Abstract
Cord blood transplantation is being used with increasing frequency for patients with high-risk hematologic malignancies. Myeloablative preparative regimens provide anti-tumor efficacy and facilitate engraftment but are associated with higher morbidity and non-relapse mortality than nonablative regimens. We evaluated three sequential myeloablative regimens in the cord blood transplant setting. Regimen-1 melphalan, fludarabine and thiotepa produced prompt engraftment and minimal engraftment failure but was associated with a high non-relapse mortality. Regimen-2 busulfan and fludarabine was very well tolerated but was associated with a high rate of engraftment failure and relapse. Regimen-3 busulfan, clofarabine, fludarabine and low-dose total body irradiation given 9 days after the chemotherapy was associated with a low rate of engraftment failure but was logistically difficult to administer. Finally, regimen-3 with the total body irradiation given immediately following the chemotherapy was well tolerated, with prompt engraftment and tumor control. This latter regimen appears to be effective in preliminary studies and warrants further evaluation.
Keywords: myeloablative conditioning regimen, cord blood transplant, treatment related mortality, stem cell transplant
Introduction
Despite several unique advantages,1 cord blood transplant (CBT) is associated with a higher risk of graft rejection and delayed immune reconstitution compared to peripheral blood (PB) or bone marrow (BM) transplants.2–4 The choice of a conditioning regimen prior to the hematopoietic stem cell transplant (HSCT) has significant impact on these outcomes. In addition to exerting cytotoxic effects on any residual malignant cells, a conditioning regimen also “creates space” for the donor stem cells in the recipient bone marrow to allow engraftment and provides immunosuppression to host T-cells thereby preventing graft rejection.5 Several types of myeloablative conditioning (MAC) regimens have been used in the CBT setting, with varying outcomes.6,7 Although high-dose total body irradiation (TBI)-based preparative regimens have potent anti-tumor activity, they are typically restricted primarily to patients 45 years old or younger.8 A MAC regimen, in general, is associated with less risk of relapse or graft rejection but higher treatment related morbidity and non-relapse mortality (NRM) when compared to non-myeloablative regimens, particularly for adult patients.5,8,9 The latter fact was later challenged by introduction of the reduced-toxicity regimens based on nucleoside analog(s)-intravenous (i.v.) busulfan combinations, that yield safe and efficacious myelo- and immunoablation without increasing the rate of NRM beyond what is commonly seen with reduced-intensity regimens10. This paper briefly summarizes our experience of developing a safe and effective MAC regimen at MD Anderson Cancer Center for CBT.
Materials and Methods
We evaluated three MAC regimens for patients up to age 65 receiving CBTs for high-risk hematologic malignancies. The first regimen included melphalan (140 mg/m2 on day -8), thiotepa (10mg/kg on day -7) and fludarabine (40mg/m2 on days-6 through days-3) (Mel-Flu-Thio). As discussed in the results section, this regimen was associated with a high NRM rate, stimulating us to search for a less toxic alternative regimen.
Our long-term success with prompt engraftment and acceptable toxicity using intravenous busulfan and fludarabine in the related and unrelated allogeneic donor settings11 led us to explore the Bu-Flu (BF) regimen-2 in CBT patients. A test dose of busulfan 32mg/m2 i.v. was given on day-8. Further doses of busulfan were pharmacokinetically adjusted to achieve an AUC (average daily systemic exposure, represented by the area-under-the concentration vs. time curve, AUC) of 5,500–6,000 μMol-min, and each dose was administered immediately following the fludarabine doses (40 mg/m2) on day -6 through day -3.
A high rate of graft failure prompted the development of the third regimen, which included busulfan (average daily AUC of 5,000 μMol-min) administered immediately following each dose of fludarabine (10 mg/m2) and clofarabine (30 mg/m2) for four days from day -13 through day -10 with TBI 2Gy given on day -1 (Bu-Flu-Clo-delayed TBI). This approach resulted in significantly longer time to engraftment than in previous studies. Further, there was a higher than expected rate of grade 3 mucositis, which together with logistical difficulties of administering the TBI following a long delay after chemotherapy prompted us to move TBI up to immediately following the last dose of the conditioning chemotherapy. Therefore, busulfan (average daily AUC 5,000 μMol-min), fludarabine (10 mg/m2) and clofarabine (30 mg/m2) were administered from day-7 through day-4 with 2 Gy TBI 2Gy given on day-3 (Bu-Flu-Clo-early TBI).
