Abstract
Background
Bone marrow derived mesenchymal stem cells (MSCs) are a population of multipotent progenitors which have the capacity of proliferation and differentiation into mesenchymal lineage cells. Hypoxia could promote the proliferation of MSCs. Micro-RNAs are endogenous RNAs that can play an important role in some processes such as proliferation and differentiation. MiR-210 could help for better proliferation of MSCs since this miRNA could activate HIF pathway. In current study we investigated if MSCs can preserve their differentiation and proliferation ability under normoxic conditions by upregulation of miR-210.
Materials and Methods
MSCs isolated from C57 BL/6 mice by flushing it's femurs into the cell culture media. After 72 hours, MSCs which are plastic adherent cells were attached to the flask and non-adherent cells were removed. Subsequently, MSCs induced to differentiate into osteocytes and adipocytes with specific differentiation media in order to confirm their identity and multipotency. Then miR-210 was inserted in Lentiviruse vectors and affected MSCs. In each passage, the number and viability of cells were evaluated.
Results
Comparison between miR-210 infected MSCs with control cells showed that miR-210 has ability to increase proliferation of MSCs significantly.
Conclusion
We showed that miR-210 has ability to induce proliferation of MSCs without any negative effect on their differentiation abilities. Further studies are needed for evaluation of probable effects of miR-210 mechanisms on MSCs proliferation.
Keywords: Mesenchymal stem cells, MiR-210, HIF-1α
INTRODUCTION
MSCs are multipotent progenitors which reside in bone marrow, fat and some other tissues and can be isolated from various adult and fetal tissues.1–3 In vivo and in vitro, they are able to proliferate and differentiate into osteoblasts, adipocytes, chondrocytes and a variety of cell lineages.4–6 Self-renewal potential7 and multipotency are the hallmarks of MSCs.8 MSCs comprise about 0.001% of bone marrow mononuclear cells.1 For clinical purposes, we need a large-scale of MSCs, thus we must induce proliferation in them to generate adequate number of cells. Hypoxia could promote the proliferation of MSCs. This finding were reported in different sources derived MSCs. Hypoxia enhances proliferation of human MSCs,9 rat MSCs10, PB-MSCs11 and adipose-derived MSCs.12
MicroRNAs (miRNAs) are small conserved family of short (approximately 18–22 nucleotides) endogenous non-coding RNAs which are post-transcriptional negative regulators of protein-coding gene expression at the mRNA level via translational repression and/or mRNA degradation.13, 14 MiRNAs regulate gene expression via inhibition of RNA translation through binding to the 3′ untranslated regions (UTRs) of their target mRNAs by its seed sequence, 2–7 nucleotides in 5′ UTR, which is complementary to their target genes’ 3′ UTR.15–18 MicroRNAs genes are involved in regulation of a wide variety of cellular mechanisms including proliferation, differentiation, apoptosis, and metabolism13, 19–26 as well as angiogenesis and tumorigenesis.20, 27–31
Hypoxamirs are a group of hypoxia-induced miRNA molecules which are highly upregulated by hypoxia. MiR-210, one member of hypoxamirs family, is a direct transcriptional target of Hypoxia-inducible factor-1α (HIF-1α) which is robustly and ubiquitously expressed in a wide range of both normal and transformed hypoxic cells.15, 32–38
Hypoxia-inducible factor (HIF) is a heterodimeric transcription factor, which regulate the cellular response to hypoxia. HIF is composed of two α subunit (oxygen sensitive) and a β subunit (constitutively active). In normoxia, HIF-α is transcribed and translated but is rapidly degradated by the Von Hippel Lindau (VHL) protein.39–41 In hypoxia, HIF-1α is stabilized and accumulates for dimerization with HIF-β; this complex can bind to the specific sites in gene promoters named hypoxia response elements (HREs), and it transcriptionally regulates a variety of target genes.40–48 HIF1α directly binds to a highly conserved HRE on the proximal miR-210 promoter49 and upregulated miR-210 gene. A wide spectrum of miR-210 physiological roles includes cell proliferation, angiogenesis, mitochondrial metabolism, DNA repair and cell cycle regulation.34, 35 Due to the role of miR-210 in stem cell survival and proliferation,44 in current study, we investigated induction of mir-210 upregulation on proliferation of mouse mesenchymal stem cells in normoxic conditions.
