Abstract
Obesity is an important public health problem and major risk factor for postmenopausal breast cancer. Adipose tissue is the major component involved in the control of the metabolism through energy homeostasis, adipocyte differentiation, insulin sensitivity and the activation of anti-inflammatory metabolic and immune pathways. Leptin and Adiponectin pathways are involved in proliferation process in breast cancer. Current review describes potential relationship between the molecular actions and clinical significance of leptin and adiponectin in breast cancer.
Keywords: Obesity, Breast Cancer, Adiponectin, Leptin, Cytokine
INTRODUCTION
Obesity has emerged as an important public health problem worldwide. It is well recognized that obesity is the major risk factor for certain diseases. Obesity can develop adipose tissue stores in the body. The quantity of body fat could be a significant source of hundreds of biologically active molecules, the “adipokines”, including more than 50 cytokines, chemokines, hormone-like factors and other mediators [leptin, adiponectin, visfatin, apelin, vaspin, hepcidine, chemerin, omentin including tumor necrosis factor alpha (TNF), monocyte chemoattractant protein-1 (MCP-1), and plasminogen activator protein (PAI).1 Adipose tissue is one of the largest tissues in the human body and total amount deposited will have a detrimental impact on regular body functions.2 Current review describes the adipokines leptin and adiponectin molecular actions and clinical significance in breast cancer. The two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT) present in mammals have a different cellular composition and localization.3 WAT is the major component is involved in the control of metabolism through energy homeostasis, adipocyte differentiation, insulin sensitivity and the activation of anti-inflammatory metabolic and immune pathways.1, 4 Adiponectin and leptin adipokines are WAT derived proteins.
Adiponectin (ADIPOQ)
Adiponectin is a 244 amino acid protein synthesised by adipocytes, most abundant gene transcript (ApM1) found on chromosome 3 locus 3q27 in adipose tissue.5 It consists of three exons and two introns which share similarity with leptin encoding gene.6 Insulin-like growth factor-1 (IGF-1), peroxisome proliferator activated receptors (PPAR) are not only the triggers behind adiponectin gene expression in adipose tissue but are also involved in the regulation of adiponectin synthesis.7, 8 Some studies have shown that bone, mammary glands, salivary glands and cardiac tissue may express adiponectin in limited quantities.9–12 Adiponectin is a single monomer composed of four structurally distinct domains. It contains amino terminal sequence, a variable region, a collagen-like domain and an amino terminal globular domain.13 Adiponectin and globular fragments are structurally similar to complement C1q protein and TNF-α.7, 14 studies in mice and humans have discovered that circulating adiponectin is a disulfide-linked oligomer composed of trimers, hexamers and a high molecular mass multimers (HMW), containing up to 18 monomers and also globular form of protein.15, 16 Some biochemical analyses and in vivo studies have led to a conclusion that adiponectin complexes do not interconvert after secretion and in circulating plasma it exists as full-length multimeric forms.17
Adiponectin Receptors
Adiponectin biological actions facilitate through two distinct receptors called AdipoR1 and AdipoR2. AdipoR2 expression is mostly restricted to the liver18 whereas AdipoR1 (mRNA) was detected in human platelets. The expression of these receptors (AdipoR1 and AdipoR2) have been identified in monocytes, and megakaryocyte cell lines.19, 20 Both AdipoR1 and AdipoR2 are seven-transmembrane domains that are structurally functionally and topologically similar to heptahelical G protein-coupled receptors (GPCRs).18 Adiponectin receptors N-terminus is found to be cytoplasmic and the C terminus is extra cellular an orientation opposite to GPCRs. Once activated AdipoR1 and AdipoR2 can form both homo and heterodimeric complexes, although the biological significance of dimerization is unknown.18, 21 In 2004, the glycosyl phosphatidylinositol-anchored extra cellular protein, T-cadherin a third receptor was identified as a binding protein for the hexameric and high molecular weight forms of adiponectin although T-cadherin lacks known biological function.22 These adiponectin receptors existence can be explained by different isoforms of adiponectin which require different receptor conformations to ensure a high binding affinity and by the wide biological actions of adiponectin in different tissues.
