Abstract
The introduction of combination antiretroviral therapy significantly reduced the prevalence of the most severe form of HIV-associated neurocognitive disorders (HAND). Despite this decline, 35–70% of HIV-infected patients continue to develop mild motor and cognitive impairments. Although neuropsychological studies have shown that HAND affects a wide array of cognitive functions, a formal diagnosis is still based on the exclusion of opportunistic infections and other common ailments, as no specific tests or biomarkers are currently available. In this study, we used magnetoencephalography (MEG) to measure neural activity during the resting-state in 15 HIV-infected older patients and a demographically-matched group of 15 uninfected controls. MEG is a noninvasive and direct measure of neural activity with excellent spatiotemporal resolution. All MEG data were coregistered to structural MRI, corrected for head motion, fitted to a regional-level source model, and subjected to spectral analyses to quantify population-level neural oscillatory activity. We found that HIV-infected persons exhibited decreased beta oscillations in the supplementary motor area bilaterally, paracentral lobule, posterior cingulate, and bilateral regions of the superior parietal lobule relative to healthy controls. Beta oscillations in the posterior cingulate, a critical component of the default mode network, were also positively correlated with patient scores on the memory recall aspect of the Hopkins Verbal Learning Test-Revised. These results demonstrate that chronic HIV infection does not uniformly disturb cortical function, and that neuronal populations in dorso-medial motor and parietal cortices are especially affected. These findings also suggest that resting-state MEG recordings may hold significant promise as a functional biomarker for identifying HAND and monitoring disease progression.
Keywords: HAND, cognitive disorders, biomarker, AIDS, magnetoencephalography, default mode, gamma, theta, alpha
Introduction
Prior to the advent of combination antiretroviral therapy (cART), persons infected with the human immunodeficiency virus type one (HIV) often developed severe cognitive impairment, which was generally referred to as HIV-associated dementia (HAD; Adamson et al, 1999; Bouwman et al, 1998). The prevalence of HAD has sharply decreased since cART became widely available, although HIV-infected patients remain at an increased risk for developing cognitive deficits with two milder forms of HAD widely recognized (Antinori et al, 2007). Collectively, these disorders are referred to as HIV-associated neurocognitive disorders (HAND), and overall they have persisted in the era of cART as recent studies have shown that 35–70% of HIV-infected patients exhibit at least some form of neuropsychological impairment (Heaton et al, 2010; Heaton et al, 2011; Simioni et al, 2010). The underlying source of this persistence remains uncertain. Furthermore, the basic neural substrates of HAND have not been fully established, and to date the diagnosis of HAND continues to be made by the exclusion of psychiatric disorders, opportunistic infections, drug toxicities, and other factors. In short, there is currently no definitive diagnostic test for HAND.
Clinical indices of disease severity such as CD4+ T-cell count have been used in conjunction with neuropsychological testing, and structural MRI to better understand the association between immune dysfunction, brain atrophy, and cognitive decline (Cohen et al, 2010; Jernigan et al, 2011; Thompson et al, 2005). Cohen et al. examined the association between disease profiles, CD4+ count, duration of infection, and brain atrophy in asymptomatic HIV-infected participants. They found that lower nadir CD4+ was correlated with reduced white matter and that the duration of HIV infection was correlated with reduced gray matter, indicating that the severity of immune suppression is coupled with brain injury. Similarly, several studies have found that HIV-infected participants exhibit diminished cortical gray matter thickness and cerebral volumes relative to non-infected controls (Jernigan et al, 2011; Thompson et al, 2005). Furthermore, these volumetric disparities were directly linked with the severity of cognitive decline, with HAD patients displaying greater volumetric disparities than asymptomatic or mildly impaired patients (Jernigan et al, 2011; Thompson et al, 2005). Studies using functional magnetic resonance imaging (fMRI) have examined neuronal activity during attention and working memory tasks in HIV-infected and un-infected participants, and found greater activation in the frontal and parietal cortices of HIV-infected compared with control participants (Chang et al, 2001; Chang et al, 2004; Chang et al, 2008; Ernst et al, 2002; Ernst et al, 2009). In particular, Chang et al (2008) used a visual attention task with a variable attentional load during fMRI, and revealed that HIV-infected participants had similar task accuracy and reaction times relative to controls during less attention-demanding trials, but were less accurate and exhibited altered blood-oxygen-level-dependent (BOLD) responses during the more demanding trials. Finally, fMRI studies have also shown reduced activation and lower resting cerebral-blood-flow in the primary visual cortices of HIV-infected participants compared with controls (Ances et al, 2011; Ances et al, 2009; Ances et al, 2010).