All patients treated with these regimens received rabbit thymoglobulin (ATG) 1.25 mg/Kg on day -4 and 1.75 mg/Kg on day -3. Tacrolimus and mini-methotrexate (5 mg/m2 days 1, 3, 6 and 11) (23%) or mycophenolate mofetil (MMF) 1 gram orally twice daily (77%) were administered for graft-versus-host disease (GVHD) prophylaxis, tapering the MMF at day 100 and tacrolimus at 6 months if no GVHD was present. The vast majority of patients (91%) received double CB units and had one of those units expanded ex-vivo prior to infusion with either a liquid culture system (41%)12 or in mesenchymal stem cells (MSC) co-cultures (33%) as previously described.13
Results
As summarized in Table 1, the median age of the entire cohort was 33years (range 1–64). The patients had high-risk hematologic malignancies, such as acute myeloid leukemia (AML) (41%), acute lymphoblastic leukemia (ALL) (23%), lymphoma (17%) and myelodysplastic syndrome (MDS) (9.6%).
Table 1.
Patients characteristics and clinical outcomes
| Regimen 1 Mel/Flu/Thio |
Regimen 2 Bu/Flu |
Regimen 3 Bu/Flu/Clo + TBI |
p-value | |
|---|---|---|---|---|
|
| ||||
| (n=107) | (n=24) | (n=17) | ||
| Age (years) | 0.62* | |||
| Median (Range) | 34 (1–64) | 26 (10–56) | 33 (7–54) | |
| Disease (%) | 0.001** | |||
| AML (secondary) | 35.5 (2.8) | 58.4 (4.2) | 46.9 (5.9) | |
| ALL | 29.9 | 8.3 | ||
| MDS | 7.5 | 16.7 | 11.8 | |
| Lymphoma | 19.6 | 4.2 | 29.4 | |
| CML/CLL | 2.8/2.8 | 20.8 | 0 | |
| Others | 1.9 | 0 | 0 | |
| Prior Response (%) | 0.015** | |||
| CR | 56.1 | 37.5 | 52.9 | |
| PR/SD | 5.6/6.5 | 0/4.2 | 0/5.9 | |
| NR/PD | 11.2/9.3 | 37.5/0 | 23.5/0 | |
| MRD | 0.9 | 4.2 | 11.8 | |
| CCyR/MMR | 2.8 | 12.5 | ||
| Untreated | 7.5 | 8.3 | ||
| Engrafted (%) | <0.0001** | |||
| Yes | 91.6 | 62.5 | 100 | |
| No | 0.0 | 37.5 | 0 | |
| Early Death | 8.4 | 0.0 | 0 | |
| Time to engraftment (days) | 0.36* | |||
| Median (Range) | 19 (6– 46) | 22 (12– 68) | 20 (13– 31) | |
| Acute GVHD (%) | 0.538** | |||
| Yes | 49.5 | 50 | 64.7 | |
| No | 50.4 | 50 | 35.3 | |
| Acute GVHD grade (%) | 0.933** | |||
| Grade I | 9.3 | 8.3 | 11.8 | |
| Grade II | 25.2 | 29.1 | 17.7 | |
| Grade III/IV | 13.1 | 12.5 | 5.9 | |
| Chronic GVHD (%) | 0.425** | |||
| None | 34.7 | 33.3 | 88.2 | |
| Limited/Extensive | 13.1/26.2 | 8.3/8.3 | 0/11.8 | |
| NRM Day 100 and One-year (%) | 21.5/49 | 12.5/33 | 5.9/6.25 | 0.239/0.001** |
| One-year Survival (%) | 45.8 | 29.1 | 88.2 | 0.001** |
| OS (%) | 29.9 | 8.3 | 88.2 | <0.0001** |
| Overall NRM (%) | 51.4 | 33.3 | 5.8 | 0.001** |
K-sample equality of median test
Fischer-exact/ Chi-square test
Absolute Neutrophil Count ≥ 0.5 x 109/L for three days
Abbreviations: Mel=melphalan; Flu=fludarabine; Thio=thiotepa; Bu=busulfan; Clo=clofarabine; TBI=total body irradiation; AML=acute myeloid leukemia (s=secondary); ALL=acute lymphoblastic leukemia; MDS=myelodysplastic syndrome; CML=chronic myeloid leukemia; CLL=chronic lymphocytic leukemia; CR=complete remission; NR=non response; PR=partial response; SD=stable disease; PD=progressive disease; MRD=molecular residual disease; CCyR=complete cytogenetic response; MMR=major molecular response; NRM=non relapse mortality; NE= Not Evaluable; OS=overall survival. (Patients follow-up updated at the time of writing the manuscript, 5.21.2013)
For patients treated with the regimen-1, the median time to neutrophil engraftment was 19 (range 6–46) days and 92% successfully engrafted (absolute neutrophil count >0.5 x 109/L for three days). The day 100 NRM rate was higher than expected at 21%, with one-year survival of 46%. The NRM included a high-rate of delayed multi-organ failure involving diffuse alveolar hemorrhage and renal failure, attributed in part to the unpredictable and highly variable pharmacokinetic profiles of thiotepa and melphalan. Despite reducing the dose of thiotepa from 10 mg/kg to 5 mg/kg, excessive regimen- related toxicities persisted and stimulated our interest in developing a less toxic conditioning regimen.