Adiponectin Molecular Action
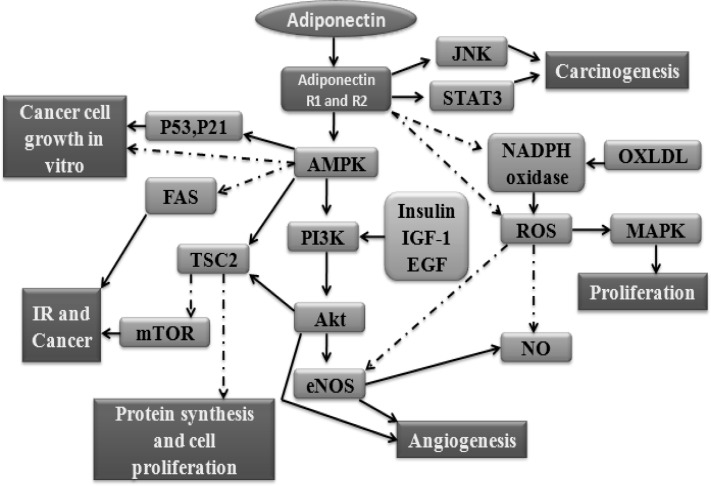
Adiponectin is considered a starvation hormone under fasting conditions, high adiponectin levels stimulate central and peripheral AMPK leading to increased food intake and decreased energy expenditure, promoting fat storage.23 The molecular pathways downstream of AdipoR remain to be fully elucidated. Studies have shown that activation of the pleiotropic AMPK is part of the signaling cascade downstream of adiponectin receptor. AMPK is an evolutionarily conserved serine/ threonine kinase with a catalytic α-subunit and regulatory β- and γ-subunits, forming a heterotrimeric complex.24 Activated AMPK stimulates catabolic processes that produce ATP and switches off ATP consuming processes, thus restoring the AMP: ATP ratio. It also phosphorylates and inhibits ACC1 and HMG-CoA, decreases fatty acid synthase (FAS) expression and activates malonyl-CoA carboxylase, glycerol phosphate acyltransferase (GPAT) thereby leading to a decrease in fatty acid and cholesterol synthesis25–27 which are key regulators of protein, fatty acid and glycerolipid synthesis, respectively. These enzymes have been associated with breast cancer.28 The enzyme downstream of AMPK plays a key role in regulating lipid metabolism. The regulation of TSC2 and mTOR by AMPK might have special implications because the PI3K-Akt signaling pathway is constitutively active in many cancers. The activation of this pathway is most notable in cancers that have over expression of an activated member of the epidermal growth factor (EGF) receptor family (e.g. Her2/neu in breast cancer).29 EGF stimulates the mTOR pathway by activating PI3-kinase and in turn Akt and the subsequent phosphorylation of TSC2 by Akt, leading to the inhibition of TSC2.30, 31 In the absence of growth-promoting stimuli, TSC2 binds to TSC1 to form a tumor suppressor complex, which has growth-inhibitory activity via suppression of Mtor.32 Activation of AMPK by adiponectin also activates endothelial NO synthase (eNOS) and increases nitric oxide (NO) production. eNOS activation is also dependent on signaling through Akt kinase (Akt).33, 34 These signaling pathways are constitutively active in breast cancer (Fig. 1).
Fig. 1.
Multiple potential signaling pathways for adiponectin. Modified from I Kelesidis et al 2006 34
Adiponectin and Breast Cancer- Clinical significance
Obesity is a risk factor for breast cancer, as obesity has been associated with the development of breast cancer, adipocytokines, a group of polypeptide growth factors and cytokines which are produced exclusively by adipose tissue, may underlie the association between obesity and breast cancer risk. Adiponectin is a protein hormone secreted exclusively by adipocytes,35 and circulating adiponectin levels are inversely associated with the risk of obesity-related malignancies, including breast cancer36–38 which implies that low blood concentrations of adiponectin are associated with high incidence and poor prognosis of breast cancer. Obesity and estrogens have long been implicated in the pathogenesis of breast cancer.39, 40 Adipose tissue serves as the site of peripheral aromatization of adrenal androgens to estrogens, which induce mitogenic activity in mammary tissue by binding to estrogen receptors.
