Abstract
Objective
We sought to assess the impact of myocardial scar burden (MSB) on the association between implantable cardioverter defibrillator (ICD) implantation and mortality in patients with ischaemic cardiomyopathy (ICM) and left ventricular EF ≤40%. In addition, we sought to determine the impact of gender on survival benefit with ICD implantation.
Design
Retrospective observational study.
Setting
Single US tertiary care centre.
Patients
Consecutive patients with significant ICM who underwent delayed hyperenhancement-MRI between 2002 and 2006.
Interventions
ICD implantation.
Main outcome measures
All-cause mortality and cardiac transplantation.
Results
Follow-up of 450 consecutive patients, over a mean of 5.8 years, identified 186 deaths. Cox proportional hazard modelling was used to evaluate associations among MSB, gender and ICD with respect to all-cause death as the primary endpoint. ICDs were implanted in 163 (36%) patients. On multivariable analysis, Scar% (χ2 28.21, p<0.001), Gender (χ2 12.39, p=0.015) and ICD (χ2 9.57, p=0.022) were independent predictors of mortality after adjusting for multiple parameters. An interaction between MSB×ICD (χ2 9.47, p=0.009) demonstrated significant differential survival with ICD based on MSB severity. Additionally, Scar%×ICD×Gender (χ2 6.18, p=0.048) suggested that men with larger MSB had significant survival benefit with ICD, but men with smaller MSB derived limited benefit with ICD implantation. However, the inverse relationship was found in women.
Conclusions
MSB is a powerful independent predictor of mortality in patients with and without ICD implantation. In addition, MSB may predict gender-based significant differences in survival benefit from ICDs in patients with severe ICM.
Keywords: CORONARY ARTERY DISEASE
Introduction
Implantable cardioverter defibrillators (ICDs) have been shown to reduce mortality in patients with ischaemic cardiomyopathy (ICM) and significant LV systolic dysfunction (LVD).1–3 Currently, echocardiographically-assessed LVEF and New York Heart Association class are the only established criteria for ICD implantation for primary prevention. However, LVEF≤35% has been shown to be a non-specific criterion for ventricular tachycardia/ventricular fibrillation (VT/VF) risk,4 and previous studies have demonstrated that a significant proportion of patients who underwent ICD implantation did not experience life-threatening arrhythmias after up to 5 years of follow-up.5 6 Thus, improved risk stratification is needed to identify optimal candidates for ICD implantation.
Delayed hyperenhancement cardiac magnetic resonance (DHE-CMR) quantified myocardial scar burden (MSB) has been shown to correlate with VT/VF7 and has been shown to be a powerful predictor of mortality in patients with ICM.8 9 Hence, we hypothesised that DHE-CMR may enhance risk stratification in patients with severe ICM being considered for ICD implantation. We sought to determine how scar burden (SB) impacts the association between ICD implantation and mortality.
Methods
We examined 450 consecutive patients with severe ICM (defined as LVEF≤40% with ≥70% stenosis in ≥1 epicardial coronary vessel on angiography and/or history of myocardial infarction or revascularisation) who were referred for DHE-MRI between January 2002 and December 2006. We expanded our previous published studies, which previously included patients who were referred for DHE-MRI between 2003 and 2006.9 10 With the addition of 101 patients and longer follow-up, our expanded study population resulted in 1956 additional patient-years and 135 additional primary outcome events. Telephone call follow-up was also performed to capture patients who underwent ICD implantation outside of our institution. Patients with standard CMR contraindications were not imaged.
Clinical and demographic variables were entered prospectively into electronic medical records. The use of cardiac medications, post-CMR revascularisation (percutaneous or surgical), and placement of ICD or cardiac resynchronisation therapy (CRT) were recorded. Echocardiographic data were obtained within 1 month of the DHE-MRI study. Mitral regurgitation (MR) severity was assessed by echocardiography using the vena contracta method. Telephone follow-up was conducted to capture patients undergoing procedures (revascularisation and ICD implantation) outside of our institution. Cardiac MRI viability assessment was used clinically to decide whether to revascularise patients. In patients who did not undergo revascularisation, cardiac MRI LVEF was clinically used to determine candidacy for ICD implantation.
All-cause mortality, ascertained by social security death index, was used as the primary endpoint. This study was approved by the institutional review board.
CMR protocol
CMR examinations were performed on 1.5-T MR scanners (Sonata and Avanto, Siemens Medical Solutions, Erlangen, Germany), using 40–45 mT/m maximum gradient strength, 200 T/m/s maximum slew rate with electrocardiographic gating. For assessment of global cardiac function, steady-state free precession (SSFP) images were acquired (slice thickness 8–10 mm in contiguous short-axis images). LV volumes and LVEF were calculated on short-axis SSFP images. DHE-CMR images were obtained in long- and short-axis orientations, approximately 15–20 min after injection of 0.2 mmol/kg of Gadolinium dimenglumine, with segmented inversion-recovery (IR) gradient echo sequences (GRE) for studies performed in 2002–2003 and phase-sensitive IR spoiled GRE sequence for studies performed after 2003 (spatial resolution of 1.5–2.1×1.1–1.4 mm).
