Abstract
Statins and nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most commonly prescribed medications. In vitro studies suggest that statins and NSAIDs may have potential as anti-cancer therapies in low grade non-Hodgkin lymphomas including chronic lymphocytic leukemia (CLL) and a recent observational study found statin use was associated with improved event free survival in patients with follicular lymphoma. Other studies have suggested that statins reduce the efficacy of rituximab by inhibiting binding to CD20. We therefore conducted an observational cohort study of 686 patients with newly diagnosed Rai stage 0 CLL to evaluate whether statin or NSAID use was related to their clinical outcome or influenced the efficacy of rituximab therapy. At diagnosis, 136 (20%) patients took statins and 230 (34%) scheduled daily aspirin, ibuprofen, or naproxen. No difference in time to treatment was observed based on statin or NSAID use. Among patients receiving a rituximab containing first-line therapy, no difference in time to salvage treatment was observed based on statin use. Although previous studies suggested statins may improve event free survival among patients with follicular lymphoma, we find no impact of statins on time to initial therapy in this large study of patients with Rai stage 0 CLL. The in vitro observation that statins reduce rituximab efficacy does not appear to have clinical significance in CLL care.
BACKGROUND
Statins and nonsteroidal anti-inflammatory drugs (NSAID) are among the most commonly prescribed medications in Western countries1. Statins are used primarily for treatment of hyperlipidemia with an estimated 1 in 4 individuals age 60 and older taking statin therapy2, while an estimated 17 million Americans use NSAIDs on a daily basis3. Since the median age at the time of diagnosis of chronic lymphocytic leukemia (CLL) is 65-70, it is anticipated that a larger portion of patients with CLL take statins and NSAIDs.
Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme-A reductase (HMG-CoAR) which is the rate-limiting enzyme in the mevalonate pathway required for the synthesis of isoprenoids such as cholesterol4,5. Inhibition of isopreniod synthesis by statins could have a direct antitumor effect by interfering with the formation of cholesterol-rich lipid micro-domains (“lipid rafts”) within the cell membrane6 as well as by impairing protein prenylation7. These processes are important for signaling activity of proteins known to be related to lymphomagenesis and tumor survival5. Other studies have suggested that statins may exert effects on immune cells and the tumor microenvironment8,9. These findings have led to in vitro testing of statins as an anticancer therapy for a variety of tumor types including CLL10-12. In addition, CLL cells have been reported to have less low-density lipoprotein receptor activity and higher HMG-CoAR activity than normal mononuclear blood cells. Other studies suggest lipoprotein lipase mRNA or protein levels identify CLL patients at higher risk of progression13-16. These results suggest that CLL cells may depend on de-novo cholesterol synthesis for their survival and proliferation 11. This hypothesis is supported by the finding that in vitro treatment of CLL cells with statins induces apoptosis via a caspase-dependent mechanism but the clinical relevance of this finding remains uncertain10.
NSAIDs have also been extensively studied as both as chemoprevention agents as well as an anti-cancer therapies17. NSAIDs inhibit cyclo-oxygenase (COX) and decrease prostaglandin synthesis. Other reported anti-tumor effects of NSAIDs include caspase activation, activation of NFKB, down regulation of BCL2, effects on DNA mismatch repair and generation of reactive oxygen species as previously reviewed17. The magnitude of these effects may differ depending on both tissue characteristics (e.g. expression of cyclooxygenase) and the properties of the various NSAIDs. CLL cells have been shown to have constitutive COX-2 expression that increases with CD40 ligand stimulation18and NSAIDs appear to induce cell death by both COX dependent and independent mechanisms in vitro 18-20. Limited in vitro data suggests that NSAIDS may enhance the effects of purine nucleoside analogue (PNA) and rituximab based treatments in CLL21,22.
