Abstract
Objective
The use of fetal hemoglobin (HbF) inducer drugs is considered as a novel approach in treatment of β-hemoglobinopathies, especially β- thalassemia and sickle cell disease. HbF inducers including hydroxyurea, histone deacetylase (HDAC) inhibitor agents such as sodium butyrate, azacitidine, decitabine and new immunomodulator drugs like pomalidomide, lenalidomide and thalidomide can reduce α-globin chain production in erythroid progenitors and improve α: β chain imbalance, the most crucial complication of β-thalassemia.
Materials and Methods
In this article, we reviewed more than 40 articles published from 1979 to 2012 in the field of fetal hemoglobin augmentation.
Results
Recent studies suggest the synergistic effect of drug combinations in efficient induction of fetal hemoglobin and gene over-expression.
Conclusion
It seems that drugs which act with different molecular and epigenetic mechanisms have proper synergistic effects in fetal hemoglobin induction and gene over-expression.
Keywords: Fetal hemoglobin, β-Hemoglobinopathies, Histone deacetylase
INTRODUCTION
β- hemoglobinopathies are among in the monogenic blood disorders with autosomal recessive inheritance and a high mortality worldwide. β-thalassemia and sickle cell disease (SCD) are considered the most common β- hemoglobinopathies. β-thalassemia has a high prevalence in regions bordering the Mediterranean Sea, Middle East, Central Asia, India, south China, Far East, northern Africa and South America. According to investigations, the highest prevalence of β-thalassemia carriers has been reported in Cyprus (14 %), Sardinia (10.3%) and Southeast Asian regions.1, 2 β-thalassemia is the result of defective hemoglobin production due to reduced or absent expression of β- globin gene. This phenomenon is due to a wide range of point mutations and some deletions in this gene, causing impaired production of β- globin chain. So far, more than 200 point mutations causing β-thalassemia have been reported.3, 4 Based on studies, IVS-II-1 mutation is considered as the most common type of β- thalassemia mutation in the Iranian population.5 Following mutation in β-globin gene, polypeptide chains of α-globin are not able to participate in the structure of hemoglobin, and they precipitate in erythroid precursors resulting in ineffective erythropoiesis. In fact, lack of balance between α- globin and β-globin chains is the major factor in the pathology of β- thalassemia.6–13 In SCD, polymerization of hemoglobin S (HbS) due to lack of oxygen causes disorders such as ischemia, vascular occlusion, tissue scarring and other acute and chronic complications in patients.14 The cause of SCD is a point mutation of β-globin gene in amino acid at position 6, giving rise to defective β-globin chain and formation of HbS.15
Currently, the main focal point of therapy in β-thalassemia patients is a regular blood transfusion schedule and use of iron chelating agents. Allogeneic transplantation of hematopoietic stem cell is of course the only basic therapy available for thalassemia. In Class 1 and Class 2 thalassemic patients who are younger than 17 years of age, BMT (Bone Marrow Transplantation) from a donor with compatible HLA has cured the disease in most patients. BMT is associated with a number of problems including finding the donor with compatible HLA and requiring long-term use of immunosuppressive drugs to prevent or treat GVHD (Graft Versus Host Disease).16
Due to these limitations and severe therapeutic complications of routine therapeutic strategies, novel therapeutic methods for treatment of β-thalassemia seem to be necessary. In recent years, the use of fetal hemoglobin (HbF) inducing drugs is regarded as the appropriate therapy for hemoglobinopathies. HbF expression in β-thalassemia will decrease the accumulation and precipitation of α-globin chains, and thus reduces the ineffective erythropoiesis. The high level of HbF has a direct relationship with acute clinical status in SCD including pain crises, acute chest syndrome and death. Therefore, HbF expression induction is an important therapeutic strategy in reducing the clinical morbidity and mortality in patients with β-thalassemia and SCD.2, 17 The use of pharmacological agents inducing expression of γ-globin gene can be important in treatment of β-globin disorders, and can be a subject for more extensive review. The origin of this idea is the observation that high levels of HbF at birth are associated with milder clinical symptoms in patients with β-globin disorders. With this interpretation, following induction of HbF in such patients, partial compensation for the oxygen deficiency problem due to lack of HbF is expected along with reduced side effects resulting from the accumulation of α chains.15–17