DHE-CMR analysis
DHE-CMR images were analysed using a custom analysis multi-vendor package (Qi Imaging, Redwood City, California, USA). Endocardial and epicardial myocardial edges were manually delineated on DHE-CMR images. Scar was defined by intensity ≥2 SDs above user-defined viable myocardium. Peri-infarct areas were defined as areas 2–3 SD above the user-defined viable myocardium.11 Areas that were identified as scar by the software but not deemed to be scar by the user were excluded manually by the user. MSB and peri-infarct% were automatically determined as percentage of total myocardium (infarct volume/mass divided by total LV volume/mass). Each study was also semiquantitatively graded using the standard American Heart Association 17-segment, 5-point scale model (0: no DHE; 1: DHE of 1%–25% of LV segment; 2: DHE extending to 26%–50%; 3: DHE extending to 51%–75%; and 4: DHE extending to 76%–100%). CMR analysis was blinded from the clinical analysis.
Statistical analysis
Baseline demographic data, risk factors and clinical variables were descriptively summarised with continuous variables expressed as mean±SD and categorical data presented as percentage frequency. Groups were compared with the Student t test and analysis of variance for continuous variables and the χ2 test for categorical variables. All-cause mortality was the primary endpoint.
Propensity analysis, performed to correct for non-randomised treatment assignment, used logistic regression modelling and included age, gender, risk factors, LVEF/RVEF, LV/RV volumes, SB, revascularisation history, medical therapy, QRS duration, MSB and scar location. Cox proportional hazard (CPH) modelling was used to assess the impact of (1) MSB and (2) patient gender on the association between post-test ICD use and outcomes after adjusting for baseline differences and possible confounders. Covariate selection for model inclusion was based on clinical experience and prior publications. ICD implantation was modelled as a time dependent intervening event and was determined to satisfy the proportional hazard assumption. Survival functions stratified by key CMR parameters were plotted using the Kaplan–Meier method and compared using log-rank tests. Predicted survival was graphically depicting for predefined covariate values of interest while holding the remaining covariates constant at typical values. The added value of preidentified interactions to the model (ICD×Scar%, LV end systolic volume index (ESVI)×Scar%, Gender×Scar%, RV ESVI×MR, and ICD×Scar%×Gender) was examined using the likelihood ratio test. The models were examined, when applicable, for proportional hazards assumption, multicollinearity and the additive value of the terms.
Statistical comparisons were performed with SPSS V.16.0 (SPSS Inc, Chicago, Illinois, USA). S-PLUS 2000 (Release 2) software package (Insightful Corp, Seattle, Washington, USA) with supplemental libraries (Hmisc, Design) was used for multivariable analyses. A p<0.05 was considered significant.
Results
Our population (n=450) was predominantly male (75%), with an age of 62.8±10.7 years. The vast majority of ICDs were implanted for primary prevention, 145/164 (89%). Patients with versus without ICD implantation (table 1) had similar prevalence of risk factors; however, patients who underwent subsequent ICD implantation had larger body surface areas and were more likely to have had previous revascularisations. There was no difference between groups with regard to subsequent revascularisation. Patients with post-test ICD implantation were more likely to have hyperlipidaemia, and taking β-blockers, ACE inhibitors/ARBs, and spironolactone. Of the 163 ICDs implanted, 99 (61.0%) were implanted within 6 months of the index test, 22 (13%) were implanted between 6 months and 1 year, 11 (7%) between 1 and 2 years, and 31 (19%) were implanted beyond 2 years postindex test.
Table 1.
Baseline demographics and clinical/imaging characteristics in patients with and without ICD
| Variables | Total study N=450 |
No ICD N=287 |
ICD N=163 |
p Value |
|---|---|---|---|---|
| Age at MRI | 62.8±10.7 | 63.4±11.2 | 61.8±9.7 | 0.116 |
| Female gender | 111 (25%) | 74 (26%) | 37 (23%) | 0.467 |
| BSA | 1.92±0.24 | 1.91±0.23 | 1.96±0.24 | 0.039 |
| Previous CABG/PCI | 200 (44%) | 123 (43%) | 88 (54%) | 0.028 |
| HTN | 232 (52%) | 140 (49%) | 91 (56%) | 0.172 |
| DM | 168 (37%) | 109 (38%) | 60 (37%) | 0.863 |
| Hyperlipidaemia | 223 (50%) | 129 (45%) | 95 (58%) | 0.008 |
| Number of vessels with severe CAD | 2.27±0.87 | 2.27±0.86 | 2.26±0.91 | 0.948 |
| β-Blocker | 361 (80.2%) | 218 (76%) | 143 (88%) | 0.001 |
| ACE-i/ARB | 355 (78.5%) | 215 (75%) | 140 (86%) | 0.006 |
| Statin | 342 (76%) | 218 (76%) | 135 (83%) | 0.058 |
| Spironolactone | 79 (18%) | 40 (14%) | 37 (23%) | 0.015 |
| Post-MRI CABG/PCI | 265 (59%) | 163 (57%) | 55 (62%) | 0.319 |
| Imaging variables | ||||
| Scar% | 29.56±17.15 | 29.1±17.5 | 30.6±16.6 | 0.380 |
| Peri-infarct% | 5.75±3.90 | 5.65±3.46 | 5.94±4.58 | 0.437 |
| EDVI | 146.8±50.0 | 144.8±49.1 | 150.4±51.4 | 0.247 |
| ESVI | 115.3±49.5 | 111.8±48.2 | 121.5±49.8 | 0.04 |
| LVEF | 23.1±9.0 | 24.5±9.1 | 20.7±8.1 | <0.001 |
| RVEF | 42.6±13.9 | 43.6±13.8 | 49.1±29.9 | 0.024 |
| RV ESVI | 46.2±28.1 | 44.5±26.9 | 49.1±29.9 | 0.97 |
| MR (VC) | 0.33±0.27 | 0.30±0.26 | 0.37±0.27 | 0.016 |
ACE-i/ARB, ACE inhibitor/angiotensin receptor blocker; BSA, body surface area; CABG/PCI , coronary artery bypass grafting/percutaneous coronary intervention; CAD, coronary artery disease; DM, diabetes mellitus; EDVI, end diastolic volume index; ESVI, end systolic volume index; HTN, hypertension; ICD, implantable cardioverter defibrillator; MR, mitral regurgitation; VC, vena contracta.
