1. INTRODUCTION
Place may influence health through several pathways; stress is one potential mediator that is frequently invoked (Diez Roux and Mair, 2010, Anisman and Zacharko, 1992). For example, living in a blighted urban neighborhood may increase exposure to stressors such as violence, noise, and crowding. These exposures may elicit repeated activations of the stress response system, which in turn may lead to eventual dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, a primary stress regulatory system. HPA axis dysregulation has been associated with a range of mental, cardiovascular, immunologic, and metabolic disorders (Anisman and Zacharko, 1992, Seeman et al., 2004). Although recent studies have found associations between neighborhood conditions and stress biomarkers (Bird et al., 2010, Chen and Paterson, 2006, Do et al., 2011, Nazmi et al., 2010), there has been limited research on links between neighborhood conditions and stress biomarkers in children or adolescents.
Cortisol is a hormone involved in the HPA axis (McEwen, 2007) that has been used in several contexts. Adverse conditions in neighborhood and family environments have been linked to both cortisol levels and cortisol reactivity, although the evidence is mixed. In adults, some studies have yielded associations between neighborhood- and individual-level low socioeconomic status (SES) and cortisol diurnal levels—specifically lower waking levels (Hajat et al., 2010), higher average levels (Cohen et al., 2006b, Cohen et al., 2006a), and less steep declines over the course of the day (Do et al., 2011, Hajat et al., 2010, Agbedia et al., 2011, Karb et al., 2012), though others have found null or opposite results (Agbedia et al., 2011, Cohen et al., 2006a, Hajat et al., 2010). Do et al. also found that neighborhood violence was associated with lower cortisol levels at awakening and less steep initial declines (Do et al., 2011). In children, studies have reported associations between individual-level disadvantage (including low SES, exposure to stressful life events, and family adversity) and lower morning cortisol levels (Bevans et al., 2008, Repetti et al., 2002), higher average cortisol levels (Bevans et al., 2008, Fernald and Gunnar, 2009, Kelly et al., 2008, Repetti et al., 2002), and less steep declines (Kelly et al., 2008, Gustafsson et al., 2010, Martin et al., 2012). In addition, some have suggested a curvilinear (upside-down u-shaped) association; children and adolescents exposed to the most stressful conditions have cortisol levels that resemble those of non-disadvantaged individuals (Bevans et al., 2008, Gustafsson et al., 2010).
The link between individual- and neighborhood-level adverse conditions and cortisol reactivity is likely complex. Some studies have shown that adverse conditions in childhood are associated with greater cortisol reactivity in adulthood (Goldman-Mellor et al., 2012, Pesonen et al., 2010, Mangold et al., 2010), but lifetime adversity is associated with blunted reactivity (Goldman-Mellor et al., 2012, Lovallo et al., 2012). Others have found no association (Steptoe et al., 2005). Relatedly, moderate adversity has been associated with heighted reactivity in children and adolescents (Gutteling et al., 2005, Repetti et al., 2002), whereas more severe forms of adversity, such as prolonged child maltreatment, has been associated with blunted reactivity (MacMillan et al., 2009). The timing and duration of exposure to adverse conditions may also be influential (Bosch et al., 2012, Steptoe et al., 2005).
The evidence for an independent association between adverse neighborhood conditions and salivary cortisol in adolescents is extremely limited. Studies conducted to date provide preliminary evidence that neighborhood disadvantage is associated with higher average resting cortisol levels (Brenner et al., 2012, Chen and Paterson, 2006) and greater cortisol reactivity (Hackman et al., 2012). However, the studies have been based on small, racially homogeneous samples in single urban areas (Chen and Paterson, 2006, Brenner et al., 2012, Hackman et al., 2012). The present study was motivated to address this gap in the literature. We used the National Comorbidity Survey Replication Adolescent Supplement (NCS-A) to estimate the association between neighborhood disadvantage and salivary cortisol levels in adolescents. The NCS-A consists of a nationally representative, ethnically diverse sample of adolescents in the United States. Cortisol measurements are available for 2490 of the adolescents, making it the largest sample of cortisol in U.S. children or adolescents. Our analyses of these data utilize a propensity score approach coupled with regression adjustment designed to address a key threat to internal validity—non-random neighborhood assignment and consequent imbalance of confounding variables, including those particularly influential to cortisol measurement.
2. METHODS
2.1. Study sample
The NCS-A is a nationally representative, cross-sectional survey of U.S. adolescent mental health. The background, design, sampling, and field procedures are presented elsewhere (Kessler et al., 2009b, Kessler et al., 2009a, Kessler et al., 2009c, Merikangas et al., 2009). Participants ages 13–17 were recruited from a dual-frame sample consisting of household and school subsamples. Face-to-face, computer-assisted interviews (which included a modified Composite International Diagnostic Interview) were conducted in the adolescent’s home by professional interviewers from the Survey Research Center at the Institute for Social Research at the University of Michigan. The interviews took place between February 2001 and January 2004. While the adolescent was interviewed, his/her parent or parent surrogate was given a self-administered questionnaire. A short-form version of the questionnaire was administered to parents who did not complete the long-form version. Each participating adolescent and his/her parent or guardian provided informed assent and consent. Recruitment and consent procedures were approved by the Human Subjects Committees of Harvard Medical School and the University of Michigan.
2.2. Contextual measures
2.2.1. Neighborhood Disadvantage
The Survey Research Center at the Institute for Social Research at the University of Michigan geocoded residential addresses to U.S. Census tracts. Neighborhood SES, defined at the Census tract level, is a summary measure created by Diez-Roux et al., 2001 that has been used previously (Diez Roux et al., 2004, Henderson et al., 2005, Borrell et al., 2006, Nordstrom et al., 2007). We defined neighborhoods in the lowest SES tertile as disadvantaged, and those in the middle and upper tertiles as non-disadvantaged.
Neighborhood SES is made up of six indicators from the U.S. Census Short Form 3 (SF3): log median household income; % households with interest, dividend, or rental income; log median value of housing units; % persons over age 25 with high school degree; % persons over age 25 with college degree; % persons in executive, managerial, or professional specialty occupations (Diez Roux et al., 2001). The normally distributed summary score results from summing the z-score of each indicator. In the NCS-A sample, the summary score has a median value of −0.36 (range: −13.6, 17.8) and a Cronbach’s alpha of 0.83.
2.3. Individual Measures
2.3.1. Outcome measures
Cortisol levels in ng/mL were measured using saliva samples. Saliva samples were collected in a salivette by passive drool after the participant chewed on a piece of sugarless gum immediately before and after the interview, while the interviewer was present. The interviewer’s laptop automatically recorded the time and date of each sample collection, and interviews lasted an average of 146 minutes (standard deviation: 32 minutes, range: 28–237 minutes, interquartile range: 124–169 minutes). Salivettes were treated with sodium azide at Harvard University, centrifuged, and pre-labeled with subject identification numbers and study information prior to sample collection. After collection, samples were mailed to NIH where they were stored at −80°C until testing. Quantification of cortisol levels was done by a radioimmunoassay (Siemens Diagnostic). The sensitivity of the assay was 0.0165 ng/mL. Intra- and inter-assay coefficients of variation were 5.4% and 26.0%, respectively. Similar coefficients of variation for this method have been reported previously (Wirth et al., 2006).