The mechanism through which adiponectin modulate breast cancer risk is currently unknown. It has been noted that adiponectin stimulates the sensitivity of peripheral tissue to insulin, and decreased levels of adiponectin are associated with increased serum insulin levels, which accompany insulin resistance.41 Insulin has been shown to stimulate the proliferation of breast cancer cells by binding and signaling through the insulin and insulin-like growth factor I (IGF-I) receptors.42 In addition, insulin may synergize with the mitogenic effects of estrogen43 and may also upregulate the expression of vascular endothelial growth factor (VEGF), a potent angiogenic agent that is secreted by breast cancer cells.44 Moreover, adiponectin has been inversely associated with estrogen levels.45 It remains possible that adiponectin may influence breast cancer risk by altering circulating estrogen levels. It is thus reasonable to hypothesize that the reduced adiponectin levels increase the risk of postmenopausal breast cancer and ER-positive breast tumors through a mitogenic effect of hyper insulinemia and increased IGFs and estrogen levels as well as by upregulating VEGF. Furthermore, it has been shown that adiponectin potently inhibits endothelial cell proliferation and migration, and induces a cascade of activation of caspases-8, -9, and -3, which leads to cell death.46 Additionally, it has been found that treatment with adiponectin is able to modulate apoptosis of human breast cancer cells in vitro and may be involved in cell signaling pathways associated with carcinogenesis.47, 34 Several studies have demonstrated that low serum adiponectin levels are associated with increased risk for breast cancer,48 data for which is summarised in Table 1.
Table 1.
Adiponectin Levels and Breast Cancer Studies and their Implications
| Author & Year | Significant | |
|---|---|---|
| Adiponectin and breast cancer | Gulcelik MA et al 201249 | low serum adiponectin level may be associated with both breast and colon cancer |
| Sonmez B et al 201150 | serumadiponectin levels were inversely correlated with tumor tissue adiponectin levels. Adiponectin levels in breast tumor tissue increase while serum adiponectin levels decrease | |
| M. Seker, F et al 2009 51 | The low serum adiponectin and high normal and tumor tissue adiponectin levels detected in breast cancer patients and serum adiponectin levels inversely associated with tumor tissue adiponectin levels | |
| Virginia G et al 200852 | The adiponectin axis may emerge as an important modifier of breast cancer risk | |
| Karaduman M et al 200753 | high tissue adiponectin levels significantly detected in breast cancer patients and associated with an increased risk for breast cancer | |
| Shelley S et al 2007 54 | adiponectin may be inversely associated with postmenopausal breast cancer risk, particularly in a low-estrogen environment | |
| Kang JH et al 2007 55 | low serum adiponectin levels associated with increased breast cancer risk in Korean women | |
| Hou WK et al 2007 56 | Decreased serum adiponectin levels are risk factors of breast cancer | |
| Mantzoros C et al 2004 37 | significant association between adiponectin and breast cancer was found among premenopausal women | |
| Miyoshi Y et al 2003 36 | low serum adiponectin levels are significantly associated with an increased risk for breast cancer |
Leptin
Leptin, a product of the obgene discovered in 1994, is a 16 kDa molecular mass of single-chain proteohormone which is localized on the 7 alpha chromosome and consists of three exons separated by two introns.57, 58 Structurally-similar to members of the long-chain helical cytokine family, including IL-6 (interleukin-6), IL-1159. The 167-amino-acid leptin molecule three-dimensional structure is based on four antiparallel a-helices, connected by two long crossover links and one short loop arranged in a left-handed helical bundle, which forms a two-layer packing.60 Leptin is expressed in a variety of tissues counting the placenta, ovaries, mammary epithelium, bone marrow and lymphoid tissues.61, 62 It acts through specific receptors in the hypothalamus to modulate appetite and thermogenesis, Increased levels suppress appetite and increase thermogenesis.