In this study, we utilized magnetoencephalography (MEG) to examine cortical function in HIV-infected and uninfected older persons during a resting-state task. We have previously reported activity during visual processing, and finger tapping (Wilson et al, 2013a; Wilson et al, 2013c). MEG is a noninvasive, silent, and direct measure of neurophysiological activity with excellent temporal and spatial precision. The method quantifies the minute magnetic fields that naturally emanate from electrical activity in populations of active neurons. Our primary goal in the current study was to identify cortical regions where there was reduced oscillatory neuronal firing in HIV-infected participants compared with controls, and to evaluate whether activity in these regions was predictive of neuropsychological functioning. Such reductions in neural activity would likely indicate cortical atrophy in HIV-infected patients (i.e., reduction in the number of local pyramidal cells), and/or reduced neuronal synchronization within specific brain areas. Potentially, such functional indices of neural activity may be more sensitive to early cortical atrophy than structural-based measures, and thus provide improved options for early detection of HAND. As the asymptomatic stage of HAND appears to be highly prevalent and difficult to diagnose, it is of critical importance to understand and delineate a profile of disease progression with respect to structural and functional brain abnormalities that precede cognitive decline (Cysique and Brew, 2009; Gannon et al, 2011; Simioni et al, 2010). Based on the available structural and functional neuroimaging literature, we hypothesized that HIV-infected patients would show reduced neurophysiological activity in the bilateral precentral gyrus, medial motor areas such as the supplementary motor area (SMA), parietal cortices, and visual cortices. Moreover, we hypothesized that neural activity in these regions would correlate with CD4+ count and neuropsychological measures of cognitive function in the patient group.
Methods
We evaluated 15 HIV-infected adults (4 females) and 15 uninfected healthy controls (4 females). Controls were individually-matched to patients in regards to age, sex, and ethnicity. At enrollment, HIV-infected participants were receiving effective cART and had undetectable viremia. Exclusionary criteria included any pre-existing major psychiatric or neurological disorder, active brain infection (except HIV-1), presence of brain neoplasm or space-occupying lesion, history of head trauma, current substance abuse, and the MEG Laboratory’s standard exclusion criteria (e.g., dental braces, metal implants, pacemakers, etc.). Written informed consent was obtained following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board, who reviewed and approved the study protocol.
Neuropsychological Assessments
All HIV-infected patients underwent a battery of neuropsychological testing. This battery was sensitive, tested multiple domains, yet was relatively brief and convenient, and adhered to the recommendations of the Frascati consensus (Antinori et al. 2007). The battery assessed multiple functional domains including, gross motor (timed gait), fine motor (grooved pegboard), language (WRAT 4 reading), verbal learning (Hopkins Verbal Learning Test – Revised), verbal memory (HVLT-R), speed of processing (Trailmaking-A, digit symbol), attention and working memory (Paced Auditory Serial Addition Task), and executive functioning (verbal fluency, Stroop, and Trailmaking-B). All raw scores were transformed to demographically-adjusted z-scores for the assessment of HAND and other analyses. Correlation analyses were conducted on a subset of these neuropsychological metrics and the MEG data (see below). Selection of this subset was based on the distribution of scores across patients and the cognitive faculty probed by individual measures.
Experimental Paradigm
Throughout the paradigm, participants were seated within the magnetically-shielded room with both arms resting on a tray attached to the chair body. Participants were instructed to relax, remain still, and fixate on a centrally-presented cross hair for one continuous six-minute block.
Structural Magnetic Resonance Imaging (MRI)
High-resolution neuroanatomic images were acquired using a Philips Achieva 3T X-series scanner. The T1-weighted sagittal images were obtained with an eight channel head coil using a 3D fast field echo sequence with the following parameters: field of view, 24 cm; slice thickness, 1 mm with no gap; in-plane resolution, 1.0 × 1.0 mm; sense factor, 1.5. The structural volumes were aligned parallel to the anterior and posterior commissures and used for MEG coregistration.