Several recent observations raise further questions regarding the clinical effects of statins in patients with CLL. First, laboratory studies have suggested that B lymphocytes obtained from patients on statin therapy have conformational changes in CD20 which reduces rituximab binding and could thus reduce rituximab efficacy23. Since the addition of rituximab to purine nucleoside analogue based chemotherapy has been shown to prolong both treatment-free and overall survival for CLL in randomized phase 3 trials24-26, such an interaction could have important clinical consequences. Indeed some physicians have advocated discontinuation of statin during treatment with rituximab containing regimens27,28. Although a recent observational study of patients with follicular lymphoma (n=293) and diffuse large B-cell lymphoma (n=228) found no impact of statin use on response to rituximab containing chemotherapy regimens29, a small effect of statins on rituximab binding may have greater consequences in CLL since CLL B-cells usually have only dim CD20 expression. The same study made the unexpected finding that statin use was associated with improved event free survival among patients with follicular lymphoma including those asymptomatic patients initially managed with observation29. Notably, these apparent benefits of statin use persisted after adjusting for the follicular lymphoma international prognostic index (FLIPI) score and type of therapy, raising the question of whether the observed in vitro effects of statins on NHL and CLL cells had clinical relevance29. The conflicting in vivo and in vitro data for statins and NSAIDs in B-cell malignancies the common use of these drugs prompted us to evaluate whether statin or NSAID use affected clinical outcome in patients with newly diagnosed, early stage (Rai 0) CLL. This analysis was conducted an observational cohort study using the Mayo Clinic CLL Database which contains a large set of clinical information on an early stage CLL cohort.
METHODS
Patients
The Mayo Clinic CLL Database includes all patients with a diagnosis of CLL seen in the Division of Hematology at Mayo Clinic Rochester (MCR) who permit their records to be used for research purposes30-35. Clinical information regarding date of diagnosis, physical examination, clinical stage (Rai), prognostic parameters, treatment history, and disease-related complications are abstracted from clinical records on all patients at the time of inclusion and maintained on an ongoing, prospective basis. Results of prognostic testing performed as part of clinical or research studies are also included in the database. This includes immunoglobulin variable heavy chain region (IGHV) gene mutation analysis, ZAP-70 status, CD38 status, and cytogenetics abnormalities by interphase fluorescence in situ hybridization (FISH) testing using methods previously described by our group.36-38
With the approval of the Mayo Clinic Institutional Review Board and in accord with federal regulations and the Declaration of Helsinki, we used this database to identify all Rai stage 0 patients diagnosed with CLL between 1/1/1995 and 12/31/2008 who were seen in the Mayo Clinic Division of Hematology within 3 months of diagnosis. Use of statins and/or scheduled daily aspirin, ibuprofen, and naproxen (the most widely used NSAIDs1) at the time of diagnosis was abstracted from medical records. Among patients taking statins or daily NSAID, the specific statin (e.g. simvastatin, atorvastatin, lovastatin, pravastatin, fluvastatin, rosuvastatin) or NSAIDs (e.g. aspirin, ibuprofen, naproxen) used was recorded. Statin and NSAID use at the time of first treatment was also abstracted for those patients who went on to require salvage therapy.
Statistical methods
Overall survival (OS) was defined as the time between the date of diagnosis to the date of death or last follow-up. Time to first treatment (TFT) was defined as the time between date of diagnosis and the date of initiation of first treatment or date of last follow-up at which patient was known to be untreated. The accepted indications to initiate treatment were based on the NCI-WG 1996 criteria during the study interval3. Time to salvage therapy was defined as the time between initiation of first line treatment and the date of initiation of salvage treatment or date of last follow-up. Patients receiving early treatment as part of experimental protocols prior to meeting NCI-WG 1996 criteria to initiate therapy were censored as untreated on the date that experimental therapy was administered. OS and TFT analyses were performed with results being displayed using Kaplan-Meier curves and p-values calculated using a log-rank test. Chi-square and Fisher exact tests, where appropriate, were used to assess the association of statin use with other clinical and laboratory characteristics. Since not all patients had all prognostic tests performed, the association of prognostic test results with statin and NSAID use was evaluated for each assay individually among those with test results available. All statistical tests were two-sided and considered significant at the alpha=0.05 level; all analyses were performed in SAS version 9.1 (SAS Institute, http://www.sas.com).
