Abstract
Stem cell therapy could have great potential for the treatment of a wide variety of diseases. Stem cells might have the ability to differentiate into a widespread cell types, and to repopulate and revitalize the damaged cells with healthy tissue, and improve its performance. We provide here the evidence supporting the critical use of stem cell as a treatment in disease conditions existing with high glucose complaint such as diabetes. The reduction of glucose stimulated cell proliferation and high glucose enhanced apoptosis in rat model, which may be a problem in therapeutic strategies based on ex vivo expansion of stem cell, and may also propagate the development of osteoporosis in high glucose complaint such as diabetes. This leads to the hypothesis that, high glucose could be more deleterious to stem cell therapy that may be due to the aggravation of oxidative stress triggered by high glucose. These findings may help to understand the possible reasons associated with high glucose induced detrimental effects on stem cells as well as provide novel therapeutic strategies for preventing the adverse effects of glucose on the development and progression of stem cells in patients with diabetes.
Keywords: Stem cell therapy, Glucose, Regulation, Proliferation, Differentiation
INTRODUCTION
Stem cell therapy could have great potential for the treatment of a wide variety of diseases.1 Stem cells might have the ability to differentiate into a widespread cell types, and to repopulate and revitalize the damaged cells with healthy tissue, and improve its performance.2 Stem cells are also expand and differentiate to different cell entities for regenerative therapies by applying culture media of different compositions.3 Common media contain diverse elements such as glucose to ensure the stable maintenance of cell differentiation after prolonged culture in vitro. The applied culture media components may influence stem cell proliferation, replicative senescence, and apoptosis.4
Glucose is an essential source of cellular energy and is an important substrate for protein and lipid synthesis. Glucose enters eukaryotic cells through two different membrane associated carrier proteins, glucose transporter facilitators (GLUT) and the Na+-coupled glucose transporters (SGLT).5 Glucotoxicity or high glucose concentrations may impair β-cell function and finally induce apoptosis.6 Elevated glucose concentrations affect pancreatic β-cells and can lead to oxidative damage in the major organs in the body, such as eyes, kidneys, nerves, and blood vessels.7 In stem cell obtained from rats high glucose induced cellular senescence, while reduction of glucose enhanced proliferation, decreased apoptosis, and increased the number of colony forming units.8, 9
We provide here the evidence supporting the critical use of stem cell therapy in the disease conditions existing with high glucose complaint such as diabetes. This leads to the hypothesis that, high glucose could be more deleterious to stem cell therapy that may be due to the aggravation of oxidative stress triggered by high glucose.
Evidences
Experimental Evidence
Reducing glucose stimulated cell proliferation and high glucose enhanced apoptosis in rat model,8 which may be a problem in the stem cells-based therapeutic strategies,10 and may also propagate developing osteoporosis in high glucose conditions such as diabetes.11 Some but not all studies suggested that β-cell mass is significantly reduced in diabetic patients and thus increased apoptosis.12–14 Both human and animal studies have found that increasing of β-cell apoptosis is an important reason of insulin deficiency in type 2 diabetic patients.15, 16 So far, several lines of evidence have suggested that chronic persistent hyperglycemia results in β-cells dysfunction and ultimately apoptosis, called β-cell glucotoxicity.17