Switching in β- Globin Gene Family
β-globin like gene family is located on chromosome 11, and includes five coding regions from 5’ to 3’ comprising ε, γG, γA, d and β genes, respectively. The placement of genes from 5’ to 3’ direction is based on the evolution of gene expression in fetus with predominant expression of ε gene in yolk sac blood islands, γG and γA genes during embryonic period in the liver as well as δ and β genes in the bone marrow in postnatal period.18 Following switching of γ-globin gene expression to β-globin after birth, complications of β-thalassemia and SCD are manifested. Thus, the use of drugs affecting the silencing of γ-globin gene or preventing it can be regarded as an effective therapeutic approach. Several studies indicate the important role of epigenetic changes in changing expression pattern of various genes including β- globin like genes. The change in methylation pattern in DNA level especially in CpG islands along with lysine residue acetylation in N-terminal Histone regions is among the therapeutic approaches to change the pattern of gene expression. Using medications that decrease the methylation in DNA level and increase Histone acetylation in γ- globin gene especially in promoter region can induce γ- globin gene expression and thus increase HbF level in patients with β-thalassemia and SCD.18
Fetal Hemoglobin Gene Inducing Agents
Hydroxyurea (HU) is considered as an inducer of HbF production. Since HU has the potential to induce γ-globin gene and reduce the expression of β-globin gene and because of its anti-sickling effect, prescription of HU is indicated in SCD treatment. In addition, HU reduces the number of white blood cells and prevents their activation, and thereby inhibits vascular occlusion in these patients. The effect of HU in treating patients with β-thalassemia major and intermedia has been very disappointing, and it has not made any significant improvement in the anemia either. In fact, this drug results in regeneration of erythroid series through its cytotoxic effects, and releases nitric oxide (NO) through metabolic effects.18–20 The NO induced HbF via recruitment of P38 Mitogen-activated protein kinase (p38 MAPK) pathway.21
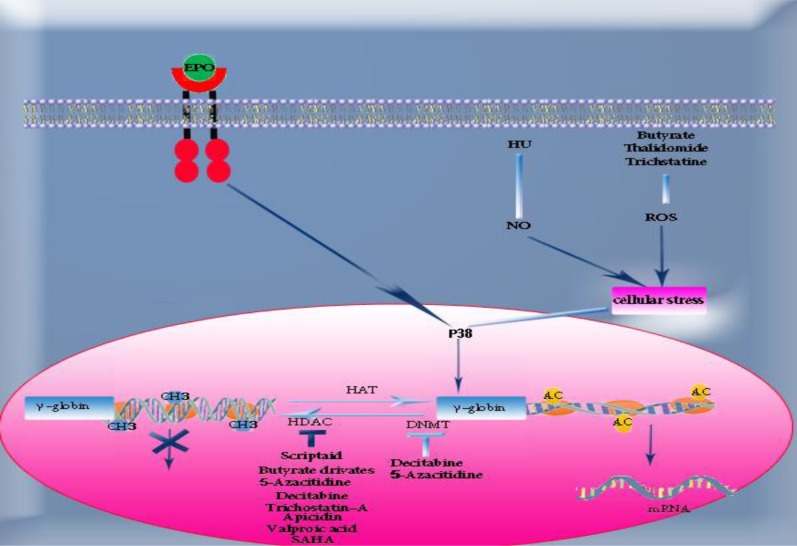
Histone deacetylase (HDAC) inhibitors including butyrate derivatives, azacitidine, decitabine (5-aza 2-deoxycytidine) and trichostatin-A play a role in γ- globin gene induction by changing the epigenetic patterns of β-globin cluster such as increasing histone H3 acetylation and decreasing methylation in γ- globin gene promoter. Azacitidine is considered an inducer drug with a high potential to increase production of fetal hemoglobin. Azacitidine and decitabine act as HDAC and DNA methyl transferase (DNMT) inhibitors that result in increased HbF.22–27 Decitabine, the analogous drug of azacitidine, has a higher potential in DNMT enzyme inhibition compared with azacitidine, as well as in activation of tumor suppressor genes. (Figure 1) This drug has had proper therapeutic effects in patients with sickle cell anemia, even in patients resistant to HU.16, 26 In addition, butyrate derivative drugs such as sodium butyrate and arginine butyrate have been considered low risk and highly effective in treatment of sickle cell anemia and thalassemia. The studies have shown that the level of HbF is increased by the same rate following intermittent and continuous administration of butyrate, and therefore the effect of butyrate in induction of γ-gene expression appears to be heritable. Butyrate has also been capable of increasing the expression of β-globin gene, while azacitidine has no effect on β-globin gene expression. In addition, butyrate can increase histone acetylation in ε gene, but there is no increase in expression of ε gene following this phenomenon. This is due to other factors such as specific transcription factors and epigenetic changes in LCR.26, 28, 29
Figure 1.
