Abstract
Choline dihydrogen phosphate (CDHP) is an ionic liquid reported to increase thermal stability of model proteins. The current work investigated CDHP effect on structural integrity and biological activity of recombinant human interleukin-2 (rhIL-2), a therapeutic protein used for treating advanced melanoma. In vitro CDHP biocompatibility was also evaluated using primary cell cultures, or B16-F10 cell line, chronically exposed to the ionic liquid. Formulation of rhIL-2 in an aqueous 680mM CDHP pH 7.4 solution resulted in a 12.5°C increase in the Tm of rhIL-2 compared to a basic buffer formulation, and provided conformational rhIL-2 stabilization when the solution was heated to 23.3°C above the Tm. CDHP solutions (≤80mM), exhibited no cytotoxic activity toward primary splenocytes or B16-F10 cells in culture. However, a 10-fold loss in biological activity was observed when rhIL-2 was used in a 30mM CDHP aqueous solution with NaHCO3 (pH≥7.2) compared to controls without CDHP. While increased Tm is associated with a diminished rhIL-2 biological activity, the therapeutic protein remains structurally intact and functional.
Keywords: Ionic liquids, Interleukin-2, Structural integrity, Biological activity, Biocompatibility
INTRODUCTION
The range of stabilizing agents routinely employed as pharmaceutical excipients has, to date, been limited to a relatively small number of sugars, amino acids, polymers, salts, and detergents.1 Recently, various low melting point organic salts have been reported to stabilize proteins and DNA, decrease protein aggregation, and protect against thermal denaturation, raising the possibility of their use as additional excipients in pharmaceutical protein formulations.2–5 Some of these salts can be considered ionic liquids as their melting points are less than 100°F and others are rendered liquid with the addition of a small amount of water and thus are referred to as hydrated ionic liquids. It has been reported that a combination of kosmotropic (water structure-making) anions and chaotropic (water structure-breaking) cations can stabilize proteins in aqueous solution, and this criteria can be employed to design ionic liquids that stabilize therapeutic proteins in liquid formulation.6–8 One such organic salt, choline dihydrogen phosphate (CDHP), is reported to extend the shelf life of several model proteins (including cytochrome C, lysozyme, and ribonuclease A) when formulated with 20% (v/w) water, supporting the retention of structure and activity of these proteins.2,4,7 Stabilization effects have also been observed in model proteins when using lower concentrations (<40 wt %) of this salt.8,9
Interleukin-2 (IL-2) is a cytokine produced by the body during immune processes. While classically synthesized and released in response to infection, IL-2 production can also be triggered by the presence of transformed, cancerous cells. Following release, IL-2 can act as an autocrine factor to promote expansion of tumor-specific helper and cytotoxic T-cells. It can also act as a paracrine-signaling factor on B-cells, natural killer cells, and lymphokine-associated killer cells, to aid in the removal of tumorigenic cells.10,11 In addition to the removal of damaged and/or transformed cells; IL-2 is implicated in maintaining peripheral tolerance via augmentation of regulatory T-cell (Treg) survival and function. For example, Tregs act to maintain homeostasis by limiting IL-2 availability via constitutive expression of high-affinity IL-2 receptors.12
Recombinant human IL-2 (rhIL-2) is an approved therapy for treating unresectable metastatic renal cell carcinoma and stage IV melanoma.13,14 Using rhIL-2 therapy, durable cancer remission is reported in a significant number of patients (5–10%). Due to widespread distribution of the cancer at late disease stages and high clearance rate of rhIL-2, bolus intravenous injections of rhIL-2 are employed to maintain a circulating therapeutic concentration. The use of high dose-bolus regimes is limited however, due to adverse, often severe, side effects that include malaise, hypotension, hyperthermia, vomiting, diarrhea, edema, and in severe cases, capillary leakage syndrome.13–15 In vivo studies report that continuous, local rhIL-2 delivery to the tumor site is more efficacious, and is associated with lower toxicity. Several drug delivery systems aimed at delivering a sustained, lower dose of rhIL-2 targeted to the tumor site(s), or relevant immune cells, have been investigated as a means of improving clinical outcome while minimizing side effects associated with bolus administration.16,17 However, because manufacturing and formulation processes for these types of drug delivery systems may affect native protein structure required for clinical efficacy, stabilizing excipients are necessary. Interleukin-2 is thus a good candidate therapeutic protein for evaluating the potential of specific candidate ionic liquids as excipients for use in formulations intended for delivery into physiologic environments.
Choline DHP extends the shelf life of model proteins when used in high concentrations as a solvent, and increases thermal stability of model proteins at lower concentrations. Further studies are needed to understand the mechanism of stabilization in different concentration ranges, to identify possible differences between the use of CDHP with relatively stable model proteins and less robust therapeutic proteins, and to extend biocompatibility studies to better anticipate possible complications of in vivo injection. The aim of the current study was to investigate applicability of CDHP as an excipient for use in rhIL-2 formulation, at concentrations that elicit no adverse cytotoxic effects in vitro following chronic exposure. In doing so we sought to define the effect of low concentrations of CDHP on IL-2 structure and function, as well as the biological activity of CDHP, using complementary in vitro models of splenocyte and melanoma cell cultures.