Diabetes is associated with reduced numbers and functional viability of stem cells in vivo, which lead to degenerative pathologies of the musculoskeletal system. Stolzing et al., elucidated the effects of elevated glucose levels on the proliferation of bone marrow mesenchymal stem cells, and found that culture in high-glucose-containing medium had a negative effect on the colony formation and differentiation.18 Human adipose-derived mesenchymal stem cells may be ideal source of cells for musculoskeletal system regeneration, but the harsh chemical microenvironment may significantly influence the biological and metabolic vitality of this stem cell and impair their repair potential. Liang et al. harvested and cultured Human adipose-derived mesenchymal stem cells under low glucose condition for two weeks, and reported that low glucose is a positive factor that affects the survival and biological behaviors of such stem cells.19 Elseberg et al., compared high- with low-glucose medium showing that high-glucose has positive effects on stem cell proliferation.20 Kim et al., identified miR-486-5p, which is progressively expressed in human adipose tissue-derived mesenchymal stem cells, and showed that high glucose increases miR-486-5p expression.21 Bone marrow-derived mesenchymal stem cells have multilineage differentiation potential and can be designated for the treatment of diabetic patients. Though, high-glucose levels can adversely affect the function of stem cells and justifies the need for a strategy to overcome this problem. Khan et al., showed that growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells in animal model.22 Elevated glucose concentrations may harm function of the cells and induce apoptosis. Li et al., assessed the effects of high glucose concentrations on human mesenchymal stem cells in vitroand reported that proliferation and osteogenic differentiation are stimulated by high glucose.23 Keats and Khan, investigated whether stem cells show cellular activation and dysfunction to high- glucose levels, and showed that though endothelial progenitor cells are resistant to high- glucose levels, but high- glucose levels may cause reduced growth and altering the differentiation potential.24 Although low glucose concentration (5.5mM) is physiologically maintained in vivo, high glucose levels (25mM) induced forming embryoid body, which is an important step in the differentiation of pluripotent stem cells, in vitro. Mochizuk et al., investigated the effect of glucose concentration during stem cells embryoid body formation, and showed that low-glucose concentration was suitable.25 Howard et al., investigated whether diabetes induces a repair defect in skeletal muscle myocytes, and showed that this repair defect was mimicked in cultured cells by prolonged exposure to high-glucose.26 Kim et al., examined the effects of high-glucose on stem cell proliferation, and concluded that high-glucose levels can increase the rate of stem cells growth.27
Clinical Evidence
In a group of metabolic diseases in which a person experienced high-glucose condition either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced by the pancreas, high-glucose produces three big diabetes signs including polyuria, polydipsia and polyphagia. 28 High-glucose levels also can lead to severe complications such as vision loss, cardiovascular diseases, kidney disorder, and nerve damage .29–33 Clinical studies have shown that high-glucose level leads to endothelial cell dysfunction in diabetes (Table 1).34–36
Table 1.
Relationship between Glucose Levels and Stem Cells Function
| Glucose concentration | Stem cell type | Human / Rat | Effects | Ref. |
|---|---|---|---|---|
| High level glucose | Non adherent bone- marrow-derived MSC | Rat | Negative effect on SC colony formation and differentiation | 18 |
| Low level glucose | Adipose-derived MSC | Human | Positive effect on survival and biological behaviors | 19 |
| High level glucose | MSC | Diabetic mice | Induce premature senescence and apoptosis, reduce colony forming activity | 22 |
| High level glucose | MSC | Human | Stimulate proliferation and osteogenic differentiation | 23 |
| High level glucose | Adult vascular stem/progenitor cell | Human | Reduce growth, alter differentiation potential | 24 |
| High level glucose | ESC | mice | Increase SC growth | 27 |
Abbreviations: MSC, Mesenchymal stem cell. SC, Stem cell. ESC, Embryonic stem cell