Imaging findings
Patients had severe LVD, mild RV systolic dysfunction, moderate MR and severely dilated ventricles (table 1). The total scar score was 1.33±0.76; the mean scar% on DHE-MR was 29.6±17.1%.
As expected, patients with ICDs had larger LVs and more severe LVD. Patients with ICDs also had greater MR than patients without ICDs. However, there was no difference between groups with regard to MSB or peri-infarct%. Additionally, patients with ICDs had slightly higher RV EFs, but no significant difference in RV volumes.
Outcome events
Over a mean follow-up of 5.8±2.7 years, there were 186 deaths. A total of 126 deaths occurred in patients without ICD and 60 among patients with ICDs. Although there was a significant difference in medical therapy in patients with and without ICD implantation, medical therapy in our study population was not associated with mortality on unadjusted analysis.
Propensity score
Logistic regression modelling identified ESVI, LVEF, MR, β-blocker use and QRS duration as the significant predictors of ICD implantation (C-index 0.73, p<0.003, χ2 53).
Multivariable survival analysis
CPH identified multiple independent predictors of death. Age and Scar% demonstrated the strongest association (table 2). As previously demonstrated, LVEF was not an independent predictor of mortality in this selected population of patients with advanced ICM.9 12 Several interactions examined emerged as independent predictors including ICD×Scar%, Gender×ICD and Gender×ICD×Scar%.
Table 2.
Baseline demographics and clinical/imaging characteristics in men versus women
| Variables | Men N=339 |
Women N=111 |
p Value |
|---|---|---|---|
| Age at MRI | 62.6±10.6 | 63.2±11.0 | 0.643 |
| BSA | 1.99±0.22 | 1.72±0.18 | <0.001 |
| Previous CABG/PCI | 151 (45%) | 49 (44%) | 0.949 |
| HTN | 172 (51%) | 60 (54%) | 0.545 |
| DM | 125 (37%) | 43 (39%) | 0.725 |
| Hyperlipidaemia | 165 (49%) | 58 (52%) | 0.442 |
| Number of vessels with severe CAD | 2.31±0.86 | 2.13±0.91 | 0.071 |
| β-Blocker | 268 (79%) | 93 (84%) | 0.279 |
| ACE-i/ARB | 266 (79%) | 89 (80%) | 0.702 |
| Statin | 262 (77%) | 80 (72%) | 0.185 |
| Spironolactone | 65 (19%) | 14 (13%) | 0.115 |
| ICD | 126 (37%) | 37 (33%) | 0.467 |
| Post-MRI CABG/PCI | 207 (61%) | 55 (50%) | 0.1 |
| Scar% | 29.4±16.80 | 30.01±18.2 | 0.730 |
| Peri-infarct% | 5.68±3.89 | 5.97±3.93 | 0.503 |
| EDVI | 149.9±46.8 | 137.4±57.7 | 0.022 |
| ESVI | 118.1±46.6 | 106.1±54.8 | 0.022 |
| LVEF by CMR | 22.6±8.7 | 24.8±9.4 | 0.021 |
ACE-i/ARB, ACE inhibitor/angiotensin receptor blocker; BSA, body surface area; CABG/PCI, coronary artery bypass grafting/percutaneous coronary intervention; CAD, coronary artery disease; CMR, cardiac magnetic resonance; DM, diabetes mellitus; EDVI, end diastolic volume index; ESVI, end systolic volume index; HTN, hypertension; ICD, implantable cardioverter defibrillator.