MEG Data Acquisition
All recordings were conducted in a one-layer magnetically-shielded room (MSR) with active shielding engaged. With an acquisition bandwidth of 0.1 – 330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta Neuromag system with 306 magnetic sensors, including 204 planar gradiometers and 102 magnetometers (Elekta, Helsinki, Finland). Using MaxFilter (v2.1.15; Elekta), MEG data from each session and subject were individually-corrected for head motion, coregistered to structural MRI, and subjected to noise reduction using the signal space separation method with a temporal extension (tSSS; (Taulu and Simola, 2006; Taulu et al, 2005)
MEG Source Analyses
Following tSSS and head-motion correction, the sensor-level time series was divided into epochs of 4096 ms duration (4096 points) and artifact rejection was performed using a fixed threshold method supplemented with visual inspection. Artifact-free epochs were transformed into a regional source model per participant via inverse spatial filtering using the Brain Electrical Source Analysis software (BESA version 6.0; see Fig. 1; (Franzen et al, 2013; Hoechstetter et al, 2004; Wilson et al, 2013b). Essentially, a 29-point grid with dual orthogonal orientations per point was constructed, and each orientation was used as an inverse spatial filter on the continuous sensor-level time series data of the entire six-minute recording, per participant (Franzen et al, 2013; Wilson et al, 2013b). After transformation into source space, the current-amplitude (nAm) time series for each of the two orthogonal orientations per source was transformed into the frequency domain using Fourier analyses (i.e., 4096 data points per window). Average spectra across the six minute recording were then computed for each orientation per brain region by averaging the ~90 Fourier-transformed epochs. Subsequently, for each of the 29 regional sources, the amplitude per band was summed across the two orthogonal orientations to yield the total current-amplitude per frequency-band for the particular brain region. We focused on the local spectral amplitude within the five traditional frequency bands of quantitative electroencephalography (qEEG; i.e., delta: 1–4 Hz, theta: 4–7 Hz, alpha: 8–14 Hz, beta: 14–30 Hz, and gamma: 30–56 Hz) using a repeated measures ANOVA analysis. Initially, we evaluated group differences using an omnibus mixed-model ANOVA with brain region and frequency-band as within-subjects factors, and group (HIV-infected, uninfected) as a between-subjects factor. All statistical analyses were two-tailed and conducted in SPSS (Release 21.0.0). All MEG pre-processing and source modeling used the BESA software (version 6.0), and MEG-MRI coregistration and visualization used the BESA MRI (Version 2.0) software.
Figure 1.
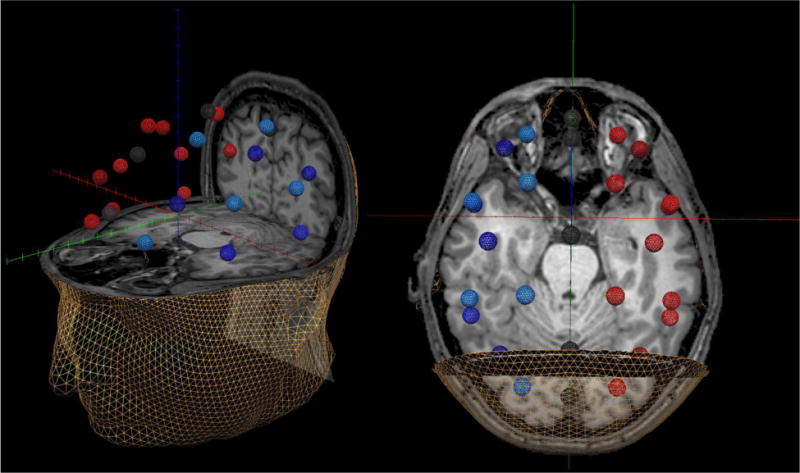
Representative Example of the 29-node Regional Source Model. For each participant, a 29-node (grid-point) model with dual orthogonal orientations was fitted to the T1-weighted MRI following coregistrati on. This model was used to estimate regional neuronal activity during the resting-state MEG recording using inverse spatial filtering. In the figure above, the model can be seen overlaid on the MRI of a HIV-infected participant. The same 3D rendition is shown in both the left and right panels, although the orientation of the image differs between the two panels to facilitate visualization of the spatial location of each source. For example, using the left panel one can discern that the regional sources that appear to be in the orbits on the right panel are actually located in the ventral prefrontal cortices. Likewise, the nodes that appear to be located within the sinuses on the right panel can be discerned as medial frontal sources using the left panel. The different colors are only meant to aid in visually distinguishing the regional sources. Note that the regional sources are spaced equidistant apart and that each represents activity over an extended cortical area (i.e., > 1cm3). Thus, the time series of each node reflects the average neuronal activity over that brain region, and not the amount of activation at a precise neuroanatomical coordinate (e.g., a voxel in MNI space). Following spectral analyses, the current amplitude (in nAm) was summed across the dual orthogonal orientations to yield the total amplitude for the given brain region.