Stem cells offer the greatest potential for developing an abundant source of pancreatic islets, hence Insulin-producing cells for transplantation can be generated from both embryonic and adult stem cells.37 Phadnis et al., isolated stem cells from diabetic patients, to investigate the effect of diabetic microenvironment on human bone marrow-derived mesenchymal stem cells, and claimed that diabetic high-glucose level plays a major role in inducing the differentiation of human stem cells.38 Gu et al., enrolled type 1 diabetes patients, and harvested hematopoietic stem cells. 39 They showed that hematopoietic stem cells to be an effective long-term treatment for insulin dependence that achieved a greater efficacy in patients without diabetic ketoacidosis at diagnosis. Fadini et al., explored whether circulating pericyte progenitor cells levels are affected by glucose control in a poorly controlled type 2 diabetic patients.40 They concluded that glucose control transiently mobilizes PPCs diabetic patients with micro angiopathy. Increase in pericyte progenitor cells may represent a vasoregenerative event or may be a consequence of ameliorated glucose control on microvascular lesions. Li et al., examined the impact of autologous hematopoietic stem cell transplantation on lymphocytes and pancreatic β-cell function, in thirteen patients with new onset of type 1 diabetes.41 They reported that autologous hematopoietic stem cell transplantation modulated lymphocytes and preserved β-cell function in Chinese patients with new onset of type 1 diabetes and diabetic ketoacidosis. Ruiz-Salmeron et al., compared the neovasculogenesis and clinical improvement of diabetic patients with peripheral artery disease, at baseline and at 3 and 12 months after autologous bone marrow-derived stem cell transplantation. They reported that in diabetic patients with critical limb ischemia, autologous bone marrow-derived stem cell transplantation is a safe procedure that generates a significant increase in the vascular network in ischemic areas and promotes remarkable clinical improvement.42 Zhao et al., developed a procedure for stem cell therapy in which a patient's blood is circulated through a closed-loop system that separates lymphocytes from the whole blood and briefly co-cultures them with adherent in 15 patients with type 1 diabetes.43 They concluded that stem cell therapy is safe, and in individuals with moderate or severe type 1 diabetes, a single treatment produces lasting improvement in metabolic control. Jiang et al., studied the therapeutic effect of human placenta-derived stem cells in ten type 2 diabetes patients with longer duration, islet cell dysfunction, high insulin doses as well as poor glycemic control in order to evaluate the safety, efficacy and feasibility of this treatment.44 This pilot clinical trial indicates that transplantation of stem cells represents a simple, safe and effective therapeutic approach for type 2 diabetes patients with islet cell dysfunction. Bhansali et al., studied the efficacy of autologous bone marrow-derived stem cell transplantation in ten patients with type 2 diabetes for more than 5 years, failure of triple oral anti-diabetic drugs, currently on insulin at least for one year, and glutamic acid decarboxylase antibody negative.45 Their observations indicated that stem cells therapy is a safe and effective modality of treatment to improve beta-cell function in patients with type 2 diabetes.
DISCUSSION
Stem cells are defined as cells that have clonogenic and self-renewing capabilities and differentiate into multiple cell lineages. Stem cells therapy offers the greatest potential for developing an abundant source of pancreatic islets. Insulin-producing cells for transplantation can be generated from both embryonic and adult stem cells. Before stem cell therapeutic strategies for diabetes mellitus can be transferred to clinical application in humans, stem cell biologists have to address several pressing issues related to appropriate differentiation protocols, functional aspects of insulin secretion, its regulation, cell-maturation and control of proliferation, with ethical norms and safety. Not only blood glucose control is the most important target in managing patients with diabetes, but also high glucose may lead to several adverse effects in such patients and subsequent stem cell therapy.46–50
Line of evidences showed that diabetes is now curable by transplantation therapy, and stem cells offer a potential starting material from which to generate the large numbers of cells required.51, 52 While some argue, the generation of the large numbers of cells by stem cell therapy such as perfect beta-cell may not necessarily lead to the most suitable tissue for transplantation in patients with diabetes.53 It is also possible that stem cell-derived insulin-producing cell clusters within these experiments are more efficient at producing insulin than conventionally isolated islets, based on the cells in vitro high-glucose environment. Accordingly, Fujikawa et al., characterized embryonic