MATERIALS AND METHODS
Isolation, Culture, and Proliferation of MSC
Mesenchymal stem cells were collected from male C57Bl/6 mice by flushing the femurs with Dulbecco's modified Eagle's medium-low glucose (DMEM, Gibco) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin, and 100 µg/mL streptomycin (Invitrogen). The obtained suspension was centrifuged at 300× g for 5 min. The pellet was resuspended in growth medium and incubated in 5% CO2 at 37 °C for 72h. After 3 days, the medium containing the non-adherent hematopoietic cells was removed from the flask, and fresh medium was replaced. After approximately 4 days, 7 days from isolation, the primary cells were passaged when BMSCs reached 80% confluency. Adherent cells were retrieved by trypsinization and then replated.
Differentiation Assays
MSCs were plated in duplicate in 24-well tissue culture plate, 6*104 cells per well, and then induced to differentiate. The cells were chosen from third passage. For adipogenic differentiation of MSCs, cells were cultured in adipocytic differentiation medium (100nM dexamethasone, 50µm indomethacine, 0.5mM isobutyl-1-methyl xanthine) for 14 days and stained with Oil Red O (Sigma). For osteogenic differentiation of MSCs, cells were cultured in osteogenic differentiation medium (50µM ascorbic acid, 10mM beta-glycerol-3-phosphate, and 100nM dexamethasone) for 21 days and stained with Alizarin Red S (Sigma).
Cloning pre- miR-210
pLenti-III-mir-GFP containing the precursor of miR-210 were prepared from stem cell technology institute. (Figure 1: Map of plasmid)
Fig. 1.
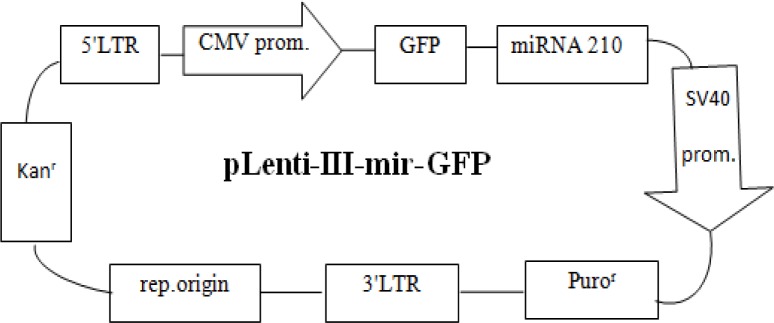
The map of plasmid used in this study
Lentivirus Production & Infection of Target Cells
Lentiviral vectors expressing the transgene were produced by transfecting three plasmid systems (Packaging plasmid ps-PAX2, envelope plasmid pMD2.G and pLenti-III-mir-GFP vector plasmids with insert fragments) into the producer cells (HEK-293) using the calcium–phosphate method. Culture medium was replaced 16 hours post-transfection. Vector-containing supernatants were collected 24h, 48h and 72h post-transfection, then centrifuged, filtered and pooled. One day before transduction, MSCs (from passage 4) were plated in the 24-well plates (35× 103 cells/ well) and then transduced with 500µl of lentiviral vectors containing pLenti-miR-210 and pLenti (without miR-210) as control; one well remained as negative control. The supernatant containing vectors that collected from HEK239 was added directly to MSCs without using any reagent for transduction. After 24h, the medium was replaced with fresh complete medium; and after 48h, the efficiency of transduction was evaluated by an invert fluorescent microscope. The infected cells were selected by means of puromycin with optimal effective dose of 2µg/ml. 2µg/ml of puromycin was add into the passage 1-3 in order to kill non-transfected cells.
