Abstract
Multiple Sclerosis is an inflammatory disease of the central nervous system in which T cells experience a second phase of activation, which ultimately leads to axonal demyelination and neurological disability. The recent advances in stem cell therapies may serve as potential treatments for neurological disorders. There are broad types of stem cells such as neural, embryonic, mesenchymal and hematopoietic stem cells with unprecedented hope in treating many debilitating diseases. In this paper we will review the substantial literature regarding experimental and clinical use of these stem cells and possible mechanisms in the treatment of MS. These results may pave the road for the utilization of stem cells for the treatment of MS.
Keywords: Multiple sclerosis, Stem cells therapy, Human embryonic stem cells, Hematopoietic stem cells, Mesenchymal stem cells, Neural stem cells
INTRODUCTION
Multiple Sclerosis is an inflammatory disease of the central nervous system in which T cells experience a second phase of activation, which ultimately leads to axonal demyelination and neurological disability.1 MS in most patients is characterized with axonal loss underlying long-term progressive disability. Disease-modifying treatments reduce the progression rate of the disease, but do not stop it. Both drug therapy and neurorehabilitation have shown to ease the burden of some symptoms, though neither influences disease progression.2–4
Stem cells are unspecialized cells in the body that have the ability to proliferate or reproduce, and differentiate into other type of body cells with specialized functions.5, 6 Stem cell therapies may serve as potential treatments for neurodegenerative disease.6, 7
There are broad types of stem cells such as neural (NSCs), embryonic (ESCs), mesenchymal (MSCs) and hematopoietic stem cells (HSCs) with unprecedented hope in treating many debilitating diseases. In this paper, we will review the substantial literature regarding experimental and clinical use of these stem cells and possible mechanisms in the treatment of MS.
MATERIALS AND METHODS
Study Selection
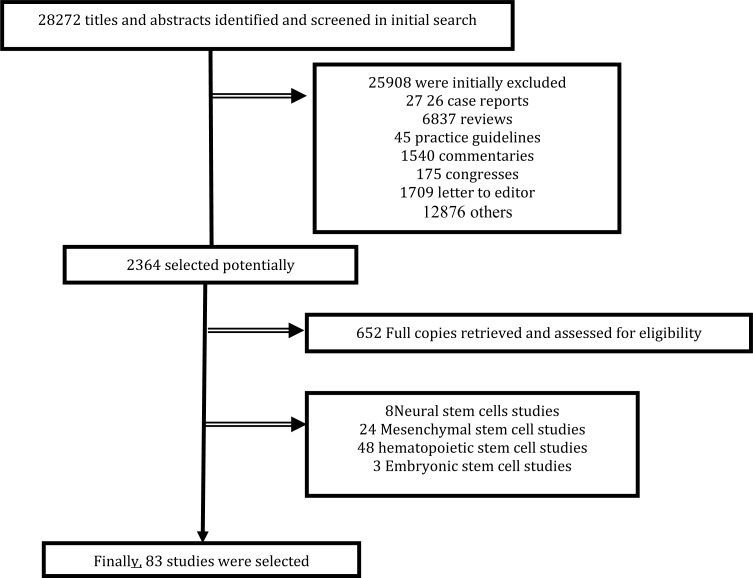
We performed a comprehensive electronic search on the Pub Med and ISI web of science for all studies of Multiple Sclerosis (MS) based on the cell therapy using following terms: “Tissue Therapy”, “Neural stem cells”, “Mesenchymal stem cell”, “hematopoietic or haematopoietic peripheral blood stem cell”, “Multiple Sclerosis” and all possible combinations between 1/1/1990 and 31/12/2012. These search terms were confirmed with a MeSH database. Out of 28272 studies, 77 that met our primary criteria of interest were selected (Fig. 1). Finally, 11 titles and abstracts of articles were screened.
Figure 1.
Flowchart of eligible studies
Inclusion Criteria
Study design: All trial studies were included in the evaluation since these study designs are essential for the systematic review.
Participants: Studies that included tissue therapy and Multiple Sclerosis conditions were included in the evaluation.
Exclusion criteria
The studies that showed not enough data for analysis were excluded after contacting corresponding author twice.
Data Extraction
Two reviewers independently screened all titles and abstracts. Full paper manuscripts of any titles/abstracts that appeared to be relevant were obtained and the relevance of each study was independently assessed by two reviewers according to the inclusion and exclusion criteria. Two authors collected data and reached an agreement on all of the eligible items, including author, journal and year of publication, location of study and selection.
RESULTS AND DISCUSSION
Neural Stem Cells (NSCs) for the Treatment of MS
Overall, 8 studies included different models of NSCs applications in MS were selected through the search process (Table 1). NSCs can be isolated from the adult central nervous system (CNS). The sub-ventricular zone (SVZ) of lateral ventricle wall is a major germinal region that is used for isolation of NSCs.8, 9 The migratory properties of NSCs are self-renewing, multipotent and long-distance migrants within the inflamed CNS.10–15 These properties make NSCs suitable for cellular therapy in brain.16 However, there is an increasing evidence that NSCs have neuroprotective and immunomodulatory effects.17–21 Moreover, multiple recent studies showed the beneficial effects of NSCs therapy in neurologic disorders such as Huntington's disease, Parkinson's disease (PD), MS, Stroke, Spinal cord injuries and amyotrophic lateral sclerosis.22
Table 1.
Available Studies Related to Use of Neural Stem Cell in MS
| Authors | Country | Neural Stem cell | Model | Findings |
|---|---|---|---|---|
| Heffernan et al., 2012 | Australia | glial cells | Human | new therapeutic strategy for the treatment of as MS(101) |
| Payne et al., 2012 | Australia | 46C-NS cells | Mouse | Improving the efficiency at which NSCs home to inflammatory sites may enhance their therapeutic potential in MS(102) |
| Song et al., 2012 | Australia | induced pluripotent stem (iPS) cells | Human | A novel approach for the study of MS pathophysiology and potential drug discovery(103) |
| Rasmussen et al., 2011 | USA | Sub-ventricular zone cells | Mouse | treatments targeting chronic microglial activation have the potential for enhancing repair in MS(104) |
| Huang et al., 2011 | UK | oligodendrocyte precursor cells (OPCs) | Human | might be useful pharmacological targets to overcoming remyelination failure in MS(105) |
| Giannakopoulou et al., 2011 | Greece | neural precursor cell (NPC) | Mouse | NPC intraventricular transplantation should be accountable for their therapeutic effect in MS(106) |
| Carbajal et al., 2011 | USA | oligodendrocyteprogentior cells (OPCs) | Mouse | highlight the importance of the CXCL12:CXCR4 pathway in regulating homing of engrafted stem cells to sites of tissue damage in the MS(107) |
| Yip et al., 2003 | USA | oligodendrocyteprogentior cells (OPCs) | Human | Emerging knowledge of the molecules that may be involved in such responses may help in the design of future stem cell-based treatment of demyelinating diseases such as multiple sclerosis(108) |
Thus, today NSCs therapy is a useful therapeutic approach, which can be defined as the use of cells that need to differentiate into both oligodendrocytes and neurons to treat disease like MS. Several investigations have shown that NSCs can differentiate into mature oligodendrocytes in animal models of dysmiyelination.18, 23–28 and neurons cerebral degeneration.29 Recent studies reported therapeutic potential of adult neural stem cells (aNSCs) in MS.14, 17, 18, 30. Another type of NSCs is bone marrow-derived NSCs (BM-NSCs), which have neurogeneration potential and immunomodulatory effects.31, 32 BM-NSCs are ethically preferred types of NSCs. Neural progenitor cells (NPCs) are other types of NSCs that are capable to differentiate into oligodendrocytes.10 Furthermore, NPCs have anti-inflammatory properties by producing a variety of cytokines and neutrophils.33, 34
Although these findings clearly confirmed tremendous potential of NSCs therapy for patients with MS (Table 1), a lot of work still needs to be done to prove their clinical effectiveness and safety.
Mesenchymal Stem Cells as a Therapeutic Strategy for MS
Overall, 24 studies included applications of Mesenchymal stem cells (MSCs) in MS were selected through the search process (Table 2). MSCs are capable of transdifferentiation into cells of the endodermal and ectodermal origin.35–38 These cells derived from various sources such as bone marrow, amniotic fluid, deciduous teeth, adipose tissue, umbilical cord, synovial membranes, peripheral blood and etc. However, the main source of MSCs is the bone marrow.39–44 Recently, numerous studies have focused on MSCs for cell therapy in many neurodegenerative disorders such as MS.45
Table 2.
