Abstract
Aims
To determine the prevalence of polypoidal choroidal vasculopathy (PCV) in patients with presumed neovascular age-related macular degeneration (AMD) who were considered poor responders to ranibizumab.
Methods
Caucasian patients with suspected neovascular AMD, presumed to be choroidal neovascularisation, previously treated with ≥8 intravitreal injections of ranibizumab 0.5 mg (Lucentis; Novartis AG, Basel, Switzerland) administered as required during optical coherence tomography-guided dosing were retrospectively included. Eyes were categorised according to the time from injection 1 to injection 6 (group 1: <12 months; group 2: ≥12 months). Indocyanine green angiography (ICGA) was used to re-evaluate eyes for PCV. Suitable candidates received reduced-fluence photodynamic therapy/ranibizumab combination therapy supplemented by ranibizumab monotherapy, as required.
Results
202 eyes were included (group 1: 73.8%; group 2: 26.2%). The prevalence of PCV in group 1 (21.5%) was significantly higher than in group 2 (3.8%; p=0.003). After initiation of combination therapy, 16 eyes with PCV received 3.1±2.5 ranibizumab injections/year vs 8.4±2.4 injections/year before initiation of combination therapy (p<0.001).
Conclusions
In Caucasian patients with presumed neovascular AMD, PCV prevalence is increased in eyes that respond poorly to ranibizumab monotherapy. ICGA improved PCV diagnosis in poor responders; combination therapy may be beneficial for eyes with PCV.
Keywords: Neovascularisation, Treatment Medical, Imaging, Macula
Introduction
Antivascular endothelial growth factor (VEGF) agents, including ranibizumab, aflibercept and bevacizumab, are currently the standard option for the treatment of neovascular age-related macular degeneration (AMD).1 The widespread use of ranibizumab intravitreal injection for the treatment of this condition was based on results from the pivotal Phase III Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA) and Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularisation in AMD (ANCHOR) studies.2–4 Data from open-label studies using optical coherence tomography (OCT)-guided re-treatment strategies have shown that improvements in best-corrected visual acuity (BCVA) and OCT-assessed central retinal thickness can be achieved with fewer ranibizumab injections than the fixed monthly dosing regimens used in studies such as MARINA and ANCHOR. However, BCVA improvements with OCT-guided re-treatment indications are generally smaller than those achieved with fixed monthly dosing.3–7 OCT images have shown that, in most patients, resolution of intraretinal and subretinal fluid (the presence of which corresponds with a decrease in BCVA) can be achieved 1–3 months after the start of monthly ranibizumab injections, often followed by an injection-free interval of several months before fluid begins to reaccumulate.5 7 8 However, some patients continue to require frequent injections.5 7 8 Why some patients have a poor response to ranibizumab is not well understood, but use of indocyanine green angiography (ICGA) has revealed the presence of polypoidal choroidal vasculopathy (PCV) in several patients with neovascular AMD who did not respond to repeated injections of anti-VEGF agents.9 10 Additionally, patients with retinal angiomatous proliferation (RAP) have been reported to require more anti-VEGF treatments than patients with non-RAP lesions.5
Neovascular AMD may be caused by different types of neovascularisation, the most common of which is choroidal neovascularisation (CNV).11 PCV, characterised by a branching network of inner choroidal vessels with terminal aneurysmal dilations, is most common in Asian individuals and other pigmented races (reported in 22–55% of Asian patients with neovascular AMD), but has also been observed in 4–14% of Caucasian patients.12 13 Despite some debate as to whether PCV is a separate clinical entity, it is increasingly considered to be a subtype of occult CNV, particularly in Caucasian individuals.12 14–17 RAP, which begins as proliferation of new vessels within the retina and may merge with the choroidal circulation as CNV in late-stage disease, is another relatively recently recognised subtype of neovascular AMD that may represent up to 15% of newly diagnosed cases.11 18 19
The primary purpose of this study was to use ICGA to determine the prevalence of PCV in Caucasian patients with presumed neovascular AMD who were considered to have poor response to ranibizumab, as indicated by the need for frequent intravitreal injections during OCT-guided dosing. The prevalence of RAP was also investigated, as was the frequency of ranibizumab injections during the 12 months before and after the start of combination therapy.