Outcomes and ICD implantation
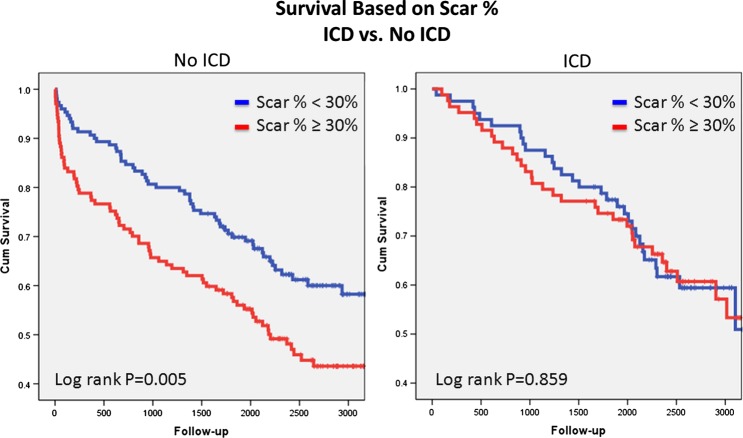
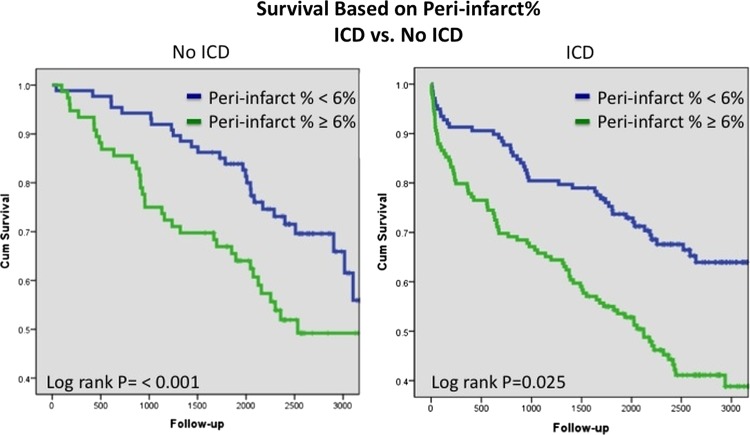
Examination of the unadjusted relationship between MSB and ICD implantation identified increasing risk with increasing MSB in patients without ICD implantation. However, this was not present in patients with ICD implantation (figure 1). ICD implantation was an independent predictor of survival in the CPH model and interacted with SB (p=0.009) (figure 2). Patients without ICD had superior survival compared with patients with ICD in the setting of less scar (≤25%). Equipoise between ICD and no ICD use occurred at ∼30% SB. However, increasing survival benefit was present in patients with versus without ICD with increasing values of scar% (figure 3). On the other hand, peri-infarct% was associated with increased unadjusted risk regardless of the presence of subsequent ICD implantation (figure 4). This relationship persisted after risk-adjustment on multivariate CPH analysis.
Figure 1.
Unadjusted Kaplan–Meier survival analysis comparing survival based on myocardial scar burden in patients with and without implantable cardioverter defibrillator (ICD). Access the article online to view this figure in colour.
Figure 2.
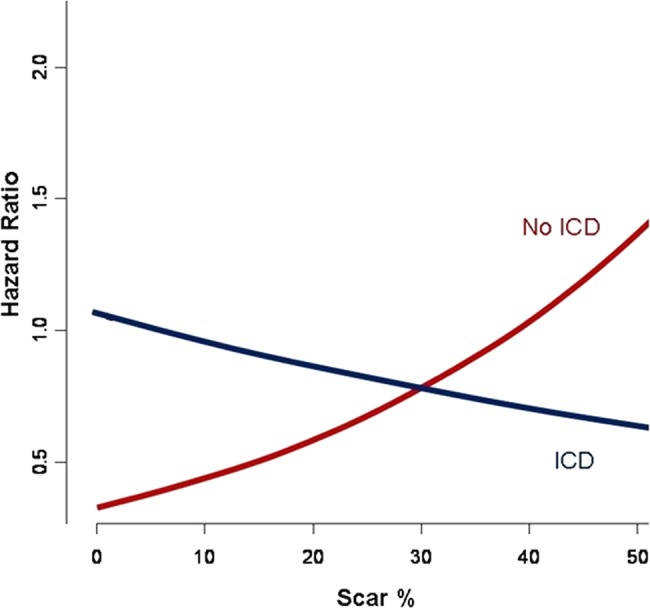
Adjusted hazard analysis based on the Cox proportional hazard model illustrating prediction of risk based on myocardial scar burden and presence of implantable cardioverter defibrillator (ICD). Access the article online to view this figure in colour.
Figure 3.
Unadjusted Kaplan–Meier survival analysis comparing overall survival based on myocardial scar burden and presence of implantable cardioverter defibrillator. Access the article online to view this figure in colour.
Figure 4.
Unadjusted Kaplan–Meier survival analysis comparing survival based on peri-infarct% in patients with and without implantable cardioverter defibrillator (ICD). Access the article online to view this figure in colour.
Impact of gender on outcomes
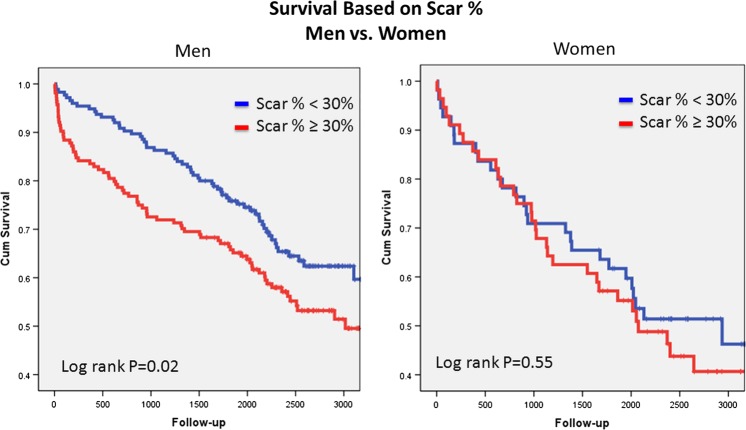
Among the patients without ICDs, 83/215 men (39%) and 43/74 women (58%) died, and 45/126 men (36%) and 15/37 (41%) women with ICDs died. There were no differences between men and women with regard to age, comorbidities, medical treatment, total SB or peri-infarct% (see table 2). Unadjusted analyses revealed that increasing scar% was associated with increasing risk in men. In women, however, increasing MSB was not associated with increased risk (figure 3). After risk-adjusted analysis in the final CPH model, Scar%×ICD×Gender (table 3; figure 5) demonstrated a survival advantage with ICD in women with low and intermediate values of Scar% with equipoise reached at ∼40%–50% Scar%. Alternatively, increasing survival benefit was present in men with ICD with increasing Scar%, while survival was superior in men without ICD with decreasing Scar%. Equipoise between ICD and no ICD use occurred at ∼30% Scar% in men.