Available Studies Related to Use of Mesenchymal Stem Cell in MS
| Authors | Country | Mesenchymal Stem cell | Model | Findings |
|---|---|---|---|---|
| Bonab et al., 2012 | Iran | Autologous bone marrow derived mesenchymal stem cell (BM-MSC) | Human | MSC therapy can improve/stabilize the course of the disease in progressive MS in the first year after injection with no serious adverse effects(109) |
| Payne et al., 2012 | Australia | bone marrow derived mesenchymal stem cell (BM-MSC) | Mouse | MSCs as a cell therapeutic that may be used to treat MS patients(110) |
| Cobo et al., 2012 | Spain | allogenicmesenchymal stem cells (MSCs) | Mouse | Unmodified MSCs were not therapeutic when administer at the peak of disease(111) |
| Al Jumah et al., 2012 | Saudi Arabia | Mesenchymal stem cells (MSCs | Mouse | effectiveness of MSCs in modulating the immunopathogenic process and in providing neuroprotection in MS(112) |
| Fisher-Shoval et al., 2012 | Israel | human placental MSCs (PL-MSCs) | Mouse | PL-MSCs have a therapeutic effect in the EAE mice modelof MS(113) |
| Bai et al., 2012 | USA | Mesenchymal stem cells (MSCs) | Mouse | MSC-stimulated functional recovery in animal models of MS(114) |
| Payne et al., 2012 | Australia | human adipose-derived MSCs (Ad-MSCs) | Mouse | Ad-MSCs express anti-inflammatory cytokines may provide a rational approach to promote immunomodulation and tissue protection in MS(115) |
| Connick et al., 2012 | UK | Autologous mesenchymal stem cells | Human | The evidence of structural, functional, and physiological improvement after treatment in some visual endpoints is suggestive of neuroprotection in MS(116) |
| Zhang et al., 2012 | China | NT-3 gene-modified MSC | Rat | genetically modified MSCs could be a potential therapeutic avenue for improving the efficacy of stem cell treatment for neurodegenerative diseases such as MS(117) |
| Harris et al., 2012 | USA | bone marrow mesenchymal stem cell-derived neural progenitors (MSC-NPs) | Human | MSC-NPs may influence the rate of repair through effects on endogenous progenitors in the spinal cord in MS(118) |
| Odinak et al., 2011 | Russia | autologicmultipotentmesenchymal stem cells (MSC) | Human | safety of the elaborated protocol of treatment and the moderate clinical efficacy of treatment in MS patients or those with poor response to treatment(119) |
| Mohajeri et al., 2011 | Iran | bone marrow derived mesenchymal stem cells | Human | support the potential of bone marrow derived MSC for treatment of MS patients(120) |
| Grigoriadis et al., 2011 | Greece | Autologous bone marrow stromal cells (BMSCs) | Mouse | substantial relevance for clinical trials in MS, particularly regarding the possibility that transplanted BMSCs entering the inflamed central nervous system(121) |
| Cristofanilli et al., 2011 | USA | embryonic-derived oligodendrocyte progenitor cells (OPCs)- Mesenchymal stem cells (MSCs) | Mouse | combining the immunomodulatory and trophic properties of MSCs with the myelinating ability of OPCs might be a suitable strategy for promoting neurological regeneration in MS(122) |
| Karussis et al., 2010 | Israel | autologous mesenchymalstem cells (MSCs) | Human | Transplantation of MSCs in patients with MS is a clinically feasible and relatively safe procedure and induces immediate immunomodulatory effects(49) |
| Yamout et al., 2010 | Lebanon | autologous bone marrow derived mesenchymal stem cells (BM-MSCs) | Human | clinical but not radiological efficacy and evidence of safety with no serious adverse events in MS(50) |
| Darlington et al., 2010 | Canada | bone marrow-derived hMSCs | Human | importance of further preclinical work and immune-monitoring to define hMSC effects on disease-relevant immune responses under variable conditions in MS(123) |
| Rice et al., 2010 | UK | autologous bone marrow-derived mesenchymal stem cells (MSCs) | Human | therapeutic potential of autologous MSCs which primarily utilize MSCs from individuals without MS, and relevance to clinical studies extrapolating from these scientific findings(124) |
| Mallam et al., 2010 | UK | human MSCs (hMSC) | Human | implications for the development of new therapeutic interventions designed to mobilize endogenous cells to enhance repair in MS(125) |
| Barhum et al., 2010 | Israel | Bone marrow mesenchymal stem cells (MSCs) | Mouse | NTFCs-transplanted ICV delay disease symptoms of EAE mice, possibly via neuroprotection and immunomodulation, and may serve as a possible treatment to MS(126) |
| Constantin et al., 2009 | Italy | adipose-derived MSCs (ASCs) | Mouse | ASCs represent a valuable tool for stem cell-based therapy in chronic inflammatory diseases of the CNS such as MS(127) |
| Liang et al., 2009 | China | mesenchymal stem cells | Human | mesenchymal stem cells have a potent immunosuppressive effect in MS(128) |
| Bai et al., 2009 | USA | human bone marrow-derived MSCs (BM-hMSCs) | Mouse | BM-hMSCs represent a viable option for therapeutic approaches in MS(129) |
| Mohyeddin et al., 2007 | Iran | Autologous Mesenchymal stem cells (MSCs) | Human | emphasizes on the feasibility of autologous MSC for treatment of MS patients(130) |
MSCs have a potential for migration into inflamed CNS tissue and differentiate into cells expressing neuronal and glial cell markers.46 Indeed, MSCs can differentiate into neuronal cells, which is confirmable with molecular, biomedical, anatomical and electrophysiological characteristics.47
Harris et al., investigated potential role of MSCs on promotion of repair and recovery after intrathecal injection into mice with experimental autoimmune encephalomyelitis (EAE). They showed improvement in neurological functions compared with controls, and suggested that MSCs can influence the rate of repair through effects on endogenous progenitors in the spinal cords. Thus, MSCs can use in MS patient for promoting CNS repair.48
Reduction of expanded disability status scale (EDSS) were observed when Karussis et al., injected autologous MSCs intrathecally and intravenously in patients with MS.49 They showed a clinical improvement in treated MS patients.50 Neurotrophin-3 (NT-3)-modified MSCs via recombinant adenoviral vector40 implanted into a region of ethidium bromide (EB)-induced demyelination in the rats with demyelinated spinal cord. Results were shown that AdvNT-3-MSC implants upgrade the endogenous remyelinating cells to participate directly in myelination. These data suggests that genetically modification of MSCs could be a potential therapeutic approach for elevating the efficacy of such treatment for MS and other neurodegenerative diseases.51 However, our literature survey about the use of MSCs in MS patients has revealed the feasibility and safety of MSC therapy (Table 2).
Hematopoietic Stem Cell Transplantation in MS
A total of 48 studies including different models of hematopoietic stem cell (HSC) applications in MS were selected through the search (Table 3). HSCs are multipotent stem cells that give rise to all the blood cell types from the lymphoid to myeloid lineages. There is increasing use of HSC transplantation over the last years for the treatment of hematological and non-hematological neoplasms and several autoimmune diseases, including MS.52 In MS, T cells experience a second phase of activation, which ultimately leads to axonal demyelination and neurological disability.53
Table 3.
Available Studies Related to Use of Hematopoietic Stem Cell in MS
| Authors | Country | Mesenchymal Stem cell | Model | Findings |
|---|---|---|---|---|
| Shevchenko et al., 2012 | Russia | autologous hematopoietic stem cell transplantation (AHSCT) | Human | support the feasibility of AHSCT with reduced-intensity conditioning in MS patient(131) |
| Saccardi et al., 2012 | Italy | Haematopoietic stem cell transplantation (HSCT) | Human | HSCT indeed leads to extensive renewal of the T-cell repertoire provided crucial evidence to document that autologous HSCT goes beyond a profound and long-lasting immunosuppression, which can be achieved by conventional treatment in MS(132) |
| Lutterotti et al., 2012 | Germany | Autologous hematopoietic stem cell transplantation (aHSCT) | Human | Support the use of aHSCT for treatment of MS(133) |
| Atkins et al., 2012 | Canada | Autologous hematopoietic stem cell transplantation (HCT) | Human | The promising data that is emerging may establish these diseases as standard indications for HCT(134) |
| Chen et al., 2012 | China | Autologous haematopoietic stem cell transplantation (AHSCT) | Human | AHSCT is a feasible treatment for severe MS and its long-term efficacy is favorable(135) |
| Mancardi et al., 2012 | Italy | Autologous haematopoieticstem cell transplantation (AHSCT) | Human | This study shows that AHSCT with a BEAM/ATG conditioning regimen has a sustained effect in suppressing disease progression in aggressive MS cases unresponsive to conventional therapies(136) |
| Capobianco et al., 2012 | Italy | autologous haematopoietic stem cell transplantation (HDC-AHSCT) | Human | Use of HDC-AHSCT could be effective and safe, but the very long-term risk of adverse events due to sequential aggressive immunosuppression has to be established(137) |
| Fassas et al., 2011 | Greece | hemopoietic stem cell transplantation (HSCT) | Human | HSCT also resulted in a significant reduction in the number and volume of gadolinium-enhancing lesions on MRI of MS patient(138) |
| Reston et al., 2011 | USA | autologous hematopoietic cell transplantation | Human | Patients with secondary progressive MS refractory to conventional medical treatment have longer progression-free survival following autologous stem cell transplantation with intermediate-intensity conditioning regimens than with high-intensity conditioning regimens(139) |
| Xu et al., 2011 | China | autologous peripheral blood stem cell transplantation (APBCST) | Human | Progressive OSMS has a higher relapse rate than CMS following APBSCT(140) |
| Guimarães et al., 2010 | Brazil | autologous hematopoetic stem cell transplantation (autoHSCT) | Human | In spite of the high risk of complications of the procedure, the HSCT had positive impact in the health related quality of life(141) |