Drugs and their induction mechanism of γ-globin gene. For details refer to text
Abbreviation: EPO, erythropoietin; HU, hydroxyurea; SCF, stem cell factor; NO, nitric oxide; ROS, reactive oxygen species; P38 MAPK, p38 mitogen activation protein kinase; CH3, methyl group; HAT, histone acetyl transferase; HDAC, histone deacetylase; DNMT, DNA methyl transferase; AC, acetyl group; SAHA, suberoylanilide hydroxaminc acid.
There is another compound called hemin, which has a similar function as butyrate in changing the pattern of gene expression but in a lower extent. This compound is an inducer of erythropoiesis with contradictory effects on α- globin gene expression, just like butyrate. Studies have shown that these two compounds increase the level of α- globin gene mRNA in β-thalassemia patients while reducing its level in SCD patients. Therefore, promising effects of increased HbF level following treatment with these two compounds is accounted for as a result of their effect in disrupting the α:β balance in patients with β- thalassemia. However, some studies show that using these two drugs has reduced the globin imbalance in a number of β-thalassemia patients.30
Nicotinic acid and its derivatives have been assessed as HbF inducers. These compounds can stimulate erythroid differentiation in K562 cell line similar to butyrate and hemin, but as they reduce glycophorin A (GphA) expression, they seem to have a mechanism different than the mentioned compounds in inducing erythroid differentiation.31
Compared with HU, Pomalidomide is a stronger inducer of HbF production. It has been demonstrated that the epigenetic mechanisms of this drug in increasing HbF expression is related to acetylation of H3K9 and H3K14 at LCR region of γ-globin gene. It has been also shown that this epigenetic pattern is locus specific without any global changes in H3 acetylation pattern. In addition, pomalidomide has a synergistic effect with HU in the induction of HbF.32–34 In this drug family, thalidomide used for multiple myeloma treatment has appropriate effects in HbF induction with similar mechanism of action with other immunomodulatory agents. The exact mechanism of the therapeutic effect of this factor is still unknown.
However, the effects of thalidomide may be due to suppression of NF-KB induction by inflammatory cytokines such as Tumor Necrosis Factor (TNF- α), Vascular Endothelial Growth Factor (VEGF) and prostaglandin E2 synthesis (PG-E2) associated with increased release of reactive oxygen species (ROS). ROS can launch P38 MAPK, which results in increased HbF levels.(Figure 1) Teratogenic property of thalidomide is still debatable, although it has been speculated that the teratogenic properties of thalidomide may be due to ROS damage.35–38 In a case report published in 2010, appropriate treatment of a patient with β- thalassemia major using thalidomide has been reported.39 In addition, recent studies indicate a synergistic effect of different concentrations of sodium butyrate and thalidomide in induction of β- and γ-globin genes in erythroid cells derived from CD133+ cells in cord blood. Other findings also indicate a high potential of this drug combination in inducing the production of erythroid precursors compared with single-drug treatments. Comparison of the effects of sodium butyrate and thalidomide in gene expression induction suggests higher capacity of the latter in increasing production of β- and γ-globin genes.34, 38, 40–42 Epigenetic studies in this field show that as H3K27 methylation is regarded as an index of heterochromatin and reduced gene expression, combining thalidomide and sodium butyrate has less effect in reduction of histone methylation than thalidomide. Therefore, since these two drugs have a synergistic effect in induction of globin gene expression, the combination drug thalidomide and sodium butyrate exerts this synergistic effect in inducing gene expression through another epigenetic mechanism.43 Azacitidine and Decitabine are also known as DNMT inhibitors.44
In other studies, cytokines like stem cell factor (SCF) and transforming growth factor-beta (TGF-β) have been shown to have HbF induction potential. It is seen that MAPK signaling pathway is associated with fetal hemoglobin induction using SCF.29 This signaling pathway affects the regulatory region in β-globin gene cluster through increased expression of NF-E2 transcription factor. TGF-β also induces the expression of this gene through activation of fetal Krüppel-like transcription factor (FKLF) through recognition of its direct effect in γ-globin gene expression. Another study reports the synergistic effect of SCF, TGF-β and erythropoietin (EPO) in fetal hemoglobin expression induction in erythroid progenitor cells in vitro.45 Table 1 shows molecular and/or epigenetic mechanisms of some other drugs in HbF induction, which have been recently studied.
Table 1.