Evaluating the Effect of miR-210 on MSCs Proliferation
Every 72h, cells were tripsinized from each well (plasmid with and without miRNAs, and negative control) and counted with hemocytometer; their viability was quantified with trypan- blue assay.
Statistical analysis
Data were analyzed by Student's t test using SPSS version 16.00. P value less than 0.01 was considered as statistically significant.
RESULTS
MSCs Isolation, Culture and Differentiation
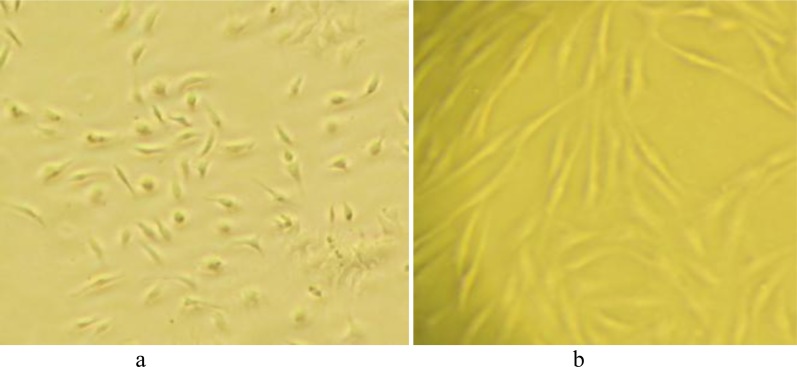
MSCs were isolated from the femurs of the C57Bl/6 mice by flushing. Non-adherent cells were removed after 3 days and fresh medium was added to the adherent cells. Approximately 4 days after isolation, spindle-shaped morphology was seen (Figure 2).
Fig. 2.
Mesenchymal stem cells 96h after isolation (a), Mesenchymal stem cells 14 days after isolation-3th passage (b)
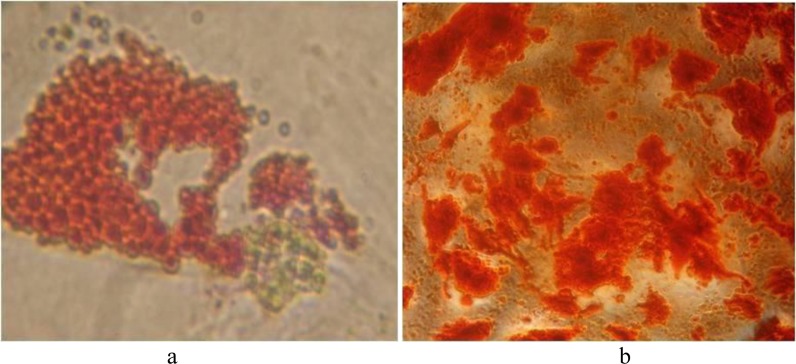
Differentiation assays, were carried out in order to confirm the osteogenic and adipogenic potential of cells. Alizarin Red staining for osteocytes and Oil Red staining for adipocytes were performed. Intracellular lipid vesicles in mature adipocytes were stained bright red with Oil Red stain; and extracellular calcium deposits in osteocytes were stained bright orange-red with Alizarin Red stain. (Figure 3)
Fig. 3.
Oil Red O staining, intracellular lipid accumulation were stained bright red in adipocytes at day 14 (a), Alizarin Red S staining, calcium deposition were stained bright orange-red in osteocytes at day 21 (b)
The isolated cells were identified as MSCs by their adherence to plastic flasks, spindle-shaped morphology and differentiation potential.