| Lu et al., 2010 | Canada | allogeneic hematopoietic stem cell transplantation (allo-HSCT) | Human | Allo-HSCT fails to halt the demyelination and inflammation of MS(142) |
| Krasulová et al., 2010 | Czech Republic | autologous haematopoietic stem cell transplantation (ASCT) | Human | ASCT represents a viable and effective treatment option for aggressive multiple sclerosis(143) |
| Tappenden et al., 2010 | UK | autologous haematopoietic stem cell transplantation (HSCT) | Human | HSCT could potentially achieve an acceptable level of cost-effectiveness(144) |
| Rogojan et al., 2009 | Denmark | haematopoietic stem cell transplantation (HSCT) | Human | Relatively young patients with active inflammatory lesions of relatively short duration and rapidly progressive disease, but still low disability scores, unresponsive to conventional therapy seem the best candidates for transplantation(145) |
| Burt et al., 2009 | USA | Autologous non-myeloablativehaemopoietic stem cell transplantation | Human | Non-myeloablative autologous haemopoietic stem cell transplantation in patients with relapsing-remitting MS reverses neurological deficits(146) |
| Lu et al., 2009 | Canada | allogeneic hematopoietic cell transplantation (HCT) | Human | Despite high-dose, cytotoxic, immunosuppressive therapy and exchange of a presumed autoreactive immune system with a healthy immune system, MS in this patient continued to be active(80) |
| Fassas et al., 2008 | Greece | autologous transplantation of hemopoietic stem cells (ASCT) | Human | ASCT does not only cause debulking of autoreactive clones but it also brings about qualitative immunological changes that might eventually establish immunologic self-tolerance; the progression of brain atrophy appears to slow down with time; with the implementation of proper patient-selection criteria, the risks of morbidity and mortality can be minimized(147) |
| Fagius et al., 2009 | Sweden | autologous hematopoietic stem cell transplantation (HSCT) | Human | HSCT to be an effective treatment option for this relatively rare disease course in MS(148) |
| Saiz et al., 2008 | Spain | Autologous hematopoietic stem cell transplantation (AHSCT) | Human | AHSCT cannot be deemed a curative treatment but may cause prolonged stabilisation or change the aggressive course of the disease(149) |
| Shevchenko et al., 2008 | Russia | autologous hematopoietic stem cell transplantation (auto-HSCT) | Human | Auto-HSCT treatment strategies based on the level of disability, namely “early,” “conventional,” and “salvage/late” transplantation, appears to be feasible to improve treatment outcomes(150) |
| Rocca et al., 2007 | Italy | autologous hematopoietic stem cell transplantation (AHSCT) | Human | After AHSCT, the rate of brain tissue loss in patients with MS declines dramatically after the first 2 years(151) |
| Portaccio et al., 2007 | Italy | autologous hematopoietic stem cell transplantation (AHSCT) | Human | Cases with very active, relapsing-remitting (RR) MS, who underwent AHSCT, and obtained a dramatic resolution to disease activity(152) |
| Roccatagliata et al., 2007 | Genoa | autologous hematopoietic stem cell transplantation (AHSCT) | Human | AHSCT is associated to a longlasting suppression of inflammation and to a marked decrease of the rate of brain atrophy after the second year following treatment(153) |
| Metz et al., 2007 | Germany | autologous hematopoietic stem cell transplantation (AHSCT) | Human | Continued clinical disease progression in multiple sclerosis patients with high expanded disability system scores despite autologous stem cell transplantation(154) |
| Xu et al., 2006 | China | autologous haematopoietic stem cell transplantation (ASCT) | Human | ASCT as a therapy is safe and available. It can improve or stabilize neurological manifestations in most patients with progressive MS following failure of conventional therapy(74) |
| Loh et al., 2007 | USA | autologous hematopoietic stem cell transplantation (auto-HSCT) | Human | Peripheral blood stem cells were not found to be significantly associated with development of a secondary autoimmune disorder(155) |
| Su et al., 2006 | China | autologous hematopoietic stem cell transplantation (auto-HSCT) | Human | Auto-HSCT proved to be safe and beneficial for some MS patients. Further studies are needed to establish the merit of this procedure for MS patients(156) |
| Ni et al., 2006 | China | autologous hematopoietic stem cell transplantation (auto-HSCT) | Human | Autologous HSCT seems beneficial to PMS. However, more patients and longer follow up would be required to assess the risk/benefit ratio(157) |
| Daumer et al., 2006 | Germany | autologous hematopoietic stem cell transplantation (auto-HSCT) | Human | The estimated probability of MS progression, defined as an increase in EDSS score by > or = 1.0 sustained for at least 180 days, was 5% after one year, 14% after two years, 22% after three years, 38% after five years, 57% after 10 years, and >80% after 20 years of observation(158) |
| Papadaki et al., 2005 | Greece | Bone marrow (BM) hematopoietic progenitorsstem cell | Human | provide support for the use of autologous stem cell transplantation in MS patients(159) |
| Blanco et al., 2005 | Spain | peripheral blood mononuclear cells (PBMC) | Human | Our study suggests that AHSCT can reduce BDNF levels to values associated with lower activity. This decrease does not seem to correlate with the brain atrophy measures observed in the MRI in MS(160) |
| Blanco et al., 2005 | Spain | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | The course of MS seems to be stabilized after autologous HSCT, especially in ambulatory patients with evidence of active disease like MS(161) |
| Saccardi et al., 2004 | Italy | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | Significant transplant-related morbidity and mortality have been observed. This is primarily due to complications related to either the stage of the disease at transplant or due to infections. The number of deaths related to cardiac toxicity is low(162) |
| Blanco et al., 2004 | Spain | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | ASCT as a therapy is safe and available. It can improve or stabilize neurological manifestations in most patients with progressive MS following failure of conventional therapy(163) |
| Healey et al., 2004 | USA | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | Inflammation parameters and functional disability findings raising questions about optimal future stem cell transplantation strategies for MS(164) |
| Inglese et al., 2004 | Italy | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | In MS, progressive loss of tissue can occur independently of concomitant MRI-visible inflammation(165) |
| Sun et al., 2004 | USA | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | Findings have important implications in the understanding of the role of HSCT as a potential treatment for multiple sclerosis(166) |
| Saiz et al., 2004 | Spain | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | Findings have important implications in the understanding of the role of HSCT as a potential treatment for multiple sclerosis(167) |
| Saccardiet al., 2004 | Italy | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | Allogeneic HSCT improved the clinical course of MS(168) |
| Burt et al., 2003 | USA | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | a total body irradiation (TBI)-based regimen and hematopoietic stem cell transplantation (HSCT) are not effective for MS patients with progressive disease and high pretransplantation disability scores(169) |
| Nash et al., 2003 | USA | autologous haematopoietic-stem-cell transplantation (HSCT) | Human | The clinical role of autologous HSCT will require a comparison with conventional treatment of MS(170) |
| Carreras et al., 2003 | Spain | autologous peripheral blood stem cell | Human | conditioning regimen has an acceptable toxicity and clearly reduces the progression of MS(171) |
| Fassas et al., 2002 | Greece | autologous peripheral blood stem cell | Human | Autologous HSCT suggest positive early results in the management of progressive MS and is feasible(77) |
| Rossiev et al., 2002 | Russia | autologous peripheral blood stem cell | Human | Autologous HSCT suggest positive early results in the management of progressive MS and is feasible(172) |
| Ouyang et al., 2001 | China | autologous peripheral blood stem cell transplantation (Auto-PBSCT) | Human | Auto-PBSCT is effective and safety for PMS, hence the duration of remission remains to be decided in long-term follow up(173) |
| Burt et al., 1998 | USA | hematopoietic stem cells (HSC) | Human | Stem cell transplantation has resulted in modest neurologic improvements for the first time since onset of progressive MS(57) |
| Fassas et al., 1997 | Greece | hematopoietic stem cells (HSC) | Human | Autologous HSCT appears feasible in MS; it does not aggravate disability and seems to offer a clinical benefit. However, these observations need confirmation and long-term outcomes will show if benefits counterbalance toxicity and cost(56) |
Treatment of multiple sclerosis (MS) has 2 aspects: immunomodulatory therapy for the underlying immune disorder and therapies to relieve or modify symptoms. Hence, first-line immunomodulatory therapies for multiple sclerosis (MS) reduce the relapse rate and slow progression of disability, but are not successful for all patients. Some patients cannot tolerate these therapies or have a suboptimal response and therefore require changes in therapeutic management. Early recognition of suboptimal response and prompt intervention are necessary to limit future impairment.54 Patients with relapse have good response to allogenic or autologous HSC transplantation, as a viable therapeutic option.55–57 Several studies in animal models of MS and human revealed that HSC transplantation can induce MS remission.58–60 However, a few studies present that HSC transplantation has no effect on MS improvement.