stem cell-derived insulin-expressing cells and assessed their relevance for treating type I diabetes, the clusters in their system may be applied to the marginal mass transplant theory.54 Long and relatively high-glucose levels in the culture media may have stimulated a greater insulin response than would be expected from normal islet cells. However, the risk of teratoma formation would need to be eliminated before embryonic stem cell-based therapies for treating diabetes are considered. Unexpectedly, Keats and Khan found the progenitor population most affected by high glucose is the mesenchymal cell type. When they cultured bone marrow-derived progenitor cells in high glucose media, and noted a significant reduction in cell numbers at day one. Besides, this effect was normalized upon continued exposure.24 Other recent study found that in vitro stimulation with advanced glycation products induced generation of reactive oxygen species, and inhibited dose-dependently proliferation and migration of mesenchymal stem cells.55 Furthermore, recent work has shown that exposure of vascular smooth muscle cells to high glucose activates several signal transduction networks responsible for mediating the proliferative and growth-promoting response in patients with diabetes.56
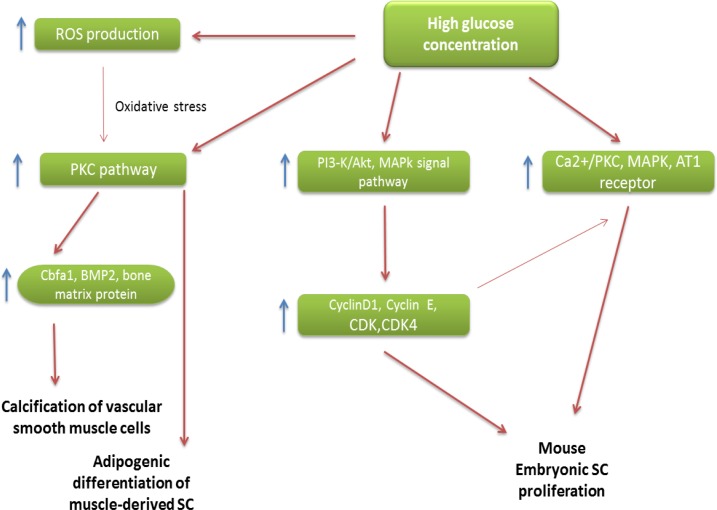
The adverse effects of high glucose on stem cell therapy have been suggested through different mechanisms include, induces adipogenic differentiation of muscle-derived stem cells,50 has an effect in stimulating mouse embryonic stem cell proliferation through the Ca2+/PKC, MAPKs, and the AT1 receptor,49 insulin and estradiol are able to contain the deleterious effect of high glucose on stem cells-derived osteoblast proliferation and function,48 increase cell cycle regulatory proteins of mouse embryonic stem cells by PI3-K/Akt and MAPKs signal pathways,27 and increases the expression of Cbfa1, BMP-2 and bone matrix protein and enhances the calcification of vascular smooth muscle cells (Figure 1).56
Figure 1.
Adverse effects of high glucose on stem cell therapy
Abbreviations: SC, Stem cell; AT1, ANG II type 1; BMP2, Bone morphogenic protein; CDK, Cyclin dependent kinase; ROS, Reactive oxidative stress; PKC, Protein kinase C; Cbfa1, Core binding factor alpha-1
Although the protection and expansion of all forms of stem cell therapy, which offer great hope for a cure and better treatments for patients who suffered from diabetes, but better understand high glucose pathogenesis could prevent the treatment or improve the survival, and ultimately to expand therapeutics. These findings may help to understand the possible reasons associated with high glucose induced detrimental effects on stem cells as wellas provide novel therapeutic strategies for preventing the adverse effects of glucose on the development and progression of stem cells in patients with diabetes.
ACKNOWLEDGEMENT
We wish to thank all our colleagues in Shafa Hospital and Allied Health Sciences School, Ahvaz Jundishapur University of Medical Sciences.
REFERENCES
- 1.Larijani B, et al. Stem cell therapy in treatment of different diseases. Acta Med Iran. 2012;50(2):79–96. [PubMed] [Google Scholar]
- 2.Brehm M, Stanske B, Strauer B.E. Therapeutic potential of stem cells in elderly patients with cardiovascular disease. Exp Gerontol. 2008;43(11):1024–32. doi: 10.1016/j.exger.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Hassiotou F, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30(10):2164–74. doi: 10.1002/stem.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding V, Choo A.B, Oh S.K. Deciphering the importance of three key media components in human embryonic stem cell cultures. Biotechnol Lett. 2006;28(7):491–5. doi: 10.1007/s10529-006-0005-8. [DOI] [PubMed] [Google Scholar]
- 5.Scheepers A, Joost H.G, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28(5):364–71. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, et al. High Levels of Glucose Induced the Caspase-3/PARP Signaling Pathway, Leading to Apoptosis in Human Periodontal Ligament Fibroblasts. Cell Biochem Biophys. 2012 doi: 10.1007/s12013-012-9470-y. [DOI] [PubMed] [Google Scholar]