Patients and methods
We retrospectively studied the medical records of patients with presumed neovascular AMD, initially visualised by fluorescein angiography and presumed to be occult or classic CNV, diagnosed between May 2006 and September 2010. ICGA had not been performed at baseline because it is not standard practice in Europe. Patients were treated with intravitreal injections of ranibizumab 0.5 mg (Lucentis; Novartis AG, Basel, Switzerland) for ≥1 year in a routine clinical setting at Vista Klinik, Binningen, Switzerland. Patients initially received three consecutive monthly ranibizumab injections and were then re-treated pro re nata if there was OCT evidence of CNV and intraretinal or subretinal fluid ≥1 month after the last injection or a new macular haemorrhage. To be eligible for this analysis, patients must have received ≥8 ranibizumab injections and were required to be re-evaluated with ICGA. All patients who received ≥8 ranibizumab injections underwent ICGA, except those with a known indocyanine green allergy.
In previous clinical studies with pro re nata ranibizumab treatment, on average 5–6 ranibizumab injections/year were required to achieve significant visual acuity improvements and OCT central retinal thickness reductions compared with baseline.5 7 8 Therefore, we chose to categorise eyes according to the time from injection 1 to injection 6 (group 1: <12 months; group 2: ≥12 months). Group 1 eyes were considered to be relatively poor responders to ranibizumab, with injection frequency also reflecting CNV activity on OCT. Reinjection criteria were based on signs of CNV activity on OCT or new intraretinal or subretinal haemorrhages.
Ophthalmological examinations performed in this study included BCVA assessment, dilated fundus examination with a 90 D lens, colour fundus photography, spectral-domain OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany), as well as fluorescein angiography and ICGA by scanning laser ophthalmoscopy (Heidelberg Retina Angiography-2; Heidelberg Engineering). An ICG movie was used in each lesion for ≥30 s after filling to show pulsating PCV. OCT was used to evaluate changes in exudation, including intraretinal or subretinal fluid, serous or haemorrhagic retinal pigment epithelial detachments and intraretinal or subretinal haemorrhage. ICGA was not performed at initial diagnosis of AMD because it is currently not a standard primary diagnostic examination in Europe, where the population is largely Caucasian and data previously have indicated a low prevalence of PCV in this group. Conversely, PCV is known to be common in Asian populations; therefore, ICGA is generally included in initial clinical examination in Asia.20
PCV was defined as evidence of at least one focal polypoidal lesion in the inner choroid during early-phase ICGA. Only clearly identified PCV lesions were counted as such; differentiation was possible from the pulsation of polyps that could be observed using an ICGA movie. Furthermore, diagnosis of RAP lesions and identification of CNV feeder vessels were facilitated by ICGA. Areas of geographic atrophy observed on fluorescein angiography were also recorded during ophthalmological examination.
Once PCV was diagnosed, patients were offered reduced-fluence photodynamic therapy (PDT) with verteporfin (Visudyne; Novartis AG, Basel, Switzerland), followed 1 h later by intravitreal ranibizumab 0.5 mg injection, if they had no lesions near the fovea in combination with well-preserved BCVA (16/20–20/20) and no evidence of high-pigment epithelial detachment. Patients with lesions located very close to the optic nerve without any margin to the papilla were not offered combination treatment, nor were patients with good response to monotherapy—defined as >2 months with complete resolution of intraretinal or subretinal fluid following the last ranibizumab injection. Generally, patients considered eligible for PDT also had ≥6 ranibizumab injections/year after initial diagnosis and before ICGA and diagnosis of PCV. PDT was administered by a Zeiss VISULAS 690s laser (Carl Zeiss Meditec AG; Jena, Germany) with a reduced fluence of 25 J/cm2 for 83 s (300 mW/cm2) 15 min after initiation of verteporfin infusion according to standard protocol.21 However, laser spot size was determined by the greatest linear dimension of the lesion, which was measured with ICGA, as described by Chan et al22 and in the efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy (EVEREST) study by Koh et al.23 The laser spot size was chosen to cover the polyps and the surrounding abnormally dilated choroidal vessels seen on ICGA by adding 500–1000 µm to the greatest linear diameter, if required. Lesions too close to the optic nerve were excluded. When recurrent or residual PCV lesions were associated with exudative fluid during follow-up, patients could be re-treated with combined PDT and ranibizumab every 3 months, whereas intravitreal ranibizumab injections could be administered on a monthly basis.
