HIV = human immunodeficiency virus.
Prevalence of active tuberculosis symptoms and stratification by CD4 count
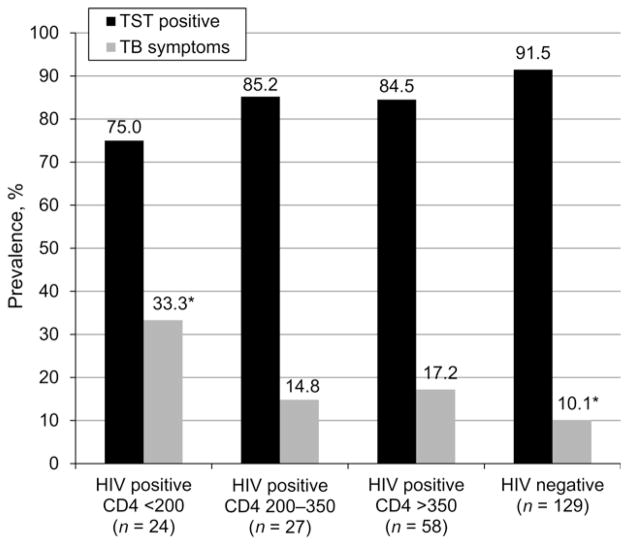
Symptoms suggestive of active TB occurred in 13.6% of the samples and there was a trend toward significantly higher prevalence among HIV-infected prisoners (16.9% vs. 10.1%, P = 0.105). Those screening positive for active TB symptoms were significantly older (aOR 1.07, 95%CI 1.01–1.13, P < 0.05) and had a lower BMI (aOR 0.82, 95%CI 0.70–0.96, P < 0.05) (Table 3). TST-negative participants, the minority in the sample (12.4%), were 3.5-fold more likely to have positive TB screening symptoms (aOR 3.46, 95%CI 1.20–9.97, P < 0.05). Screening symptoms consistent with active TB among HIV-infected participants tended to be significantly higher (Figure 2) among those with CD4 < 200 cells/ml than those with CD4 > 350 (33.3% vs. 17.2%, P = 0.066) and significantly higher than non-HIV-infected participants (10.1%, P = 0.005).
Table 3.
Correlates of positive TB symptom screening (AIC = 183.2)
| Total (n = 265)* n (%) |
Negative screening results (n = 229, 86.4%) n (%) |
Positive screening results (n = 36, 13.6%) n (%) |
Unadjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value | |
|---|---|---|---|---|---|---|---|
| Characteristic | |||||||
| Age (continuous), years, mean ± SD | 33.4 ± 7.2 | 32.9 ± 6.7 | 36.2 ± 9.5 | 1.06 (1.01–1.11) | 0.014 | 1.07 (1.01–1.13) | 0.013 |
| BMI, kg/m2, mean ± SD | 22.4 ± 3.4 | 22.6 ± 3.4 | 21.0 ± 2.6 | 0.82 (0.72–0.94) | 0.005 | 0.82 (0.70–0.96) | 0.014 |
| ≤18.5 | 24 (9.1) | 18 (7.9) | 6 (16.7) | 2.34 (0.86–6.37) | 0.095 | ||
| >18.5 | 241 (90.9) | 211 (92.1) | 30 (83.3) | Referent | — | ||
| Health history | |||||||
| History of alcohol use | 231 (87.2) | 204 (89.1) | 27 (75.0) | 0.37 (0.16–0.87) | 0.023 | 0.38 (0.14–1.06) | 0.064 |
| HIV status† | |||||||
| Infected | 136 (51.3) | 113 (49.3) | 23 (63.9) | 1.82 (0.88–3.76) | 0.108 | ||
| Non-infected | 129 (48.7) | 116 (50.7) | 13 (36.1) | Referent | |||
| HIV status stratified by CD4 count, cells/μl (n = 238)‡ | |||||||
| HIV-infected, CD4 ≥200 | 85 (35.7) | 71 (35.0) | 14 (40.0) | 1.78 (0.79–3.99) | 0.165 | 1.47 (0.41–5.27) | 0.552 |
| HIV-infected, CD4 <200 | 24 (10.1) | 16 (7.9) | 8 (22.9) | 4.80 (1.71–13.47) | 0.003 | 1.13 (0.45–2.85) | 0.803 |
| Non-HIV-infected | 129 (54.2) | 116 (57.1) | 13 (37.1) | Referent | — | Referent | — |
| History of active TB | 27 (10.2) | 19 (8.3) | 8 (22.2) | 3.16 (1.26–7.89) | 0.014 | 1.84 (0.58–5.88) | 0.303 |
| TST results | |||||||
| Positive | 32 (12.4) | 23 (10.4) | 9 (25.0) | Referent | — | Referent | — |
| Negative | 227 (87.6) | 199 (89.6) | 27 (75.0) | 2.88 (1.21–6.88) | 0.017 | 3.46 (1.20–9.97) | 0.021 |
One participant did not complete the TB symptom screening.