We examined three outcomes in the present study: (1) point-in-time pre-interview cortisol level, (2) point-in-time post-interview cortisol level, and (3) cortisol rate of change (slope) over the course of the interview, calculated as the difference in post versus pre-interview levels divided by the length of the interview in hours. These outcomes do not directly map onto specific HPA axis dimensions. For example, a Trier stress test (Kirschbaum et al., 1993) measures stress reactivity, but such a test was deemed inappropriate for children by the NCS-A investigators. Instead, pre- and post-interview samples measure cortisol in slightly different naturalistic settings. In the case of the pre-interview sample, the adolescent is interrupted from his/her normal routine for the interview (the adolescent may have been active—not sitting quietly), and the adolescent is anticipating the new experience of being interviewed by a stranger as part of a survey of mental health. In the case of the post-interview sample, the adolescent has been seated for an average of 2 ½ hours and has finished answering questions that were designed not to be stressful. We hypothesized that the extent to which neighborhood disadvantage is associated with cortisol may differ slightly in these two settings. In addition, we hypothesized that cortisol levels would decline over the course of the interview due to cortisol’s circadian rhythm, the seated nature of the interview, and the fact that the questions were designed not to be stressful, and that the rate of decline may also be associated with neighborhood disadvantage.
Cortisol was measured on the first 2490 adolescents enrolled in the NCS-A. Budget limitations prevented cortisol testing on the full sample. Participants with and without cortisol measures were similar across most demographic characteristics (see Supplementary Table 1). They differed slightly in terms of where they were sampled (e.g., region of the country, urbanicity, neighborhood disadvantage status), when they were sampled (e.g., season, time of interview), and in terms of family income, maternal education, current parental employment, small for gestational age, and smoking status. Five of the 2490 adolescents with cortisol data were excluded, because they resided in Census tracts with missing or inestimable SF3 indicators, which precluded characterization of their neighborhood. Removing extreme outlying cortisol values resulted in the exclusion of six additional participants. A small proportion of adolescents were not able to complete the interview in one sitting. In an effort to reduce heterogeneity in the post-interview measure, we excluded those adolescents who took more than four hours to complete the interview (2.4%) from the post-interview and rate of change outcome analyses. However, inclusion of those adolescents did not change the inferences (results not shown).
Cortisol peaks in the morning during the cortisol awakening response (CAR) and then declines over the rest of the day (Chida and Steptoe, 2009). Research suggests that the association between salivary cortisol levels and SES may be in opposite directions depending on whether cortisol is measured during the CAR or afterward (Do et al., 2011). To avoid this source of heterogeneity, we limited our analysis to those adolescents with measures taken during the late decline portion of the cortisol diurnal cycle (Golden et al., 2011). We operationalized this by excluding adolescents whose first sample was taken prior to 10am on a weekday during the school year and prior to noon on a weekend or during summer vacation (we did not have information on the time the adolescent awoke on the day of the interview). Participants with cortisol measures during versus after the CAR were similar across most demographic characteristics (see Supplementary Table 2). There were slight differences in season, weekend versus weekday, typical weekend bedtime, and family structure.
2.3.2.Covariate measures
We included covariates that have been recommended (Leventhal and Brooks-Gunn, 2000) as potentially important covariates to control for in assessing relationships between neighborhood and mental health in addition to covariates hypothesized to confound the association between neighborhood and cortisol.
By definition, confounding variables are not affected by the exposure of interest. In contrast, mediators (sometimes called explanatory variables) lie on the causal pathway between exposure and outcome. With this distinction in mind, we controlled for confounding through the following variables: adolescent age (in years), race/ethnicity, urbanicity, immigrant generation, citizenship, English as a second language, maternal age at birth (in years), maternal level of education, region, time of cortisol collection, weekend versus weekday, and season. Although maternal age at birth and maternal education may have been affected by prior neighborhood residence (which may be related to current residence), we decided to include these variables to control for two important measures of family socioeconomic status (Leventhal and Brooks-Gunn, 2000). Household income (log-transformed) and parental employment may be thought of as confounders or mediators. We controlled for these variables in a separate model. Potentially mediating variables included: small for gestational age status, body-mass index (BMI), typical bedtime, and typical hours of sleep (Rosmalen et al., 2005, Randler and Schaal, 2010, Bouma et al., 2009).
Although some variables (e.g., adolescent’s age) had no missing data, others had missing data due to non-response. The two variables with the most missing data were maternal education (19% missing) and current parental employment (30% missing). Following recommendations for addressing missing data (Stuart et al., 2009), we performed multiple imputation by chained equations (van Buuren and Groothuis-Oudshoorn, 2011), creating 10 imputed datasets. This approach may be less biased than one that restricts analyses to those with complete data (Stuart et al., 2009, Hernan et al., 2004). The imputation model included variables used in the analysis as well as those predictive of non-response.
2.4. Exclusion criteria
In addition to exclusions described in the Outcome Measures section, we excluded participants who were currently pregnant (0.3%), diagnosed with type-1 diabetes (0.9%), taking psychiatric medication (4.8%), possibly taking steroid mediation for asthma (3.6%), taking oral contraceptives (3.7%), current smokers (5.7%), and current illicit drug users (8.4%). After applying the exclusion criteria, there were 1646 adolescents in the sample. Four adolescents with a pre-interview measure did not have a post-interview measure. All adolescents that had a post-interview cortisol measure also had a pre-interview measure.
2.4. Analytic Approach
2.4.1. Matching
We used a propensity score approach to match adolescents living in disadvantaged neighborhoods with those in non-disadvantaged neighborhoods to create two similar groups based on the time and day of cortisol collection as well as demographic characteristics. We followed the recommendations of Rubin and Thomas and combined propensity score matching (with replacement) with the more stringent matching requirements of exact and caliper matching on a subset of particularly influential covariates (Rubin and Thomas, 2000). Because research suggests that time of day, race, and weekend versus weekday are important determinants of cortisol levels and reactivity (Randler and Schaal, 2010, Dickerson and Kemeny, 2004, Hajat et al., 2010, DeSantis et al., 2007), we exact matched on race/ethnicity and weekend/weekday and matched within calipers of time of day (0.20 standard deviations).