Table 3.
Multivariate survival analysis based on CPH for prediction of all-cause mortality*
| Variables | χ2 score | HR (95% CI) | p Value |
|---|---|---|---|
| Age | 39.12 | 1.71 (1.46 to 2.01) | <0.001 |
| Scar% | 28.21 | 1.34 (1.15 to 1.55) | <0.001 |
| ESVI | 6.54 | 0.95 (0.91 to 0.99) | 0.038 |
| ESVI×Scar% | 6.44 | 0.011 | |
| Female gender | 12.39 | 2.09 (1.27 to 3.46) | 0.015 |
| Female gender×ICD | 6.20 | 0.045 | |
| Female gender×ICD×Scar% | 6.18 | 0.013 | |
| RV ESVI | 14.94 | 1.12 (1.05 to 1.20) | <0.001 |
| MR (VC) | 14.24 | 1.14 (1.06 to 1.22) | <0.001 |
| Peri-infarct% | 8.52 | 1.05 (1.02 to 1.09) | 0.004 |
| MR×RV ESVI | 8.12 | 0.004 | |
| ICD | 9.57 | 0.048 | |
| ICD×Scar% | 9.47 | 0.008 |
*Stratified to the presence of post-MRI revascularisation.
CPH, Cox proportional hazard; ESVI, end systolic volume index; ICD, implantable cardioverter defibrillator; VC, vena contracta.
Figure 5.
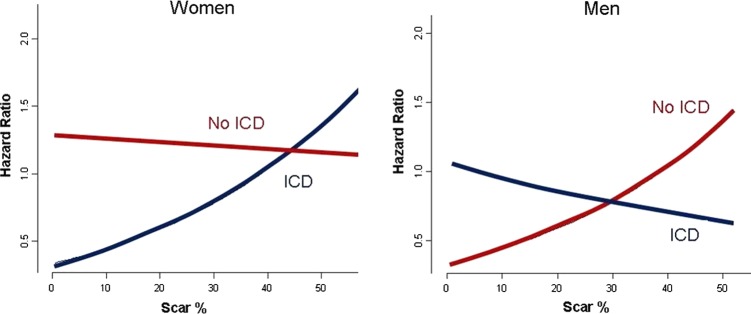
Adjusted hazard analysis based on the Cox proportional hazard model illustrating prediction of risk based on myocardial scar burden and presence of implantable cardioverter defibrillator (ICD). Access the article online to view this figure in colour.
Discussion
Our results demonstrate that infarct size, gender and ICD implantation are significant independent predictors of mortality. Although MSB is a powerful predictor of mortality in our overall study population without subsequent ICD implantation, the risk associated with myocardial scarring appeared to be mitigated in patients with subsequent ICD implantation on unadjusted analysis. While increasing MSB was associated with increasing risk in men on unadjusted analysis, this association was not present in women. After adjustment for potential confounders with CPH modelling, significant interactions among MSB, gender and subsequent ICD implantation emerged. In our total study population with severe LVD due to ICM, patients with MSB≥30% derived the most mortality benefit from ICD implantation, and had significantly improved survival compared with those with no ICD implantation in our study. However, ICD implantation did not appear to result in significant risk reduction in patients with SB <30%.
Impact of scar and ICD implantation
Isolated viable myocytes intertwined with fibrous scar tissue can create re-entry circuits, thus resulting in malignant ventricular arrhythmias. This concept was evaluated using DHE-MRI by Bello et al.7 In this study, the extent of MSB was a more powerful predictor of inducible monomorphic VT on electrophysiological testing than LVEF in patients with coronary artery disease (CAD). Yan et al11 also demonstrated that assessment of peri-infarct area was a powerful predictor of mortality in patients with CAD. Therefore, there is compelling evidence that infarct size and infarct heterogeneity, identified by DHE-CMR, may offer significant additional risk stratification over the traditionally accepted criteria for ICD implantation.
The current approach to identifying which patients may benefit from ICD implantation has focused on LVEF by echocardiography. However, LVEF by echocardiography has been shown to be a relatively insensitive measure of risk, as most patients suffering sudden cardiac death have a preserved LVEF, and many patients with poor LVEF do not benefit from ICD prophylaxis.4 13 Gao et al14 recently demonstrated that MSB, assessed by DHE-MRI, predicts arrhythmic events in patients being evaluated for ICD implantation. Among the 124 patients who were studied, 59 (48%) patients had severe ICM, and all patients with ICM had significant myocardial scarring. MSB was significantly higher in those who experience a primary outcome versus those without (23% vs 35%, p=0.001). In addition, Klem et al15 also demonstrated the powerful prognostic ability of MSB to predict adverse outcomes, and suggested that quantification of MSB was superior to LVEF≤30% in the identification of high-risk patients. In their study of 137 patients (53% with ICM), 25/65 patients with LVEF≤30% died or had an appropriate ICD discharge during follow-up. Patients with MSB≤5% had an event rate that was below or similar to that of the entire group with low-risk LVEF>30%. However, it is important to note that both of these studies included mixed populations of non-ICM (NICM) and ICM patients. Additionally, the limited number of appropriate shocks and deaths in these studies resulted in limited ability to conduct robust multivariate analysis.