In this paper, prevalence data are presented as percentages and all other variables as mean±SD. Statistical analyses were performed with SPSS V.17.0 for Windows (SPSS; Chicago, Illinois, USA). We used a two-sided t test to compare differences between groups. A p value of <0.05 was considered to be statistically significant.
Results
Of 753 eyes (675 patients) diagnosed with neovascular AMD and treated with ranibizumab for ≥1 year, 213 eyes received ≥8 injections. Inability to perform ICGA because of known allergies to iodine or severely reduced general condition in 11 patients meant that 11 eyes were excluded from the analysis. The total population for analysis comprised 202 eyes from 180 Caucasian patients (table 1).
Table 1.
Baseline characteristics and details of PRN ranibizumab injections overall, and for group 1 and 2 eyes, and eyes with and without PCV
| Characteristics and injection intervals* | Total eyes (N=202) | Group 1† (N=149) | Group 2‡ (N=53) | Eyes with PCV (N=34) | Eyes without PCV (N=168) |
|---|---|---|---|---|---|
| Male patients | 67 (33.2) | 48 (32.2) | 19 (35.8) | 10 (29.4) | 55 (32.7) |
| Female patients | 135 (66.8) | 101 (67.8) | 34 (64.2) | 24 (70.6) | 113 (67.3) |
| Mean age (years) | 77.9±6.4 (60–91) | 78.1±6.5 (60–91) | 77.4±6.2 (65–91) | 76.2±7.0 (62–87) | 78.2±6.3 (60–91) |
| Interval between injections 1 and 6 (months) | 10.4±4.6 (5–30) | 8.3±1.7 (5–11) | 16.4±4.9 (12–30) | 8.8±2.3 (6–16) | 10.8±4.8§ (6–28) |
| Interval between injections 1 and 8 (months) | 15.4±5.7 (5–28) | 12.9±3.0 (8–21) | 22.4±5.6 (15–34) | 13.8±3.9 (8–22) | 15.7±5.9 (8–34)¶ |
*Data are presented as n (%) or mean±SD (range).
†<12 months between injections 1 and 6.
‡≥12 months between injections 1 and 6.
§p=0.02 versus eyes with PCV.
¶p=0.08 versus eyes with PCV.
PCV, polypoidal choroidal vasculopathy; PRN, pro re nata (as needed).
The total population received a mean of 12.8±3.9 injections over 27.8±10.8 months, with ICGA performed at 15.1±10.2 months (group 1:13.1±9.1 months; group 2:20.7±1.2 months). The interval between injections 1 and 6 was <12 months in 149 eyes (73.8%; group 1) and ≥12 months in 53 eyes (26.2%; group 2).
Prevalence of PCV, RAP and CNV as evaluated by ICGA
Overall, 16.8% of eyes had evidence of PCV on ICGA (figure 1). The prevalence of PCV in group 1 (21.5%) was significantly higher than in group 2 (3.8%; p=0.003) (table 2). ICGA also showed RAP lesions in 11.4% of eyes (group 1: 10.7%; group 2: 13.2%) and AMD-type CNV in 71.8% of eyes (group 1: 67.8%; group 2: 83.0%). There were no significant differences between the two groups in the prevalence of RAP or CNV, including CNV feeder vessels and large areas of geographic atrophy in association with small occult CNV (table 2).
Figure 1.
Examples of evidence of polypoidal choroidal vasculopathy observed on indocyanine green angiography not diagnosed with fluorescein angiography. (A) Typical peripapillar location; (B) subfoveal location with extended subfoveal haemorrhage.
Table 2.
Prevalence of PCV, RAP and CNV in eyes with presumed neovascular AMD requiring ≥8 intravitreal ranibizumab injections during PRN treatment
| Prevalence, n (%) | Overall (n=202) | Group 1* (n=149) | Group 2† (n=53) |
|---|---|---|---|
| PCV | 34 (16.8) | 32 (21.5) | 2 (3.8)‡ |
| RAP | 23 (11.4) | 16 (10.7) | 7 (13.2) |
| AMD-type CNV | 145 (71.8) | 101 (67.8) | 44 (83.0) |
| CNV feeder vessels | 60 (29.7) | 46 (30.9) | 14 (26.4) |
| Geographic atrophy with small occult CNV | 9 (4.5) | 6 (4.0) | 3 (5.7) |
*<12 months between injections 1 and 6.