Not included in the final model as HIV status stratified by CD4.
Excludes HIV-infected participants who did not complete CD4 testing, but underwent TB symptom screening.
TB = tuberculosis; AIC = Akaike Information Criterion; OR = odds ratio; CI = confidence interval; SD = standard deviation; BMI = body mass index; HIV = human immunodeficiency virus; TST = tuberculin skin test.
Figure 2.
Prevalence of TB symptoms and latent tuberculous infection among inmates of Pengkalan Chepa prison. * P = 0.005. TST = tuberculin skin test; TB = tuberculosis; HIV = human immunodeficiency virus.
DISCUSSION
LTBI prevalence among prisoners in northeastern Malaysia is remarkably high (87.6%), and to our knowledge the highest reported among prisoners. Although much debate remains about the utility of TST in bacille Calmette-Guérin (BCG) vaccinated populations, the immunogenic effects of BCG wane after 8–10 years.19 While numerous studies have shown that the majority of TST-positive, interferon-gamma release assay-negative discordances occur in BCG-vaccinated populations,20,21 these are relatively infrequent. The WHO therefore supports the use of TST to detect LTBI in settings where BCG is used.22
LTBI among Malaysian prisoners remains higher than the 52.1% and 59.0% prevalence reported among at-risk health care workers from two other studies using similar methods.13,14 The higher prevalence in our sample, when viewed alongside other high-risk, BCG-vaccinated Malaysians, suggests that false-positives are not responsible for the high LTBI prevalence among prisoners. LTBI prevalence in prison settings elsewhere, in Spain (40.3%)23 and Nigeria (54.2%),24 is lower than our observed prevalence. These comparisons with Malaysian prisoners and among prisoners elsewhere suggest that uncharacterized factors are driving the exceedingly high LTBI prevalence among Malaysian prisoners.
Kelantan’s prison is constructed with ‘open air’ architecture and makes use of extensive natural ventilation. While this measure might seem to reduce TB transmission,25 the LTBI burden in this prison is very high, and similar (84%) to those reported among HIV-infected inmates in a prison with poor ventilation in the Selangor State of Malaysia.26 These results highlight the urgent need for routine and pro-active measures to screen for TB, initiate preventive therapy and ultimately control TB transmission on prison entry. Additional public health incentives derive from the knowledge that TB negatively impacts both on prison staff and on the communities to which prisoners return.27,28 Proactive and routine TB surveillance and preventive measures include isoniazid preventive therapy (IPT) for TST-reactive individuals, and symptom-based TB screening, followed by sputum culture, and isolation for symptomatic inmates.29 IPT is now recommended for all HIV-infected persons, particularly those with LTBI.30 It is inexpensive, but requires treatment for a minimum of 6 months (longer if HIV-infected), which could be problematic in detention settings where transition to the community is particularly challenging, as noted in a recent systematic review.31,32 New trials document the efficacy of shorter IPT regimens, including a 3-month course of weekly rifapentine plus isoniazid, which is equivalent to 9-month IPT in non-HIV-infected populations.33 IPT options with shorter courses are likely to have a greater impact on TB prevention strategies where prison sentences are short.
Although our data do not justify the use of negative TST results to identify active TB cases, our findings of a negative TST in this setting with a particularly high prevalence of TST positivity among HIV-positives may have important implications. For example, in this setting where TST reactivity among non-HIV-infected inmates was 91.5%, a negative TST in the HIV-infected might potentially be an indication of advanced HIV disease and be a marker of cutaneous anergy. This observation, coupled with the high prevalence of TB-related symptoms among those with CD4 < 200 cells/ml, is compelling. HIV-infected prisoners who were TST-negative in our sample were over three fold more likely to have TB-related symptoms, underscoring the importance not only of relying on TST to indicate TB exposure, but also of incorporating other measures to detect active TB, including symptom surveys, chest radiographs and sputum testing for smear and culture.
The cross-sectional nature of this study makes it impossible to confirm whether the prison environment is responsible for increased LTBI or if previous incarceration portends a higher LTBI risk for reasons that were not measured. Regardless, proactive measures to reduce the numbers of PLWHA who are incarcerated will likely improve the health of this population, and our data support the vast body of literature that show that prison conditions are correlated with high TB prevalence and lead to poor health outcomes. Many HIV-infected prisoners were convicted solely for drug use, suggesting that expanding community-based opioid substitution treatment (OST) could reduce incarceration rates among this vulnerable population.34 Alternatively, introducing prison-based methadone maintenance before prison release and continuing in the community could serve as an important conduit to continuity of care,34,35 including treatment for active TB or LTBI.