MATERIAL AND METHODS
Assurances
Male C57BL/6J mice (10–12 weeks old; Jackson laboratories, Bar Harbor, ME) were used for these studies. All experiments were approved by the Institutional Animal Care and Use Committee (CMC) and conformed to NIH Guidelines for the Care and Use of Animals.
Materials
Choline dihydrogen phosphate (CDHP), and CDHP pH 7.4, were prepared in the MacFarlane laboratory (Monash University, Australia) as previously reported.7,18,19 Lyophilized rhIL-2 (Des-alanyl-1, ser-125 interleukin-2) was purchased from GenScript USA Inc. (Piscataway, NJ). The B16F10 mouse melanoma cell line was purchased from the National Cancer Institute Tumor Repository (Frederick, MD) and the IL-2 dependent CTLL-2 cell line was purchased from ATTC (Manassas, VA). RPMI 1640 culture medium and calcium/magnesium free phosphate buffer saline (PBS) were purchased from Mediatech (Manassas, VA). Dulbecco’s modified Eagle medium mixture F12 (DMEM), resazurin, sodium bicarbonate (NaHCO3), sodium pyruvate, 2-β-mercaptoethanol, 4-(2-hydroxyethyl)-1-piperazineethasulfonic acid (HEPES), carboxy-fluorescein succinimidyl ester (CFSE), choline chloride, and red blood cell lysis buffer were purchased from Sigma-Aldrich (St. Louis, MO). Sodium dihydrogen phosphate and sodium chloride were purchased from VWR International. Trypan blue, L-Glutamine, non-essential amino acids, 0.25% trypsin/EDTA, and propidium iodide (PI) were purchased from Life Technologies (Rockville, MA). Interleukin-2 culture supplement from rat with concanavalin A (T-stim) was purchased from BD Biosciences (San Jose, CA). Fraction V bovine serum albumin (BSA) was purchased from MP Biomedicals (Solon, OH). Fetal Bovine serum (FBS) and a bicinchoninic acid (BCA) protein assay kit were purchased from Thermo-Fisher Scientific (Waltham, MA).
Solution Properties
Osmolality of solutions was measured using a vapor pressure osmometer (Wescor Vapro 5520, Logan, UT, USA).
Cell culture
Spleen tissue from male C57BL/6J mice was harvested and disrupted in RPMI 1640 culture medium through a 70µm nylon cell strainer as previously reported.20 Splenocyte suspensions were washed in RPMI 1640 and centrifuged (200×g, 7min, 25°C). Red blood cell lysis buffer was used to remove any remaining erythrocytes prior to washing cells (x2) in RPMI 1640. The resulting splenocytes, or CTLL-2 cells, were cultured in complete RPMI medium [RPMI 1640 supplemented with 10% (v/v) FBS, 1% (v/v) non-essential amino acids, 1mM sodium pyruvate, 10mM HEPES, 50µM β-mercaptoethanol, penicillin (100U/ml), and streptomycin (50µg/ml)] at 37°C in a 5%/95% (CO2/air) humidified chamber. Growth of CTLL-2 cells was maintained by the addition of 3% (v/v) T-stim and subcultures obtained by detachment and reseeding (1:5 ratio). B16F10 cells were cultured in complete DMEM medium as previously reported.20 subcultures were obtained by detachment and reseeding (1:100 ratio).
Resazurin reduction assay
Splenocytes (1×105 cells/well) or B16F10 cells (1×104 cells/well) were cultured in 96-well plates in complete culture medium containing 0–80mM CDHP or CDHP supplemented with 0.15 to 0.33% (w/v) NaHCO3 to ensure pH≥7.2. To maintain cell proliferation splenocyte cultures were also supplemented with rhIL-2 (500U/ml). Either 4 hours (splenocyte) or 18 hours (B16F10) after initial plating, resazurin was added (final concentration: 43.7µM) and 24 hours after initial plating resazurin reduction was measured colorimetrically (530/590nm).
Trypan blue exclusion assay
Splenocytes (5×105 cells/well) or B16F10 cells (2×105 cells/well) were plated in 96-well plates as described for the resazurin reduction assay. Twenty-four hours later cells were detached and stained with 0.04% (w/v) trypan blue and total cell number/ viability (trypan blue exclusion) was measured using a Countess® automatic cell counter (Life Technologies, Rockville, MA).