†≥12 months between injections 1 and 6.
‡p=0.003 versus group 1.
AMD, age-related macular degeneration; CNV, choroidal neovascularisation; PCV, polypoidal choroidal vasculopathy; PRN, pro re nata (as needed); RAP, retinal angiomatous proliferation.
Eyes with PCV before and after PDT
Baseline characteristics, such as age at diagnosis and sex, were not significantly different between eyes with or without PCV (table 1). The mean interval between injections 1 and 6 was 8.8±2.3 months for eyes with PCV, which was significantly shorter than for eyes without PCV (10.8±4.8 months; p=0.02). Between injections 1 and 8, the mean interval was 13.8±3.9 months for eyes with PCV and 15.7±5.9 months for eyes without PCV, but the between-group difference did not quite reach statistical significance (p=0.08).
Before eyes were evaluated with ICGA, patients with PCV had 6.7±3.1 ranibizumab injections over 12.1±7.2 months. After diagnosis of PCV, 16 of 34 (47.1%) eyes were treated with reduced-fluence PDT plus ranibizumab combination therapy (figures 2 and 3). Combination therapy was not performed in 18 (52.9%) eyes with PCV because of good response to monotherapy (9 eyes), lesion location near the fovea and good BCVA (3 eyes), pigment epithelial rupture (2 eyes), large pigment epithelial detachment (2 eyes), and refusal to receive combination therapy (1 eye). Combination therapy was planned in one additional eye, but no follow-up was available. No differences in characteristics were observed between PCV lesions that required monotherapy versus those that required combination therapy.
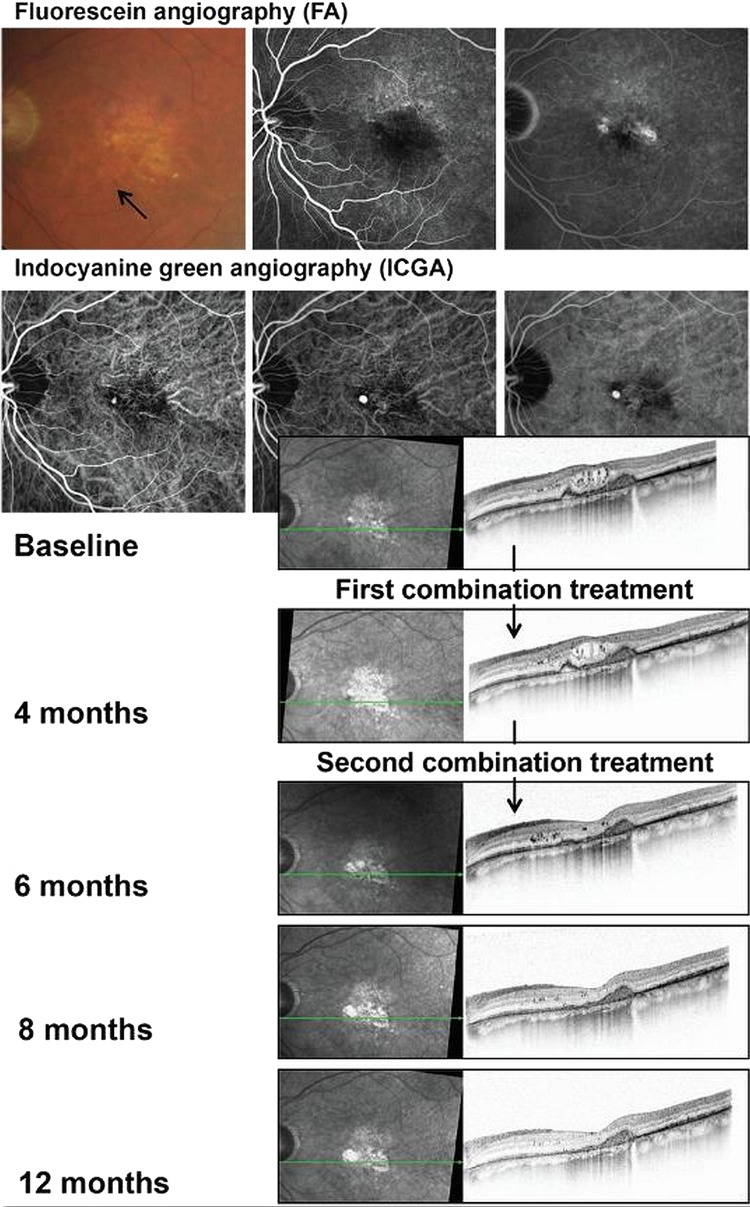
Figure 2.