RESULTS
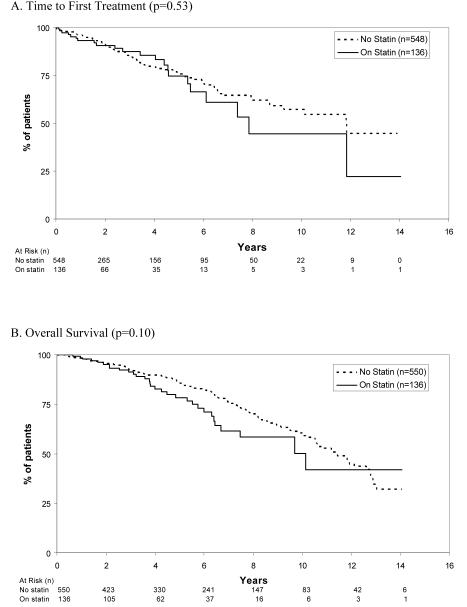
We identified 686 consecutive, newly diagnosed, Rai stage 0 patients with CLL seen at Mayo Clinic within three months of diagnosis since January 1995. Patient characteristics are outlined in Table 1. At the time of diagnosis, 136 (20%) patients were taking statin therapy. Patients who were on statin therapy at diagnosis were older (median age 69 versus 66; P = 0.01) and more likely to be male (68% vs. 59%; p=0.05) but less likely to be ZAP-70 positive (14% vs. 27%; p=0.02) or IGHV unmutated (15% vs. 33%; p=0.004). After a median follow-up of 4.9 years, 125 (18%) patients progressed to require treatment and 173 (25%) died. TFT and OS did not differ based on whether or not patients were on a statin at diagnosis. No difference in TFT was observed by type of statin used. TFT and OS based on statin use are shown in Figure 1A and B. Statin use also remained unrelated to TFT when women and men were analyzed separately (p>0.05).
Table 1.
Participant Characteristics
| Statin Use at Diagnosis | Daily NSAID Use at Diagnosis | |||||
|---|---|---|---|---|---|---|
| Yes (n=136) |
No (N=550) |
P value | Yes (n=230) |
No (N=456) |
P value | |
| Age at diagnosis (median, years) | 69 | 66 | 0.01 | 69 | 65 | <0.0001 |
| Male | 92 (68%) | 322 (59%) | 0.05 | 156 (68%) | 258 (57%) | 0.005 |
| CD38 |
0.32 |
0.46 |
||||
| Positive | 29 (24%) | 82 (20%) | 42 (22%) | 69 (20%) | ||
| Negative | 93 (76%) | 335 (80%) | 146 (78%) | 282 (80%) | ||
| Missing | 14 | 133 | 42 | 105 | ||
| ZAP-70 |
0.02 |
0.94 |
||||
| Positive | 11 (14%) | 73 (27%) | 30 (24%) | 54 (25%) | ||
| Negative | 66 (86%) | 194 (73%) | 94 (76%) | 166 (75%) | ||
| Missing | 59 | 283 | 106 | 236 | ||
| IGHV |
0.004 |
0.61 |
||||
| Unmutated | 10 (15%) | 80 (33%) | 28 (27%) | 62 (30%) | ||
| Mutated | 57 (85%) | 163 (67%) | 75 (73%) | 145 (70%) | ||
| Missing | 69 | 307 | 127 | 249 | ||
| FISH |
0.20 |
0.64 |
||||
| 13q- | 27 (39%) | 96 (49%) | 39 (42%) | 84 (49%) | ||
| Normal | 22 (32%) | 60 (30%) | 31 (33%) | 51 (30%) | ||
| Trisomy 12 | 15 (22%) | 22 (11%) | 15 (16%) | 22 (13%) | ||
| 11q- | 1 (1%) | 10 (5%) | 3 (3%) | 8 (5%) | ||
| 17p- | 3 (4%) | 6 (3%) | 5 (5%) | 4 (2%) | ||
| Other | 1 (1%) | 3 (2%) | 1 (1%) | 3 (2%) | ||
| Missing | 67 | 353 | 136 | 284 | ||
| Time to First Treatment (median, years) | 7.9 | 11.8 | 0.52 | Not reached |
11.8 | 0.88 |
| Type of First Treatment | ||||||
| Purine analogue monotherapy | 1 (4%) | 6 (6%) | 0.43 | 1 (3%) | 6 (8%) | 0.81 |
| Rituximab containing purine analogue combination | 11 (44%) | 29 (31%) | 15 (38%) | 25 (32%) | ||
| Alkylating agent monotherapy (+/- steroid) | 4 (16%) | 31 (33%) | 12 (31%) | 23 (29%) | ||
| Rituximab containing alkylating agent combination | 3 (12%) | 5 (5%) | 3 (8%) | 5 (6%) | ||
| Antibody therapy with chemo | 5 (20%) | 16 (17%) | 7 (18%) | 14 (18%) | ||
| Other | 1 (4%) | 6 (6%) | 1 (3%) | 6 (8%) | ||
| Survival (median, years) | 10.1 | 11.4 | 0.10 | 10.6 | 11.9 | 0.02 |
Figure 1.