The regimen-2 was better tolerated than Mel-Flu-Thio. The group receiving the therapy had a 12% NRM rate at day 100, slightly higher than the typical 2–4% that we see in the standard allograft setting with matched related or unrelated donors using the Bu-Flu based regimens 11,14,15. Somewhat surprisingly, however, 38% of the patients failed to engraft and nearly 64% of deaths were due to primary disease recurrence. We therefore decided to explore another ablative regimen that would provide cytoreductive efficacy and optimize engraftment.
We investigated regimen-3 based on our busulfan, fludarabine, clofarabine regimen for related and unrelated adult donors15,16 with the addition of 2 Gy TBI to quickly necrose host T-cells and optimize the chance for early engraftment. With the original version of regimen -3, the day 100 NRM was only 8% and one-year overall survival was 83%. However, the delayed administration of TBI led to a perceived aggravation of mucositis (including grade 2–3 in two-thirds of the patients). This was the major observed toxicity in 10/12 (83%) patients. All recipients in this cohort received MSC-expanded CB cells, and experienced relatively delayed neutrophil engraftment at a median of 23 (range 13–31) days, whereas our historical controls engrafted in 15 days.13 We hypothesized that the delayed TBI was responsible for the increased mucositis and delayed engraftment. Additionally, the logistics of a nine day interval between the chemotherapy and the TBI made this regimen cumbersome to deliver. Thus, we revised regimen-3 and administered the TBI immediately following the chemotherapy as discussed above. To date, five patients with hematologic malignancies and a median age of 33 years (range 29–46) have received this modified regimen. Collectively for patients treated with both variants of regimen-3, the NRM at day 100 is less than 6%, and their overall survival close to 90%. (Figure 1) Eleven of the twelve patients (92%) who received the original version engrafted, while all 5 patients receiving the latest modified regimen-3 have engrafted at a median time of 17 days (range 13–19). All 5 patients receiving the modified regimen tolerated the therapy well with only one grade 2 and one grade 3 mucositis as the major complication and without any other serious adverse events.
Figure 1.