Molecular and/or Epigenetic Mechanisms of some HbF Inducer Drugs
| Drug | Molecular mechanism | Epigenetic mechanism | Reference |
|---|---|---|---|
| Trichostatin-A | Activation of P38 MAPK signaling pathway | HDAC inhibitor | (46) |
| adipicin | Activation of P38 MAPK signaling pathway | HDAC inhibitor | (47) |
| Valproic acid | Activation of P38 MAPK signaling pathway | HDAC inhibitor | (48, 49) |
| SDMB | ------------------------- | HDAC inhibitor | (50) |
| Scriptaid | Activation of P38 MAPK signaling pathway | HDAC inhibitor | (51) |
| EPO | Activation of STAT5, Src family kinase and ERK-1/ERK-2 MAPK signaling pathways | ------------------------- | (52) |
| Fructus trichosanthis | Activation of ERK and P38 MAPK signaling pathway | ------------------------- | (53) |
| SAHA | ------------------------- | HDAC inhibitor | (54) |
Abbreviations: SDMB, Sodium 2,2 dimethyl butyrate; EPO, erythropoietin; SAHA, suberoylanilide hydroxaminc acid; HDAC, histone deacetylase; MAPK, p38 mitogen activation protein kinase.
DISCUSSION
The use of drugs with a high potential to stimulate erythropoiesis appears to induce HbF expression by increasing proliferation of erythroid precursors. Thus, activation of signaling pathways involved in erythroid differentiation may play an important role in increased expression of γ-globin gene. In addition, in different investigations in recent years on epigenetic induction of fetal hemoglobin expression in clinical and laboratory conditions, various combinations have been used for this purpose. Thalidomide increases HbF via P38 MAPK and is a strong HbF inducer. However, despite a high HbF induction capacity, it has limited application due to teratogenic effects and some neurological complications. In view of reduced dosage, it is a very important drug in diseases in which exploiting its induction effect in increasing production of HbF is desired.35–37 Butyrate increases the level of α- globin gene mRNA in β-thalassemia patients while reducing its level in SCD patients. Therefore, favorable effects of increased HbF level following treatment with this drug are completely covered as a result of its effect in disrupting the α: β balance in patients with β- thalassemia. However, some studies show that using this drug has reduced the globin imbalance in a number of β-thalassemia patients.28 Moreover, its use is limited because of suppressive effect on erythropoiesis,55, 56 and so it has limited capacity for HbF induction.57 DNMT inhibitors such as azacitidine and decitabine are potent HbF inducers and are used in SCD patients resistant to HU44. These drugs are useful for patient with severe SCD and β-thalassemia.58, 59 DNMTs have been administered to these patients with good results, but their use is limited because of concerns about potential carcinogenic effects.25, 26 HU has the potential of γ-globin gene induction as well as reduction of the expression of β- globin gene and has anti-sickling effects, but it is effective in approximately 50% of SCD, and is less effective in increasing HbF for β-thalassemia patients.60–62 All these HbF inducers have mutagenic and carcinogenic effects and cause clinical toxicity, among which only HU has been useful for long term clinical treatment. In a prospective view, recent studies suggest new approaches for HbF induction such as targeting BCL11A63 and ELKF64 (transcription factors involved in Hb switching). Other researches focus on miRNAs (for example, miR 144 that targets α-globin to prevent its precipitation) as therapeutic goals with less side effects.65
ACKNOWLEDGEMENT
The authors thank Dr. Najmaldin Saki for preparation of the figure and for critically reading the manuscript.