Time to First Treatment and Survival Among Rai Stage 0 CLL Patients Based on Statin Use
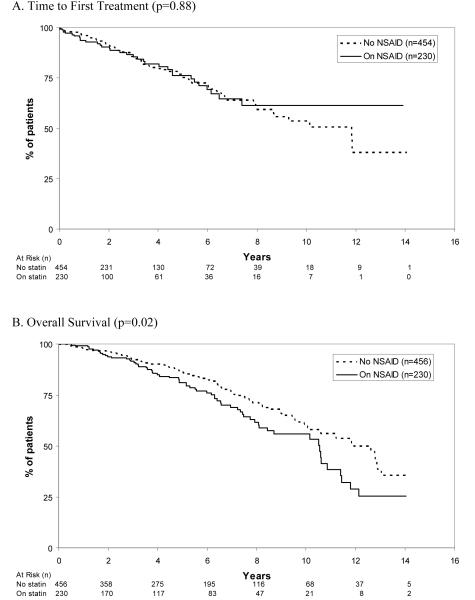
With respect to NSAID use, 230 (34%) patients were taking daily aspirin, ibuprofen, or naproxen therapy at the time of diagnosis (Table 1). Patients taking daily NSAIDs were older (median age 69 vs. 65; P < 0.0001) and more likely to be male (68% vs. 57%; p=0.005). No differences in novel prognostic parameters were observed by daily NSAID use. While TFT did not differ based on whether or not patients were on daily NSAIDs at diagnosis, NSAIDs use was associated with shorter overall survival (median 10.6 years vs. 11.9 years; p=0.02). No difference in TFT was observed by type of NSAID used. TFT and OS based on daily NSAID use are shown in Figure 2A and B.
Figure 2.
Time to First Treatment and Survival Among Rai Stage 0 CLL Patients Based on NSAID Use
Given concern that statin use may alter the efficacy of rituximab-based therapy, we next explored the relationship between statin use at the time of treatment initiation and time to salvage therapy among patients receiving rituximab-containing chemoimmunotherapy regimen. First-line therapy selection among Rai stage 0 patients who progressed to require treatment did not differ based on statin use. Among patients who received a rituximab containing first-line therapy, there was no difference in time to salvage therapy based on whether or not patients received statin treatment at the time of treatment initiation (Figure 3). First-line therapy selection among Rai stage 0 patients who progressed to require treatment also did not differ based on NSAID use. Daily NSAID use at the time of treatment initiation had no relationship to time to salvage therapy among all treated patients or patients who received a rituximab containing first-line therapy.
Figure 3.