Results
Participant Demographics, Laboratory Test Results, & Neuropsychological Measures
Mean age was 56.79 years (range: 50–71) in the HIV-infected group and 58.47 years-old (range: 50–75 years) in the control group. This difference was not significant (p = 0.24). The mean duration of HIV diagnosis was 17 years (range: 10–22), the average CD4+ T-cell count was 772 cells/mm3 (range: 267–1391), and all patients had undetectable viremia. Of the 15 HIV-infected patients, seven were impaired in at least two domains and thus were considered impaired according to the Frascati criteria (Antinori et al, 2007).
MEG Data
The omnibus 29 × 5 × 2 mixed-model ANOVA with location (29 brain regions) and frequency bin (5 levels) as within-subjects factors, and group as a between-subjects factor indicated that the three-way, brain region-by-frequency-by-group interaction effect was significant F(112, 3136) = 1.37 (p < 0.01). In addition, the two-way brain region-by-group interaction was significant F(28, 784) = 1.6 (p < 0.05), as was the brain region-by-frequency interaction F(112, 3136) = 22.58 (p < 0.0001). Finally, the main effects of brain region and frequency-band were significant (both p’s < 0.0001), but not the main effect of group (p = 0.52). No other effects were significant.
Given the focus of this study, follow-up testing was conducted on the significant three-way and the two-way brain region-by-group interaction effects. Post-hoc t-tests (independent-sample) of the brain region-by-frequency-by-group interaction term indicated that HIV-infected participants had significantly weaker beta activity in the left SMA t(28) = 2.01 (p < 0.05; Cohen’s d = 0.76), left superior parietal lobule t(28) = 2.36 (p < 0.05; d = 0.89), paracentral lobule t(28) = 2.6 (p < 0.05; d = 0.98), posterior cingulate t(28) = 2.02 (p < 0.05; d = 0.76), and the right superior parietal lobule t(28) = 2.53 (p < 0.05; d = 0.96; see Fig. 2). A marginal decrease in beta activity was also detected in the right SMA of HIV-infected participants, t(28) = 1.79 (p = 0.08; d = 0.68; Fig. 2). To evaluate whether impairment status modulated beta activity in these brain regions, we split the HIV-infected group into impaired and unimpaired subgroups and performed an exploratory analysis which is shown in Table 1. Although preliminary, this analysis clearly suggested that impairment status is a critical factor in the amplitude of beta oscillations in these brain regions. In regard to the brain region-by-group interaction effect, post-hoc independent-sample t-tests showed that no individual brain area was significantly different between groups when the amplitude of neuronal activity was collapsed across all five frequency bands, but was marginally stronger in the paracentral lobule t(28) = 1.93 (p = 0.06; d = 0.73) and the right superior parietal lobule t(28) = 1.86 (p = 0.07; d = 0.70) of uninfected controls.
Figure 2.
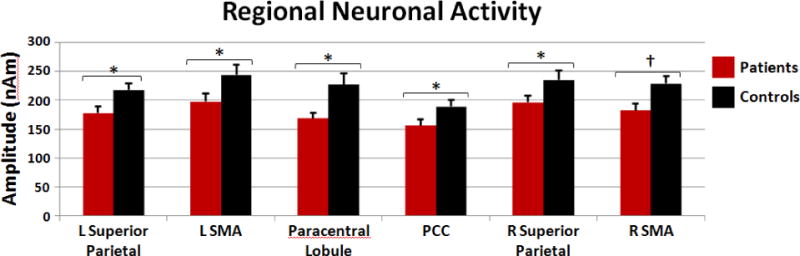
HIV-related Neuronal Activity Differences during the Resting-State. Neuronal activity in the beta-band (14–30 Hz) was significantly reduced in superior parietal areas, medial motor regions, and the posterior cingulate of HIV-infected patients (red) compared to uninfected controls (black) during the awake resting-state. Neuronal activity in the other frequency bins did not significantly differ between patients and controls. Error bars indicate one standard error of the mean. PCC: posterior cingulate; SMA: supplementary motor area; * = p < 0.05; † = p < 0.08
Table 1.