Recombinant lentivirus production and transduction of MSCs
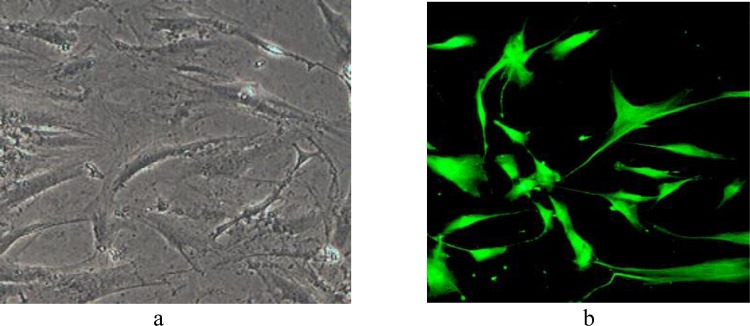
In order to produce the lentiviral particles expressing pre-miR-210, packaging plasmid psPAX2, envelope plasmid pMD2.G and pLenti-III-mir-GFP vector with inserted fragments were co-transfected into the HEK-293 using the calcium-phosphate method. 24, 48, and 72h after transfection, Lentiviral particles were collected from HEK293 supernatant. MSCs from 4th passage were cultured in 24-well plates, 35 × 103 cells/well and infected by 500µl of supernatants. 48h later, the GFP positive cells were seen by fluorescent microscope, about 50% of MSCs were GFP positive. Because the infected cells were puromycin resistance, cells were cultured in medium containing 2µg/ml of puromycin in order to select infected cells. At the end of passage 3, 90% of cells were GFP positive. (Figure 4)
Fig. 4.
The GFP positive mesenchymal stem cells that infected with vectors without fluorescent microscope (a) and with fluorescent microscope (b)
The number of passages, proliferation and viability of MSCs
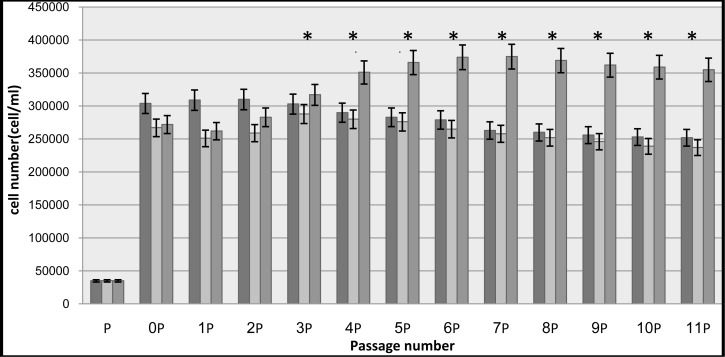
Cell counting performed every 72h using hemocyometer; and cell viability of were assessed using trypan-blue assay. Cells proliferation evaluation has been continued up to 40 days. Results are shown in Table 1 and Fig. 5.
Table 1.
Cell Number (the average of duplicate pellets) and Viability of Mesenchymal Stem Cells in Control Group, Cells Containing Plasmid without miR-210 Group and Cells Containing Plasmid with miR-210 Group, in Each Passage.
| Passage number | Control cells | Plasmid without miR-210 | Plasmid with miR-210 | Viability of cells containing Plasmid with miR-210 |
|---|---|---|---|---|
| P | 35000 | 35000 | 35000 | 90% |
| P0 | 304000 | 267000 | 272000 | 70% |
| P1 | 309000 | 251000 | 262000 | 75% |
| P2 | 310000 | 259000 | 283000 | 85% |
| P3 | 303000 | 288000 | 317000 | 85% |
| P4 | 290000 | 280000 | 351000 | 90% |
| P5 | 283000 | 276000 | 366000 | 85% |
| P6 | 279000 | 265000 | 374000 | 85% |
| P7 | 263000 | 258000 | 375000 | 85% |
| P8 | 260000 | 252000 | 369000 | 85% |
| P9 | 256000 | 246000 | 362000 | 85% |
| P10 | 253000 | 239000 | 359000 | 80% |
| P11 | 252000 | 237000 | 355000 | 80% |
Fig. 5.
Cell number (cell/ml). The control groups are presented in black, cells without miR-210 in white and with miR-210 in gray.