Time to Salvage Therapy Among Patients Receiving Rituximab Containing First-line Therapy Based on Statin Use At Time of First Treatment (p=0.60)
DISCUSSION
This study provides important observations regarding statin and NSAID use in patients with CLL. First, although previous studies suggested statin use may delay disease progression among patients with follicular lymphoma29, we find no such relationship in CLL. Whether this discrepancy is due to a different biologic effect of statins in follicular lymphoma or unidentified confounding factors in the prior study of follicular lymphoma patients is unknown. Unlike a recently reported abstract, we do not find a differential effect of statins in CLL patients by gender in our series39. Second, in vitro studies suggest statins may inhibit rituximab binding to CD20 and reduce rituximab efficacy23. Although recently published study found no clinical evidence that statin use reduces the efficacy of rituximab based therapy in follicular or diffuse large cell lymphoma (both of which have bright CD20 expression)29, the very low level of CD20 expression on CLL B cells raised concern that a subtle effect of statins on rituximab binding may be more clinically relevant in CLL. We find no evidence to support this supposition, an important negative result since ~20% of CLL patients use statin therapy.
NSAIDs have also undergone in vitro testing as a potential therapy for CLL and in vitro data suggests some NSAIDs may actually enhance the effects of PNA and rituximab based treatments21,22. In this observational study, we found no delay in TFT or time to salvage therapy among patients with CLL receiving daily aspirin, ibuprofen, or naproxen. The shorter OS observed among patients on daily NSAIDs could be caused by the more advanced age of patients on NSAID therapy and the underlying health problems for which NSAIDs were used. Daily NSAID use also did not appear to affect the efficacy of CLL therapy. There was no relationship between NSAID use and time to salvage therapy among either all treated patients or patients receiving a rituximab containing first-line therapy.
Our study is subject to a number of limitations. First, the observational study design cannot exclude the possibility that unmeasured confounding factors influence the results. Second, treatment and follow-up were based on routine clinical practice as opposed to standardized observation as would occur in a clinical trial. In this regard, it is particularly worth noting that the lack of association between daily aspirin, ibuprofen, and/or naproxen use and TFT in our observational study does not mean that other NSAID derivatives currently in clinical testing for patients with CLL will not be useful agents40,41. It is also possible there may be a relationship between NSAID dose and clinical effects in CLL patients which is an aspect not evaluated in our study. Third, statin and NSAID use were abstracted from medical records at the time of diagnosis. While this data would be expected to have reasonable validity, all use of these medications may not have been captured and we do not know how compliant patients were with prescribed medications. In this regard, only scheduled daily aspirin, ibuprofen, and naproxen use listed in the medical record was considered in our analysis where intermittent, over the counter NSAIDs are a frequently used pain reliever. Intermittent NSAIDs were undoubtedly used by some patients in the “no NSAID” group and may have masked a beneficial effect of NSAIDs. Fourth, since only 18% of patients progressed to require therapy and 25% died, it is possible difference may become apparent with longer follow-up. Finally, this study was not population based although the distribution of clinical variables, prognostic parameters, and treatments suggest the results may be applicable to CLL patients in general.
In summary, statins and conventional NSAID use among patients with newly diagnosed, Rai stage 0 CLL do not appear to affect disease progression. It also does not appear that either of these commonly used medications influences the efficacy of current therapies including rituximab-containing chemotherapy regimens for CLL patients.