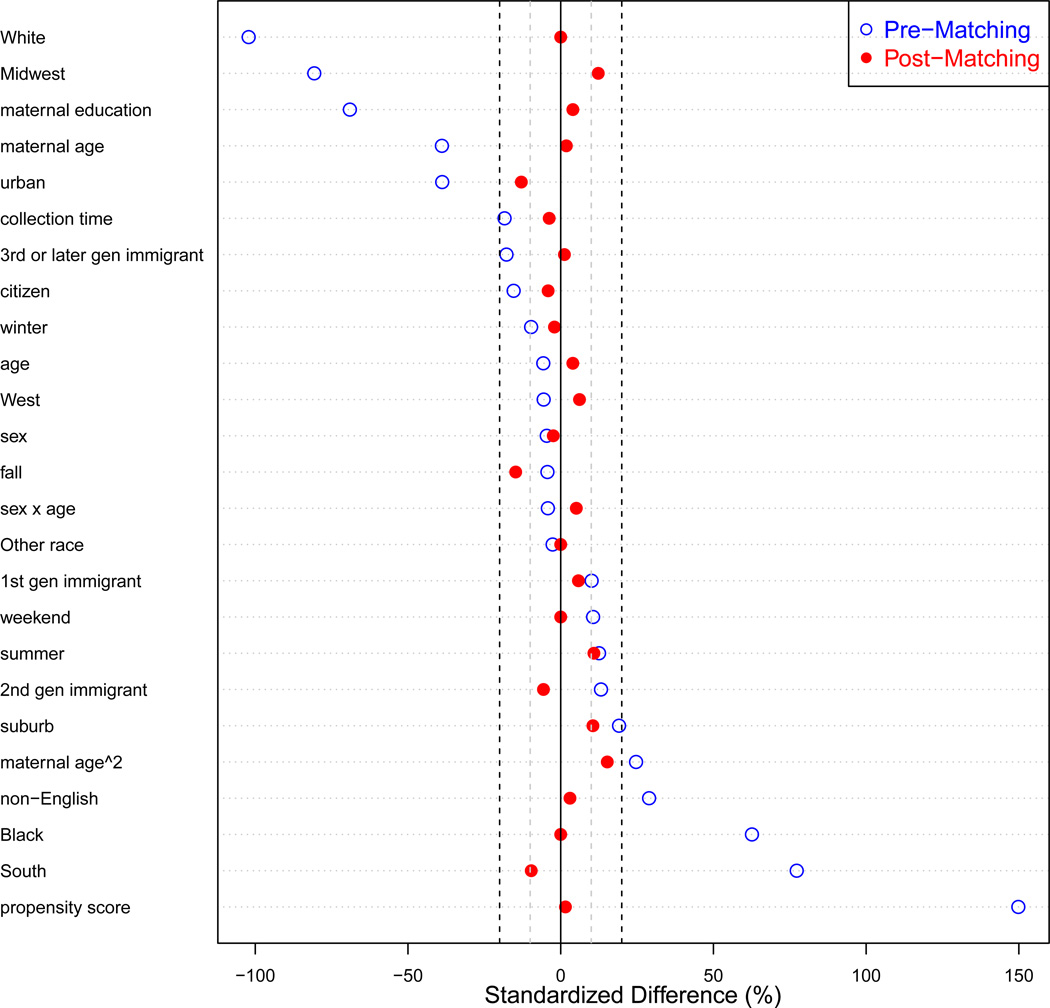
After exact and caliper matching, adolescents were matched based on their estimated propensity scores. We estimated a propensity score (Rosenbaum and Rubin, 1983) for each adolescent from a logistic regression model of living in a disadvantaged neighborhood at the time of the study as a function of the covariates detailed in Figure 1; the propensity scores are the resulting predicted probabilities from this model. Covariate balance between the disadvantaged versus non-disadvantaged neighborhood groups was achieved in the final matched dataset (all standardized mean differences less than 15%, Figure 1).
Figure 1.
Covariate balance pre- and post-matching. Plotted points represent the standardized mean differences (difference in means between the disadvantaged neighborhood group and non-disadvantaged neighborhood group standardized by the standard deviation in the disadvantaged group) for each covariate. Open dots represent standardized mean differences in the pre-matched data. Closed dots represent standardized mean differences in the post-matched data. Vertical grey and black dashed lines indicate standardized mean differences of 10% and 20%, respectively. Participants were exact matched on race/ethnicity and weekend/weekday, caliper matched on time of sample, and matched on propensity score that was a function of the covariates listed on the y-axis of the figure.
2.4.2. Regression
After matching, between 852 and 894 adolescents remained in the sample, depending on imputation number. Table 1 describes the final matched sample by neighborhood disadvantage status. These adolescents resided in between 531 and 550 Census tracts, depending on imputation number. One way to address clustering of people in neighborhoods is through multilevel models; however, this strategy requires having enough adolescents within the same neighborhood to accurately estimate neighborhood parameters. Typical guidance suggests using multilevel models if there are at least 15–30 residents per neighborhood (Leventhal and Brooks-Gunn, 2000). In this sample, clustering of participants within neighborhoods was low: there was an average of 1.6 adolescents per neighborhood, and only 10% of neighborhoods had more than two adolescent participants. Because multilevel modeling is not recommended in such scenarios, we accounted for clustering by using the survey package in R to incorporate sampling strata and cluster variables in standard error estimation using Taylor linearization (Lumley, 2004). This strategy produced similar results as calculating cluster robust standard errors using a sandwich estimator (results not shown but available from the first author upon request).
Table 1.