*: P value less than 0.01 was considered as statistically significant (P < 0.01).
DISCUSSION
Most previous studies investigated mesenchymal stem cell proliferation in hypoxic conditions.9–12 Hypoxia can ragulates cell proliferation by the means of hypoxia-inducible factors (HIFs).15 Induction of miR-210, major hypoxia-inducible miRNAs, is a feature of the hypoxic response in both normal and transformed cells.15, 34, 50–52 MiR-210 functioned by targeting MNT, a known antagonist of MYC-dependent transcriptional activation and cell growth. Inhibition of MNT leads to activation of C-MYC indirectly. C-MYC is a basic-helix-loop-helix/leucine zipper (bHLH/LZ) transcription factor that controls the cell-cycle progression. The ability of MYC to promote cell proliferation depends on its dimerization with MAX.49, 53–56 MYC-MAX heterodimers bind to Miz1 at the transcription initiator element (INR) of the CDKN1A gene and consequently inhibit CDKN1A (coding p21) which results in cell cycle progression. Additionally, MYC-MAX heterodimers can bind to E-box element (CACGTG) in the intron directly, and to the GC-rich region of the promoter through transcription factor Sp1 which leads to CDC25A expression and subsequently cell cycle progress. In contrast with former researches, in the current study, we investigated miR-210 over expression effects on mesenchymal stem cells proliferation in normoxic condition.56–58 MiR-210 may mediate the proliferation and survival of MSCs by targeting MNT, a known MYC antagonist.53
Several studies indicated that MSCs are highly sensitive to oxygen pressure and hypoxia facilitates their proliferation. Ren et al (2006) described that under low oxygen tension (8% O2), Balb/c mice derived MSCs exhibited high proliferation potential.59 In Grayson et al (2007) study on human mesenchymal stem cells (hMSCs) under hypoxic atmospheres (2% O2), an approximate 9-fold expansion was observed at each passage for hypoxic cells, whereas under normoxic atmospheres (20% O2) only 5-fold expansion was seen in each passage.9 In 2007 Fehrer et al., determine the proliferation capacity of MSCs at 3% and 20% pO2 in long-term culture (100 days) and represented that under ambient oxygen tension cell growth declined after several passages, whereas cells cultured in hypoxic conditions maintain the ability to proliferate.60 Another study done by Lee et al., (2008) indicated that Adipose-derived stem cells (ADSCs) which exposed to low O2 concentration demonstrated better survival and proliferation in comparison with ADSCs under ambient O2 concentration.12 Dos Santos et al., (2009) indicated that human bone marrow (BM) MSC exhibited an early start of the exponential growth phase and subsequently a higher fold increase under hypoxia than normoxia.61 Wei-li et al., (2011) illustrated that hypoxia could enhance proliferation of PB-MSCs.11 Wang et al in 2012 demonstrated that hypoxia (3% O2 treatment) can increase rat MSC proliferation by upregulation of phosphorylated p38 MAPK.10
In the present study, we showed that miR-210 over expression leads to induction of mesenchymal stem cells proliferation in normoxic condition. Our results were in agreement with most studies in this area unless they investigated cell proliferation in hypoxic conditions and we just induced miR-210 in mesenchymal stem cells and studied its effects on MSCs expansion.
ACKNOWLEDGEMENT
This study was done in faculty of allied medical sciences of Tehran University of medical sciences and supported by grants from the Tehran University of medical sciences (research project number 91-02-3116939). The authors appreciatively thank the research committee of the faculty, all authorities and office worker of the research assistance of Tehran University. The authors would like to thank Dr. Soleimani for the kind gift of pLenti-III-mir-GFP plasmid.