Experimental autoimmune encephalomyelitis (EAE)-diseased mice have shown that allogenic HSC transplantation during acute phase of MS lead to full remission.61, 62 Moreover, autologous HSC transplantation in EAE mice resulted in complete remission.63, 64
In this regard, Takahashi et al., transduced TREM-2 (an innate immune receptor) in bone marrow-derived myeloid precursor cells and intravenously injected to mice with EAE. They observed that TREM-2 transduced myeloid precursors ameliorate clinical symptom of MS in mice with EAE by clearance of nervous tissue debris and degenerated myelin.65
Resident perivascular macrophage and microglia in central CNS physiologically derived from myeloid progenitors of hematopoietic cells not only during development, but also in life span.66–68 Moreover, it has been presented that some hematopoietic cells are recruited to sites of neurological damage to become functional perivascular macrophage and microglia like dells.69, 70 Although macrophages play harmful or beneficial roles in CNS injury, they are able to remove the cellular debris in acute phase of injury.71–73
Juan et al., evaluated clinical and neurological outcomes after autologous HSC transplantation in 22 patients with progressive MS. They showed that it can improve or stabilize neurological manifestations in most patients with progressive MS, following failure of conventional therapy.74 Proposed mechanism for improvement of MS symptoms by autologous HSC transplantation is immunity system alteration.75 Fassas et al., reported the outcomes of 15 patients with progressive MS and a median EDSS of 6.0 by HSC transplantation after conditioning. During 6 months of follow- up, no death and worsening of neurological symptoms were observed and EDSS was improved in 7 of 15 patients.56
In the study conducted by Saiz et al., 5 patients with progressive MS and median EDSS of 6.5 underwent HSCT after BCNU, cyclophosphamide and ATG conditioning. Based on MRI findings, 4 patients showed improvement, whereas neurological symptoms worsened in the fifth one. 76 Large series of MS patients including 85 cases were evaluated by the European Group for Blood and Marrow Transplantation (EBMT) Working Party on Autoimmune Diseases. The study included patients with secondary progressive MS (70%) and primary progressive MS (26%). The median EDSS of patients was 6.5 (ranging from 4.5 to 8.5), so the patients were subjected to HSCT after conditioning. At a median follow-up of 16 months, the chance of progression -free survival was 74% at 3 years. Five patients died of treatment-related complications including infection and cardiac failure.77
Patients with both hematological neoplasms and autoimmune diseases inconsistently respond to HSC transplantation.78 Mandalfino et al., reported neurological improvement in 4 patients with MS, following HSCT with follow-up of 6-48 months.79 Whereas, Lu et al., reported that activities of MS persisted after allo-HSCT in a 39-year-old woman with CML affected by MS.80 Another study on 5 autopsy cases in patients with MS that cured by autologous hematopoietic stem cell transplantation showed that MS activity continued in spite of high-dose cytotoxic/immunosuppressive therapy.81 However, these studies included heterogeneous group of patients, follow-up duration, status of MS symptoms and conditioning regimen. But, results suggest that HSC transplantation could improve MS symptoms in progressive phase.
Embryonic Stem Cell Application in MS Treatment
Only three studies were reviewed in detail on the use of Embryonic stem cells (ESCs) in MS. ESCs are pluripotent stem cells that derived from the inner cell mass of an early stage embryo called blastocyst.82–84 They are able to develop into any type of cell in the body. The actual limitation in preparation of sufficient human oligodendrocyte precursor cells obligate research in getting tissue-specific progenitor cells from human embryonic stem cells (hESCs). Many studies have tried to differentiate mouse embryonic stem cells (mESCs) into oligodendrocyte with myelogenic properties.85–87 Moreover, studies have revealed that hESCs can be directed into neural cells.84, 88–90 Interestingly, recent studies discovered several systems such as small molecules and specific transcription factors that control ESC fate to produce neurons91–94 and oligodendrocytes.95, 96 hESC-derived oligodendrocytes are capable of remyelination.95, 97 However, there are always risk of tumorigenesisin neural cells derived from ESCs, limiting the potentialities of science and therapy in such studies.55 hESC-based therapies can give rise to specific specialty cells such as, dermatomes from undifferentiated ESCs or incompletely differentiated neural cells.98, 99
Aharonowiz et al., transplanted hESC-derived neural progenitors into the mice with EAE.100 They observed that clinical symptoms of EAE remarkably reduced after transplantation. Histological evaluation revealed that transplanted neural progenitors migrate to the mice brain, especially in the host white matter. However, remyelination and production of mature oligodendrocytes were not clearly observed.
Besides, they concluded that the therapeutic effect of neural progenitor's transplantation was mediated by an immunosuppressive neuroprotective mechanism. Further studies are required to define the efficacy of ESC-derived neural cell therapy in MS patients.
CONCLUSION
Nowadays, Stem cell therapy in axonal demyelination and neurological disability (Specially MS) had been accelerated growth in animal model as well as human patient clinical treatment. A new way that promotes this procedure is tissue engineering which uses synthesis of natural polymer that simulates extra cellular matrix for better response of body to grafted cells.
REFERENCES
- 1.Al Jumah MA, Abumaree MH. The Immunomodulatory and Neuroprotective Effects of Mesenchymal Stem Cells (MSCs) in Experimental Autoimmune Encephalomyelitis (EAE): A Model of Multiple Sclerosis (MS) Int J Mol Sci. 2012;13(7):9298–331. doi: 10.3390/ijms13079298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med. [Review] 2002;53:285–302. doi: 10.1146/annurev.med.53.082901.103909. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. [News] 2001 Sep;2(9):762–4. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 4.Chandran S, Compston A. Neural stem cells as a potential source of oligodendrocytes for myelin repair. J Neurol Sci. [Review] 2005 Jun 15;233(1-2):179–81. doi: 10.1016/j.jns.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Keirstead HS. Stem cells for the treatment of myelin loss. Trends Neurosci. [Review] 2005 Dec;28(12):677–83. doi: 10.1016/j.tins.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Silani V, Corbo M. Cell-Replacement Therapy with Stem Cells in Neurodegenerative Diseases. Current Neurovascular Research. 2004 Apr;1(3):283–9. doi: 10.2174/1567202043362243. [DOI] [PubMed] [Google Scholar]
- 7.Su P, Loane C, Politis M. Review Paper, The Use of Stem Cells in the Treatment of Parkinson's Disease. Insciences J. [Case Reports] 2011 Feb;1(3):136–56. [Google Scholar]
- 8.Pluchino S, Martino G. The therapeutic plasticity of neural stem/precursor cells in multiple sclerosis. J Neurol Sci. [Research Support, Non-U.S. Gov't Review] 2008 Feb 15;265(1-2):105–10. doi: 10.1016/j.jns.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. [Review] 2008 Apr;65(4):452–6. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 10.Brundin L, Brismar H, Danilov AI, Olsson T, Johansson CB. Neural stem cells: a potential source for remyelination in neuroinflammatory disease. Brain Pathol. [Comparative Study Research Support, Non-U.S. Gov't] 2003 Jul;13(3):322–8. doi: 10.1111/j.1750-3639.2003.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. [Research Support, Non-U.S. Gov't] 2007 Mar 13;104(11):4694–9. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, et al. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp Neurol. [Comparative Study Research Support, Non-U.S. Gov't] 2006 Apr;198(2):275–84. doi: 10.1016/j.expneurol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Hur T, van Heeswijk RB, Einstein O, Aharonowiz M, Xue R, Frost EE, et al. Serial in vivo MR tracking of magnetically labeled neural spheres transplanted in chronic EAE mice. Magn Reson Med. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2007 Jan;57(1):164–71. doi: 10.1002/mrm.21116. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, et al. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. [Research Support, Non-U.S. Gov't] 2003 Jan;41(1):73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- 15.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. [Research Support, Non-U.S. Gov't]. 1996 Dec 1;16(23):7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garzon-Muvdi T, Quinones-Hinojosa A. Neural stem cell niches and homing: recruitment and integration into functional tissues. Ilar J. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2009;51(1):3–23. doi: 10.1093/ilar.51.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. [Research Support, Non-U.S. Gov't] 2007 Mar;61(3):209–18. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. [Review] 2008 Feb 15;265(1-2):102–4. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, et al. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol. [Research Support, Non-U.S. Gov't] 2009 Sep;66(3):343–54. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- 20.Quesenberry PJ, Dooner G, Colvin G, Abedi M. Stem cell biology and the plasticity polemic. Exp Hematol. [Research Support, U.S. Gov't, P.H.S. Review] 2005 Apr;33(4):389–94. doi: 10.1016/j.exphem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. [Review] 2006 Jun 29;441(7097):1094–6. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 22.Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't. P.H.S. 1999 Jun 8;96(12):7029–34. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammang JP, Archer DR, Duncan ID. Myelination following transplantation of EGF-responsive neural stem cells into a myelin-deficient environment. Exp Neurol. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 1997 Sep;147(1):84–95. doi: 10.1006/exnr.1997.6592. [DOI] [PubMed] [Google Scholar]