MEG Results by Impairment Status in the HIV-Infected Group
| Brain Region | Impaired Mean(SD) | Unimpaired Mean(SD) | t-value | p-value |
|---|---|---|---|---|
| L SMA | 163.31(40) | 227.35(53) | −2.620 | 0.021 |
| L Superior Parietal | 146.50(20) | 205.23(49) | −2.971 | 0.011 |
| Paracentral Lobule | 147.50(32) | 186.60(37) | −2.149 | 0.051 |
| PCC | 127.36(23) | 180.75(39) | −3.158 | 0.008 |
| R SMA | 163.56(33) | 224.78(40) | −3.245 | 0.006 |
| R Superior Parietal | 152.13(22) | 208.71(53) | −2.598 | 0.022 |
Neuropsychological, CD4, & MEG Correlations
To examine possible relationships between MEG measures of beta activity, CD4 T-cell counts, and neuropsychological metrics, we conducted a series of Pearson-correlation analyses in the HIV-infected participants. To this end, we used the beta amplitude value in each brain region where significant group differences were found, and the participant scores on individual neuropsychological assessments of fine motor control (Grooved Pegboard: z-transformed dominant-hand score), psychomotor speed (z-transformed time score on Trail Making Test A-B), memory function (z-transformed delayed-recall and retention indices of the HVLT-R), speed of processing (z-transformed digit symbol), and executive functioning (z-transformed color-word interference time on the Stroop task). Since these analyses were restricted to brain areas where significant group differences were observed, we did not correct p-values for multiple comparisons (e.g., using the Bonferroni method). Nonetheless, these results should still be considered preliminary and interpreted with caution.
These analyses showed that performance on the delayed recall measure of the HVLT-R was significantly correlated with the amplitude of beta responses in the posterior cingulate, r(15) = 0.52 (p < 0.05, two-tailed). In addition, the normalized color-word interference time on the Stroop task was correlated with the amplitude of beta activity in the left SMA, r(15) = 0.59 (p < 0.05, two-tailed), and the right SMA, r(15) = 0.64 (p < 0.01, two-tailed). The amplitude of beta oscillations was not correlated with performance measures on any other neuropsychological assessment. Likewise, CD4+ T-cell counts at the time of the MEG recording were not correlated with the amplitude of beta oscillations in brain regions where group differences were observed.
Discussion
We examined regional neurophysiological activity during the awake resting-state in HIV-infected older adults and a demographically-matched sample of uninfected healthy controls using high-density MEG. Our results showed that HIV-infected participants had abnormally reduced resting-state beta oscillations in the SMA bilaterally, paracentral lobule, posterior cingulate, and bilateral regions of the superior parietal lobule when compared to healthy uninfected controls. While we hypothesized that HIV-infected patients would show diminished neural activity in the association, motor and visual cortices, we found that differences in beta oscillations were primarily localized to dorsal motor and parietal areas, along with one critical component of the default-mode network (Fox and Raichle, 2007; Greicius and Menon, 2004; Raichle et al, 2001), the posterior cingulate. Interestingly, patients’ scores on the HVLT-R delayed-recall measure were positively correlated with beta oscillatory amplitudes in the posterior cingulate, which indicates that patients with the highest HVLT-R recall scores were more similar to healthy controls. Likewise, neural activity in the SMA bilaterally was correlated with performance on the Stroop task in HIV-infected patients, with better performers having levels of neural activity similar to the controls. Beyond theses brain regions, neither neuropsychological measures nor CD4+ count was correlated with neural activity in any of the remaining brain regions. Below, we discuss the implications of these findings for neuroimaging studies of HAND and current understanding of the pathophysiological basis of these cognitive disorders.