REFERENCES
- 1.Flint J, Harding RM, Boyce AJ, Clegg JB. 1 The population genetics of the haemoglobinopathies. Baillière's clinical haematology. 1998;11(1):1–51. doi: 10.1016/s0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 2.Atweh GF, DeSimone J, Saunthararajah Y, Fathallah H, Weinberg RS, Nagel RL. Hemoglobinopathies. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2003:14–39. doi: 10.1182/asheducation-2003.1.14. PubMed PMID: 14633775. [DOI] [PubMed] [Google Scholar]
- 3.Giardine B, van Baal S, Kaimakis P, Riemer C, Miller W, Samara M, et al. HbVar database of human hemoglobin variants and thalassemia mutations: 2007 update. Human Mutation. 2007;28(2):206. doi: 10.1002/humu.9479. [DOI] [PubMed] [Google Scholar]
- 4.Hardison RC, Chui DH, Riemer CR, Miller W, Carver MF, Molchanova TP, et al. Access to a syllabus of human hemoglobin variants (1996) via the World Wide Web. Hemoglobin. 1998 Mar;22(2):113–27. doi: 10.3109/03630269809092136. PubMed PMID: 9576329. [DOI] [PubMed] [Google Scholar]
- 5.Karimi M, Yarmohammadi H, Farjadian S, Zeinali S, Moghaddam Z, Cappellini MD, et al. β-Thalassemia intermedia from southern Iran: IVS-II-1 (G→ A) is the prevalent thalassemia intermedia allele. Hemoglobin. 2002;26(2):147–54. doi: 10.1081/hem-120005452. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MH, Rodgers GP. Pharmacologic modulation of fetal hemoglobin. Medicine. 2001;80(5):328–44. doi: 10.1097/00005792-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Perrine SP. Fetal globin stimulant therapies in the beta-hemoglobinopathies: principles and current potential. Pediatric annals. 2008;37(5):339. doi: 10.3928/00904481-20080501-10. [DOI] [PubMed] [Google Scholar]
- 8.Gallo E, Massaro P, Miniero R, David D, Tarella C. The importance of the genetic picture and globin synthesis in determining the clinical and haematological features of thalassaemia intermedia. British journal of haematology. 1979;41(2):211–21. doi: 10.1111/j.1365-2141.1979.tb05850.x. [DOI] [PubMed] [Google Scholar]
- 9.Schrier SL. Pathobiology of thalassemic erythrocytes. Current opinion in hematology. 1997 Mar;4(2):75–8. doi: 10.1097/00062752-199704020-00001. PubMed PMID: 9107522. [DOI] [PubMed] [Google Scholar]
- 10.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Experimental hematology. 2005;33(3):259–71. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centis F, Tabellini L, Lucarelli G, Buffi O, Tonucci P, Persini B, et al. The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with β-thalassemia major. Blood. 2000;96(10):3624–9. [PubMed] [Google Scholar]
- 12.Mathias LA, Fisher TC, Zeng L, Meiselman HJ, Weinberg KI, Hiti AL, et al. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Experimental hematology. 2000 Dec;28(12):1343–53. doi: 10.1016/s0301-472x(00)00555-5. PubMed PMID: 11146156. [DOI] [PubMed] [Google Scholar]
- 13.Pootrakul P, Sirankapracha P, Hemsorach S, Moungsub W, Kumbunlue R, Piangitjagum A, et al. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in thai patients with thalassemia. Blood. 2000 Oct 1;96(7):2606–12. PubMed PMID: 11001918. [PubMed] [Google Scholar]
- 14.Ataga KI. Novel therapies in sickle cell disease. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2009:54–61. doi: 10.1182/asheducation-2009.1.54. PubMed PMID: 20008182. [DOI] [PubMed] [Google Scholar]
- 15.Wayengera M. Zinc finger nucleases for targeted mutagenesis and repair of the sickle-cell disease mutation: An in-silico study. BMC blood disorders. 2012;12:5. doi: 10.1186/1471-2326-12-5. PubMed PMID: 22583379. Pubmed Central PMCID: 3407482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galanello R, Origa R. Beta-thalassemia. Orphanet journal of rare diseases. 2010;5:11. doi: 10.1186/1750-1172-5-11. PubMed PMID: 20492708. Pubmed Central PMCID: 2893117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trompeter S, Roberts I. Haemoglobin F modulation in childhood sickle cell disease. Br J Haematol. 2009 Feb;144(3):308–16. doi: 10.1111/j.1365-2141.2008.07482.x. PubMed PMID: 19036119. [DOI] [PubMed] [Google Scholar]