Images from a patient with no response to antivascular endothelial growth factor monotherapy who was treated with combined reduced-fluence photodynamic therapy and intravitreal ranibizumab until month 15 to maintain stable visual acuity (20/40 to 20/32).
Figure 3.
Images from a patient with no response to antivascular endothelial growth factor monotherapy who was treated with combined reduced-fluence photodynamic therapy and intravitreal ranibizumab. Complete resolution of polypoidal choroidal vasculopathy on optical coherence tomography was achieved after the second treatment.
The 16 eyes with PCV that were treated with combination therapy received 8.4±2.4 ranibizumab injections/year before the start of combination therapy compared with 3.1±2.5 injections/year after combination therapy (p<0.001). Outcomes included prolonged stabilisation of visual acuity and resolution of PCV (figures 2 and 3).
Discussion
Despite improvements in visual acuity with ranibizumab treatment in the MARINA and ANCHOR studies, a subset of patients with neovascular AMD continued to lose vision and to display leakage from CNV, even with continued intravitreal anti-VEGF therapy.2–4 Improvements in visual acuity in response to ranibizumab were greatest in patients with predominantly classic CNV in these trials; however, ICGA was not used to investigate the possible influence of PCV on treatment response.2–4 ICGA fluorescence can penetrate blood, fluid and retinal pigment epithelium to reveal underlying abnormalities of the inner choroidal vasculature and is essential for making a definitive diagnosis of PCV.13 24
In this study, ICGA revealed PCV in 16.8% of patients. When analysed according to the frequency of ranibizumab injections, which reflect the quality of response as assessed by OCT, the prevalence of PCV was relatively high (21.5%) in patients requiring ≥6 ranibizumab injections within the first 12 months of therapy. Evidence of PCV on ICGA was only observed in 3.8% of patients who received their first six injections over a ≥12-month interval. This relatively low prevalence was similar to the overall 4% prevalence of PCV previously reported in a large case series (374 eyes) of VEGF inhibitor-naive Caucasian patients initially diagnosed with occult CNV.25
In two small case series, each involving 12 eyes, Caucasian patients with neovascular AMD receiving regular intravitreal anti-VEGF treatment also showed persistent exudation related to PCV.9 10 In one of these case series, eight eyes underwent baseline ICGA evaluation but did not show any evidence of PCV.9 In the present study, baseline ICGA was only available in one patient diagnosed with PCV. On average, patients had already received a mean of 6.7 ranibizumab injections over 12.1 months before being diagnosed with PCV, so it was not possible to determine whether PCV was present at the start of treatment. We hypothesise that, in most cases, PCV is not recognised at baseline simply because ICGA is unavailable. Better access to ICGA for early diagnosis of PCV is important so that the most appropriate treatment options can be started before irreversible disease damage occurs.
It is becoming increasingly clear that combination therapy may be more effective than anti-VEGF monotherapy in patients with PCV.24 Outcomes from the EVEREST study showed that complete regression of polyps was significantly higher in patients treated with standard-fluence verteporfin PDT alone (71.4%) or in combination with ranibizumab (77.8%) compared with ranibizumab monotherapy (28.6%; p=0.0037). Combining verteporfin with ranibizumab resulted in fewer re-treatments with ranibizumab over 5 months compared with ranibizumab monotherapy and also led to an observed trend towards improved BCVA outcomes.23 Current guidelines developed from a recent roundtable meeting of PCV experts recommend either ICGA-guided verteporfin PDT or verteporfin PDT together with three monthly 0.5 mg ranibizumab intravitreal injections as the initial treatment of juxtafoveal and subfoveal PCV.20 In the present study, 16 patients received combination therapy with reduced-fluence verteporfin PDT plus ranibizumab after they were diagnosed with PCV. Compared with the 12-month period before the start of combination therapy, the need for ranibizumab injections during the 12-month period after combination therapy (8.4 vs 3.1 injections) was significantly reduced. We did not systematically evaluate the impact of combination therapy on lesion characteristics. However, in a case series of 12 eyes with PCV lesions treated with multiple injections of anti-VEGF agents, Cho et al9 reported that eight eyes went on to be treated with combination verteporfin PDT and an anti-VEGF agent, with complete resolution of the PCV lesion and associated macular exudation over 1.5–3 months in 75% of cases. Despite stabilisation of disease in all patients, final visual acuity generally did not improve.9 The authors stated that this finding probably was a result of permanent photoreceptor damage from chronic oedema and recurrent haemorrhage.