NCSA matched sample characteristics by neighborhood disadvantage status.1
| Variable | Disadvantaged neighborhood (N=602) Mean (SE) |
Non-disadvantaged neighborhood (N=265) Mean (SE) |
P-value2 (2-sided) |
|---|---|---|---|
| Female, % | 48.01 (1.90) | 48.50 (4.15) | 0.92 |
| Age, y | 14.98 (0.10) | 14.90 (0.15) | 0.59 |
| Race/ethnicity, % | 1.00 | ||
| Hispanic | 30.73 (3.37) | 30.73 (6.32) | |
| Black | 37.21 (3.49) | 37.21 (6.54) | |
| Other | 4.82 (1.13) | 4.82 (1.82) | |
| White | 27.24 (3.37) | 27.24 (2.95) | |
| Urbanicity, % | 0.69 | ||
| Urban center | 30.40 (4.13) | 35.22 (5.57) | |
| Suburb | 42.02 (3.40) | 38.37 (5.28) | |
| Non-urban | 27.58 (3.00) | 26.41 (7.60) | |
| Region, % | 0.55 | ||
| Northeast | 10.13 (2.65) | 11.79 (2.08) | |
| Midwest | 9.80 (1.61) | 6.15 (1.85) | |
| South | 60.80 (3.69) | 64.29 (5.39) | |
| West | 19.27 (2.80) | 17.77 (3.48) | |
| Household income (log), dollars | 10.40 (0.09) | 10.63 (0.14) | 0.17 |
| Maternal age at birth, y | 24.82 (0.30) | 24.77 (0.40) | 0.92 |
| Maternal level of education, % | 0.67 | ||
| Less than high school | 19.27 (1.59) | 18.94 (5.03) | |
| High school graduate | 49.67 (2.44) | 54.65 (5.49) | |
| Some college | 21.43 (1.48) | 16.61 (2.22) | |
| College graduate | 9.63 (1.42) | 9.80 (2.05) | |
| Family structure, % | |||
| Lived with mother whole life | 87.71 (1.07) | 86.05 (3.67) | 0.68 |
| Lived with father whole life | 47.34 (1.93) | 46.35 (4.23) | 0.83 |
| Immigrant generation, % | 0.87 | ||
| 1st | 8.64 (1.19) | 9.63 (2.99) | |
| 2nd | 16.11 (1.51) | 17.11 (3.35) | |
| 3rd or greater | 75.25 (2.17) | 73.36 (4.02) | |
| Small for gestational age3, % | 8.80 (0.79) | 6.64 (1.73) | 0.31 |
| Physical abuse by parent, % | 15.45 (1.54) | 17.61 (4.06) | 0.59 |
| Three or more parental adversities, % | 3.16 (0.61) | 1.33 (0.69) | 0.11 |
| Season, % | 0.78 | ||
| Spring | 11.13 (2.22) | 9.63 (2.60) | |
| Summer | 42.19 (4.00) | 39.87 (4.20) | |
| Fall | 33.39 (3.15) | 38.21 (4.52) | |
| Winter | 13.29 (1.94) | 12.29 (3.09) | |
| Weekend, % | 27.24 (2.43) | 27.24 (4.72) | 1.00 |
| Interview time, hr | 15:37 (0:08) | 15:38 (0:14) | 0.97 |
| Pre-interview cortisol, ng/mL (×10−2) | 25.20 (0.82) | 20.68 (1.30) | <0.01 |
| Post-interview cortisol, ng/mL (×10−2) | 14.07 (0.49) | 16.21 (1.47) | 0.15 |
| Cortisol slope, ng/mL/hr (×10−2) | −4.69 (0.33) | −1.84 (0.72) | <0.01 |
Matched sample from the first imputation, matching weights used, clustering by neighborhood accounted for.
P-values were calculated from the t statistic for continuous covariates and from the chi-squared statistic for categorical covariates.
Defined using guidelines in Alexander et al., 1999.
We ran fully parameterized outcome regression models using the final matched dataset and weighting by the matching frequency weights (to account for matching with replacement) to estimate associations between living in a disadvantaged versus non-disadvantaged neighborhood and (1) pre- and (2) post-interview cortisol levels and (3) cortisol slope, conditional on potential confounders. Although researchers may address confounding by either (1) including confounders in the propensity score matching model or (2) by including confounders in the regression model, the propensity score literature recommends combining propensity score matching with regression (including the same confounders in both models) in order to best control for confounding (Ho et al., 2007). This type of analysis has the advantage of being doubly robust, because estimates are consistent if either the propensity score model is correct or if the outcome model is correct (Ho et al., 2007). Gamma regression models with a log link were used for the point-in-time cortisol measures. Gamma regression is appropriate for positive, skewed data, as was the case for these cortisol data, and gamma regression coefficients have a straightforward multiplicative interpretation (Faraway, 2006). The exponentiated neighborhood regression coefficient from each of the pre- and post-interview cortisol models estimates the conditional multiplicative effect of living in a disadvantaged neighborhood on point-in-time cortisol level. Linear regression was used to model cortisol slope. The neighborhood regression coefficient for this model estimates the conditional additive effect of living in a disadvantaged neighborhood on cortisol slope.
We ran one unadjusted and three adjusted models for each cortisol outcome. Adjusted Model 1 contained potential confounding variables (listed in Figure 1) that we believe preceded the adolescent’s neighborhood residence. Adjusted Model 2 added variables that could be thought of as confounders or mediators: household income and current and historical parental employment. Adjusted Model 3 added hypothesized mediators of the neighborhood disadvantage-cortisol association: sleep, BMI, and small for gestational age. Results for each outcome model were combined across the ten imputed datasets using Rubin’s combining rules (Rubin, 1987). All analyses were performed using the R statistical language (version 2.15.1).
3. RESULTS
Table 1 describes the demographic characteristics of adolescents included in the final matched dataset by neighborhood disadvantage status. The propensity score matching procedure described above resulted in adolescents having similar demographic characteristics between disadvantaged and non-disadvantaged neighborhoods. The mean age was just under 15 years, and 48% were female. Hispanic, African American, and White racial/ethnic categories were well represented (31%, 37%, and 28%, respectively), as were urban, suburban and rural areas (32%, 41%, and 27%, respectively). In this matched dataset, 62% of adolescents were from the Southern region of the U.S., 51% had mothers with a high school education, and 41% were interviewed in the summer.
We estimated the conditional expected ratios of pre- and post-interview cortisol levels and conditional expected differences in cortisol rate of change between those living in disadvantaged versus non-disadvantaged neighborhoods running regression-adjusted models on matched datasets balanced on covariates of interest. As described in the Methods section, cortisol measures were limited to those occurring after the cortisol awakening response, so inference is limited to the late decline portion of cortisol’s circadian rhythm. Unadjusted model results are included for comparison.