- 18.Kiefer CM, Hou C, Little JA, Dean A. Epigenetics of beta-globin gene regulation. Mutation research. 2008 Dec 1;647(1-2):68–76. doi: 10.1016/j.mrfmmm.2008.07.014. PubMed PMID: 18760288. Pubmed Central PMCID: 2617773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkitt MJ, Raafat A. Nitric oxide generation from hydroxyurea: significance and implications for leukemogenesis in the management of myeloproliferative disorders. Blood. 2006 Mar 15;107(6):2219–22. doi: 10.1182/blood-2005-08-3429. PubMed PMID: 16282342. [DOI] [PubMed] [Google Scholar]
- 20.Ma Q, Wyszynski D, Farrell J, Kutlar A, Farrer L, Baldwin C, et al. Fetal hemoglobin in sickle cell anemia: genetic determinants of response to hydroxyurea. The pharmacogenomics journal. 2007;7(6):386–94. doi: 10.1038/sj.tpj.6500433. [DOI] [PubMed] [Google Scholar]
- 21.Liu K, Xing H, Zhang S. Cucurbitacin D induces fetal hemoglobin synthesis in K562 cells and human hematopoietic progenitors through activation of p38 pathway and stabilization of the γ-globin mRNA. Blood Cells, Molecules, and Diseases. 2010;45(4):269–75. doi: 10.1016/j.bcmd.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 22.DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proceedings of the National Academy of Sciences. 1982;79(14):4428–31. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabaera R, Lowrey CH. Response: 5-azacytidine induction of human fetal hemoglobin. Blood. 2008;111(4):2486. doi: 10.1182/blood-2007-06-093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunthararajah Y, Hillery CA, Lavelle D, Molokie R, Dorn L, Bressler L, et al. Effects of 5-aza-2’-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003 Dec 1;102(12):3865–70. doi: 10.1182/blood-2003-05-1738. PubMed PMID: 12907443. [DOI] [PubMed] [Google Scholar]
- 25.Ley TJ, DeSimone J, Anagnou NP, Keller GH, Humphries RK, Turner PH, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. The New England journal of medicine. 1982 Dec 9;307(24):1469–75. doi: 10.1056/NEJM198212093072401. PubMed PMID: 6183586. [DOI] [PubMed] [Google Scholar]
- 26.Fathallah H, Atweh GF. Induction of fetal hemoglobin in the treatment of sickle cell disease. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2006:58–62. doi: 10.1182/asheducation-2006.1.58. PubMed PMID: 17124041. [DOI] [PubMed] [Google Scholar]
- 27.Fard A, Kaviani S, Noruzinia M, Saki N. Epigenetic modulations on the fetal hemoglobin induction. International Journal of Hematology-Oncology and Stem Cell Research. 2012;6(1) [PMC free article] [PubMed] [Google Scholar]
- 28.Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, et al. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. The New England journal of medicine. 1993 Jan 14;328(2):81–6. doi: 10.1056/NEJM199301143280202. PubMed PMID: 7677966. [DOI] [PubMed] [Google Scholar]
- 29.Hagh MF, Fard AD, Saki N, Shahjahani M, Kaviani S. Molecular Mechanisms of hemoglobin F induction. International Journal of Hematology-Oncology and Stem Cell Research. 2011;5(4) [Google Scholar]
- 30.Marianna P, Kollia P, Akel S, Papassotiriou Y, Stamoulakatou A, Loukopoulos D. Valproic acid, trichostatin and their combination with hemin preferentially enhance gamma-globin gene expression in human erythroid liquid cultures. Haematologica. 2001 Jul;86(7):700–5. PubMed PMID: 11454524. [PubMed] [Google Scholar]
- 31.Ida C, Ogata S, Okumura K, Taguchi H. Induction of differentiation in k562 cell line by nicotinic acid-related compounds. Bioscience, biotechnology, and biochemistry. 2009 Jan;73(1):79–84. doi: 10.1271/bbb.80483. PubMed PMID: 19129652. [DOI] [PubMed] [Google Scholar]
- 32.Sekeres MA, List A. Lenalidomide (Revlimid, CC-5013) in myelodysplastic syndromes: Is it any good? Current hematologic malignancy reports. 2006 Mar;1(1):16–9. doi: 10.1007/s11899-006-0012-9. PubMed PMID: 20425326. [DOI] [PubMed] [Google Scholar]
- 33.Moutouh-de Parseval LA, Verhelle D, Glezer E, Jensen-Pergakes K, Ferguson GD, Corral LG, et al. Pomalidomide and lenalidomide regulate erythropoiesis and fetal hemoglobin production in human CD34+ cells. The Journal of clinical investigation. 2008 Jan;118(1):248–58. doi: 10.1172/JCI32322. PubMed PMID: 18064299. Pubmed Central PMCID: 2117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehghanifard A, Kaviani S, Noruzinia M, Soleimani M, Abroun S, Zonoubi Z. Evaluation the effect of thalidomide and sodium butyrate on cord blood stem cell differentiation induction to erythroid lineage; Genetics in the 3rd Millennium; 2011. [Google Scholar]