- 24.Milward EA, Lundberg CG, Ge B, Lipsitz D, Zhao M, Duncan ID. Isolation and transplantation of multipotential populations of epidermal growth factor-responsive, neural progenitor cells from the canine brain. J Neurosci Res. [Research Support, Non-U.S. Gov't] 1997 Dec 1;50(5):862–71. doi: 10.1002/(SICI)1097-4547(19971201)50:5<862::AID-JNR22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Kohama I, Lankford KL, Preiningerova J, White FA, Vollmer TL, Kocsis JD. Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. J Neurosci. [In Vitro Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S. 2001 Feb 1;21(3):944–50. doi: 10.1523/JNEUROSCI.21-03-00944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnett SC, Alexander CL, Iwashita Y, Gilson JM, Crowther J, Clark L, et al. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain. [Research Support, Non-U.S. Gov't] 2000 Aug;123(Pt 8):1581–8. doi: 10.1093/brain/123.8.1581. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 2000 May;30(3):209–18. doi: 10.1002/(sici)1098-1136(200005)30:3<209::aid-glia1>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imaizumi T, Lankford KL, Burton WV, Fodor WL, Kocsis JD. Xenotransplantation of transgenic pig olfactory ensheathing cells promotes axonal regeneration in rat spinal cord. Nat Biotechnol. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. 2000 Sep;18(9):949–53. doi: 10.1038/79432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. [Research Support, Non-U.S. Gov't] 2003 Apr 17;422(6933):688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 30.Magalon K, Cantarella C, Monti G, Cayre M, Durbec P. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. Eur J Neurosci. 2007 Feb;25(3):761–71. doi: 10.1111/j.1460-9568.2007.05335.x. [DOI] [PubMed] [Google Scholar]
- 31.Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. [Review] 2008 Feb 15;265(1-2):131–5. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 2005 Sep;195(1):16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Pluchino S, Zanotti L, Brambilla E, Rovere-Querini P, Capobianco A, Alfaro-Cervello C, et al. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PLoS One. [Research Support, Non-U.S. Gov't] 2009;4(6):e5959. doi: 10.1371/journal.pone.0005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci. [Research Support, U.S. Gov't, Non-P.H.S. 2008 Jul 15;270(1-2):70–6. doi: 10.1016/j.jns.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Portmann-Lanz CB, Schoeberlein A, Portmann R, Mohr S, Rollini P, Sager R, et al. Turning placenta into brain: placental mesenchymal stem cells differentiate into neurons and oligodendrocytes. Am J Obstet Gynecol. [Research Support, Non-U.S. Gov't] 2010 Mar;202(3):294 e1–e11. doi: 10.1016/j.ajog.2009.10.893. [DOI] [PubMed] [Google Scholar]
- 36.Lue J, Lin G, Ning H, Xiong A, Lin CS, Glenn JS. Transdifferentiation of adipose-derived stem cells into hepatocytes: a new approach. Liver Int. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2010 Jul;30(6):913–22. doi: 10.1111/j.1478-3231.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2009 Dec 15;183(12):7787–98. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najmadin Saki, Saeid Abroun, Majid Farshdusti Hagh, Farahnaz Asgharei. Neoplastic Bone Marrow Niche: Hematopoietic And Mesenchymal Stem Sells. Cell Journal (Yakhteh) 2011;13(3):131–6. [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson WM, Nesti LJ, Tuan RS. Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells. Expert Opin Biol Ther. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't Review] 2010 Apr;10(4):505–17. doi: 10.1517/14712591003610606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehghani Fard A, Saki N, Ahmadvand M, Mahmoodinia Maymand M, Mosahebi Mohammadi M, et al. Mesenchymal stem cell; biology, application and its role in regenerative medicine. Sci J Blood Transfus Organ. 2012;8(4):306–20. [Google Scholar]
- 41.Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. [Research Support, Non-U.S. Gov't] 2007 Oct;16(5):797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 42.Reger RL, Tucker AH, Wolfe MR. Differentiation and characterization of human MSCs. Methods Mol Biol. 2008;449:93–107. doi: 10.1007/978-1-60327-169-1_7. [DOI] [PubMed] [Google Scholar]
- 43.Delorme B, Nivet E, Gaillard J, Haupl T, Ringe J, Deveze A, et al. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. [Research Support, Non-U.S. Gov't]. 2010 Jun;19(6):853–66. doi: 10.1089/scd.2009.0267. [DOI] [PubMed] [Google Scholar]
- 44.in ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003 Aug;88(8):845–52. [PubMed] [Google Scholar]
- 45.Dazzi F, Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract Res Clin Haematol. [Review] 2011 Mar;24(1):49–57. doi: 10.1016/j.beha.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Slavin S, Kurkalli BG, Karussis D. The potential use of adult stem cells for the treatment of multiple sclerosis and other neurodegenerative disorders. Clin Neurol Neurosurg. [Review] 2008 Nov;110(9):943–6. doi: 10.1016/j.clineuro.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. [Review] 2005 Jun 10;306(2):330–5. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Harris VK, Yan QJ, Vyshkina T, Sahabi S, Liu X, Sadiq SA. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J Neurol Sci. 2011 Sep 28; doi: 10.1016/j.jns.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 49.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. [Clinical Trial, Phase I Clinical Trial, Phase II Research Support, Non-U.S. Gov't] 2010 Oct;67(10):1187–94. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. [Clinical Trial, Phase I Comparative Study Research Support, Non-U.S. Gov't] 2010 Oct 8;227(1-2):185–9. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Zhang YJ, Zhang W, Lin CG, Ding Y, Huang SF, Wu JL, et al. Neurotrophin-3 gene modified mesenchymal stem cells promote remyelination and functional recovery in the demyelinated spinal cord of rats. J Neurol Sci. 2011 Oct 12; doi: 10.1016/j.jns.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 52.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2006 Mar 2;354(9):942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 53.Tabatabai G, Bahr O, Mohle R, Eyupoglu IY, Boehmler AM, Wischhusen J, et al. Lessons from the bone marrow: how malignant glioma cells attract adult haematopoietic progenitor cells. Brain. [Research Support, Non-U.S. Gov't] 2005 Sep;128(Pt 9):2200–11. doi: 10.1093/brain/awh563. [DOI] [PubMed] [Google Scholar]
- 54.McCormack PL, Scott LJ. Interferon-beta-1b: a review of its use in relapsing-remitting and secondary progressive multiple sclerosis. CNS Drugs. [Review] 2004;18(8):521–46. doi: 10.2165/00023210-200418080-00004. [DOI] [PubMed] [Google Scholar]
- 55.van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. [Multicenter Study] 2011 Apr 1;29(10):1342–8. doi: 10.1200/JCO.2010.30.2596. [DOI] [PubMed] [Google Scholar]
- 56.Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transplant. 1997 Oct;20(8):631–8. doi: 10.1038/sj.bmt.1700944. [DOI] [PubMed] [Google Scholar]
- 57.Burt RK, Traynor AE, Cohen B, Karlin KH, Davis FA, Stefoski D, et al. T cell-depleted autologous hematopoietic stem cell transplantation for multiple sclerosis: report on the first three patients. Bone Marrow Transplant. [Clinical Trial] 1998 Mar;21(6):537–41. doi: 10.1038/sj.bmt.1701129. [DOI] [PubMed] [Google Scholar]
- 58.Kewalramani T, Nimer SD, Zelenetz AD, Malhotra S, Qin J, Yahalom J, et al. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin's disease or aggressive non-Hodgkin's lymphoma. Bone Marrow Transplant. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 2003 Oct;32(7):673–9. doi: 10.1038/sj.bmt.1704214. [DOI] [PubMed] [Google Scholar]
- 59.Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. [Research Support, Non-U.S. Gov't] 2010 Dec 2;116(23):4934–7. doi: 10.1182/blood-2010-05-282756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zelenetz AD, Hamlin P, Kewalramani T, Yahalom J, Nimer S, Moskowitz CH. Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin's lymphoma. Ann Oncol. [Review]. 2003;14(Suppl 1):i5–10. doi: 10.1093/annonc/mdg702. [DOI] [PubMed] [Google Scholar]
- 61.van Bekkum DW. Stem cell transplantation for autoimmune disorders. Preclinical experiments. Best Pract Res Clin Haematol. [Review] 2004 Jun;17(2):201–22. doi: 10.1016/j.beha.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 62.van Gelder M, van Bekkum DW. Treatment of relapsing experimental autoimmune encephalomyelitis in rats with allogeneic bone marrow transplantation from a resistant strain. Bone Marrow Transplant. [Research Support, Non-U.S. Gov't] 1995 Sep;16(3):343–51. [PubMed] [Google Scholar]