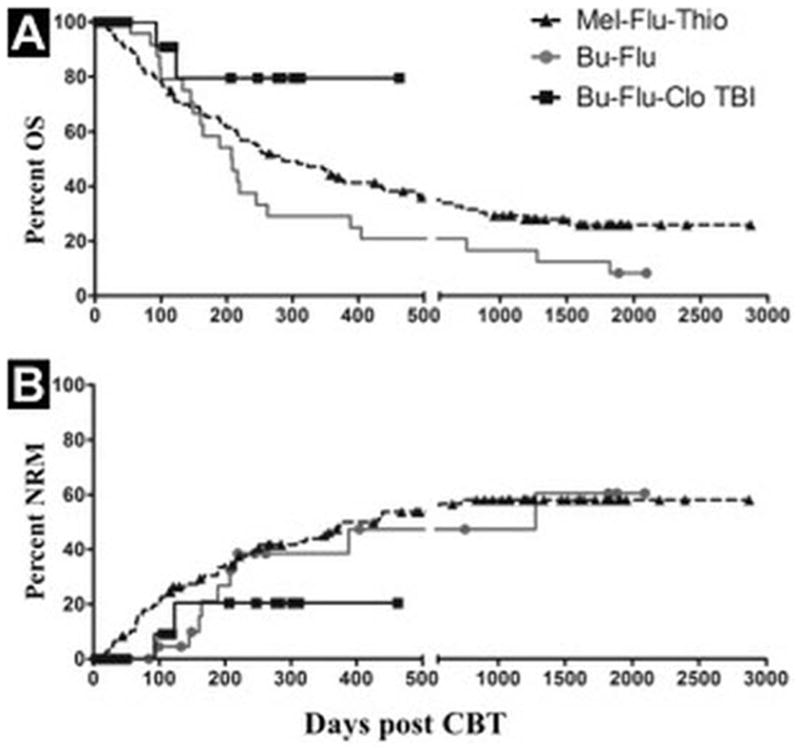
(a–b): Kaplan-Meyer estimates of overall survival (OS) (a) and non-relapse mortality (NRM) (b) for the indicated cohort. Symbols indicate censoring (death or progression).
Discussion
We initially used regimen-1 (Mel-Flu-Thio) which, despite a good engraftment rate, yielded a high rate of NRM, likely attributed in part to the unpredictable pharmacokinetic behavior of thiotepa and melphalan. This safety problem was unfortunately not improved by reducing the thiotepa dose by 50%. Additionally, an inconsistent supply of thiotepa in the United States contributes to a lack of enthusiasm for this regimen. Further, although regimen-2 Bu-Flu was very well tolerated, an unacceptably high fraction of patients failed to engraft. This was probably due to the fact that both agents act through induction of apoptosis, which is a comparatively slow process after the conditioning program. This is an advantage, since this provides protection against infection, but only if the graft source contains a large dose of progenitor- and ancillary cells, that will overcome the rejection mediated by host T-cells. However, it is a major disadvantage in a situation of a low-cell dose graft (CBT), when there is need for rapid removal of the majority of the host’s T-cells prior to graft infusion to secure engraftment. We suggest that long-term stable engraftment is maintained by the alkylating-agent-mediated elimination of host stem cells, which prevents regeneration of allo-reactive T-cells. Engraftment problems that were similar to our experience with regimen-2 were also reported by Horwitz and colleagues17 with a variant busulfan-fludarabine combination. Based on available information from both our own experience and that of others,7,18–20 we now suggest that the early “immunoablative part” of conditioning (i.e. host T-cell elimination) should be addressed as a separate issue in the conditioning program. Several strategies can be contemplated to accomplish this, such as including (low-dose) TBI21,22, or an agent such as Thiotepa that eliminates lymphocytes rapidly, (partly) through necrosis rather than apoptosis.23 Alternatively, some European investigators opted for a more selective T-cell ablation, using rabbit anti-thymocyte globulin (ATG, 1.5 mg/Kg daily on days -12 to -9) prior to starting the busulfan-nucleoside analog-based conditioning chemotherapy. This has met with prompt engraftment when using single cord transplants in mostly pediatric patients (Boehlens JJ, personal communication, 2012). We elected to utilize 2Gy TBI to necrose the host T-cells in our regimen-3 and observed a consistent rapid ablation of total white blood cell counts (WBC) and lymphocytes on the day after TBI administration. Therefore, we hypothesized that leukodepletion, as a surrogate marker for T-cell elimination, could potentially explain the improved engraftment with regimen-3. Indeed, the analysis of total WBC data from day -10 to day10 post-transplant for all the regimens showed that leukodepletion was more rapid and profound with regimen-3 Bu-Flu-Clo-TBI, as compared to regimen-2 Bu-Flu (Figure 2). In the regimen-1 Mel-Flu-Thio, thiotepa also contributed to a quicker onset of leukodepletion; however, with this regimen the toxicity profile outweighed the potential benefits as discussed above. Finally, we postulated that this early added TBI-dose would be associated with consistent CB engraftment, while retaining the undiminished cytoreductive capability and high level of safety for the regimen-3. In its first permutation we delayed the TBI to allow for chemotherapy “wash-out” (Bu-Fu-Clo-delayed TBI), which resulted in an acceptable engraftment rate. However, when compared with our previous program the time to engraftment was long and the incidence of serious mucositis (grades 2–3) appeared quite high. The delay between the chemotherapy and TBI also made the regimen logistically cumbersome. By moving the TBI to immediately following the chemotherapy, the modified regimen-3 (Bu-Flu-Clo-early TBI) demonstrated an optimized safety profile. Engraftment has been prompt in all patients, with one patient each having grade 2 and 3 mucositis, respectively as the only extra-hematologic toxicity. Accrual to this latest regimen continues and will hopefully confirm the safety and effectiveness of this myeloablative CBT regimen for patients with high-risk hematologic malignancies.