Our current study demonstrates the significant independent predictive power of DHE-MRI in predicting differential survival in patients with severe ICM undergoing subsequent ICD implantation, using a primary endpoint of all-cause mortality. The mortality rate was 41% (186 deaths) over a mean follow-up of 5.8 years, reflecting the high-risk nature of our patient population, and allowing sufficient endpoints for robust multivariable analysis. The results of our study demonstrate that total MSB provides further risk stratification in patients with severe ICM, and may identify which patients will derive the most survival benefit with ICD implantation. Risk associated with increased total SB appeared to be neutralised after ICD implantation. On the other hand, risk associated with peri-infarct% was not mitigated by ICD implantation in our study population. Because our patients in our study did not consistently return to our institution for device interrogation at regular intervals, accurate shock therapy was not available. However, we speculate that patients with significantly increased per-infarct% may have experienced significantly increased number of appropriate ICD discharges. Because recent studies have demonstrated that appropriate defibrillation can result in myocardial damage and increased mortality,16–19 further studies are needed to evaluate the relationship and interaction among total SB, peri-infarct%, increased appropriate shock therapy and survival.
Interaction of scar, gender and ICD implantation
Scar%×ICD×Gender emerged as a significant independent predictor of mortality. Men appeared to have significant survival benefit with ICD implantation with increased MSB, compared with men with smaller infarct size. On the other hand, women in our study derived the most benefit with ICD implantation in the setting of smaller MSB, unlike their male counterparts. Although increasing risk reduction with ICD versus medical therapy was seen in men with MSB>30%, risk reduction was greatest in women with MSB<40%–50%. Women with large MSB did not appear to experience improved outcomes with ICD implantation. Our study results may reflect that higher MSB in women leads to progressive heart failure, while increased scar in men likely results in increased arrhythmogenic substrate. In addition, our study suggests that smaller MSB is more arrhythmogenic in women versus men. The mechanism of this finding may be due to differential susceptibility to arrhythmia based on gender differences in repolarisation, potassium-channel kinetics, calcium-channel density and sensitivity, autonomic modulation, and sodium–calcium exchanger.20–27 Future studies are needed to explore potential mechanisms of our study's finding.
Although several multi-centre randomised controlled trials have demonstrated the survival benefit associated with ICD implantation in patients with ICM and severe LVD,1–3 several meta-analyses and substudies have suggested that women do not derive the same benefit that men experience.28–31 However, previous meta-analysis have all included women with mostly NICM. In the SCD-HeFT trial,1 there was no significant survival benefit with ICD therapy in patients with NICM, which may partially account for the limited benefit seen in women, given the higher prevalence of NICM as the aetiology of LVD in the women. The pattern of myocardial scarring in NICM and ICM is considerably different, which likely represents differences in arrhythmogenic substrate and susceptibility to malignant arrhythmias.
Previous studies have suggested that women may be less prone to experience VT and/or sudden cardiac death (SCD) after myocardial infarction, compared with men.32 33 These findings suggest that the mode of death in women versus men may be progressive congestive heart failure rather that malignant arrhythmias. In a meta-analysis of five large myocardial infarction trials, Yap et al demonstrated that all-cause mortality was high and remained high for 2 years following myocardial infarction in the patients in the placebo arm. The rate of SCD was the major mode of death for 2 years following the index myocardial infarction in men. On the other hand, women were more likely to die of SCD for only 6 months following their index myocardial infarction.34 Furthermore, women have been previously shown to experience a greater frequency of adverse advents (worsening heart failure, chest pain, VT, supra-VT, dyspnoea, haematoma and lead migration/dislodgement) compared with men.35 Therefore, there is need for more precise gender-specific risk stratification to determine which patients will derive the most survival benefit from ICD.
In our study population with significant LV dysfunction purely due to ICM, female gender was independently associated with increased mortality compared with men with severe ICM. Interestingly, there were significant gender-based differences in risk based on MSB in our study population. Although this is the largest study of patients with advanced ICM undergoing viability assessment with cardiac MRI prior to device therapy, larger studies with a higher proportion of women are needed to determine the validity of the findings from our study.
Clinical relevance
Although LVEF is accepted as the most powerful predictor of SCD among traditional clinical parameters, DHE-MRI can identify myocardial scar, the substrate for the development of potentially fatal ventricular arrhythmias. While ICDs have demonstrated significant mortality reduction in patients at risk for VT/VF, inappropriate ICD discharges are not uncommon, and may result in increased mortality.18 Furthermore, up to 47% of patients with ICD implantation may not experience life-threatening arrhythmias after 5 years of follow-up.6 Therefore, more selective criteria are needed to identify which patients will derive the most survival benefit with ICD implantation.
Our data demonstrate that quantification of MSB offers further risk stratification in patients with advanced LV dysfunction due to ICM, and can identify the patients who would benefit from ICD implantation. Patients with severe LV dysfunction, but minimal MSB, did not experience decreased mortality with ICD insertion; conversely, patients with severe LV dysfunction and large MSB derived the most survival benefit after ICD implantation. In addition, our results also suggest that women with significant MSB might not benefit from ICD therapy, suggesting that their mode of death is more likely to be from heart failure (HF), rather than VT/VF. Future randomised controlled trials should be conducted to determine if DHE-MRI, in association with traditional clinical risk factors, can improve the accuracy of the selection criteria to identify patients who would significantly benefit from ICD implantation.