In this study, HIV-infected patients exhibited abnormally reduced beta band oscillations in dorsal and medial motor regions. Thompson et al (2005) found significantly decreased cortical thickness in these same brain regions in HIV-infected patients relative to healthy controls, and reported that the greatest differences in cortical thickness were in the SMA, medial motor areas, and primary visual regions. Critically, with the exception of primary visual regions, we found significantly decreased beta oscillations in the same brain regions (i.e., medial sensorimotor and SMA). Such observations of brain atrophy and aberrant neural activity (current study) in the sensorimotor cortices of the HIV-infected brain makes intuitive sense, since less severe cases of HAD were originally referred to as minor cognitive motor disorder (MCMD). Furthermore, our recent MEG study of motor performance in HIV-infected patients found that HIV-infected participants exhibited increased beta activity in the prefrontal cortices, as well as decreased beta responses in the SMA and precentral gyrus relative to age- and sex-matched controls (Wilson et al, 2013c). This study also found that the activation magnitudes in these motor regions were correlated with scores on the Trail Making Test – B and Grooved Pegboard Test. Interestingly, recent neuropsychological studies of HAND have also revealed that measures of psychomotor speed and fine motor control are among the most sensitive assessments for HAND (Joska et al, 2010; Robertson et al, 2007; Woods et al, 2005), which further implicates these brain areas as critically deficient in HIV infection. In the current study we also found a significant correlation between beta activity in the SMA and color-word inference time during the Stroop task in HIV-infected patients. Presumably, this finding reflects the motor programming component of this task, although it is unclear why other motor assessments were not correlated with MEG activity in these brain regions and this warrants future studies.
Sensory and motor information is integrated and manipulated in the parietal lobes so any alteration to their functional or structural integrity may impair performance and result in poor visuo-spatial processing (Koenigs et al, 2009). Thompson et al (2005) found that cortical thickness in parietal regions was strongly correlated with neuropsychological impairment in HIV-infected persons. In addition, proton magnetic resonance spectroscopy findings from Ernst et al (2010) revealed that HIV-infected patients who exhibited poorer performance on cognitive tests of attention had decreased glutamate levels in parietal gray matter relative to healthy uninfected controls. Previous neuroimaging studies have also suggested that neural activity patterns in prefrontal (supplementary motor areas) and parietal cortices of HIV-infected patients are selectively altered during attentional and working memory tasks in a load-dependent manner, arising during more difficult tasks requiring greater attention and integration of complex stimuli (Chang et al, 2004; Chang et al, 2008; Ernst et al, 2002; Ernst et al, 2009). The aforementioned studies utilized an fMRI visual attention task requiring subjects to mentally track two, three, or four balls as they traveled around a screen among 10 randomly moving balls. Essentially, every 10 seconds a set of target balls, either the target set or a new set, was briefly highlighted and participants were required to push a response button if the highlighted balls were the same as the original targets. Using this task, Ernst et al (2009) recorded the BOLD responses of HIV-infected patients over a one year period and found that although HIV-infected patients showed no change in task performance, relative to healthy controls, they exhibited increased BOLD responses in posterior parietal cortices. Ernst proposed that HIV-infected patients’ increased neural activity represents a compensatory response involving the increased usage of the brain’s reserve capacity in the dorsal attention network (DAN). A recent functional connectivity study also found abnormalities in the DAN in HIV-infected patients (Thomas et al (2013); described below).