- 7.Robertson R.P, Harmon J.S. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41(2):177–84. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9(1):31–5. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, et al. Adiponectin prevents diabetic premature senescence of endothelial progenitor cells and promotes endothelial repair by suppressing the p38 MAP kinase/p16INK4A signaling pathway. Diabetes. 2010;59(11):2949–59. doi: 10.2337/db10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassem M. Stem cells: potential therapy for age-related diseases. Ann N Y Acad Sci. 2006;1067:436–42. doi: 10.1196/annals.1354.062. [DOI] [PubMed] [Google Scholar]
- 11.Strotmeyer E.S, et al. Middle-aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care. 2006;29(2):306–11. doi: 10.2337/diacare.29.02.06.dc05-1353. [DOI] [PubMed] [Google Scholar]
- 12.Butler A.E, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 13.Yoon K.H, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88(5):2300–8. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 14.Sakuraba H, et al. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45(1):85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 15.Marinkovic T, Sysi-Aho M, Oresic M. Integrated Model of Metabolism and Autoimmune Response in beta-Cell Death and Progression to Type 1 Diabetes. PLoS One. 2012;7(12):51909. doi: 10.1371/journal.pone.0051909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414(6865):792–8. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 17.Robertson R.P, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–24. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 18.Stolzing A, Bauer E, Scutt A. Suspension cultures of bone-marrow-derived mesenchymal stem cells: effects of donor age and glucose level. Stem Cells Dev. 2012;21(14):2718–23. doi: 10.1089/scd.2011.0406. [DOI] [PubMed] [Google Scholar]
- 19.Liang C, et al. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med. 2012;10:49. doi: 10.1186/1479-5876-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elseberg C.L, et al. Microcarrier-based expansion process for hMSCs with high vitality and undifferentiated characteristics. Int J Artif Organs. 2012;35(2):93–107. doi: 10.5301/ijao.5000077. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y.J, et al. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells Dev. 2012;21(10):1749–60. doi: 10.1089/scd.2011.0429. [DOI] [PubMed] [Google Scholar]
- 22.Khan M, et al. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 2011;20(1):67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 23.Li Y.M, et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun. 2007;363(1):209–15. doi: 10.1016/j.bbrc.2007.08.161. [DOI] [PubMed] [Google Scholar]
- 24.Keats E, Khan Z.A. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS One. 2012;7(6):e38752. doi: 10.1371/journal.pone.0038752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochizuki H, Ohnuki Y, Kurosawa H. Effect of glucose concentration during embryoid body (EB) formation from mouse embryonic stem cells on EB growth and cell differentiation. J Biosci Bioeng. 2011;111(1):92–7. doi: 10.1016/j.jbiosc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Howard A.C, et al. A novel cellular defect in diabetes: membrane repair failure. Diabetes. 2011;60(11):3034–43. doi: 10.2337/db11-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.H, Heo J.S, Han H.J. High glucose increase cell cycle regulatory proteins level of mouse embryonic stem cells via PI3-K/Akt and MAPKs signal pathways. J Cell Physiol. 2006;209(1):94–102. doi: 10.1002/jcp.20706. [DOI] [PubMed] [Google Scholar]
- 28.McCance D.R, et al. Diagnosing diabetes mellitus--do we need new criteria? Diabetologia. 1997;40(3):247–55. doi: 10.1007/s001250050671. [DOI] [PubMed] [Google Scholar]
- 29.Wasfy J.H. Childhood vaccination and type 1 diabetes. N Engl J Med. 2004;351(3):298. doi: 10.1056/NEJM200407153510319. [DOI] [PubMed] [Google Scholar]
- 30.Hviid A, et al. Childhood vaccination and type 1 diabetes. N Engl J Med. 2004;350(14):1398–404. doi: 10.1056/NEJMoa032665. [DOI] [PubMed] [Google Scholar]
- 31.DeStefano F, et al. Childhood vaccinations, vaccination timing, and risk of type 1 diabetes mellitus. Pediatrics. 2001;108(6):E112. doi: 10.1542/peds.108.6.e112. [DOI] [PubMed] [Google Scholar]
- 32.Milne L.M. Difficulties in assessing the relationship, if any, between mumps vaccination and diabetes mellitus in childhood. Vaccine. 2000;19(9-10):1018–25. doi: 10.1016/s0264-410x(00)00243-7. [DOI] [PubMed] [Google Scholar]
- 33.Dahlquist G, Gothefors L. The cumulative incidence of childhood diabetes mellitus in Sweden unaffected by BCG-vaccination. Diabetologia. 1995;38(7):873–4. doi: 10.1007/BF03035306. [DOI] [PubMed] [Google Scholar]