Limitations
Our patient cohort represents the patient population seen at a tertiary referral centre; therefore, selection biases and missing/unmeasured variables may impact the findings in this study. Furthermore, patients with prior CRT±ICD were excluded from this study due to contraindications for MRI. Of the 163 patients who underwent ICD implantation, 31 patients had ICDs implanted more than 2 years after scar assessment with DHE-MRI. Interceding events resulting in increased SB may have occurred which were not captured by electronic chart review. Additionally, because multivariable modelling of our observational data was conducted to assess the impact of MSB on survival benefit with ICD implantation, our findings are limited. Future randomised controlled trials using DHE-MRI to guide ICD implantation should be conducted to confirm our findings.
CMR findings were used to guide revascularisation therapy and may have also impacted the referral for ICD implantation. Although we used propensity scoring to assess for significant associations with post-ICD treatment, propensity methods can only account for variables that are measured. Patients who were not referred for ICD may have been at higher risk in ways that were not measured. Furthermore, comparisons of post-CMR survival by treatment are based on retrospective categorisation of patients, which has limitations. Because we capture actual treatment received by the patient from retrospective review, but not any planned treatments that were not carried out due to intercedent adverse events, misclassification bias of events may have occurred. However, recent analysis from a multi-centre registry demonstrated that there were no identified factors associated with ICD referral.36
The number of women (n=111) in our study population is relatively limited; however, this is currently the largest cohort of women undergoing MRI and subsequent ICD implantation for severe ICM in the literature. Due to the observational nature of this study, our findings are hypothesis generating, and future prospective studies should be conducted to confirm our findings.
Conclusions
Total MSB predicts survival in patients with and without ICD implantation. In addition, MSB predicts gender-based significant differences in mortality benefit with ICD in patients with severe ICM. Future randomised control trials should be conducted to determine the ability of DHE-MRI to guide selection of patients for ICD therapy.
Key messages.
What is already known on this subject?
Although LVEF is accepted as the most powerful predictor of SCD among traditional clinical parameters, delayed hyperenhancement-MRI can identify myocardial scar, the substrate for the development of potentially fatal ventricular arrhythmias. While implantable cardioverter defibrillators (ICDs) have demonstrated significant mortality reduction in patients at risk for ventricular tachycardia/ventricular fibrillation, inappropriate ICD discharges are not uncommon, and may result in increased mortality. Furthermore, up to 47% of patients with ICD implantation may not experience life-threatening arrhythmias after 5 years of follow-up. Therefore, more selective criteria are needed to identify which patients will derive the most survival benefit with ICD implantation.
How might this impact on clinical practice?
Improved risk stratification in identifying which patients will derive the most survival benefit with implantable cardioverter defibrillator implantation.
What this study adds?
Our data demonstrate that quantification of myocardial scar burden (MSB) offers further risk stratification in patients with advanced LV dysfunction due to ischaemic cardiomyopathy, and can identify the patients who would benefit from implantable cardioverter defibrillator (ICD) implantation. Patients with severe LV dysfunction, but minimal MSB, did not experience decreased mortality with ICD insertion; conversely, patients with severe LV dysfunction and large MSB derived the most survival benefit after ICD implantation. In addition, our results also suggest that women with significant MSB might not benefit from ICD therapy, suggesting that their mode of death is more likely to be from HF, rather than ventricular tachycardia/ventricular fibrillation. Future randomised controlled trials should be conducted to determine if delayed hyperenhancement-MRI, in association with traditional clinical risk factors, can improve the accuracy of the selection criteria to identify patients who would significantly benefit from ICD implantation.
Footnotes
Contributors: RH and TM contributed significantly to the conception and design of the analysis, interpretation of the data and revision of the manuscript for important intellectual content. RH, BN and ZBP significantly contributed to the multivariable Cox proportional hazard modelling. AA contributed to the acquisition of demographic variables and conducted the telephone call follow-up. MYD contributed to the initial study design. BLW implanted a significant number of the ICDs and contributed to the study design and analysis. SDF contributed to the optimisation of the delayed hyperenhancement-MRI (DHE-MRI) technique and analysis as well as revision of the manuscript for important intellectual content. As the corresponding author, DHK conceived the study hypothesis, constructed the study design, individually analysed all 450 DHE-MRI studies, performed statistical analysis and drafted the manuscript.
Competing interests: None.