- 35.Aerbajinai W, Zhu J, Gao Z, Chin K, Rodgers GP. Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood. 2007 Oct 15;110(8):2864–71. doi: 10.1182/blood-2007-01-065201. PubMed PMID: 17620452. Pubmed Central PMCID: 2018668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens TD, Fillmore BJ. Hypothesis: thalidomide embryopathy-proposed mechanism of action. Teratology. 2000 Mar;61(3):189–95. doi: 10.1002/(SICI)1096-9926(200003)61:3<189::AID-TERA6>3.0.CO;2-W. PubMed PMID: 10661908. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson T, Bjorkman S, Hoglund P. Clinical pharmacology of thalidomide. European journal of clinical pharmacology. 2001 Aug;57(5):365–76. doi: 10.1007/s002280100320. PubMed PMID: 11599654. [DOI] [PubMed] [Google Scholar]
- 38.Fard AD, Kaviani S, Saki N, Mortaz E. The emerging role of immunomodulatory agents in fetal hemoglobin induction. International Journal of Hematology-Oncology and Stem Cell Research. 2012;6(4):35–6. [Google Scholar]
- 39.Masera N, Tavecchia L, Capra M, Cazzaniga G, Vimercati C, Pozzi L, et al. Optimal response to thalidomide in a patient with thalassaemia major resistant to conventional therapy. Blood transfusion = Trasfusione del sangue. 2010 Jan;8(1):63–5. doi: 10.2450/2009.0102-09. PubMed PMID: 20104280. Pubmed Central PMCID: 2809513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmadvand M, Norouznia M, Soleimani M, Kaviani, Abroun S, Dehghanifard A. Invitro induction of the gama glubin gene in erithroid cells derived from CD133+ by thalidomid and sodium butyrate; Genetic in the 3RD Millennium; 2011. [Google Scholar]
- 41.Dehghanifard A, Kaviani S, Noruzinia M, Soleimani M, Abroun S, Hajifathali A, et al. Synergistic Effect of Sodium Butyrate and Thalidomide in the Induction of Fetal Hemoglobin Expression in Erythroid Progenitors Derived from Cord Blood CD133+ Cells. Zahedan Journal of Research in Medical Sciences. 2012;14(7):29–33. [Google Scholar]
- 42.Fard AD, Kaviani S, Noruzinia M, Soleimani M, Abroun S, Chegeni R, et al. Evaluation of H3 Histone Methylation and Colony Formation in Erythroid Progenitors Treated with Thalidomide and Sodium Butyrate. Laboratory Hematology. 2013;19(1):1–5. doi: 10.1532/LH96.12003. [DOI] [PubMed] [Google Scholar]
- 43.Fard AD, Kaviani S, Noruzinia M, Soleimani M, Abroun S, Hajifathali A. Changing the pattern of histone H3 methylation following treatment of erythroid progenitors derived from cord blood CD133+ cells with sodium butyrate and thalidomide; [Google Scholar]
- 44.Koshy M, Dorn L, Bressler L, Molokie R, Lavelle D, Talischy N, et al. 2-deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood. 2000;96(7):2379–84. [PubMed] [Google Scholar]
- 45.Atashi A, Soleimani M, Kaviani S, Hajifathali A, Arefian E. In vitro induction of fetal hemoglobin in erythroid cells derived from CD133+ cells by transforming growth factor-and stem cell factor. Iranian Journal of Biotechnology (IJB) 2008;6(3) [Google Scholar]
- 46.Sangerman J, Lee MS, Yao X, Oteng E, Hsiao C-H, Li W, et al. Mechanism for fetal hemoglobin induction by histone deacetylase inhibitors involves γ-globin activation by CREB1 and ATF-2. Blood. 2006;108(10):3590–9. doi: 10.1182/blood-2006-01-023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao H, Stamatoyannopoulos G, Jung M. Induction of human γ globin gene expression by histone deacetylase inhibitors. Blood. 2004;103(2):701–9. doi: 10.1182/blood-2003-02-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witt O, Mönkemeyer S, Kanbach K, Pekrun A. Induction of fetal hemoglobin synthesis by valproate: modulation of MAPkinase pathways. American journal of hematology. 2002;71(1):45–6. doi: 10.1002/ajh.10161. [DOI] [PubMed] [Google Scholar]
- 49.Chu BF, Karpenko MJ, Liu Z, Aimiuwu J, Villalona-Calero MA, Chan KK, et al. Phase I study of 5-aza-2’-deoxycytidine in combination with valproic acid in non-small-cell lung cancer. Cancer chemotherapy and pharmacology. 2013 Jan;71(1):115–21. doi: 10.1007/s00280-012-1986-8. PubMed PMID: 23053268. [DOI] [PubMed] [Google Scholar]