- 63.van Gelder M, Kinwel-Bohre EP, van Bekkum DW. Treatment of experimental allergic encephalomyelitis in rats with total body irradiation and syngeneic BMT. Bone Marrow Transplant. [Research Support, Non-U.S. Gov't] 1993 Mar;11(3):233–41. [PubMed] [Google Scholar]
- 64.van Gelder M, van Bekkum DW. Effective treatment of relapsing experimental autoimmune encephalomyelitis with pseudoautologous bone marrow transplantation. Bone Marrow Transplant. [Research Support, Non-U.S. Gov't] 1996 Dec;18(6):1029–34. [PubMed] [Google Scholar]
- 65.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. [Research Support, Non-U.S. Gov't]. 2007 Apr;4(4):e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 1988 Jan 15;239(4837):290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 67.Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. [Research Support, Non-U.S. Gov't] 2001 Dec;7(12):1356–61. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 68.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. Faseb J. [Research Support, Non-U.S. Gov't] 2004 Jun;18(9):998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 69.Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. [Research Support, Non-U.S. Gov't] 2001 Oct 1;66(1):74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- 70.Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simburger E, Naftolin F, et al. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. Faseb J. [Research Support, Non-U.S. Gov't] 2005 Apr;19(6):647–9. doi: 10.1096/fj.04-2599fje. [DOI] [PubMed] [Google Scholar]
- 71.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. [Letter] 1997 Nov 27;390(6658):350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 72.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. [Research Support, Non-U.S. Gov't Review] 2002 Dec;2(12):965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 73.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] 2005 Dec;6(12):1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 74.Xu J, Ji BX, Su L, Dong HQ, Sun XJ, Liu CY. Clinical outcomes after autologous haematopoietic stem cell transplantation in patients with progressive multiple sclerosis. Chin Med J (Engl). [Research Support, Non-U.S. Gov't] 2006 Nov 20;119(22):1851–5. [PubMed] [Google Scholar]
- 75.Tyndall A, Matucci-Cerinic M. Haematopoietic stem cell transplantation for the treatment of systemic sclerosis and other autoimmune disorders. Expert Opin Biol Ther. [Review] 2003 Oct;3(7):1041–9. doi: 10.1517/14712598.3.7.1041. [DOI] [PubMed] [Google Scholar]
- 76.Saiz A, Carreras E, Berenguer J, Yague J, Martinez C, Marin P, et al. MRI and CSF oligoclonal bands after autologous hematopoietic stem cell transplantation in MS. Neurology. [Research Support, Non-U.S. Gov't] 2001 Apr 24;56(8):1084–9. doi: 10.1212/wnl.56.8.1084. [DOI] [PubMed] [Google Scholar]
- 77.Fassas A, Passweg JR, Anagnostopoulos A, Kazis A, Kozak T, Havrdova E, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol. [Clinical Trial Clinical Trial, Phase I Clinical Trial, Phase II Multicenter Study Research Support, Non-U.S. Gov't] 2002 Aug;249(8):1088–97. doi: 10.1007/s00415-002-0800-7. [DOI] [PubMed] [Google Scholar]
- 78.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. [Research Support, N.I.H, Extramural Review] 2008 Nov 1;112(9):3543–53. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mandalfino P, Rice G, Smith A, Klein JL, Rystedt L, Ebers GC. Bone marrow transplantation in multiple sclerosis. J Neurol. 2000 Sep;247(9):691–5. doi: 10.1007/s004150070112. [DOI] [PubMed] [Google Scholar]
- 80.Lu JQ, Storek J, Metz L, Yong VW, Stevens AM, Nash RA, et al. Continued disease activity in a patient with multiple sclerosis after allogeneic hematopoietic cell transplantation. Arch Neurol. [Case Reports Research Support, Non-U.S. Gov't] 2009 Jan;66(1):116–20. doi: 10.1001/archneurol.2008.522. [DOI] [PubMed] [Google Scholar]
- 81.Gualandi F, Bruno B, Van Lint MT, Luchetti S, Uccelli A, Capello E, et al. Autologous stem cell transplantation for severe autoimmune diseases: a 10-year experience. Ann N Y Acad Sci. 2007 Sep;1110:455–64. doi: 10.1196/annals.1423.048. [DOI] [PubMed] [Google Scholar]
- 82.Denham M, Cole TJ, Mollard R. Embryonic stem cells form glandular structures and express surfactant protein C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol. [Research Support, Non-U.S. Gov't] 2006 Jun;290(6):L1210–5. doi: 10.1152/ajplung.00427.2005. [DOI] [PubMed] [Google Scholar]
- 83.Conley BJ, Trounson AO, Mollard R. Human embryonic stem cells form embryoid bodies containing visceral endoderm-like derivatives. Fetal Diagn Ther. [Research Support, Non-U.S. Gov't] 2004 May-Jun;19(3):218–23. doi: 10.1159/000076701. [DOI] [PubMed] [Google Scholar]
- 84.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. [Research Support, Non-U.S. Gov't] 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 85.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 1999 Jul 30;285(5428):754–6. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 86.Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 2000 May 23;97(11):6126–31. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Billon N, Jolicoeur C, Ying QL, Smith A, Raff M. Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J Cell Sci. [Research Support, Non-U.S. Gov't] 2002 Sep 15;115(Pt 18):3657–65. doi: 10.1242/jcs.00049. [DOI] [PubMed] [Google Scholar]
- 88.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000 Apr;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 89.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. [Research Support, Non-U.S. Gov't] 2001 Dec;19(12):1134–40. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 90.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. [Research Support, Non-U.S. Gov't] 2001 Dec;19(12):1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 91.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2008 Apr;26(4):886–93. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinat C, Bacci JJ, Leete T, Kim J, Vanti WB, Newman AH, et al. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2006 Feb 21;103(8):2874–9. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pomp O, Brokhman I, Ben-Dor I, Reubinoff B, Goldstein RS. Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells. [Research Support, Non-U.S. Gov't] 2005 Aug;23(7):923–30. doi: 10.1634/stemcells.2005-0038. [DOI] [PubMed] [Google Scholar]
- 94.Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. [Research Support, Non-U.S. Gov't] 2007 Dec;25(12):1468–75. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 95.Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, et al. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. [Research Support, Non-U.S. Gov't] 2007 Mar;34(3):310–23. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 96.Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. [Research Support, N.I.H, Extramural] 2009 May;136(9):1443–52. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. [Research Support, Non-U.S. Gov't] 2005 Feb;49(3):385–96. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 98.Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. [Review] 2007 Jan;25(1):24–32. doi: 10.1016/j.tibtech.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 99.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2006 Jul 20;51(2):187–99. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 100.Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One. [Research Support, Non-U.S. Gov't] 2008;3(9):e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heffernan C, Sumer H, Guillemin GJ, Manuelpillai U, Verma PJ. Design and screening of a glial cell-specific, cell penetrating peptide for therapeutic applications in multiple sclerosis. PLoS One. [Research Support, Non-U.S. Gov't] 2012;7(9):e45501. doi: 10.1371/journal.pone.0045501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Payne NL, Sun G, Herszfeld D, Tat-Goh PA, Verma PJ, Parkington HC, et al. Comparative study on the therapeutic potential of neurally differentiated stem cells in a mouse model of multiple sclerosis. PLoS One. [Research Support, Non-U.S. Gov't] 2012;7(4):e35093. doi: 10.1371/journal.pone.0035093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song B, Sun G, Herszfeld D, Sylvain A, Campanale NV, Hirst CE, et al. Neural differentiation of patient specific iPS cells as a novel approach to study the pathophysiology of multiple sclerosis. Stem Cell Res. [Research Support, Non-U.S. Gov't] 2012 Mar;8(2):259–73. doi: 10.1016/j.scr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Rasmussen S, Imitola J, Ayuso-Sacido A, Wang Y, Starossom SC, Kivisäkk P, et al. Reversible neural stem cell niche dysfunction in a model of multiple sclerosis. Ann Neurol. 2011;69(5):878–91. doi: 10.1002/ana.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang JK, Franklin RJ. Regenerative medicine in multiple sclerosis: identifying pharmacological targets of adult neural stem cell differentiation. Neurochem Int. [Review]. 2011 Sep;59(3):329–32. doi: 10.1016/j.neuint.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 106.Giannakopoulou A, Grigoriadis N, Polyzoidou E, Touloumi O, Michaloudi E, Papadopoulos GC. Inflammatory changes induced by transplanted neural precursor cells in a multiple sclerosis model. Neuroreport. [Research Support, Non-U.S. Gov't] 2011 Jan 26;22(2):68–72. doi: 10.1097/WNR.0b013e32834272eb. [DOI] [PubMed] [Google Scholar]