Our study has a number of limitations. The use of the TST in a BCG-vaccinated population raises concerns about false-positives. The age of our sample (mean 33 years) suggests that BCG contributes little to TST positivity, as the effects of BCG wane within 8–10 years;19 we were also unable to demonstrate an association between TST reactivity and lower age. Using the TST in an untreated HIV-infected population also raises concerns, as the TST relies on a functional immune response; however, TST reactivity in this study was not associated with CD4 strata. Furthermore, the sampling strategy we used to recruit the non-HIV-infected control group was a convenience sample, and was not guaranteed to represent the demographic characteristics of all HIV-infected prisoners. The majority of HIV-infected prisoners were PWID who likely had unmeasured risk factors distinguishing them from the lower proportion of non-HIV-infected inmates who were not primarily PWIDs. Lastly, although it would have been useful to examine the relationship between TB symptoms and active TB, IRB concerns and logistical constraints restricted access to this information, and further investigations are required.
CONCLUSIONS
The burden of LTBI and active TB symptoms among prisoners in northeastern Malaysia is extraordinarily high, and much higher than reported elsewhere. TB outbreaks in an HIV-infected housing unit have the potential for rapid transmission to susceptible hosts. Previous incarceration among these prisoners and the absence of HIV infection were associated with a positive TST, while increasing age and decreased BMI were associated with screening symptoms that correlated with active TB, particularly among prisoners with the most advanced HIV disease. Evidence-based control measures are urgently needed in Malaysian prisons to address these alarming findings, including proactive TB screening on prison entry, OST and alternatives to incarceration.
Acknowledgments
The authors thank M H Affendi for his efforts with data collection and translation. They also acknowledge the administrators and officers at Pengkalan Chepa Prison for their help facilitating this study, as well as the prisoners who agreed to participate. Mr Leng, the medical assistant at the prison medical clinic, was invaluable for his assistance and dedication.
Funding was provided by the National Institute on Drug Abuse, Bethesda, MD, USA (R01 DA025943), and career development awards (K24 DA017072 for FLA) and University of Malaya High Impact Research Grant (HIRGA E000001-20001) for HAAA. Additional support was provided by the Yale School of Medicine Office of Student Research, the International Disease Society of America Medical Scholars Program and the Wilber G Downs Fellowship at Yale University.
Footnotes
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2012. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 2.Banu S, Hossain A, Uddin MK, et al. Pulmonary tuberculosis and drug resistance in Dhaka central jail, the largest prison in Bangladesh. PLOS ONE. 2010;5:e10759. doi: 10.1371/journal.pone.0010759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun MM, Truman BI, Maguire B, et al. Increasing incidence of tuberculosis in a prison inmate population. Association with HIV infection. JAMA. 1989;261:393–397. [PubMed] [Google Scholar]
- 4.Niveau G. Prevention of infectious disease transmission in correctional settings: a review. Public Health. 2006;120:33– 41. doi: 10.1016/j.puhe.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Pang PT-T, Leung CC, Lee SS. Neighbourhood risk factors for tuberculosis in Hong Kong. Int J Tuberc Lung Dis. 2010;14:585–592. [PubMed] [Google Scholar]
- 6.Shetty N, Shemko M, Vaz M, D’Souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis. 2006;10:80–86. [PubMed] [Google Scholar]
- 7.Coker R, McKee M, Atun R, et al. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ. 2006;332:85–87. doi: 10.1136/bmj.38684.687940.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nava-Aguilera E, Andersson N, Harris E, et al. Risk factors associated with recent transmission of tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13:17–26. [PubMed] [Google Scholar]
- 9.Tekkel M, Rahu M, Loit H-M, Baburin A. Risk factors for pulmonary tuberculosis in Estonia. Int J Tuberc Lung Dis. 2002;6:887–894. [PubMed] [Google Scholar]
- 10.Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLOS Med. 2010;7:e1000381. doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larouzé B, Sánchez A, Diuana V. Tuberculosis behind bars in developing countries: a hidden shame to public health. Trans R Soc Trop Med Hyg. 2008;102:841–842. doi: 10.1016/j.trstmh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Tuberculosis country profiles. Geneva, Switzerland: WHO; 2013. [Accessed September 2013]. http://www.who.int/tb/country/data/profiles/en/index.html. [Google Scholar]