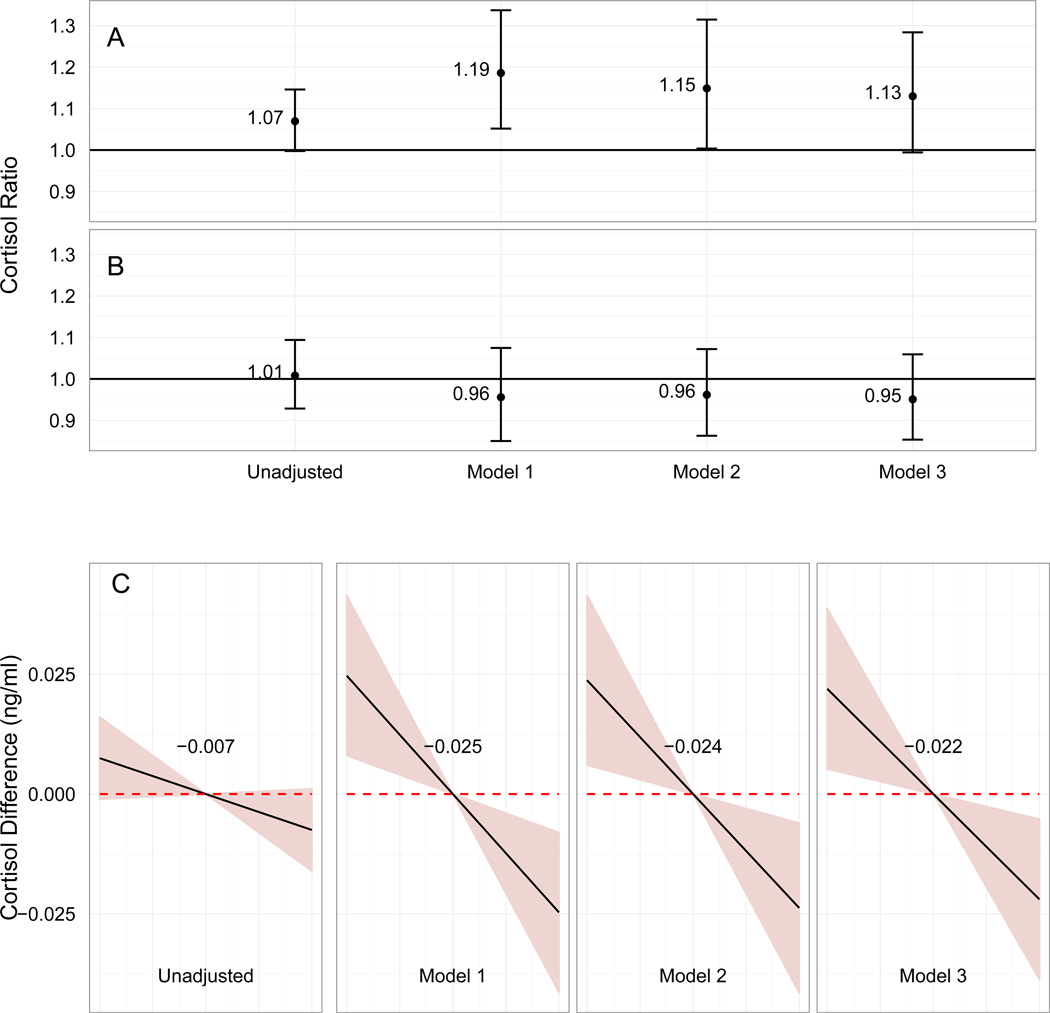
Figure 2 shows the associations between neighborhood disadvantage and (A) pre-interview cortisol, (B) post-interview cortisol, and (C) cortisol rate of decline for the unadjusted and adjusted models. In the unadjusted model, adolescents living in disadvantaged neighborhoods had 7% higher pre-interview cortisol levels than those in non-disadvantaged neighborhoods (95% CI, 1.00, 1.15), 0.8% higher post-interview cortisol levels (95% CI, 0.93, 1.09), and 0.75 (95% CI, −0.11, 1.60) ×10−2 ng/mL/hr steeper rates of decline (Figure 2).
Figure 2.
Conditional expected ratios of cortisol levels and conditional expected differences in cortisol slope during the late decline period comparing adolescents living in disadvantaged versus non-disadvantaged neighborhoods. Models were matched on and regression-adjusted for covariates listed in Figure 1. Row A: Ratios of point-in-time pre-interview cortisol levels. Error bars represent 95% CI for the mean. Row B: Ratios of point-in-time post-interview cortisol levels. Error bars represent 95% CI for the mean. Row C: Differences in cortisol slope. Shaded areas represent 95% CI for the mean.
Model 1 adjusts for covariates listed in Figure 1. In this model, adolescents in disadvantaged neighborhoods had 19% higher (95% CI, 1.05, 1.34) pre-interview cortisol levels than those in non-disadvantaged neighborhoods, 4% lower (95% CI, 0.85, 1.07) post-interview cortisol levels, and 2.47 (95% CI, 0.81, 4.13) ×10−2 ng/mL/hr steeper rates of decline (Figure 2). Model 2 adjusts for the covariates in Model 1 and also adjusts for household income and current and previous parental employment. Effect sizes attenuated slightly in this model, but neighborhood disadvantage residence remained associated with higher pre-interview cortisol levels (1.15, 95% CI: 1.00, 1.31) and steeper rates of decline (2.38, 95% CI: 0.61, 4.14).
Small for gestational age, BMI, and sleep are all potential mediators of the neighborhood disadvantage-cortisol relationship. Adjusted Model 3 added these mediating variables to the covariates included in Adjusted Model 2. The effect sizes attenuated slightly comparing Model 2 to Model 3 (Figure 2). The association between neighborhood disadvantage residence and higher pre-interview cortisol levels was no longer significant at the 95% confidence level (1.13, 95% CI: 0.99, 1.28), but the association between neighborhood disadvantage and steeper rates of decline remained statistically significant (2.20, 95% CI: 0.52, 3.87).
We also examined the potential explanatory power of each of these mediators by individually adding them to Adjusted Model 2. Neither the addition of BMI nor small for gestational age appreciably changed the size of the neighborhood disadvantage parameter for any of the cortisol outcomes, and inferences remained the same as in Adjusted Model 2. Sleep variables included the time the adolescent went to bed on weekday nights, the time the adolescent went to bed on weekend nights, hours of sleep on weekdays, and hours of sleep on weekends. The addition of sleep variables attenuated the association between neighborhood disadvantage residence and pre-interview cortisol levels by about 9% and rendered it non-significant. Adding sleep variables also attenuated the association between neighborhood disadvantage and cortisol rate by about 6%, but it remained statistically significant.
4. DISCUSSION
In a large, U.S. population-based sample, we found that adolescents living in disadvantaged neighborhoods had higher pre-interview cortisol levels and steeper declines in cortisol over the course of the interview, perhaps reflecting heightened reactivity to and recovery from the novel stimulus of the interview. There were no differences in post-interview cortisol levels. These results add to the nascent body of literature that links neighborhood context and stress in adolescents.
This study addressed several gaps in the literature. Previous research has been hampered by small sample sizes and ethnic and geographic homogeneity. We addressed this by using a large, population-based, and ethnically diverse sample of adolescents. In addition, our use of propensity score matching methods coupled with regression adjustment addressed confounding stemming from non-random neighborhood residence.
According to McEwen, there are two key dimensions of the stress response system: (1) reactivity to an acute stressor and (2) unprovoked, chronic levels of stress hormones (McEwen, 2007). Both could become dysregulated as a result of chronic stress caused by residence in a disadvantaged neighborhood. To measure these dimensions precisely requires controlled laboratory conditions that hold time, day of the week, and certain environmental variables (e.g., light, temperature, noise) and behaviors (e.g., level of physical activity, smoking, drinking) constant and protocols that administer either a standardized acute stressor (e.g., a Trier stress test) or rest period (Hamer et al., 2010). Because such a protocol was not possible, the cortisol outcomes used in this analysis do not map onto specific HPA axis dimensions.
Instead, the pre- and post-interview samples reflect slightly different naturalistic settings, as described in the Methods section. The pre-interview sample may reflect, in part, the adolescent’s anticipation of a novel interview situation and activity the adolescent was engaged in prior to the interview. We speculate that the anticipation of the interview may have provoked an HPA response for some. However, as the interview was conducted in a familiar environment, it is also possible that adolescents had no HPA response. The rate of decline in cortisol may reflect some combination of recovery from anticipation and activity as well as cortisol’s natural diurnal rhythm.