- 107.Carbajal KS, Weinger JG, Whitman LM, Schaumburg CS, Lane TE. Surgical transplantation of mouse neural stem cells into the spinal cords of mice infected with neurotropic mouse hepatitis virus. J Vis Exp. [Video-Audio Media] 2011;(53):e2834. doi: 10.3791/2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yip S, Aboody KS, Burns M, Imitola J, Boockvar JA, Allport J, et al. Neural stem cell biology may be well suited for improving brain tumor therapies. Cancer J. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.Review] 2003 May-Jun;9(3):189–204. doi: 10.1097/00130404-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 109.Bonab MM, Sahraian MA, Aghsaie A, Karvigh SA, Hosseinian SM, Nikbin B, et al. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther. 2012 Nov 1;7(6):407–14. doi: 10.2174/157488812804484648. [DOI] [PubMed] [Google Scholar]
- 110.Payne NL, Sun G, McDonald C, Layton D, Moussa L, Emerson-Webber A, et al. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant. 2012 Oct 4; doi: 10.3727/096368912X657620. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 111.Cobo M, Anderson P, Benabdellah K, Toscano MG, Munoz P, Garcia-Perez A, et al. Mesenchymal stem cells expressing vasoactive intestinal peptide ameliorate symptoms in a model of chronic multiple sclerosis. Cell Transplant. 2012 Oct 2; doi: 10.3727/096368912X657404. [Epub ahead of print] PubMed PMID: 23031550. [DOI] [PubMed] [Google Scholar]
- 112.Al Jumah MA, Abumaree MH. The Immunomodulatory and Neuroprotective Effects of Mesenchymal Stem Cells (MSCs) in Experimental Autoimmune Encephalomyelitis (EAE): A Model of Multiple Sclerosis (MS) Int J Mol Sci. 2012;13(7):9298–331. doi: 10.3390/ijms13079298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fisher-Shoval Y, Barhum Y, Sadan O, Yust-Katz S, Ben-Zur T, Lev N, et al. Transplantation of placenta-derived mesenchymal stem cells in the EAE mouse model of MS. J Mol Neurosci. 2012 Sep;48(1):176–84. doi: 10.1007/s12031-012-9805-6. [DOI] [PubMed] [Google Scholar]
- 114.Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2012 Jun;15(6):862–70. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Payne NL, Dantanarayana A, Sun G, Moussa L, Caine S, McDonald C, et al. Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adh Migr. [Research Support, Non-U.S. Gov't] 2012 May-Jun;6(3):179–89. doi: 10.4161/cam.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. [Clinical Trial, Phase II] 2012 Feb;11(2):150–6. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang YJ, Zhang W, Lin CG, Ding Y, Huang SF, Wu JL, et al. Neurotrophin-3 gene modified mesenchymal stem cells promote remyelination and functional recovery in the demyelinated spinal cord of rats. J Neurol Sci. [Comparative Study Research Support, Non-U.S. Gov't] 2012 Feb 15;313(1-2):64–74. doi: 10.1016/j.jns.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 118.Harris VK, Faroqui R, Vyshkina T, Sadiq SA. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl Med. [Research Support, Non-U.S. Gov't] 2012 Jul;1(7):536–47. doi: 10.5966/sctm.2012-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Odinak MM, Bisaga GN, Novitskii AV, Tyrenko VV, Fominykh MS, Bilibina AA, et al. Transplantation of mesenchymal stem cells in multiple sclerosis] Zh Nevrol Psikhiatr Im S S Korsak. [Clinical Trial]. 2011;111(2 Pt 2):72–6. [PubMed] [Google Scholar]
- 120.Mohajeri M, Farazmand A, Mohyeddin Bonab M, Nikbin B, Minagar A. FOXP3 gene expression in multiple sclerosis patients pre- and post mesenchymal stem cell therapy. Iran J Allergy Asthma Immunol. 2011;10(3):155–61. [PubMed] [Google Scholar]
- 121.Grigoriadis N, Lourbopoulos A, Lagoudaki R, Frischer JM, Polyzoidou E, Touloumi O, et al. Variable behavior and complications of autologous bone marrow mesenchymal stem cells transplanted in experimental autoimmune encephalomyelitis. Exp Neurol. [Research Support, Non-U.S. Gov't] 2011 Jul;230(1):78–89. doi: 10.1016/j.expneurol.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 122.Cristofanilli M, Harris VK, Zigelbaum A, Goossens AM, Lu A, Rosenthal H, et al. Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells Dev. [Research Support, Non-U.S. Gov't] 2011 Dec;20(12):2065–76. doi: 10.1089/scd.2010.0547. [DOI] [PubMed] [Google Scholar]
- 123.Darlington PJ, Boivin MN, Renoux C, Francois M, Galipeau J, Freedman MS, et al. Reciprocal Th1 and Th17 regulation by mesenchymal stem cells: Implication for multiple sclerosis. Annals of neurology, [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2010 Oct;68(4):540–5. doi: 10.1002/ana.22065. [DOI] [PubMed] [Google Scholar]
- 124.Rice CM, Scolding NJ. Adult human mesenchymal cells proliferate and migrate in response to chemokines expressed in demyelination. Cell Adh Migr. [Research Support, Non-U.S. Gov't] 2010 Apr-Jun;4(2):235–40. doi: 10.4161/cam.4.2.11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kemp K, Gray E, Mallam E, Scolding N, Wilkins A. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev. [Research Support, Non-U.S. Gov't] 2010 Dec;6(4):548–59. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 126.Barhum Y, Gai-Castro S, Bahat-Stromza M, Barzilay R, Melamed E, Offen D. Intracerebroventricular transplantation of human mesenchymal stem cells induced to secrete neurotrophic factors attenuates clinical symptoms in a mouse model of multiple sclerosis. J Mol Neurosci. [Research Support, Non-U.S. Gov't] 2010 May;41(1):129–37. doi: 10.1007/s12031-009-9302-8. [DOI] [PubMed] [Google Scholar]
- 127.Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009 Oct;27(10):2624–35. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 128.Liang J, Zhang H, Hua B, Wang H, Wang J, Han Z, et al. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler. 2009 May;15(5):644–6. doi: 10.1177/1352458509104590. [DOI] [PubMed] [Google Scholar]
- 129.Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009 Aug 15;57(11):1192–203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4(1):50–7. [PubMed] [Google Scholar]
- 131.Shevchenko JL, Kuznetsov AN, Ionova TI, Melnichenko VY, Fedorenko DA, Kartashov AV, et al. Autologous hematopoietic stem cell transplantation with reduced-intensity conditioning in multiple sclerosis. Exp Hematol. [Clinical Trial, Phase II] 2012 Nov;40(11):892–8. doi: 10.1016/j.exphem.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 132.Rabusin M, Snowden JA, Veys P, Quartier P, Dalle JH, Dhooge C, et al. Long-Term Outcomes of Hematopoietic Stem Cell Transplantation for Severe Treatment-Resistant Autoimmune Cytopenia in Children. Biol Blood Marrow Transplant. 2012 Dec 16; doi: 10.1016/j.bbmt.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 133.Lutterotti A, Jelcic I, Schulze C, Schippling S, Breiden P, Mazzanti B, et al. No proinflammatory signature in CD34+ hematopoietic progenitor cells in multiple sclerosis patients. Mult Scler. 2012 Aug;18(8):1188–92. doi: 10.1177/1352458511434067. [DOI] [PubMed] [Google Scholar]
- 134.Atkins HL, Muraro PA, van Laar JM, Pavletic SZ. Autologous hematopoietic stem cell transplantation for autoimmune disease--is it now ready for prime time? Biol Blood Marrow Transplant. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't Review] 2012 Jan;18(1 Suppl):S177–83. doi: 10.1016/j.bbmt.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen B, Zhou M, Ouyang J, Zhou R, Xu J, Zhang Q, et al. Long-term efficacy of autologous haematopoietic stem cell transplantation in multiple sclerosis at a single institution in China. Neurol Sci. 2012;33(4):881–6. doi: 10.1007/s10072-011-0859-y. [DOI] [PubMed] [Google Scholar]
- 136.Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler. [Multicenter Study Research Support, Non-U.S. Gov't] 2012 Jun;18(6):835–42. doi: 10.1177/1352458511429320. [DOI] [PubMed] [Google Scholar]
- 137.Capobianco M, Motuzova Y, Frau J, Cocco E, Mamusa E, Marrosu MG, et al. Natalizumab in aggressive multiple sclerosis after haematopoietic stem cell transplantation. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2012 Aug;33(4):863–7. doi: 10.1007/s10072-011-0848-1. [DOI] [PubMed] [Google Scholar]
- 138.Fassas A, Kimiskidis VK, Sakellari I, Kapinas K, Anagnostopoulos A, Tsimourtou V, et al. Long-term results of stem cell transplantation for MS: a single-center experience. Neurology. [Clinical Trial Research Support, Non-U.S. Gov't] 2011 Mar 22;76(12):1066–70. doi: 10.1212/WNL.0b013e318211c537. [DOI] [PubMed] [Google Scholar]
- 139.Reston JT, Uhl S, Treadwell JR, Nash RA, Schoelles K. Autologous hematopoietic cell transplantation for multiple sclerosis: a systematic review. Mult Scler. [Meta-Analysis Research Support, Non-U.S. Gov't Review] 2011 Feb;17(2):204–13. doi: 10.1177/1352458510383609. [DOI] [PubMed] [Google Scholar]
- 140.Xu J, Ji BX, Su L, Dong HQ, Sun WL, Wan SG, et al. Clinical outcome of autologous peripheral blood stem cell transplantation in opticospinal and conventional forms of secondary progressive multiple sclerosis in a Chinese population. Ann Hematol. [Clinical Trial Research Support, Non-U.S. Gov't] 2011 Mar;90(3):343–8. doi: 10.1007/s00277-010-1071-5. [DOI] [PubMed] [Google Scholar]
- 141.Guimaraes FA, Oliveira-Cardoso EA, Mastropietro AP, Voltarelli JC, Santos MA. Impact of autologous hematopoetic stem cell transplantation on the quality of life of patients with multiple sclerosis. Arq Neuropsiquiatr, [Evaluation Studies Research Support, Non-U.S. Gov't] 2010 Aug;68(4):522–7. doi: 10.1590/s0004-282x2010000400009. [DOI] [PubMed] [Google Scholar]
- 142.Lu JQ, Joseph JT, Nash RA, Storek J, Stevens AM, Metz LM, et al. Neuroinflammation and demyelination in multiple sclerosis after allogeneic hematopoietic stem cell transplantation. Arch Neurol. [Research Support, Non-U.S. Gov't] 2010 Jun;67(6):716–22. doi: 10.1001/archneurol.2010.117. [DOI] [PubMed] [Google Scholar]