Figure 2.
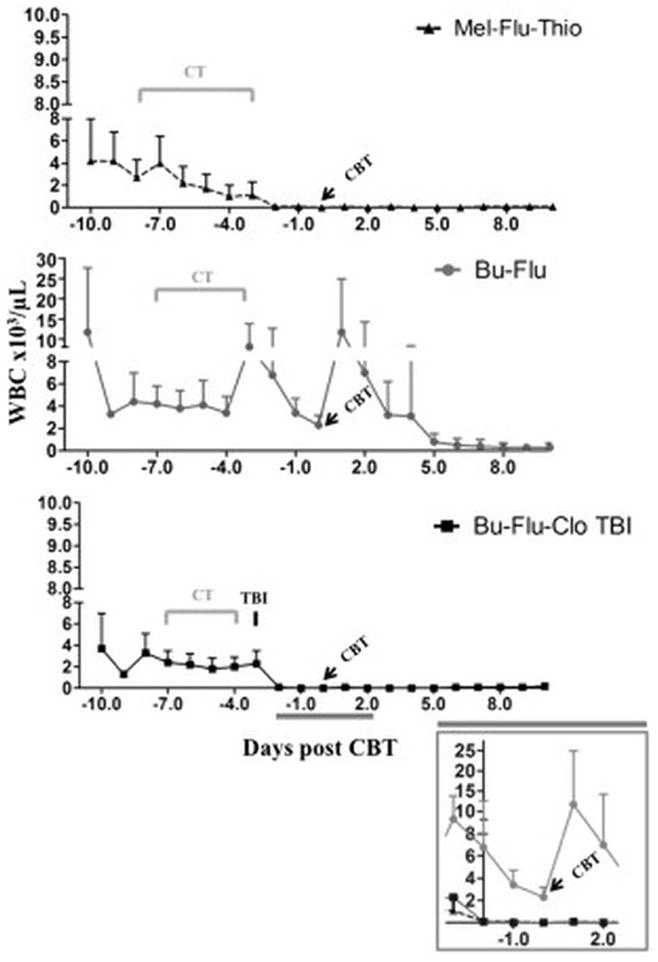
White blood cell (WBC) count per microliters of peripheral blood before and after treatment/stem cell infusion for regimens 1 to 3. Average of at least 5 patients per group + standard deviation is shown.
Abbreviations: Mel=melphalan; Flu= fludarabine; Thio= thiotepa; CBT: cord blood transplant; Bu= busulfan; Clo=clofarabine; TBI= total body irradiation; CT= chemotherapy.
In summary, we propose that the immunoablation provided by the pretransplant conditioning regimen should be considered dependent on both (immediate) elimination of functioning host T-cells to secure early engraftment, and in addition, the long-term stable engraftment will be secured by the alkylating agent-mediated stem cell elimination to prevent regeneration of allo-reactive T-cells. The described reduced toxicity nucleoside analog-IV Busulfan based platform accomplishes both, and it provides a safe and efficacious conditioning program for CB transplantation in patients with high-risk hematologic malignancies.
Acknowledgments
This work was supported in part by NCI RO1 CA061508-18 (EJS, SR), CPRIT RO1 RP100469 (EJS, SR), NCI P01 CA148600-02 (EJS, SR, CMB) and Cancer Center Core Grant CA016672 (MD Anderson investigators).
The authors are grateful to the patients and their families. The authors would like to acknowledge our PharmDs, the nursing staff, research coordinators, and cell therapy laboratory staff.
Footnotes
Conflicts of Interest:
The authors have no conflict of interests to declare.