- 13.Rafiza S, Rampal KG, Tahir A. Prevalence and risk factors of latent tuberculosis infection among health care workers in Malaysia. BMC Infect Dis. 2011;11:19. doi: 10.1186/1471-2334-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan LH, Kamarulzaman A, Liam CK, Lee TC. Tuberculin skin testing among healthcare workers in the University of Malaya Medical Centre, Kuala Lumpur, Malaysia. Infect Control Hosp Epidemiol. 2002;23:584–590. doi: 10.1086/501975. [DOI] [PubMed] [Google Scholar]
- 15.Government of Malaysia. Malaysia National Strategic Plan on HIV/AIDS 2006–2010. Putrajaya, Malaysia: Ministry of Health; 2006. [Accessed September 2013]. http://hivaidsclearinghouse.unesco.org/search/resources/1329_malaysia20062010.pdf. [Google Scholar]
- 16.Dolan K, Kite B, Black E, Aceijas C, Stimson GV. HIV in prison in low-income and middle-income countries. Lancet Infect Dis. 2007;7:32– 41. doi: 10.1016/S1473-3099(06)70685-5. [DOI] [PubMed] [Google Scholar]
- 17.Choi P, Kavasery R, Desai MM, Govindasamy S, Kamarulzaman A, Altice FL. Prevalence and correlates of community reentry challenges faced by HIV-infected male prisoners in Malaysia. Int J STD AIDS. 2010;21:416–423. doi: 10.1258/ijsa.2009.009180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbett EL, Zezai A, Cheung YB, et al. Provider-initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull World Health Organ. 2010;88:13–21. doi: 10.2471/BLT.08.055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menzies R, Vissandjee B. Effect of bacille Calmette-Guérin vaccination on tuberculin reactivity. Am Rev Respir Dis. 1992;145:621–625. doi: 10.1164/ajrccm/145.3.621. [DOI] [PubMed] [Google Scholar]
- 20.Marco Mouriño A, Orcau Palau A, Jané Galliga R, et al. Concordance of tuberculin tests and interferon gamma release assays in the prison population. Rev Esp Sanid Penit. 2011;13:15–20. doi: 10.4321/s1575-06202011000100003. [Spanish] [DOI] [PubMed] [Google Scholar]
- 21.Talati NJ, Seybold U, Humphrey B, et al. Poor concordance between interferon-gamma release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis. 2009;9:15. doi: 10.1186/1471-2334-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson K. Tuberculin testing to detect latent tuberculosis in developing countries. Epidemiology. 2007;18:348–349. doi: 10.1097/01.ede.0000259985.76928.64. [DOI] [PubMed] [Google Scholar]
- 23.Chigbu LN, Iroegbu CU. Incidence and spread of Mycobacterium tuberculosis-associated infection among Aba Federal prison inmates in Nigeria. J Health Popul Nutr. 2010;28:327–332. doi: 10.3329/jhpn.v28i4.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marco A, Solé N, Orcau A, et al. Prevalence of latent tuberculosis infection in inmates recently incarcerated in a men’s prison in Barcelona. Int J Tuberc Lung Dis. 2012;16:60–64. doi: 10.5588/ijtld.11.0007. [DOI] [PubMed] [Google Scholar]
- 25.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Darraji HAA, Kamarulzaman A, Altice F. High prevalence of TB/HIV co-infection in a Malaysian prison. Int J Tuberc Lung Dis. 2011;15 (Suppl 3):S182. [Google Scholar]
- 27.MacNeil JR, McRill C, Steinhauser G, Weisbuch JB, Williams E, Wilson ML. Jails, a neglected opportunity for tuberculosis prevention. Am J Prev Med. 2005;28:225–228. doi: 10.1016/j.amepre.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Sridhar M, Ross-Plummer R. The prevention of tuberculosis in prison staff. Occup Med (Lond) 2000;50:614–615. doi: 10.1093/occmed/50.8.614. [DOI] [PubMed] [Google Scholar]
- 29.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLOS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 31.Al-Darraji HAA, Kamarulzaman A, Altice FL. Isoniazid preventive therapy in correctional facilities: a systematic review. Int J Tuberc Lung Dis. 2012;16:871–879. doi: 10.5588/ijtld.11.0447. [DOI] [PubMed] [Google Scholar]
- 32.Nolan CM, Roll L, Goldberg SV, Elarth AM. Directly observed isoniazid preventive therapy for released jail inmates. Am J Respir Crit Care Med. 1997;155:583–586. doi: 10.1164/ajrccm.155.2.9032198. [DOI] [PubMed] [Google Scholar]
- 33.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, Altice FL. Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend. 2013;132:378–382. doi: 10.1016/j.drugalcdep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickersham JA, Marcus R, Kamarulzaman A, Zahari MM, Altice FL. Implementing methadone maintenance treatment in prisons in Malaysia. Bull World Health Organ. 2013;91:124–129. doi: 10.2471/BLT.12.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]