Leptin receptor
Leptin receptors (ObRs) are located throughout the central nervous system and several peripheral tissues.63 Six isoforms have been identified in leptin receptor (ObRa, ObRb, ObRc, ObRd, ObRe, and ObRf) all of which are products of a single leprgene.64 These isoforms vary by length and sequences due to alternative mRNA splicing, ObRs have homologous extracellular domains but distinct intracellular domains.65 ObRa and ObRc are the short isoforms which play an important role in transporting leptin across the blood–brain barrier (BBB).66 ObRb longleptin receptor isoform is responsible for leptin signaling. ObRb expressed in several organs is strongly expressed throughout the central nervous system particularly in the hypothalamus.67 Here, leptin acts on neurons that regulate levels of circulating hormones (e.g., thyroid hormone, sex steroids, growth hormone).68 It may also directly control some metabolic tissues and regulates glycemia at least partly via the central nervous system.69
Leptin Molecular Action
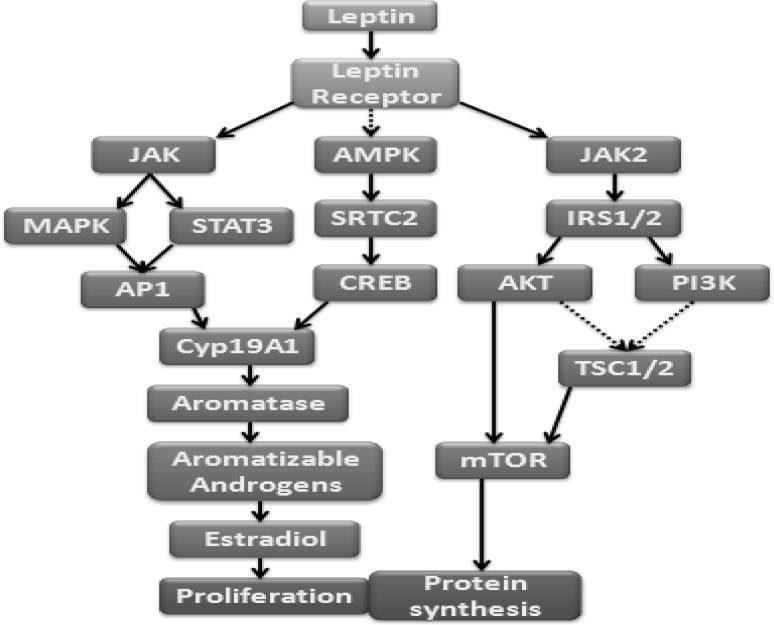
OB-Rs belong to the class I cytokine receptor family, which is known to act through JAKs (Janus kinases) and STATs (signal transducers and activators of transcription). Subsequent studies showed that OB-Rb, the full-length isoform only contains intra cellular motifs required for activation of the JAK/STAT signal transduction pathways.70 In postmenopausal obese women, adipose tissue is the only source of estrogen production by aromatization of C19 steroid androstenedione. Increased estrogen stimulation in postmenopausal obese women might be the cause of higher breast cancer risk.71 Leptin amplifies estrogen signaling in epithelial breast cancer cells which promote cell proliferation in two ways, increased STAT3 and MAPK activation of the AP1 binding site within its promoter increases the expression of CYP19A1 which in turn local estradiol production and directly activates ERα. In another way leptin down regulates STKII expression leading to a decrease in AMPK phosphorylation and increased translocation of CRTC2 to the nucleus binding of CREB and increased CYP19A1expression.72 This CYP19A1 stimulates estradiol production by increasing the conversation of aromatizable androgens (Fig. 2).
Fig. 2.
Potential signaling pathways for Leptin
Leptin and Breast Cancer Clinical significance
Leptin is a cytokine-like hormone that controls adipocyte mass and energy balance by binding to Leptin receptor (OB-R). Both leptin and OB-R are present in human breast tissue and are found to be over expressed in breast tumors.73 Both have been implicated in breast carcinogenesis through cell proliferation or tumor progression.74, 75 In postmenopausal obese women adipose tissue is the only place of estrogen production by aromatization of C19 steroid androstendione. As there is increased aromatase activity and androstendione production in obesity, the total pool of estrogens is higher in obese women and estrogen production promoted by leptin.76 The effect of leptin on breast carcinogenesis is probably mediated by the stimulation of the aromatase activity, proteolytic cleavage of intercellular matrix promoting the cancer cell invasion and angiogenic activity.76, 77 Number of studies indicates that women with breast cancer have higher leptin plasma levels and mRNA expression in adipose tissue as compared to healthy subjects, and the blood levels of estradiol increase parallel to those of leptin78 and serum leptin levels significantly correlate with total body aromatase activity in postmenopausal breast cancer patients.79 Few Leptin and Breast Cancer Clinical significance data is summarized in Table 2.