References
- 1.Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematology Am Soc Hematol Educ Program. 2012;2012:215–222. doi: 10.1182/asheducation-2012.1.215. [DOI] [PubMed] [Google Scholar]
- 2.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010 Jul;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012 Apr;18(4):565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004 Nov 25;351(22):2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 5.Gratwohl A. Principles of Conditioning. In: Apperley J, Gluckman ECE, Masszi T, editors. Hematopoietic Stem Cell Transplantation, the EBMT Handbbok. 5. France: European School of Hematology; 2008. pp. 129–142. [Google Scholar]
- 6.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005 Feb 1;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 7.Sanz J, Boluda JC, Martin C, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012 Oct;47(10):1287–1293. doi: 10.1038/bmt.2012.13. [DOI] [PubMed] [Google Scholar]
- 8.Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG. Effect of conditioning regimen intensity on acute myeloid leukemia outcomes after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011 Sep;17(9):1327–1334. doi: 10.1016/j.bbmt.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007 Oct 15;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004 Aug 1;104(3):865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 11.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004 Aug 1;104(3):857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 12.Marcos De Lima SR, McMannis John, Alousi Amin M, Saliba Rima M, Munsell Mark, Kebriaei Partow, Hosing Chitra, Parmar Simrit, Cooper Laurence, Shah Nina, Kelly Susan Staba, Rondon Gabriela, Fernandez-Vina Marcelo, Maewall Ila, Bosque Doyle, Bollard Catherine M, Chen Julianne J, McNiece Ian K, Komanduri Krishna V, Nieto Yago, Jones Roy, Andersson Borje S, Popat Uday, Champlin Richard E, Simmons Paul J, Shpall Elizabeth J. Mesenchymal Stem Cell (MSC) Based Cord Blood (CB) Expansion (Exp) Leads to Rapid Engraftment of Platelets and Neutrophils. Blood (ASH Annual Meeting Abstracts) 2010;116:362. [Google Scholar]
- 13.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012 Dec 13;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008 Jun;14(6):672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson BS, Valdez BC, de Lima M, et al. Clofarabine +/− fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011 Jun;17(6):893–900. doi: 10.1016/j.bbmt.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdez BC, Li Y, Murray D, Champlin RE, Andersson BS. The synergistic cytotoxicity of clofarabine, fludarabine and busulfan in AML cells involves ATM pathway activation and chromatin remodeling. Biochem Pharmacol. 2011 Jan 15;81(2):222–232. doi: 10.1016/j.bcp.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz ME, Morris A, Gasparetto C, et al. Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transplant. 2008 May;14(5):591–594. doi: 10.1016/j.bbmt.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worth L, Tran H, Petropoulos D, et al. Hematopoietic stem cell transplantation for childhood myeloid malignancies after high-dose thiotepa, busulfan and cyclophosphamide. Bone Marrow Transplant. 1999 Nov;24(9):947–952. doi: 10.1038/sj.bmt.1702016. [DOI] [PubMed] [Google Scholar]
- 19.Hardy NM, Hakim F, Steinberg SM, et al. Host T cells affect donor T cell engraftment and graft-versus-host disease after reduced-intensity hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007 Sep;13(9):1022–1030. doi: 10.1016/j.bbmt.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartelink IH, Bredius RG, Ververs TT, et al. Once-daily intravenous busulfan with therapeutic drug monitoring compared to conventional oral busulfan improves survival and engraftment in children undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008 Jan;14(1):88–98. doi: 10.1016/j.bbmt.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Mielcarek M, Torok-Storb B, Storb R. Pharmacological immunosuppression reduces but does not eliminate the need for total-body irradiation in nonmyeloablative conditioning regimens for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011 Aug;17(8):1255–1260. doi: 10.1016/j.bbmt.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeg HJ, Amylon ID, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7(4):208–215. doi: 10.1053/bbmt.2001.v7.pm11349807. [DOI] [PubMed] [Google Scholar]
- 23.Tran HT, Madden T, Petropoulos D, et al. Individualizing high-dose oral busulfan: prospective dose adjustment in a pediatric population undergoing allogeneic stem cell transplantation for advanced hematologic malignancies. Bone Marrow Transplant. 2000 Sep;26(5):463–470. doi: 10.1038/sj.bmt.1702561. [DOI] [PubMed] [Google Scholar]