Acknowledgment
This study was supported, in part, by the National Institute of Health (CA113408 to TDS)
References
- 1.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008;42:1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 3.Solomon D. NSAIDs: Overview of Adverse Effects. UpToDate. 2009 [Google Scholar]
- 4.Endo A. The discovery and development of HMG-CoA reductase inhibitors. Atheroscler. 1992;2004;5(Suppl):67–80. doi: 10.1016/j.atherosclerosissup.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Jakobisiak M, Golab J. Potential antitumor effects of statins (Review) Int J Oncol. 2003;23:1055–1069. [PubMed] [Google Scholar]
- 6.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Sala D, Mollinedo F. Inhibition of isoprenoid biosynthesis induces apoptosis in human promyelocytic HL-60 cells. Biochem Biophys Res Commun. 1994;199:1209–1215. doi: 10.1006/bbrc.1994.1359. [DOI] [PubMed] [Google Scholar]
- 8.Mausner-Fainberg K, Luboshits G, Mor A, et al. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis. 2008;197:829–839. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 9.Schramm R, Menger MD, Harder Y, et al. Statins inhibit lymphocyte homing to peripheral lymph nodes. Immunology. 2007;120:315–324. doi: 10.1111/j.1365-2567.2006.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman-Shimshoni D, Yuklea M, Radnay J, Shapiro H, Lishner M. Simvastatin induces apoptosis of B-CLL cells by activation of mitochondrial caspase 9. Exp Hematol. 2003;31:779–783. doi: 10.1016/s0301-472x(03)00192-9. [DOI] [PubMed] [Google Scholar]
- 11.Vitols S, Angelin B, Juliusson G. Simvastatin impairs mitogen-induced proliferation of malignant B-lymphocytes from humans--in vitro and in vivo studies. Lipids. 1997;32:255–262. doi: 10.1007/s11745-997-0032-1. [DOI] [PubMed] [Google Scholar]
- 12.Madden EA, Melnykovych G, Fiskin AM. Compactin (ML-236B) reduces the content of filipin-cholesterol complexes in the plasma membrane of chronic lymphocytic leukemia cells. Exp Cell Res. 1984;153:91–98. doi: 10.1016/0014-4827(84)90451-8. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg JB, Volkheimer AD, Mihovilovic M, et al. Apolipoprotein E genotype as a determinant of survival in chronic lymphocytic leukemia. Leukemia. 2008;22:2184–2192. doi: 10.1038/leu.2008.241. [DOI] [PubMed] [Google Scholar]
- 14.Oppezzo P, Vasconcelos Y, Settegrana C, et al. The LPL/ADAM29 expression ratio is a novel prognosis indicator in chronic lymphocytic leukemia. Blood. 2005;106:650–657. doi: 10.1182/blood-2004-08-3344. [DOI] [PubMed] [Google Scholar]
- 15.Nuckel H, Huttmann A, Klein-Hitpass L, et al. Lipoprotein lipase expression is a novel prognostic factor in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47:1053–1061. doi: 10.1080/10428190500464161. [DOI] [PubMed] [Google Scholar]
- 16.Heintel D, Kienle D, Shehata M, et al. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1216–1223. doi: 10.1038/sj.leu.2403748. [DOI] [PubMed] [Google Scholar]
- 17.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 18.Ryan EP, Pollock SJ, Kaur K, et al. Constitutive and activation-inducible cyclooxygenase-2 expression enhances survival of chronic lymphocytic leukemia B cells. Clin Immunol. 2006;120:76–90. doi: 10.1016/j.clim.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Bellosillo B, Pique M, Barragan M, et al. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92:1406–1414. [PubMed] [Google Scholar]
- 20.Ryan EP, Bushnell TP, Friedman AE, Rahman I, Phipps RP. Cyclooxygenase-2 independent effects of cyclooxygenase-2 inhibitors on oxidative stress and intracellular glutathione content in normal and malignant human B-cells. Cancer Immunol Immunother. 2008;57:347–358. doi: 10.1007/s00262-007-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robak P, Linke A, Cebula B, Robak T, Smolewski P. Cytotoxic effect of R-etodolac (SDX-101) in combination with purine analogs or monoclonal antibodies on ex vivo B-cell chronic lymphocytic leukemia cells. Leuk Lymphoma. 2006;47:2625–2634. doi: 10.1080/10428190600948147. [DOI] [PubMed] [Google Scholar]
- 22.Lindhagen E, Nissle S, Leoni L, et al. R-etodolac (SDX-101) and the related indole-pyran analogues SDX-308 and SDX-309 potentiate the antileukemic activity of standard cytotoxic agents in primary chronic lymphocytic leukaemia cells. Cancer Chemother Pharmacol. 2007;60:545–553. doi: 10.1007/s00280-006-0400-9. [DOI] [PubMed] [Google Scholar]