- 50.Perrine SP, Wargin WA, Boosalis MS, Wallis WJ, Case S, Keefer JR, et al. Evaluation of Safety and Pharmacokinetics of Sodium 2, 2 Dimethylbutyrate, a Novel Short Chain Fatty Acid Derivative, in a Phase 1, Double-Blind, Placebo-Controlled, Single-Dose, and Repeat-Dose Studies in Healthy Volunteers. The Journal of Clinical Pharmacology. 2011;51(8):1186–94. doi: 10.1177/0091270010379810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zein S, Li W, Ramakrishnan V, Lou T-F, Sivanand S, Mackie A, et al. Identification of fetal hemoglobin-inducing agents using the human leukemia KU812 cell line. Experimental Biology and Medicine. 2010;235(11):1385–94. doi: 10.1258/ebm.2010.010129. [DOI] [PubMed] [Google Scholar]
- 52.Munugalavadla V, Kapur R. Role of c-Kit and erythropoietin receptor in erythropoiesis. Critical reviews in oncology/hematology. 2005;54(1):63. doi: 10.1016/j.critrevonc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Ko CH, Tsang SY, Leung PC, Fung MC, Fung KP. The ethanol extract of Fructus trichosanthis promotes fetal hemoglobin production via p38 MAPK activation and ERK inactivation in K562 cells. Evidence-Based Complementary and Alternative Medicine. 2011;2011 doi: 10.1093/ecam/neq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hebbel RP, Vercellotti GM, Pace BS, Solovey AN, Kollander R, Abanonu CF, et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood. 2010 Mar 25;115(12):2483–90. doi: 10.1182/blood-2009-02-204990. PubMed PMID: 20053759. Pubmed Central PMCID: 2845902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Constantoulakis P, Papayannopoulou T, Stamatoyannopoulos G. alpha-Amino-N-butyric acid stimulates fetal hemoglobin in the adult. Blood. 1988;72(6):1961–7. [PubMed] [Google Scholar]
- 56.Fibach E, Prasanna P, Rodgers G, Samid D. Enhanced fetal hemoglobin production by phenylacetate and 4-phenylbutyrate in erythroid precursors derived from normal donors and patients with sickle cell anemia and beta-thalassemia. Blood. 1993;82(7):2203–9. [PubMed] [Google Scholar]
- 57.Olivieri NF, Saunthararajah Y, Thayalasuthan V, Kwiatkowski J, Ware RE, Kuypers FA, et al. A pilot study of subcutaneous decitabine in β-thalassemia intermedia. Blood. 2011;118(10):2708–11. doi: 10.1182/blood-2011-03-341909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saunthararajah Y, Molokie R, Saraf S, Sidhwani S, Gowhari M, Vara S, et al. Clinical effectiveness of decitabine in severe sickle cell disease. British journal of haematology. 2008;141(1):126–9. doi: 10.1111/j.1365-2141.2008.07027.x. [DOI] [PubMed] [Google Scholar]
- 59.Lowrey CH, Nienhuis AW. Treatment with azacitidine of patients with end-stage β-thalassemia. New England Journal of Medicine. 1993;329(12):845–8. doi: 10.1056/NEJM199309163291205. [DOI] [PubMed] [Google Scholar]
- 60.Steinberg MH, Lu Z-H, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Blood. 1997;89(3):1078–88. [PubMed] [Google Scholar]
- 61.Fucharoen S, Siritanaratkul N, Winichagoon P, Chowthaworn J, Siriboon W, Muangsup W, et al. Hydroxyurea increases hemoglobin F levels and improves the effectiveness of erythropoiesis in beta-thalassemia/hemoglobin E disease. Blood. 1996 Feb 1;87(3):887–92. PubMed PMID: 8562958. [PubMed] [Google Scholar]
- 62.Hajjar FM, Pearson HA. Pharmacologic treatment of thalassemia intermedia with hydroxyurea. The Journal of pediatrics. 1994;125(3):490–2. doi: 10.1016/s0022-3476(05)83304-9. [DOI] [PubMed] [Google Scholar]
- 63.Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of β-thalassemia. Proceedings of the National Academy of Sciences. 2008;105(5):1620–5. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perrine S. Novel therapeutic agents for HbF induction: a new era for treatment of β thalassemia? Thalassemia Reports. 2011;1(1):e7. [Google Scholar]
- 65.Fu Y-F, Du T-T, Dong M, Zhu K-Y, Jing C-B, Zhang Y, et al. Mir-144 selectively regulates embryonic α-hemoglobin synthesis during primitive erythropoiesis. Blood. 2009;113(6):1340–9. doi: 10.1182/blood-2008-08-174854. [DOI] [PubMed] [Google Scholar]