Ethics approval: IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37 [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999;341:1882–90 [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83 [DOI] [PubMed] [Google Scholar]
- 4.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–6 [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004;110:3760–5 [DOI] [PubMed] [Google Scholar]
- 6.Reichlin T, Kuhne M, Sticherling C, et al. Characterization and financial impact of implantable cardioverter-defibrillator patients without interventions 5 years after implantation. QJM 2011;104:849–57 [DOI] [PubMed] [Google Scholar]
- 7.Bello D, Fieno DS, Kim RJ, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol 2005;45:1104–8 [DOI] [PubMed] [Google Scholar]
- 8.Cheong BY, Muthupillai R, Wilson JM, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation 2009;120:2069–76 [DOI] [PubMed] [Google Scholar]
- 9.Kwon DH, Halley CM, Carrigan TP, et al. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging 2009;2:34–44 [DOI] [PubMed] [Google Scholar]
- 10.Kwon DH, Halley CM, Popovic ZB, et al. Gender differences in survival in patients with severe left ventricular dysfunction despite similar extent of myocardial scar measured on cardiac magnetic resonance. Eur J Heart Fail 2009;11:937–44 [DOI] [PubMed] [Google Scholar]
- 11.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 2006;114:32–9 [DOI] [PubMed] [Google Scholar]
- 12.Kwon DH, Hachamovitch R, Popovic ZB, et al. Survival in patients with severe ischemic cardiomyopathy undergoing revascularization versus medical therapy: association with end-systolic volume and viability. Circulation 2012;126 (11 Suppl 1):S3–8 [DOI] [PubMed] [Google Scholar]
- 13.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, et al. Out-of-hospital cardiac arrest—the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J 2003;24:1204–9 [DOI] [PubMed] [Google Scholar]
- 14.Gao P, Yee R, Gula L, et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging 2012;5:448–56 [DOI] [PubMed] [Google Scholar]
- 15.Klem I, Weinsaft JW, Bahnson TD, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol 2012;60:408–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012. 367:2275–83 [DOI] [PubMed] [Google Scholar]
- 17.Pacifico A, Hohnloser SH, Williams JH, et al. Prevention of implantable-defibrillator shocks by treatment with sotalol. d,l-Sotalol Implantable Cardioverter-Defibrillator Study Group. N Engl J Med 1999;340:1855–62 [DOI] [PubMed] [Google Scholar]
- 18.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J, Weil MH, Sun S, et al. High-energy defibrillation increases the severity of postresuscitation myocardial dysfunction. Circulation 1997;96:683–8 [DOI] [PubMed] [Google Scholar]
- 20.Haigney MC, Zareba W, Nasir JM, et al. Gender differences and risk of ventricular tachycardia or ventricular fibrillation. Heart Rhythm 2009;6:180–6 [DOI] [PubMed] [Google Scholar]
- 21.Liu XK, Katchman A, Drici MD, et al. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther 1998;285:672–9 [PubMed] [Google Scholar]
- 22.Dash R, Frank KF, Carr AN, et al. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol 2001;33:1345–53 [DOI] [PubMed] [Google Scholar]
- 23.Vizgirda VM, Wahler GM, Sondgeroth KL, et al. Mechanisms of sex differences in rat cardiac myocyte response to beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol 2002;282:H256–63 [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa M, Ooie T, Ou B, et al. Gender differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol 2005;16:278–84 [DOI] [PubMed] [Google Scholar]
- 25.Wei SK, McCurley JM, Hanlon SU, et al. Gender differences in Na/Ca exchanger current and beta-adrenergic responsiveness in heart failure in pig myocytes. Ann N Y Acad Sci 2007;1099:183–9 [DOI] [PubMed] [Google Scholar]
- 26.Hara M, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on ventricular repolarization and on the response to E4031. J Pharmacol Exp Ther 1998;285:1068–72 [PubMed] [Google Scholar]
- 27.Song M, Helguera G, Eghbali M, et al. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem 2001;276:31883–90 [DOI] [PubMed] [Google Scholar]
- 28.Ghanbari H, Dalloul G, Hasan R, et al. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:1500–6 [DOI] [PubMed] [Google Scholar]
- 29.Russo AM, Poole JE, Mark DB, et al. Primary prevention with defibrillator therapy in women: results from the Sudden Cardiac Death in Heart Failure Trial. J Cardiovasc Electrophysiol 2008;19:720–4 [DOI] [PubMed] [Google Scholar]
- 30.Russo AM, Stamato NJ, Lehmann MH, et al. Influence of gender on arrhythmia characteristics and outcome in the Multicenter UnSustained Tachycardia Trial. J Cardiovasc Electrophysiol 2004;15:993–8 [DOI] [PubMed] [Google Scholar]
- 31.Santangeli P, Pelargonio G, Dello Russo A, et al. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm 2010;7:876–82 [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB, Wilson PW, D'Agostino RB, et al. Sudden coronary death in women. Am Heart J 1998;136:205–12 [DOI] [PubMed] [Google Scholar]
- 33.Lampert R, McPherson CA, Clancy JF, et al. Gender differences in ventricular arrhythmia recurrence in patients with coronary artery disease and implantable cardioverter-defibrillators. J Am Coll Cardiol 2004;43:2293–9 [DOI] [PubMed] [Google Scholar]
- 34.Yap YG, Duong T, Bland M, et al. Temporal trends on the risk of arrhythmic vs. non-arrhythmic deaths in high-risk patients after myocardial infarction: a combined analysis from multicentre trials. Eur Heart J 2005;26:1385–93 [DOI] [PubMed] [Google Scholar]
- 35.Russo AM, Day JD, Stolen K, et al. Implantable cardioverter defibrillators: do women fare worse than men? Gender comparison in the INTRINSIC RV trial. J Cardiovasc Electrophysiol 2009;20:973–8 [DOI] [PubMed] [Google Scholar]
- 36.Hachamovitch R, Nutter B, Cerqueira MD; AdreView Myocardial Imaging for Risk Evaluation in Heart Failure investigators Predictors and frequency of ICD implantation for primary prevention in ICD eligible patients: results from a prospective multicenter registry. Circ Cardiovasc Qual Outcomes 2012;5:A107 [Google Scholar]