There has been little research on the relationship between cortisol response to novel situations and neighborhood- and individual-level SES. However, animal research demonstrates that rats stressed when young (e.g., by a lack of maternal licking/grooming) are more “neophobic,” with heightened, less controlled stress responses to novel situations that persist into adulthood (Liu et al., 2000, Liu et al., 1997, Meaney et al., 1994). In human children, Gutteling et al. found that children of anxious mothers had higher cortisol levels on the first day of kindergarten than children of non-anxious mothers (Gutteling et al., 2005), and Hackman et al. found in a sample of African American adolescents that those in disadvantaged neighborhoods had greater cortisol reactivity to a stress test than those in non-disadvantaged neighborhoods (Hackman et al., 2012).
If the anticipation of the novel interview situation provoked an HPA axis response for some, then our findings are congruent with this prior research. However, we recognize that the steeper slope found for adolescents living in disadvantaged neighborhoods may seem counterintuitive. Higher pre-interview cortisol levels and steeper slope may imply a hypervigilant HPA axis, but one that is still resilient and healthy. As a cross-sectional survey of adolescents, this study captures a particular developmental window. Over time, repeated or prolonged hypervigilant responses may succumb to allostatic load, eventually resulting in desensitization of the HPA axis (Geronimus, 1992, McEwen, 2007).
The post-interview sample may reflect a natural decline as per cortisol’s diurnal rhythm and perhaps a more restful state. Prior studies of the relationship between resting cortisol and neighborhood- and individual-level SES have yielded mixed results, possibly due to effect heterogeneity (Cohen et al., 2006a, Do et al., 2011, Hajat et al., 2010, Karb et al., 2012). Thus, while we hypothesized higher post-interview cortisol levels in adolescents who resided in disadvantaged neighborhoods, our null results are not inconsistent with prior literature. We could have obtained null results either because there is no association between neighborhood disadvantage and post-interview cortisol levels or because there is an association but our results are biased or because there is effect heterogeneity that we have not accounted for. No association between post-interview levels and neighborhood disadvantage suggests that there is no difference in cortisol levels after sitting for a 2 ½ hour interview between adolescents in disadvantaged versus non-disadvantaged neighborhoods.
4.1. Sensitivity Analyses
We performed four sensitivity analyses. First, we re-ran the analysis (1) excluding current smokers but including current drug users and (2) including both current drug users and current smokers. This tested the sensitivity of our results to the decision to exclude adolescents who are current smokers and drug users. (Such exclusion criteria are common, as these substances may affect cortisol levels and responsiveness (e.g., Kirschbaum et al., 1994, Hinkelmann et al., 2009).) With each inclusion, the effect sizes attenuate (see Supplemental Table 3). This is expected as we are including individuals whose cortisol levels may be artificially influenced and therefore may not be at-risk for being influenced by neighborhood sources of stress. When we exclude smokers only, neighborhood disadvantage remains associated with cortisol rate of decline, neighborhood disadvantage and post-interview cortisol levels remain unassociated, and the association between neighborhood disadvantage and pre-interview cortisol levels remains significant in Adjusted Model 1 but not in Adjusted Model 2. When we include both current drug users and smokers, there is no longer a statistically significant association between neighborhood disadvantage and pre-interview cortisol levels in Adjusted Models 1 or 2. The association between neighborhood disadvantage and cortisol rate remains significant at the 95% confidence level in Adjusted Model 1, but not in Model 2.
Second, we re-ran the analysis excluding adolescents who may have experienced severe adversity during childhood. We operationalized this as excluding individuals who reported ever being physically abused by a parent (including being beaten up, choked, burned, kicked, punched, bitten, or threatened with a knife or gun) or reported three or more of the following: parent suicide attempt, parent suicide completion, parent alcohol abuse, parent drug abuse, parent arrest or imprisonment. The literature indicates that timing and severity of exposure to adverse conditions alter HPA axis dynamics in adolescents (Bosch et al., 2012). Adolescents with moderate levels of childhood adversity may have a hypervigilant but resilient stress response, whereas adolescents with severe, persistent adversity may undergo a degree of desensitization leading to blunted cortisol response (Gutteling et al., 2005, MacMillan et al., 2009, Repetti et al., 2002). Failure to distinguish between these two subgroups likely results in a partial washout effect. A greater proportion of adolescents with possible severe adversity lived in disadvantaged neighborhoods (see Table 1), and we would expect from prior research that they would be more likely to have blunted HPA axis reactivity. We expected that excluding these adolescents would strengthen the associations between living in a disadvantaged neighborhood and pre-interview cortisol levels and cortisol slope. However, effect estimates changed very little and all inferences remained the same, possibly because the disadvantaged and non-disadvantaged neighborhood groups were not much different in terms of possible severe adversity after matching (see Table 1).
Third, we assessed the sensitivity of our results to our decision to use the first tertile to define neighborhoods as disadvantaged. We repeated our analysis using the 25th, 20th, 15th, 10th, and 5th percentile as cut-points (results not shown, but available from the first author upon request). The association between neighborhood disadvantage and cortisol slope remained statistically significant for Adjusted Model 1 for all cut-points except the 5th percentile. In this case, the association was no longer significant, possibly because the sample size of the matched dataset was greatly reduced in this case (from N=867 to N=148 for the first imputation). For each alternative cut-point, the parameter estimate of neighborhood disadvantage on pre-interview cortisol levels remained similar but was no longer significant at the 95% confidence level, possibly also due in part to a reduction in sample size.
We found the association between pre-interview cortisol and neighborhood disadvantage to be sensitive to the current smoker and drug user exclusion criteria in the first sensitivity analysis and sensitive to different cut-points of neighborhood disadvantage in the third sensitivity analysis. In contrast, the association between cortisol rate of change and neighborhood disadvantage was robust. We examined the robustness of this association to an unobserved confounder in a fourth sensitivity analysis (see details in Supplementary Material and Supplementary Figure 1) (Vanderweele and Arah, 2011). Assessing sensitivity to an unobserved confounder is important because although the above methods aim to approximate the comparability of exposed and unexposed groups found in a randomized control trial, the exposed and unexposed groups could differ by an unobserved confounder. Under the assumptions outlined in the Supplementary Material, a binary unobserved confounder would have to be 20% more prevalent in disadvantaged versus non-disadvantaged neighborhoods and bias the conditional mean cortisol slope by 98% to make our estimate no longer significant.
4.2. Strengths and Limitations
In terms of this study, internal validity is compromised if the adolescents who live in disadvantaged neighborhoods are still not comparable (also called exchangeable) with those who live in non-disadvantaged neighborhoods after controlling for covariates. Our analytic approach addresses this challenge in several ways not often used in studies of this kind. First, the NCS-A collected extensive information from adolescents and their parents that we were able to use to control for confounding. Second, our matching analytic design allowed us to explicitly assess covariate balance between the disadvantaged neighborhood group and non-disadvantaged neighborhood group, and perform the analysis on a subset of the data in which the two groups are balanced across our list of potential covariates. Third, using the above matching methods in conjunction with regression can be thought of as a doubly-robust approach, meaning that if either the propensity-score exposure model or outcome regression model is correct, then the estimates are unbiased in expectation (Ho et al., 2007).