Table 2.
Leptin and Breast Cancer Studies and their Implications
| Author & Year | Significant | |
|---|---|---|
| Leptin and Breast cancer | Harris HR et al 201180 | Leptin may be inversely associated with breast cancer risk |
| Rahmati-Yamchi M et al 2011 81 | Obesity could be responsible for increased incidences in breast cancer as well as its progression via enhanced production of leptin | |
| Karaduman M et al 2010 82 | Leptin levels were significantly higher in breast cancer tissue compared with normal tissue | |
| Wu MH et al 2009 83 | Leptin may have an independent role in breast tumorigenesis | |
| Han CZ et al 2008 84 | Elevated leptin should play a major role in the development of breast cancer. | |
| Chen DC et al 2006 78 | High serum leptin levels are associated with an increased risk for breast cancer | |
| Snoussi K et al 2006 85 | LEP and LEPR genes are associated with increased breast cancer risk as well as disease progress | |
| Stattin P et al 200486 | Plasma leptin is a risk factor for breast cancer | |
| Tessitore L et al 2000 87 | leptin as a clinical marker |
CONCLUSION
The data recapitulated in this review reveal a major role of leptin and adiponectin in breast cancer progression based on their molecular actions. Adiponectin and leptin antagonism has been well documented in obesity but not in human breast cancer. Leptin enhances breast cancer cell proliferation by inhibiting pro-apoptosis signaling pathways and by favoring in vitro sensitivity to estrogens although there are increasing evidences of association between metabolic syndrome and triple-negative (ER-negative/PR-negative/HER2-negative) breast cancers. The role of leptin on ER-negative breast cancers is still poorly understood and remains to be elucidated. Adiponectin displays in vitro anti-proliferative, pro-apoptotic and anti estrogen properties. Also, adiponectin is involved in tumor size reduction in animals and low plasma levels of adiponectin are associated with higher risk of breast cancer progression in women.88 Emerging data suggest that the ratio of these adipokines may be more important in breast cancer than their absolute concentrations. Increasing evidence of the advanced molecular studies reveal that targeting the adiponectin and leptin might be a new prognostic and/ or therapeutic strategy for postmenopausal breast cancer.89, 90
Thus, the understanding of molecular mechanisms of both leptin and adiponectin and their interplay in breast cancer offer the prospects for new therapeutic approaches targeting similar signaling pathways.
REFERENCES
- 1.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. “Adipose tissue: the new endocrine organ? A review article”. Digestive Diseases and Sciences. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 2.Marti A, Berraondo B, Martinez JA. ‘Obese’ protein slims mice. Scienc. 1995;269:475–476. doi: 10.1126/science.7624769. [DOI] [PubMed] [Google Scholar]
- 3.Gesta S, Tseng YH, Kahn CR. “Developmental origin of fat: tracking obesity to its source”. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Practice and Research. Clinical Endocrinology and Metabolism. 2005;19:547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J BiolChem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 6.Saito K, Tobe T, Minoshima S, Asakawa S, Sumiya J, et al. Organization of the gene for gelatin-binding protein (GBP28) Gene. 1999;229:67–73. doi: 10.1016/s0378-1119(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 7.Berg AH, Combs TP. Scherer PE,ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in Endocrinology and Metabolism. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 8.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, et al. PPAR gamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 9.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Weyermann M, Beermann C, Brenner H, Rothenbacher D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clinical Chemistry. 2006;52:2095–2102. doi: 10.1373/clinchem.2006.071019. [DOI] [PubMed] [Google Scholar]
- 11.Katsiougianni S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells - A new source of the immunoregulatory hormone adiponectin. Arthritis and Rheumatism. 2006;54:2295–2299. doi: 10.1002/art.21944. [DOI] [PubMed] [Google Scholar]