REFERENCES
- 1.Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002;195(12):1549–63. doi: 10.1084/jem.20011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr Cartilage. 2005;13:845–53. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Campagnoli C, Roberts IA, Kumar S. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood Rev. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 5.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 6.Majumdar MK, Thiede MA, Mosca JD. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol Rev. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23(4):594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–74. [PubMed] [Google Scholar]
- 9.Ma T, Grayson WL, Zhao F, Bunnell B. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Bioph Res Commun. 2007;358:948–53. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Yang J, Li H, Wang X, Zhu L, Fan M, et al. Hypoxia Promotes Dopaminergic Differentiation of Mesenchymal Stem Cells and Shows Benefits for Transplantation in a Rat Model of Parkinson's Disease. PLoS One. 2013;8(1):1–12. doi: 10.1371/journal.pone.0054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu WL, Jia ZQ, Wang WP, Zhang JY, Fu X, Duan XN, et al. Proliferation and apoptosis property of mesenchymal stem cells derived from peripheral blood under the culture conditions of hypoxia and serum deprivation. Chin Med J. 2011;124(23):3959–67. [PubMed] [Google Scholar]
- 12.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair and Regeneration. 2009;17(4):540–7. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nature reviews Molecular cell biology. 2007;8(1):23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Molecular cell. 2009;35(6):856–67. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AkbarPourfathollah A, Abroun S. Mir-155 Downregulation by miRCURY LNA™ microRNA Inhibitor Can Increase alpha Chain Hemoglobins Expression in Erythroleukemic K562 Cell Line. International Journal of Hematology-Oncology and Stem Cell Research. 2010;4(2) [Google Scholar]
- 18.Kouhkan F, Alizadeh S, Kaviani S, Soleimani M, Pourfathollah AA, Amirizadeh N, et al. miR-155 down regulation by LNA inhibitor can reduce cell growth and proliferation in PC12 cell line. Avicenna Journal of Medical Biotechnology. 2011;3(2):61. [PMC free article] [PubMed] [Google Scholar]
- 19.Calin GA, Liu C-G, Sevignani C, Ferracin M, Felli N, Dumitru CD, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11755–60. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farh KK-H, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310(5755):1817–21. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 21.Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Current opinion in structural biology. 2005;15(3):331–41. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Karp X, Ambros V. Encountering microRNAs in cell fate signaling. Science. 2005;310(5752):1288–9. doi: 10.1126/science.1121566. [DOI] [PubMed] [Google Scholar]
- 23.Miska EA. How microRNAs control cell division, differentiation and death. Current opinion in genetics & development. 2005;15(5):563–8. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Seila AC, Sharp PA. Small RNAs tell big stories in Whistler. Nature cell biology. 2008;10(6):630–3. doi: 10.1038/ncb0608-630. [DOI] [PubMed] [Google Scholar]
- 25.Guarnieri DJ, DiLeone RJ. MicroRNAs: a new class of gene regulators. Annals of medicine. 2008;40(3):197–208. doi: 10.1080/07853890701771823. [DOI] [PubMed] [Google Scholar]
- 26.Leung AK, Sharp PA. microRNAs: a safeguard against turmoil? Cell. 2007;130(4):581–5. doi: 10.1016/j.cell.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 28.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iorio MV, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer research. 2005;65(16):7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 30.Liu C-G, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9740–4. doi: 10.1073/pnas.0403293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 32.Mathew LK, Simon MC. mir-210: a sensor for hypoxic stress during tumorigenesis. Molecular cell. 2009;35(6):737–8. doi: 10.1016/j.molcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. Journal of cellular and molecular medicine. 2008;12(5a):1426–31. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer biology & therapy. 2008;7(2):255–64. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. Journal of Biological Chemistry. 2008;283(23):15878–83. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulkkinen K, Malm T, Turunen M, Koistinaho J, Ylä-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo: Ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS letters. 2008;582(16):2397–401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 37.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. MOLECULAR AND CELLULAR BIOLOGY. 2007;27(5):1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer research. 2009;69(3):1221–9. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams J, Difazio L, Rolandelli R, Lujan J, Hasko G, Csoka B, et al. HIF-1: a key mediator in hypoxia (Review) Acta Physiologica Hungarica. 2009;96(1):19–28. doi: 10.1556/APhysiol.96.2009.1.2. [DOI] [PubMed] [Google Scholar]