Our results are subject to several limitations. The summary measure of neighborhood disadvantage comprises a set of indicators measuring different components of neighborhood disadvantage, like income, assets, housing value, education, and employment, which should contain less random measurement error than any one indicator alone. However, neighborhood disadvantage is still measured with error. First, the summary measure does not capture all the characteristics that define the latent construct of neighborhood disadvantage. Second, we use Census tracts as a proxy for neighborhood—neighborhood boundaries identified by residents will likely not overlap completely with Census tract boundaries.
Another limitation is that cortisol is just one of many biomarkers of HPA axis activity. Free, unbound cortisol can be obtained from saliva samples whereas total—bound and unbound—cortisol can be obtained from serum samples. Unbound cortisol is likely the more relevant proxy, because it is thought to be the only component of cortisol to reach the “target tissue and elicit glucocorticoid effects” (Kirschbaum et al., 1994). Consistent with this idea, saliva cortisol has been found to better measure adrenal cortical function (Vining et al., 1983) and HPA axis activity (Gozansky et al., 2005), although saliva and serum cortisol are highly correlated (Woodside et al., 1991). In addition, measuring cortisol through saliva samples is non-invasive and does not induce stress—a strength because of cortisol’s sensitivity to stress (Kirschbaum et al., 1994).
In addition, the variability of cortisol presents a major challenge. Each person has her/his own activation thresholds and unique diurnal pattern. Within person, cortisol levels depend on time of day, day of the week, activities, diet, amount of sunshine, etc. Other epidemiologic studies incorporating cortisol measures in adult samples have sought to address variability within the limitation of a non-laboratory environment by modeling within- versus between-person variability of the natural diurnal rhythm of cortisol levels by collecting multiple measurements per person per day for multiple days. However, this collection scheme has been shown to be infeasible with adolescents (Halpern et al., 2012), so we instead applied exclusion criteria and restrictive matching methods to move the analysis back to a scenario that holds three of the driving sources of variability either constant or close to constant and limits the analysis to a sample whose cortisol levels are not artificially influenced by prescription or non-prescription drugs, hormones, or tobacco. This may provide an initial way forward for the practical researcher wanting to make use of cortisol measurements in large, epidemiologic studies.
Another limitation is that we cannot infer clinical significance from our results. Dysregulation of the stress response system may increase risk of mental, cardiovascular, immunologic, and metabolic disorders (Anisman and Zacharko, 1992, Seeman et al., 2004). Dysregulation of the HPA axis has also been implicated in unhealthy eating behaviors and obesity (McEwen, 2007, Sapolsky, 2004). Future research should examine dose-response relationships and possible threshold effects between cortisol measures and these health outcomes. Although the associations we found were small, seemingly small differences that persist over many years may ultimately have profound effects on neurocircuitry and glucose regulation.
In conclusion, in an ethnically and geographically diverse sample of adolescents, we found evidence of higher pre-interview cortisol levels and steeper rates of decline over the course of the interview comparing those living in disadvantaged versus non-disadvantaged neighborhoods. These findings may reflect a heightened, yet resilient, cortisol response to a novel interview situation among adolescents living in disadvantaged neighborhoods. This extends previous animal and laboratory-based research and bolsters the evidence base suggesting that place may influence the stress response system. Implications of such a conclusion argue for policies designed to improve the safety and built environment of the U.S.’s most disadvantaged neighborhoods. More research is needed to further understand individual and contextual determinants as well as to inform specific policy targets.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support: This work was supported by the Intramural Research Program of the National Institute of Mental Health at the National Institutes of Health (NIH) [Z01 MH-002808-08]. GSW’s time was supported by NIH grant K05AA02034. The National Comorbidity Survey Replication Adolescent Supplement (NCS-A) and the larger program of related National Comorbidity Surveys are supported by the National Institute of Mental Health [U01-MH60220] and the National Institute of Drug Abuse [R01 DA016558] at the NIH. The NCS-A was carried out in conjunction with the World Health Organization World Mental Health Survey Initiative. The authors wish to thank Vanya Aggarwal for help with Census data abstraction.
Role of the Funding Source: The sponsors had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
None.
Results from this paper were submitted to be presented as a poster at the 46th Annual Society for Epidemiologic Research Meeting, Boston, Massachusetts, June 18–21, 2013.
Disclaimer: The views and opinions expressed in this article are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or U.S. Government.
The authors claim no conflicts of interest.
Contributor Information
Kara E Rudolph, Email: krudolph@jhsph.edu.
Gary S Wand, Email: gwand@jhmi.edu.
Elizabeth A Stuart, Email: estuart@jhsph.edu.
Thomas A Glass, Email: tglass@jhsph.edu.
Andrea H Marques, Email: andreahorvathmarques@gmail.com.
Roman Duncko, Email: roman.duncko@gmail.com.
Kathleen R Merikangas, Email: merikank@mail.nih.gov.