- 12.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. Febs Letters. 2005;579:5163–5169. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherer PE, Williams S, Fogliano M, BaldiniLodish HF. A novel serum-protein similar to c1q, produced exclusively in adipocytes. Journal of Biological Chemistry. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 15.Kishida K, Nagaretani H, Kondo H, Kobayashi H, Tanaka S, et al. Disturbed secretion of mutant adiponectin associated with the metabolic syndrome. Biochemical and Biophysical Research Communications. 2003;306:286–292. doi: 10.1016/s0006-291x(03)00940-9. [DOI] [PubMed] [Google Scholar]
- 16.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. ProcNatlAcadSci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson F, Whitehead JP. Adiponectin-It's all about the modifications. International Journal of Biochemistry & Cell Biology. 2010;42:785–788. doi: 10.1016/j.biocel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic e.ects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 19.Weigert J, Neumeier M, Wanninger J, Wurm S, Kopp A, et al. Reduced response to adiponectin and lower abundance of adiponectin receptor proteins in type 2 diabetic monocytes. Febs Letters. 2008;582:1777–1782. doi: 10.1016/j.febslet.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Kato H, Kashiwagi H, Shiraga M, Tadokoro S, Kamae T, et al. Adiponectin acts as an endogenous antithrombotic factor. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26:224–230. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 21.Touyz RM. Endothelial cell IL-8, a new target for adiponectin: implications in vascular protection. Circ. Res. 2005;97:1216–1219. doi: 10.1161/01.RES.0000196745.09234.36. [DOI] [PubMed] [Google Scholar]
- 22.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung Thong Lim, Blerina Kola, Ma′ rtaKorbonits AMPK as a mediator of hormonal signaling. Journal of Molecular Endocrinology. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 25.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, et al. Characterization of the role of AMP-activated protein kinase in the regulation of glucoseactivated gene expression using constitutively active and dominant negative forms of the kinase. Molecular and Cellular Biology. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Lopez M, Lelliott CJ, Vidal-Puig A. Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. BioEssays. 2007;29:248–261. doi: 10.1002/bies.20539. [DOI] [PubMed] [Google Scholar]
- 28.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 29.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends PharmacolSci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 31.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTORsignalling. Nat Cell Bio. 2002;14:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 32.vanSlegtenhorst M, Nellist M, Nagelkerken B, Cheadle J, Snell R, et al. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. HumMol Genet. 1998;7:1053–1057. doi: 10.1093/hmg/7.6.1053. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J ClinEndocrinolMetab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 34.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. British Journal of Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, et al. Association of serum adiponectin levels with breast cancer risk. Clinical Cancer Research. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 37.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, et al. Adiponectin and breast cancer risk. Journal of Clinical Endocrinology and Metabolism. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 38.Tworoger SS, HeatherEliassen A, Kelesidis T, Colditz GA, Willett WC, et al. Plasma adiponectin concentrations and risk of incident breast cancer. Journal of Clinical Endocrinology and Metabolism. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 39.Lippman ME. Endocrine responsive cancers. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams textbook of endocrinology. 9th Ed. Philadelphia: Saunders; 1998. pp. 1675–1692. [Google Scholar]
- 40.Stoll BA. Upper abdominal obesity, insulin resistance and breast cancer risk. Int J ObesRelatMetabDisord. 2002;26:747–753. doi: 10.1038/sj.ijo.0801998. [DOI] [PubMed] [Google Scholar]
- 41.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 42.Lai A, Sarcevic B, Prall OW, Sutherland RL. Insulin/insulin-like growth factor-I and estrogen cooperate to stimulate cyclin E-Cdk2 activation and cell Cycle progression in MCF-7 breast cancer cells through differential regulation of cyclin E and p21(WAF1/Cip1) Journal of Biological Chemistry. 2001;276:25823–25833. doi: 10.1074/jbc.M100925200. [DOI] [PubMed] [Google Scholar]