Finally, we found decreased beta oscillations in the posterior cingulate, a critical component of the DMN, which were correlated with patient scores on the memory recall aspect of the HVLT-R, a test known to be sensitive to HAND (Carey et al, 2004; Woods et al, 2005). Several fMRI studies have implicated the medial prefrontal cortices, posterior cingulate cortices, and the mediolateral inferior parietal cortices as constituents of a cortical network known as the default-mode network (Andrews-Hanna et al, 2010; Buckner et al, 2008; Buckner and Vincent, 2007; Fox and Raichle, 2007; Greicius and Menon, 2004). The default mode network has been characterized as a subset of cortical regions which exhibit greater activation or increased neurophysiological activity during the resting-state as compared with an active task state (Chang et al, 2004; Fox and Raichle, 2007; Fox et al, 2005; Wilson et al, 2013b). Interestingly, regions of the default-mode network also maintain a strong anti-correlation with task-active regions, with activation being strongly suppressed across the default-mode network during cognitive processing (Fox et al, 2005). As mentioned above, we found that performance on the HVLT-R recall measure was positively correlated with the amplitude of beta oscillations in the posterior cingulate in HIV-infected patients. Essentially, patients with the highest scores had the strongest beta responses in this brain area (i.e., were most like controls), which is consistent with the idea that neural activity should be stronger in a “healthy” posterior cingulate during the resting-state. In accordance with our findings, a recent study by Thomas et al (2013) examined resting-state functional connectivity in HIV-infected patients using fMRI and found that patients exhibited decreased functional connectivity in the DMN relative to healthy uninfected controls and that neural activity was not correlated with either CD4+ count or cognitive impairment. Thus, our findings are largely consistent with Thomas et al (2013), with the exception that HVLT-R recall measures did correlate with the neural activity indices. As previously mentioned, Thomas et al (2013) found that HIV-infected patients exhibit aberrant resting-state neural activity in the DAN, as well as significant reduction in the strength of the anti-correlation between the DMN and DAN. Importantly, Thomas’ findings suggest that abnormalities in the DMN may compromise the functional connectivity of other brain networks resulting in cognitive impairments in integrating and processing stimuli. Examining the activity of the DMN in the HIV-infected brain will provide critical insights into understanding how HIV-related cerebral atrophy alters neuronal function. Resting state data can then be juxtaposed to current attention network data to contribute understanding to both the function of the DMN and the progression of HAND.
Beyond our recent study of motor function in HIV-infected patients (Wilson et al, 2013c), only two MEG studies and one case study have been conducted in the HIV-infected population. Nonetheless, together these studies have shown substantial promise in biomarker development for HAND. The first MEG study in HIV-infected patients showed abnormal mutual information between sensors in the anterior right part of the helmet and those in the posterior left part (Becker et al, 2012a). Measures of mutual information are thought to reflect connectivity, thus these findings suggest aberrant functional connectivity between right frontal regions and left posterior areas in HIV-infected patients (Becker et al, 2012a), which is consistent with the limited fMRI data on functional connectivity in this population (Thomas et al, 2013). The second MEG study examined measurement reliability, and provided preliminary evidence that broadband sensor-level data had good test-retest reliability after ~24 weeks in both HIV-infected patients and controls (Becker et al, 2012c). Finally, a recent case-study showed that MEG measurements of mutual information improved to the level of uninfected controls following 6-months of cART administration to a recently-diagnosed 54 year-old patient who was cART-naïve at enrollment (Becker et al, 2012b). Collectively, these studies complement the current study’s findings and provide substantial support for the potential utility of MEG as a future test for HAND. Essentially, based on our results, MEG appears to be sensitive to the degree of cognitive impairment and may afford researchers the unique opportunity to investigate the relatively unexamined asymptomatic form of HAND (ANI; Antinori et al (2007). If realized, this capacity would open up new vistas for investigating the pathogenesis of HAND. One paradoxical problem of developing MEG for this purpose is that there is no independent measure for validating impairment below the threshold for ANI; such impairments are too subtle for existing measures to detect. However, although speculative, longitudinal studies may be able to provide partial validation to these measures as presumably patients with sub-threshold ANI would progress to ANI over time. Nevertheless, MEG is a very promising technique in this domain.
In closing, it is important to identify possible limitations in generalizing our findings to other HIV neuroimaging studies. In our study, all HIV-infected patients were receiving effective cART during the study, and at this time it is unknown how such therapy modulates neuronal activity. In regards to the limited neurobehavioral and neuroimmune (e.g., CD4+) correlations in the current study, many other neuroimaging studies have also failed to find such correlations (Ances et al, 2009; Chang et al, 2008; Thomas et al, 2013). In fact, only a few previous studies have actually indicated a relationship between neuronal activity, neuropsychological deficits, and depressed CD4+ counts in HIV-infected persons (Jernigan et al, 2011; Thompson et al, 2005; Wilson et al, 2013c), and future studies should evaluate other factors that may mediate this relationship. Another possible factor that may limit the generalizability of study is that all of our patients had relatively high CD4+ counts, which may influence behavioral and neuroimaging measurements. Our cohort also consisted of older adults infected with HIV so our findings cannot be easily generalized to younger patients. Future studies should use larger sample sizes, wider age ranges, and evaluate the contributions of comorbidities to HAND in HIV-infected patients receiving cART.