REFERENCES
- Agbedia OO, Varma VR, Seplaki CL, Seeman TE, Fried LP, Li L, Harris GC, Rebok GW, Xue QL, Tan EJ, Tanner E, Parisi JM, Mcgill S, Carlson MC. Blunted diurnal decline of cortisol among older adults with low socioeconomic status. Ann N Y Acad Sci. 2011;1231:56–64. doi: 10.1111/j.1749-6632.2011.06151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Depression as a consequence of inadequate neurochemical adaptation in response to stressors. Br J Psychiatry Suppl. 1992:36–43. [PubMed] [Google Scholar]
- Bevans K, Cerbone A, Overstreet S. Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Dev Psychopathol. 2008;20:257–272. doi: 10.1017/S0954579408000126. [DOI] [PubMed] [Google Scholar]
- Bird CE, Seeman T, Escarce JJ, Basurto-Davila R, Finch BK, Dubowitz T, Heron M, Hale L, Merkin SS, Weden M, Lurie N. Neighbourhood socioeconomic status and biological 'wear and tear' in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;64:860–865. doi: 10.1136/jech.2008.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Beck JD, Heiss G. Socioeconomic disadvantage and periodontal disease: the Dental Atherosclerosis Risk in Communities study. Am J Public Health. 2006;96:332–339. doi: 10.2105/AJPH.2004.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents' cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37:1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents' cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Brenner AB, Zimmerman MA, Bauermeister JA, Caldwell CH. The Physiological Expression of Living in Disadvantaged Neighborhoods for Youth. J Youth Adolesc. 2012:1–15. doi: 10.1007/s10964-012-9838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychol. 2006;25:704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006a;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006b;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Desantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Borrell LN, Haan M, Jackson SA, Schultz R. Neighbourhood environments and mortality in an elderly cohort: results from the cardiovascular health study. J Epidemiol Community Health. 2004;58:917–923. doi: 10.1136/jech.2003.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AV, Kiefe CI, Jacobs DR, Jr, Haan M, Jackson SA, Nieto FJ, Paton CC, Schulz R. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11:395–405. doi: 10.1016/s1047-2797(01)00221-6. [DOI] [PubMed] [Google Scholar]
- Do DP, Roux AVD, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, Shea S, Seeman T. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: The Multi-Ethnic Study of Atherosclerosis. Health Place. 2011;17:625–632. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraway JJ. Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Boca Raton: Chapman & Hall/CRC; 2006. Other GLMs; pp. 135–140. [Google Scholar]
- Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Soc Sci Med. 2009;68:2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2:207–221. [PubMed] [Google Scholar]
- Golden SH, Wand GS, Malhotra S, Kamel I, Horton K. Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. Eur J Epidemiol. 2011;26:511–525. doi: 10.1007/s10654-011-9585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Mellor S, Hamer M, Steptoe A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology. 2012;37:1755–1768. doi: 10.1016/j.psyneuen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisl determined by enzyme immunoassay is preferable to serum total cortisl for assessment of hypothalamic-pituitary-adrenal axis activity. Clin Endocrinol. 2005;63:336–341. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Anckarsater H, Lichtenstein P, Nelson N, Gustafsson PA. Does quantity have a quality all its own? Cumulative adversity and up- and down-regulation of circadian salivary cortisol levels in healthy children. Psychoneuroendocrinology. 2010;35:1410–1415. doi: 10.1016/j.psyneuen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, De Weerth C, Buitelaar JK. Prenatal stress and children's cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 2012;6:277. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern CT, Whitsel EA, Wagner B, Harris KM. Challenges of measuring diurnal cortisol concentrations in a large population-based field study. Psychoneuroendocrinology. 2012;37:499–508. doi: 10.1016/j.psyneuen.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, O'donnell K, Lahiri A, Steptoe A. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. Eur Heart J. 2010;31:424–429. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- Henderson C, Diez Roux AV, Jacobs DR, Jr, Kiefe CI, West D, Williams DR. Neighbourhood characteristics, individual level socioeconomic factors, and depressive symptoms in young adults: the CARDIA study. J Epidemiol Community Health. 2005;59:322–328. doi: 10.1136/jech.2003.018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Widemann K, Kellner M, Otte C. Cognitive impairment in major depression: association with salivary cortisol. Biol Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Analysis. 2007;15:199–236. [Google Scholar]
- Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago Community Adult Health Study. Soc Sci Med. 2012;75:1038–1047. doi: 10.1016/j.socscimed.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Young R, Sweeting H, Fischer JE, West P. Levels and confounders of morning cortisol collected from adolescents in a naturalistic (school) setting. Psychoneuroendocrinology. 2008;33:1257–1268. doi: 10.1016/j.psyneuen.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Costello EJ, Green JG, Gruber MJ, Heeringa S, Merikangas KR, Pennell BE, Sampson NA, Zaslavsky AM. Design and field procedures in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Int J Methods Psychiatr Res. 2009a;18:69–83. doi: 10.1002/mpr.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Costello EJ, Green JG, Gruber MJ, Heeringa S, Merikangas KR, Pennell BE, Sampson NA, Zaslavsky AM. National comorbidity survey replication adolescent supplement (NCS-A): II. Overview and design. J Am Acad Child Adolesc Psychiatry. 2009b;48:380–385. doi: 10.1097/CHI.0b013e3181999705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Green J, Gruber MJ, Guyer M, He Y, Jin R, Kaufman J, Sampson NA, Zaslavsky AM. National comorbidity survey replication adolescent supplement (NCS-A): III. Concordance of DSM-IV/CIDI diagnoses with clinical reassessments. J Am Acad Child Adolesc Psychiatry. 2009c;48:386–399. doi: 10.1097/CHI.0b013e31819a1cbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull. 2000;126:309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry. 2012;71:344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19. [Google Scholar]
- Macmillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van Der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Horm Behav. 2010;58:637–646. doi: 10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CG, Bruce J, Fisher PA. Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: the role of parental psychosocial risk and monitoring. Horm Behav. 2012;61:661–668. doi: 10.1016/j.yhbeh.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Meaney M, Tannenbaum B, Francis D, Bhatnagar S, Shanks N, Viau V, O'donnell C, Plotsky PM. Early environmental programming hypothalamic-pituitary-adrenal responses to stress. Semin Neurosci. 1994;6:247–259. [Google Scholar]
- Merikangas K, Avenevoli S, Costello J, Koretz D, Kessler RC. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry. 2009;48:367–369. doi: 10.1097/CHI.0b013e31819996f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A, Roux AD, Ranjit N, Seeman TE, Jenny NS. Cross-sectional and longitudinal associations of neighborhood characteristics with inflammatory markers: Findings from the multi-ethnic study of atherosclerosis. Health Place. 2010;16:1104–1112. doi: 10.1016/j.healthplace.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom CK, Diez Roux AV, Schulz R, Haan MN, Jackson SA, Balfour JL. Socioeconomic position and incident mobility impairment in the Cardiovascular Health Study. BMC Geriatr. 2007;7:11. doi: 10.1186/1471-2318-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Feldt K, Heinonen K, Osmond C, Phillips DI, Barker DJ, Eriksson JG, Kajantie E. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology. 2010;35:758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Randler C, Schaal S. Morningness-eveningness, habitual sleep-wake variables and cortisol level. Biol Psychol. 2010;85:14–18. doi: 10.1016/j.biopsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Rosmalen JG, Oldehinkel AJ, Ormel J, De Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10–12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys, New York. J. Wiley & Sons; 1987. [Google Scholar]
- Rubin DB, Thomas N. Combining propensity score matching with additional adjustments for prognostic covariates. JASA. 2000;95:573–585. [Google Scholar]
- Sapolsky RM. Why Zebras Don't Get Ulcers. New York: St. Martin's Press; 2004. [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht SR, Wright C, Feldman PJ. Socioeconomic position and cardiovascular and neuroendocrine responses following cognitive challenge in old age. Biol Psychol. 2005;69:149–166. doi: 10.1016/j.biopsycho.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stuart EA, Azur M, Frangakis C, Leaf P. Multiple imputation with large data sets: a case study of the Children's Mental Health Initiative. Am J Epidemiol. 2009;169:1133–1139. doi: 10.1093/aje/kwp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- Vanderweele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology. 2011;22:42–52. doi: 10.1097/EDE.0b013e3181f74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining RF, McGinely RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20:329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Welsh KM, Schultheiss OC. Salivary cortisol changes in humans after winning or losing a dominance contest depend on implicit power motivation. Horm Behav. 2006;49:346–352. doi: 10.1016/j.yhbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Woodside DB, Winter K, Fisman S. Salivary cortisol in children: correlations with serum values and effect of psychotropic drug administration. Can J Psychiatry. 1991;36:746–748. doi: 10.1177/070674379103601011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.