- 143.Krasulova E, Trneny M, Kozak T, Vackova B, Pohlreich D, Kemlink D, et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult Scler. [Research Support, Non-U.S. Gov't] 2010 Jun;16(6):685–93. doi: 10.1177/1352458510364538. [DOI] [PubMed] [Google Scholar]
- 144.Tappenden P, Saccardi R, Confavreux C, Sharrack B, Muraro PA, Mancardi GL, et al. Autologous haematopoietic stem cell transplantation for secondary progressive multiple sclerosis: an exploratory cost-effectiveness analysis. Bone Marrow Transplant. [Comparative Study] 2010 Jun;45(6):1014–21. doi: 10.1038/bmt.2009.305. [DOI] [PubMed] [Google Scholar]
- 145.Rogojan C, Frederiksen JL. Hematopoietic stem cell transplantation in multiple sclerosis. Acta Neurol Scand. [Review] 2009 Dec;120(6):371–82. doi: 10.1111/j.1600-0404.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- 146.Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. [Clinical Trial, Phase I Clinical Trial, Phase II Research Support, Non-U.S. Gov't] 2009 Mar;8(3):244–53. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 147.Fassas A, Mancardi GL. Autologous hemopoietic stem cell transplantation for multiple sclerosis: is it worthwile? Autoimmunity. [Review] 2008 Dec;41(8):601–10. doi: 10.1080/08916930802197347. [DOI] [PubMed] [Google Scholar]
- 148.Fagius J, Lundgren J, Oberg G. Early highly aggressive MS successfully treated by hematopoietic stem cell transplantation. Mult Scler. [Clinical Trial] 2009 Feb;15(2):229–37. doi: 10.1177/1352458508096875. [DOI] [PubMed] [Google Scholar]
- 149.Saiz A, Blanco Y, Berenguer J, Gomez-Choco M, Carreras E, Arbizu T, et al. Clinical outcome 6 years after autologous hematopoietic stem cell transplantation in multiple sclerosis] Neurologia. 2008 Sep;23(7):405–7. [PubMed] [Google Scholar]
- 150.Shevchenko YL, Novik AA, Kuznetsov AN, Afanasiev BV, Lisukov IA, Kozlov VA, et al. High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Exp Hematol. [Clinical Trial, Phase II Multicenter Study] 2008 Aug;36(8):922–8. doi: 10.1016/j.exphem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 151.Rocca MA, Mondria T, Valsasina P, Sormani MP, Flach ZH, Te Boekhorst PA, et al. A three-year study of brain atrophy after autologous hematopoietic stem cell transplantation in rapidly evolving secondary progressive multiple sclerosis. AJNR Am J Neuroradiol. [Clinical Trial]. 2007 Oct;28(9):1659–61. doi: 10.3174/ajnr.A0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Portaccio E, Amato MP, Siracusa G, Pagliai F, Sorbi S, Guidi S, et al. Autologous hematopoietic stem cell transplantation for very active relapsing-remitting multiple sclerosis: report of two cases. Mult Scler. [Case Reports] 2007 Jun;13(5):676–8. doi: 10.1177/1352458506073502. [DOI] [PubMed] [Google Scholar]
- 153.Roccatagliata L, Rocca M, Valsasina P, Bonzano L, Sormani M, Saccardi R, et al. Italian GITMO-NEURO Intergroup on Autologous Stem Cell Transplantation. The long-term effect of AHSCT on MRI measures of MS evolution: a five-year follow-up study. Mult Scler. 2007;13(8):1068–70. doi: 10.1177/1352458507076982. [DOI] [PubMed] [Google Scholar]
- 154.Metz I, Lucchinetti CF, Openshaw H, Garcia-Merino A, Lassmann H, Freedman MS, et al. Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain. [Research Support, Non-U.S. Gov't] 2007 May;130(Pt 5):1254–62. doi: 10.1093/brain/awl370. [DOI] [PubMed] [Google Scholar]
- 155.Loh Y, Oyama Y, Statkute L, Quigley K, Yaung K, Gonda E, et al. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: role of conditioning regimen used. Blood. 2007 Mar 15;109(6):2643–548. doi: 10.1182/blood-2006-07-035766. [DOI] [PubMed] [Google Scholar]
- 156.Su L, Xu J, Ji BX, Wan SG, Lu CY, Dong HQ, et al. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol. 2006 Oct;84(3):276–81. doi: 10.1532/IJH97.A10516. [DOI] [PubMed] [Google Scholar]
- 157.Ni XS, Ouyang J, Zhu WH, Wang C, Chen B. Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant. [Research Support, Non-U.S. Gov't] 2006 Jul-Aug;20(4):485–9. doi: 10.1111/j.1399-0012.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 158.Daumer M, Griffith LM, Meister W, Nash RA, Wolinsky JS. Survival, and time to an advanced disease state or progression, of untreated patients with moderately severe multiple sclerosis in a multicenter observational database: relevance for design of a clinical trial for high dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation. Mult Scler. [Multicenter Study Research Support, N.I.H, Extramural] 2006 Apr;12(2):174–9. doi: 10.1191/135248506ms1256oa. [DOI] [PubMed] [Google Scholar]
- 159.Papadaki HA, Tsagournisakis M, Mastorodemos V, Pontikoglou C, Damianaki A, Pyrovolaki K, et al. Normal bone marrow hematopoietic stem cell reserves and normal stromal cell function support the use of autologous stem cell transplantation in patients with multiple sclerosis. Bone Marrow Transplant. [Research Support, Non-U.S. Gov't] 2005 Dec;36(12):1053–63. doi: 10.1038/sj.bmt.1705179. [DOI] [PubMed] [Google Scholar]
- 160.Blanco Y, Saiz A, Costa M, Torres-Peraza JF, Carreras E, Alberch J, et al. Evolution of brain-derived neurotrophic factor levels after autologous hematopietic stem cell transplantation in multiple sclerosis. Neurosci Lett. [Clinical Trial Comparative Study Research Support, Non-U.S. Gov't] 2005 May 20-27;380(1-2):122–6. doi: 10.1016/j.neulet.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 161.Blanco Y, Saiz A, Carreras E, Graus F. Autologous haematopoietic-stem-cell transplantation for multiple sclerosis. Lancet Neurol. [Research Support, Non-U.S. Gov't Review] 2005 Jan;4(1):54–63. doi: 10.1016/S1474-4422(04)00966-4. [DOI] [PubMed] [Google Scholar]
- 162.Saccardi R, Tyndall A, Coghlan G, Denton C, Edan G, Emdin M, et al. Consensus statement concerning cardiotoxicity occurring during haematopoietic stem cell transplantation in the treatment of autoimmune diseases, with special reference to systemic sclerosis and multiple sclerosis. Bone Marrow Transplant. [Consensus Development Conference Research Support, Non-U.S. Gov't]. Nov. 2004 Nov;34(10):877–81. doi: 10.1038/sj.bmt.1704656. [DOI] [PubMed] [Google Scholar]
- 163.Blanco Y, Saiz A, Carreras E, Graus F. Changes of matrix metalloproteinase-9 and its tissue inhibitor (TIMP-1) after autologous hematopoietic stem cell transplantation in multiple sclerosis. J Neuroimmunol. [Comparative Study] 2004 Aug;153(1-2):190–4. doi: 10.1016/j.jneuroim.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 164.Healey KM, Pavletic SZ, Al-Omaishi J, Leuschen MP, Pirruccello SJ, Filipi ML, et al. Discordant functional and inflammatory parameters in multiple sclerosis patients after autologous haematopoietic stem cell transplantation. Mult Scler. [Clinical Trial Research Support, Non-U.S. Gov't] 2004 Jun;10(3):284–9. doi: 10.1191/1352458504ms1022oa. [DOI] [PubMed] [Google Scholar]
- 165.Inglese M, Mancardi GL, Pagani E, Rocca MA, Murialdo A, Saccardi R, et al. Brain tissue loss occurs after suppression of enhancement in patients with multiple sclerosis treated with autologous haematopoietic stem cell transplantation. J Neurol Neurosurg Psychiatry. [Clinical Trial Clinical Trial, Phase I Clinical Trial, Phase II Research Support, Non-U.S. Gov't] 2004 Apr;75(4):643–4. [PMC free article] [PubMed] [Google Scholar]
- 166.Sun W, Popat U, Hutton G, Zang YC, Krance R, Carrum G, et al. Characteristics of T-cell receptor repertoire and myelin-reactive T cells reconstituted from autologous haematopoietic stem-cell grafts in multiple sclerosis. Brain. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. 2004 May;127(Pt 5):996–1008. doi: 10.1093/brain/awh117. [DOI] [PubMed] [Google Scholar]
- 167.Saiz A, Blanco Y, Carreras E, Berenguer J, Rovira M, Pujol T, et al. Clinical and MRI outcome after autologous hematopoietic stem cell transplantation in MS. Neurology. [Research Support, Non-U.S. Gov't] 2004 Jan 27;62(2):282–4. doi: 10.1212/wnl.62.2.282. [DOI] [PubMed] [Google Scholar]
- 168.Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. [Multicenter Study Research Support, Non-U.S. Gov't] 2005 Mar 15;105(6):2601–7. doi: 10.1182/blood-2004-08-3205. [DOI] [PubMed] [Google Scholar]
- 169.Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y, et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. [Clinical Trial Clinical Trial, Phase I Clinical Trial, Phase II] 2003 Oct 1;102(7):2373–8. doi: 10.1182/blood-2003-03-0877. [DOI] [PubMed] [Google Scholar]
- 170.Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. [Clinical Trial Multicenter Study Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S. 2003 Oct 1;102(7):2364–72. doi: 10.1182/blood-2002-12-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carreras E, Saiz A, Marin P, Martinez C, Rovira M, Villamor N, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica, [Clinical Trial Research Support, Non-U.S. Gov't] 2003 Mar;88(3):306–14. [PubMed] [Google Scholar]
- 172.Rossiev VA, Makarov SV, Aleksandrova I, Dolgikh GT, Lipshina SR, Stukalova TA, et al. Experience with high-dose immunosuppressive therapy followed by transplantation of autologous stem hematopoietic cells in patients with multiple sclerosis] Ter Arkh. [Clinical Trial] 2002;74(7):35–8. [PubMed] [Google Scholar]
- 173.Ouyang J, Ni X, Chen B. A preliminary result of treatment of progressive multiple sclerosis with autologous peripheral blood stem cell transplantation in China] Zhonghua Nei Ke Za Zhi. [Case Reports] 2001 Aug;40(8):550–2. [PubMed] [Google Scholar]