- 40.Corn PG. Role of the ubiquitin proteasome system in renal cell carcinoma. BMC biochemistry. 2007;8(Suppl 1):S4. doi: 10.1186/1471-2091-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weidemann A, Johnson R. Biology of HIF-1α. Cell Death & Differentiation. 2008;15(4):621–7. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 42.Peterson KM, Aly A, Lerman A, Lerman LO, Rodriguez-Porcel M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: role of oxidative stress. Life sciences. 2011;88(1):65–73. doi: 10.1016/j.lfs.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin BY, Jiang G, Wegiel B, Wang HJ, MacDonald T, Zhang XC, et al. Hypoxia-inducible factor 1α stabilization by carbon monoxide results in cytoprotective preconditioning. Proceedings of the National Academy of Sciences. 2007;104(12):5109–14. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. Journal of Biological Chemistry. 2009;284(48):33161–8. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovascular research. 2008;77(1):134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 46.Wang J-a, Chen T-l, Jiang J, Shi H, Gui C, Luo R-h, et al. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacologica Sinica. 2008;29(1):74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 47.Jiang B-H, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. American Journal of Physiology-Cell Physiology. 1996;271(4):C1172–C80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 48.Semenza GL, Jiang B-H, Leung SW, Passantino R, Concordet J-P, Maire P, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. Journal of Biological Chemistry. 1996;271(51):32529–37. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 49.Huang X, Le Q-T, Giaccia AJ. MiR-210–micromanager of the hypoxia pathway. Trends in molecular medicine. 2010;16(5):230–7. doi: 10.1016/j.molmed.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nie Y, Han B-M, Liu X-B, Yang J-J, Wang F, Cong X-F, et al. Identification of MicroRNAs involved in hypoxia-and serum deprivation-induced apoptosis in mesenchymal stem cells. International journal of biological sciences. 2011;7(6):762. doi: 10.7150/ijbs.7.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang W, Lee CY, Park JH, Park MS, Maeng LS, Yoon CS, et al. Survival of hypoxic human mesenchymal stem cells is enhanced by a positive feedback loop involving miR-210 and hypoxia-inducible factor 1. J Vet Sci. 2013;14(1):69–76. doi: 10.4142/jvs.2013.14.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9(6):1072–83. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, Sun H, Dai H, Walsh R, Imakura M, Schelter J, et al. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8(17):2756–68. doi: 10.4161/cc.8.17.9387. [DOI] [PubMed] [Google Scholar]
- 54.Hurlin P, Queva C, Eisenman R. C-Myc in B-Cell Neoplasia. Springer; 1997. Mnt: a novel Max-interacting protein and Myc antagonist; pp. 115–21. [DOI] [PubMed] [Google Scholar]
- 55.Hurlin PJ, Queva C, Eisenman RN. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes & development. 1997;11(1):44–58. doi: 10.1101/gad.11.1.44. [DOI] [PubMed] [Google Scholar]
- 56.Huang LE. Carrot and stick: HIF-a engages c-Myc in hypoxic adaptation. Cell Death and Differentiation. 2008;15:672–7. doi: 10.1038/sj.cdd.4402302. [DOI] [PubMed] [Google Scholar]
- 57.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2a Promotes Hypoxic Cell Proliferation by Enhancing c-Myc Transcriptional Activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.John D. Gordan, Craig B. Thompson, Simon M. Celeste. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell August. 2007;12(2):108–13. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, et al. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochemical and Biophysical Research Communications. 2006;347:12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- 60.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging cell. 2007;6(6):745–57. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 61.Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex Vivo Expansion of Human Mesenchymal Stem Cells: A More Effective Cell Proliferation Kinetics and Metabolism Under Hypoxia. J Cell Physiol. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]