- 34.Albers J.W, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–6. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratner R.E. Glycemic control in the prevention of diabetic complications. Clin Cornerstone. 2001;4(2):24–37. doi: 10.1016/s1098-3597(01)90027-4. [DOI] [PubMed] [Google Scholar]
- 36.Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- 37.Burns C.J, Persaud S.J, Jones P.M. Diabetes mellitus: a potential target for stem cell therapy. Curr Stem Cell Res Ther. 2006;1(2):255–66. doi: 10.2174/157488806776956832. [DOI] [PubMed] [Google Scholar]
- 38.Phadnis S.M, et al. Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. Rev Diabet Stud. 2009;6(4):260–70. doi: 10.1900/RDS.2009.6.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu W, et al. Diabetic ketoacidosis at diagnosis influences complete remission after treatment with hematopoietic stem cell transplantation in adolescents with type 1 diabetes. Diabetes Care. 2012;35(7):1413–9. doi: 10.2337/dc11-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fadini G.P, et al. Amelioration of glucose control mobilizes circulating pericyte progenitor cells in type 2 diabetic patients with microangiopathy. Exp Diabetes Res. 2012;2012:274363. doi: 10.1155/2012/274363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, et al. Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves beta-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab. 2012;97(5):1729–36. doi: 10.1210/jc.2011-2188. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Salmeron R, et al. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011;20(10):1629–39. doi: 10.3727/096368910X0177. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, et al. Reversal of type 1 diabetes via islet beta cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:3. doi: 10.1186/1741-7015-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang R, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med. 2011;5(1):94–100. doi: 10.1007/s11684-011-0116-z. [DOI] [PubMed] [Google Scholar]
- 45.Bhansali A, et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18(10):1407–16. doi: 10.1089/scd.2009.0164. [DOI] [PubMed] [Google Scholar]
- 46.Yue T, et al. High glucose induces differentiation and adipogenesis in porcine muscle satellite cells via mTOR. BMB Rep. 2010;43(2):140–5. doi: 10.5483/bmbrep.2010.43.2.140. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Jiang L.S, Dai L.Y. High glucose potentiates collagen synthesis and bone morphogenetic protein-2-induced early osteoblast gene expression in rat spinal ligament cells. Endocrinology. 2010;151(1):63–74. doi: 10.1210/en.2009-0833. [DOI] [PubMed] [Google Scholar]
- 48.Gopalakrishnan V, et al. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol. 2006;84(1):93–101. doi: 10.1139/o05-163. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y.H, Han H.J. Synergistic effect of high glucose and ANG II on proliferation of mouse embryonic stem cells: involvement of PKC and MAPKs as well as AT1 receptor. J Cell Physiol. 2008;215(2):374–82. doi: 10.1002/jcp.21314. [DOI] [PubMed] [Google Scholar]
- 50.Aguiari P, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008;105(4):1226–31. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanekzai J, Isenovic E.R, Mousa S.A. Treatment options for diabetes: Potential role of stem cells. Diabetes Res Clin Pract. 2012;98(3):361–8. doi: 10.1016/j.diabres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Weir G.C, Cavelti-Weder C, Bonner-Weir S. Stem cell approaches for diabetes: towards beta cell replacement. Genome Med. 2011;3(9):61. doi: 10.1186/gm277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns C.J, Persaud S.J, Jones P.M. Stem cell therapy for diabetes: do we need to make beta cells? J Endocrinol. 2004;183(3):437–43. doi: 10.1677/joe.1.05981. [DOI] [PubMed] [Google Scholar]
- 54.Fujikawa T, et al. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. 2005;166(6):1781–91. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang K, et al. Advanced glycation end products induce chemokine/cytokine production via activation of p38 pathway and inhibit proliferation and migration of bone marrow mesenchymal stem cells. Cardiovasc Diabetol. 2010;9:66. doi: 10.1186/1475-2840-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen N.X, et al. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006;21(12):3435–42. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]