In the present study, dynamic fluorescein angiography and ICGA movies during the filling phase and images taken during the mid and late phases allowed RAP lesions to be clearly differentiated. They were identified in 11.4% of eyes. However, unlike PCV, which was most prevalent in the group of patients requiring more frequent ranibizumab injections during the first year of therapy, RAP was evenly distributed between the two groups (group 1: 10.7% of eyes; group 2: 13.2% of eyes). Most RAP cases were found to be stage I/II in this study, which may be the result of the patient selection criteria, that is, early detection in an urban population combined with exclusion of patients with advanced disease before they had received eight injections. The mean number of required ranibizumab injections has previously been shown to increase significantly from 4.1/year in stage IIA RAP to 6.3/year in stage III RAP.26 These results support the observation that, relative to classic and occult subtypes of CNV, stage I–II RAP is not associated with reduced responsiveness to ranibizumab.7 27 Better response at earlier stages may provide an explanation for the even distribution of RAP lesions in this study. The selection criteria used may also explain the low prevalence of RAP observed in this study compared with other studies.28–30 The RAP prevalence among occult/minimally classic lesions was mostly determined at the same time as prevalence among all lesion types. A slightly lower prevalence of RAP lesions was previously reported in a large German population of patients with AMD, although this may be due to the investigators using only fluorescein angiography for diagnosis.31
The extent of vascular maturity can affect therapeutic response, with immature vessels having a better response to VEGF inhibitors than mature vessels, so CNV may be relatively mature and resistant to treatment in patients with chronic AMD or a history of previous anti-VEGF therapy.9 10 In this study, ICGA revealed that 29.7% of patients had CNV feeder vessels as signs of mature CNV (group 1: 30.9%; group 2: 26.4%). Large areas of geographic atrophy were observed in a relatively small proportion of patients with occult CNV in both patient groups (4.0–5.7%).
In conclusion, the relatively high prevalence of PCV in Caucasian patients with presumed neovascular AMD requiring multiple intravitreal injections of ranibizumab during the first 12 months of treatment may help to explain some of the wide variations in the need for re-treatment among patients with presumed neovascular AMD receiving OCT-guided therapy with anti-VEGF agents. Because PCV may prove to be difficult to treat with anti-VEGF therapy alone, combination therapy involving PDT may be needed. Therefore, differentiation between CNV and PCV early in the treatment sequence is important so that an alternative treatment strategy can be initiated before irreversible disease progression occurs.
Acknowledgments
The authors would like to acknowledge Chameleon Communications International, which provided medical writing services and editorial support with funding from Heidelberg Engineering.
Footnotes
Funding: Funding for medical writing support was provided by Heidelberg Engineering (Tiergartenstrasse 15, 69121 Heidelberg, Germany). The sponsor had no role in the design or conduct of this research.
Competing interests: CP is a consultant to Novartis Pharma AG, Bayer and Allergan. KH has no competing interests.