- 43.Van der Burg B, Rutteman GR, Blankenstein MA, De Laat SW, Van Zoelen EJJ. Mitogenic stimulation of human breast cancer cells in a growth factor-defined medium: synergistic action of insulin and estrogen. Journal of Cellular Physiology. 1988;134:101–108. doi: 10.1002/jcp.1041340112. [DOI] [PubMed] [Google Scholar]
- 44.Bachelder RE, Wendt MA, &Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Research. 2002;62:7203–7206. [PubMed] [Google Scholar]
- 45.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. Journal of Clinical Endocrinology and Metabolism. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 46.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. PNAS. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Körner A, Kratzsch J, Kiess W. Effect of adiponectin on proliferation of breast cancer cells. Experimental and Clinical Endocrinology and Diabetes. 2006;114:S1P03_043. [Google Scholar]
- 48.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Letters. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 49.Gulcelik MA, Colakoglu K, Dincer H, Dogan L, Yenidogan E, et al. Associations between adiponectin and two different cancers: breast and colon. Asian Pac J Cancer Prev. 2012;13(1):395–8. doi: 10.7314/apjcp.2012.13.1.395. [DOI] [PubMed] [Google Scholar]
- 50.Sonmez B, Seker M, Bilici A, YavuzErkal F, Oven Ustaalioglu BB, Gumus M, et al. Is there any correlation among adiponectin levels in serum, tumor tissue and normal tissue of the same patientswith breast cancer. J BUON. 2011;16(2):227–32. [PubMed] [Google Scholar]
- 51.Seker M, Erkal FY, Bilici A, Salman T, Ustaalioglu BO. The association of tissue and serum adiponectin levels with the parameters of insulin-resistance in breast cancer patients. J ClinOncol. 2009;27 [Google Scholar]
- 52.Virginia G. Kaklamani, Maureen Sadim, Alex Hsi, Kenneth Offit, Carole Oddoux. Variants of the Adiponectin and Adiponectin Receptor 1 Genes and Breast Cancer Risk. Cancer Res. 2008;68:3178–3184. doi: 10.1158/0008-5472.CAN-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, et al. Tissue levels of adiponectin in breast cancer patients. MedOncol. 2007;24(4):361–6. doi: 10.1007/s12032-007-0021-0. [DOI] [PubMed] [Google Scholar]
- 54.Shelley Tworoger S, Heather Eliassen A, Theodoros Kelesidis, Graham Colditz A, Walter Willett C. PlasmaAdiponectinConcentrations and Risk of Incident Breast Cancer. JClinEndocrinolMetab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 55.Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007;22(1):117–21. doi: 10.3346/jkms.2007.22.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, et al. Adipocytokines and breast cancer risk. Chin Med J (Engl) 2007;120(18):1592–6. [PubMed] [Google Scholar]
- 57.Friedman J.M, Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;22:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 58.Isse N, Ogawa Y, Tamura N, et al. Structural organization and chromosomal assignment of the human obese gene. J BiolChem. 1995;270:27728–33. doi: 10.1074/jbc.270.46.27728. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Basinski MB, Beals JM, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 60.Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995;373:13–18. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- 61.Margetic S, Gazzola C, et al. Leptin: a review of its peripheral actions and interactions. Int J ObesRelatMetabDisord. 2002;26(11):1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 62.Matarese G, Moschos S, et al. Leptin in immunology. J Immunol. 2005;174(6):3137–42. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 63.Fei H, Okano HJ, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. ProcNatlAcadSci U S A. 1997;94(13):7001–5. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee GH, Proenca R, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 65.Tartaglia LA. The leptin receptor. J BiolChem. 1997;272(10):6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 66.Hileman SM, Pierroz DD, et al. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143(3):775–83. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- 67.Elmquist JK, Bjorbaek C, et al. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neuro. 1998;395(4):535–47. [PubMed] [Google Scholar]