Acknowledgments
This work was supported by NIH grant P30 MH062261 (HSF). The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. We would like to thank our participants for volunteering. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest
KMB, EHG, HSF, JO’N, and TWW have no biomedical financial interests or potential conflicts of interest to report. KRR has served as a consultant for Abbott and GlaxoSmithKline. USS has served as a consultant for Merck and has received research grant support from Pfizer, Behring, and GlaxoSmithKline for research unrelated to this study. SS reports receiving grant support to the University of Nebraska Medical Center from GlaxoSmithKline and Pfizer for research unrelated to this study.
References
- Adamson DC, McArthur JC, Dawson TM, Dawson VL. Rate and severity of HIV-associated dementia (HAD): correlations with Gp41 and iNOS. Mol Med. 1999;5:98–109. [PMC free article] [PubMed] [Google Scholar]
- Ances B, Vaida F, Ellis R, Buxton R. Test-retest stability of calibrated BOLD-fMRI in HIV− and HIV+ subjects. Neuroimage. 2011;54:2156–62. doi: 10.1016/j.neuroimage.2010.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE, Buxton RB, Benson D, Smith DM, Little SJ, Richman DD, Moore DJ, Ellis RJ, group H Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73:702–8. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis. 2010;201:336–40. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Bajo R, Fabrizio M, Sudre G, Cuesta P, Aizenstein HJ, Lopez OL, Wolk D, Parkkonen L, Maestu F, Bagic A. Functional connectivity measured with magnetoencephalography identifies persons with HIV disease. Brain Imaging Behav. 2012a;6:366–73. doi: 10.1007/s11682-012-9149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Cuesta P, Fabrizio M, Sudre G, Vergis EN, Douaihy A, Bajo R, Schubert A, Lopez OL, Parkkonen L, Maestu F, Bagic A. Brain structural and functional recovery following initiation of combination antiretroviral therapy. J Neurovirol. 2012b;18:423–7. doi: 10.1007/s13365-012-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Fabrizio M, Sudre G, Haridis A, Ambrose T, Aizenstein HJ, Eddy W, Lopez OL, Wolk DA, Parkkonen L, Bagic A. Potential utility of resting-state magnetoencephalography as a biomarker of CNS abnormality in HIV disease. J Neurosci Methods. 2012c;206:176–82. doi: 10.1016/j.jneumeth.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC. Variable progression of HIV-associated dementia. Neurology. 1998;50:1814–20. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–6. doi: 10.1016/j.neuroimage.2007.01.010. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Grant I, Heaton RK, Group H. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004;18:234–48. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–7. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56:259–72. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Nakama H, Stokes B, Ernst T. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol. 2008;3:95–104. doi: 10.1007/s11481-007-9092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–85. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–9. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–53. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol. 2009;65:316–25. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen JD, Heinrichs-Graham E, White ML, Wetzel MW, Knott NL, Wilson TW. Atypical coupling between posterior regions of the default mode network in attention-deficit/hyperactivity disorder: a pharmaco-magnetoencephalography study. J Psychiatry Neurosci. 2013;38:120054. doi: 10.1503/jpn.120054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24:275–83. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–8. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I, Group C. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–57. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–14. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29:14980–6. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–21. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite longstanding suppression of viremia. AIDS. 2010;24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–68. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEEE Trans Signal Process. 2005;53:3359–3372. [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80:1186–93. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–52. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Fox HS, Robertson KR, Sandkovsky U, O’Neill J, Heinrichs-Graham E, Knott NL, Swindells S. Abnormal MEG Oscillatory Activity during Visual Processing in the Prefrontal Cortices and Frontal Eye-Fields of the Aging HIV Brain. PLoS One. 2013a;8:e66241. doi: 10.1371/journal.pone.0066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Franzen JD, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Hum Brain Mapp. 2013b;34:566–74. doi: 10.1002/hbm.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Robertson KR, Sandkovsky U, O’Neill J, Knott NL, Fox HS, Swindells S. Functional Brain Abnormalities During Finger-Tapping in HIV-Infected Older Adults: A Magnetoencephalography Study. J Neuroimmune Pharmacol. 2013c doi: 10.1007/s11481-013-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK, Grant I, Group HIVNRC Construct validity of Hopkins Verbal Learning Test-Revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol. 2005;20:1061–71. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]