Ethics approval: Any necessary ethics committee approval was secured for the reported study (Ethikkommission beider Basel, ref. no. 362/12).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Nguyen DH, Luo J, Zhang K, et al. Current therapeutic approaches in neovascular age-related macular degeneration. Discov Med 2013;15:343–8 [PubMed] [Google Scholar]
- 2.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116:57–65 [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–44 [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31 [DOI] [PubMed] [Google Scholar]
- 5.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007;143:566–83 [DOI] [PubMed] [Google Scholar]
- 6.Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 2011;118:663–71 [DOI] [PubMed] [Google Scholar]
- 7.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009;148:43–58 [DOI] [PubMed] [Google Scholar]
- 8.Rothenbuehler SP, Waeber D, Brinkmann CK, et al. Effects of ranibizumab in patients with subfoveal choroidal neovascularization attributable to age-related macular degeneration. Am J Ophthalmol 2009;147:831–7 [DOI] [PubMed] [Google Scholar]
- 9.Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 2009;148:70–8 [DOI] [PubMed] [Google Scholar]
- 10.Stangos AN, Gandhi JS, Nair-Sahni J, et al. Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol 2010;150:666–73 [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi LA, Negrao S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001;21:416–34 [DOI] [PubMed] [Google Scholar]
- 12.Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004;49:25–37 [DOI] [PubMed] [Google Scholar]
- 13.Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res 2010;29:19–29 [DOI] [PubMed] [Google Scholar]
- 14.Costa RA, Navajas EV, Farah ME, et al. Polypoidal choroidal vasculopathy: angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res 2005;24:560–86 [DOI] [PubMed] [Google Scholar]
- 15.Imamura Y, Engelbert M, Iida T, et al. Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol 2010;55:501–15 [DOI] [PubMed] [Google Scholar]
- 16.Khan S, Engelbert M, Imamura Y, et al. Polypoidal choroidal vasculopathy: simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina 2012;32:1057–68 [DOI] [PubMed] [Google Scholar]
- 17.Lima LH, Schubert C, Ferrara DC, et al. Three major loci involved in age-related macular degeneration are also associated with polypoidal choroidal vasculopathy. Ophthalmology 2010;117:1567–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund KB, Ho IV, Barbazetto IA, et al. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina 2008;28:201–11 [DOI] [PubMed] [Google Scholar]
- 19.Yannuzzi LA, Freund KB, Takahashi BS. Review of retinal angiomatous proliferation or type 3 neovascularization. Retina 2008;28:375–84 [DOI] [PubMed] [Google Scholar]
- 20.Koh AH, Chen LJ, Chen SJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 2013;33:686–716 [DOI] [PubMed] [Google Scholar]
- 21.Visudyne: EPAR – Product Information – European Medicines Agency. Annex1. Summary of product characteristics. http://www.ema.europa.eu/ema/ (last updated 27 Nov 2012)
- 22.Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 2004;111:1576–84 [DOI] [PubMed] [Google Scholar]
- 23.Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012;32:1453–64 [DOI] [PubMed] [Google Scholar]
- 24.de Crecchio G, Chan RV, Manzi G, et al. Polypoidal choroidal vasculopathy: recent advances in therapy. Curr Drug Targets 2011;12:206–11 [DOI] [PubMed] [Google Scholar]
- 25.Lafaut BA, Leys AM, Snyers B, et al. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol 2000;238:752–9 [DOI] [PubMed] [Google Scholar]
- 26.Reche-Frutos J, Calvo-Gonzalez C, Perez-Trigo S, et al. Ranibizumab in retinal angiomatous proliferation (RAP): influence of RAP stage on visual outcome. Eur J Ophthalmol 2011;21:783–8 [DOI] [PubMed] [Google Scholar]
- 27.Konstantinidis L, Mameletzi E, Mantel I, et al. Intravitreal ranibizumab (Lucentis) in the treatment of retinal angiomatous proliferation (RAP). Graefes Arch Clin Exp Ophthalmol 2009;247:1165–71 [DOI] [PubMed] [Google Scholar]
- 28.Parravano M, Pilotto E, Musicco I, et al. Reproducibility of fluorescein and indocyanine green angiographic assessment for RAP diagnosis: a multicenter study. Eur J Ophthalmol 2012;22:598–606 [DOI] [PubMed] [Google Scholar]
- 29.Massacesi AL, Sacchi L, Bergamini F, et al. The prevalence of retinal angiomatous proliferation in age-related macular degeneration with occult choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 2008;246:89–92 [DOI] [PubMed] [Google Scholar]
- 30.Slakter JS, Yannuzzi LA, Schneider U, et al. Retinal choroidal anastomoses and occult choroidal neovascularization in age-related macular degeneration. Ophthalmology 2000;107:742–53 [DOI] [PubMed] [Google Scholar]
- 31.Kuerzinger GR, Lang GK, Lang GE. Retinal angiomatous proliferation in age-related macular degeneration. Klin Monbl Augenheilkd 2006;223:691–5 [DOI] [PubMed] [Google Scholar]