- 68.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 69.Burcelin R, Kamohara S, Li J. Acute intravenous leptin infusion increases glucose turnover but not skeletal muscle glucose uptake in oblobmice. Diabetes. 1999;48:1264–1269. doi: 10.2337/diabetes.48.6.1264. [DOI] [PubMed] [Google Scholar]
- 70.Myers M. G., Jr Leptin receptor signaling and the regulation of mammalian physiology. Rec. Prog. Horm. Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 71.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. Journal of the National Cancer Institute. 1995;87:190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 72.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 73.O'Brien SN, Welter BH, Price TM. Presence of Leptin in Breast Cell Lines and Breast Tumors. Biochemical and Biophysical Research Communications. 1999;259:695. doi: 10.1006/bbrc.1999.0843. [DOI] [PubMed] [Google Scholar]
- 74.Kulkarni S, Saxena S, Watroba N, Groth J, Brady W, et al. Prognostic significance of leptin (L) and leptin receptor (OB-R) expression in human breast cancer (BC) Journal of Clinical Oncology. 2006;24(18S):20054. [Google Scholar]
- 75.Sulkowska M, Golaszewska J, Wincewicz A, Koda M, Baltaziak M, et al. Leptin-from regulation of fat metabolism to stimulation of breast growth. Pathology Oncology Research. 2006;12:69–72. doi: 10.1007/BF02893446. [DOI] [PubMed] [Google Scholar]
- 76.Magoffin D, Weitsman S, Aagarawal S, Jakimiuk A. Leptin regulation of aromatase activity in adipose stromal cells from regularly cycling women. Ginekologiapolska. 1999;70:1–7. [PubMed] [Google Scholar]
- 77.Castellucci M. Leptin modulates extracellular matrix molecules and metalloproteinases: possible implications for trophoblast invasion. Mol Hum Reprod. 2000;6:951–958. doi: 10.1093/molehr/6.10.951. [DOI] [PubMed] [Google Scholar]
- 78.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237(1):109–14. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 79.Geisler J, Haynes B, Ekse D, Dowsett M, Lønning PE. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels,”. Journal of Steroid Biochemistry and Molecular Biology. 2007;104(1-2):27–34. doi: 10.1016/j.jsbmb.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 80.Harris HR, Tworoger SS, Hankinson SE, Rosner BA, Michels KB. plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev Res (Phila) 2011;4(9):1449–56. doi: 10.1158/1940-6207.CAPR-11-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahmati-Yamchi M, Zarghami N, Rahbani M, Montazeri A. Plasma Leptin, hTERT Gene Expression, and Anthropometric Measures in Obese and Non-Obese Women with Breast Cancer. Breast Cancer (Auckl). 2011;5:27–35. doi: 10.4137/BCBCR.S6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, et al. Tissue leptin levels in patients with breast cancer. J BUON. 2010;15(2):369–72. [PubMed] [Google Scholar]
- 83.Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100(4):578–82. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han CZ, Du LL, Jing JX, Zhao XW, Tian FG, et al. Associations among lipids, leptin, and leptin receptor gene Gin223Arg polymorphisms and breast cancer in China. Biol Trace Elem Res. 2008;126(1-3):38–48. doi: 10.1007/s12011-008-8182-z. [DOI] [PubMed] [Google Scholar]
- 85.Snoussi K, Strosberg AD, Bouaouina N, Ben Ahmed S, Helal AN, et al. Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;20:6–38. doi: 10.1186/1471-2407-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stattin P, Söderberg S, Biessy C, Lenner P, Hallmans G, et al. Plasma leptin and breast cancer risk: a prospective study in northern Sweden. Breast Cancer Res Treat. 2004;86(3):191–6. doi: 10.1023/B:BREA.0000036782.11945.d7. [DOI] [PubMed] [Google Scholar]
- 87.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5(4):421–6. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 88.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010;121(2):479–83. doi: 10.1007/s10549-009-0591-y. [DOI] [PubMed] [Google Scholar]
- 89.Mohan Reddy N, Kalyan Kumar CH, Kaiser Jamil. Obesity, an additional burden for breast cancer patients with leptin gene polymorphisms. American journal of cancer research and clinical oncology. 2013;1:18–29. [Google Scholar]
- 90.Mohan Reddy N, Kalyana Kumar CH, Kaiser Jamil. Association of Adiponectin Gene Functional Polymorphisms (+45T/G and 276G/T) with Obese Breast Cancer. J MolBiomarkDiagn. 2012;3:138. [Google Scholar]