- 23.Winiarska M, Bil J, Wilczek E, et al. Statins impair antitumor effects of rituximab by inducing conformational changes of CD20. PLoS Med. 2008;5:e64. doi: 10.1371/journal.pmed.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallek M. Immunochemotherapy with Fludarabine (F), Cyclophosphamide (C), and Rituximab (R) (FCR) Versus Fludarabine and Cyclophosphamide (FC) Improves Response Rates and Progression-Free Survival (PFS) of Previously Untreated Patients (pts) with Advanced Chronic Lymphocytic Leukemia (CLL) Blood. 2008;112 Abstract #325. [Google Scholar]
- 25.Halleck M, Fingerle-Rowson G, Fink A, et al. First-line treatment with Fludarabine (F), Cyclophosphamide (C), and Rituixmab (R) (FCR) Improves Overall Survival (OS) in Previously Untreated Patients (pts) with Advanced Chronic Lymphocytic Leukemia (CLL): Results of a Randomized Phase III Trial On Behalf of An International Group of Investigators and theGerman CLL Study Group. Blood. 2009;114 Abstract #535. [Google Scholar]
- 26.Robak T, Moiseev SI, Dmoszynska A, et al. Rituximab, fludarabine, and cyclophosphamide (R-FC) prolongs progression free survival in relapsed or refractory chronic lymphocytic leukemia (CLL) compared with FC alone: Final results from the international randomized phase III REACH Trial. Blood. 2008;112 Abstract #1. [Google Scholar]
- 27.Rabinowitz I. Interaction between statins and rituximab in non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:5486. doi: 10.1200/JCO.2008.19.2849. author reply 5486. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein MR, Mascitelli L, Pezzetta F. Monoclonal antibody therapy and non-Hodgkin's lymphoma. N Engl J Med. 2009;360:192. doi: 10.1056/NEJMc081871. author reply 193. [DOI] [PubMed] [Google Scholar]
- 29.Nowakowski GS, Maurer MJ, Habermann TM, et al. Statin Use and Prognosis in Patients With Diffuse Large B-Cell Lymphoma and Follicular Lymphoma in the Rituximab Era. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.23.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanafelt TD, Jelinek D, Tschumper R, et al. Cytogenetic abnormalities can change during the course of the disease process in chronic lymphocytic leukemia. Journal of Clinical Oncology. 2006;24:3218–3219. doi: 10.1200/JCO.2006.06.1077. [DOI] [PubMed] [Google Scholar]
- 31.Zent CS, Ding W, Schwager SM, et al. The prognostic significance of cytopenia in chronic lymphocytic leukaemia/small lymphocytic lymphoma. Br J Haematol. 2008;141:615–621. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddocks-Christianson K, Slager SL, Zent CS, et al. Risk factors for development of a second lymphoid malignancy in patients with chronic lymphocytic leukaemia. Br J Haematol. 2007;139:398–404. doi: 10.1111/j.1365-2141.2007.06801.x. [DOI] [PubMed] [Google Scholar]
- 33.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 34.Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma. 2007;48:2412–2417. doi: 10.1080/10428190701724801. [DOI] [PubMed] [Google Scholar]
- 35.Palmer S, Hanson CA, Zent CS, et al. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br J Haematol. 2008;141:607–614. doi: 10.1111/j.1365-2141.2008.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewald G, Brockman S, Paternoster S, et al. Chromosome anomalies detected by interphase fluorscence in hybridization: correlation with significant biological features of chronic lymphocytic leukemia. British Journal of Haematology. 2003;121:287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 37.Jelinek DF, Tschumper RC, Geyer SM, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115:854–861. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- 38.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 39.Friedman DR, Harrison JD, Magura LA, Warren HA, Diehl LF, Weinberg JB. Influence of Statin Therapy on the Clinical Course of Chronic Lymphocytic Leukemia. Blood. 2009;114 Abstract 2344. [Google Scholar]
- 40.Jensen M, Engert A, Weissinger F, et al. Phase I study of a novel pro-apoptotic drug R-etodolac in patients with B-cell chronic lymphocytic leukemia. Invest New Drugs. 2008;26:139–149. doi: 10.1007/s10637-007-9106-z. [DOI] [PubMed] [Google Scholar]
- 41.Moon EY, Lerner A. Benzylamide sulindac analogues induce changes in cell shape, loss of microtubules and G(2)-M arrest in a chronic lymphocytic leukemia (CLL) cell line and apoptosis in primary CLL cells. Cancer Res. 2002;62:5711–5719. [PubMed] [Google Scholar]