Recombinant human IL-2 binding activity analysis by ELISA
To detect rhIL-2 structural integrity over increasing temperature increments, a human IL-2 enzyme-linked immunosorbent assay (ELISA) was performed (Thermo-Fisher Scientific, Waltham, MA). This assay requires two rhIL-2 conformational epitopes to remain intact for binding-detection to occur.21 Aliquots of rhIL-2 (125pg/ml) were diluted in 100µl PBS containing 0.1% (w/v) BSA and supplemented with CDHP pH 7.4 (0–680mM). Control solutions of IL-2 in 680mM NaH2PO4 (NaDHP), 680mM ChCl, and 680mM NaCl were prepared in a similar manner. Samples were incubated at 45–85°C in a dry bath incubator (Thermo Fisher Scientific), and the temperature monitored using a K-type thermocouple with a HH314A humidity temperature meter (Omega, Stamford, CT). Once samples reached desired temperature they were immediately cooled on ice prior to measurement. The rhIL-2 concentration was then determined according to manufacturer’s instructions. All samples were assayed in triplicate for each temperature point.
To determine the effect of prolonged exposure to CDHP on rhIL-2 structural integrity, 125pg/ml rhIL-2 aliquots were diluted in PBS containing 0.1% (v/v) BSA supplemented with 0–60mM CDHP, or 0–60mM CDHP supplemented with an appropriate amount of NaHCO3 to maintain the pH≥7.2, and incubated in a water bath (IsoTemp 210, Thermo Fisher Scientific) at 37°C for 48h. Sequential samples were removed and stored at −20°C prior to analysis. Structural integrity of rhIL-2, at different times and temperatures, was determined using the human IL-2 ELISA kit according to manufacturer’s instructions.
In vitro rhIL-2 activity assay
The biological activity of rhIL-2 was measured by flow cytometry using the CTLL-2 reporter cell line. CTLL-2 cells require IL-2 to survive and proliferate.22 CTLL-2 cells were initially recovered from culture (90% confluence), washed in RPMI 1640 culture medium (x2), and re-suspended in PBS. Ten million cells were then stained by incubation in 1µM CFSE for 8mins (room temperature; light protected). To stop the procedure, an equal volume of FBS was added followed by 5min incubation (in the dark), and the cells washed (x3; complete RPMI medium). CTLL-2 cells (≥1.25×105 cells/well) were placed in 96-well round-bottom culture plates in complete RPMI and stimulated with 0, 1, or 10 international units (IU) rhIL-2 in the absence or presence of 30 or 60mM buffered CDHP in NaHCO3 (pH≥7.2). Twenty-three hours later, propidium iodide was added to each well at a final concentration of 0.5µg/ml, and the cultures remained protected from light for a further hour. At the end of the 24h incubation period cells were recovered, washed (x2), and resuspended in PBS for flow cytometry analysis. Propidium iodide and CFSE fluorescence intensities were recorded using a BD FACS Aria™ II (Beckton- Dickinson, Franklin Lake, NJ) and data analysis performed using FlowJo 8.8.4 software (Treestar, Ashland, OR).
Statistical analysis
Results are expressed as means ± standard error of the mean (SEM). Paired t test or two-way ANOVA were used when appropriate to compare results from solutions supplemented with CDHP versus those not supplemented with CDHP. Statistical analyses were performed using Prism 5 software (Graphpad Software Inc., La Jolla, CA). p<0.05 was considered significant.
RESULTS
Buffered CDHP protects rhIL-2 structural integrity at high temperature
In previous work we reported on the effects of CDHP on the secondary structure of the model protein hen egg white lysozyme and rhIL-2.9 Using measurements of far-UV circular dichroism spectropolarimetry at 222nm, we observed an increase in thermal midpoint of unfolding temperature (Tm) of recombinant human IL-2 (rhIL-2) in the presence of CDHP. However, the effects on rhIL-2 bioactivity were not explored. The observed increase in thermal stability of rhIL-2 formulated with CDHP was used to establish a temperature and concentration range for structural integrity and biological activity testing, below or above Tm. The reported Tm values are included here as follows: (1) 30mM NaH2PO4: Tm = 61.7°C, (2) 30mM CDHP pH 7.4: Tm=60.2°C, (3) 185mM CDHP pH 7.4: Tm=63.0°C, and (4) 680mM CDHP pH 7.4: Tm = 74.2°C.9
To determine if these levels of CDHP also increased rhIL-2 thermal stability at the conformational level, an ELISA was performed on rhIL-2 samples formulated in 680mM CDHP, pH 7.4, that were heated from 45°C to 85°C. In order to probe the contributions of the individual choline and DHP ions to rhIL-2 stability, salt solutions of 680mM pH7.4 ChCl and NaDHP were included. Finally, a 680mM NaCl pH 7.4 solution was also analyzed to compare the stabilizing effects of CDHP to a more conventional (i.e. non-ionic liquid) excipient. Osmolality measurements of all solutions were similar, falling within 5% of one another (CDHP=1520mmol/kg, NaDHP=1530mmol/kg, ChCl=1501mmol/kg, and NaCl=1520mmol/kg). Below Tm (45–60°C), none of the 680mM pH7.4 salt solutions significantly affected rhIL-2 binding ability. Within this temperature range, similar levels of rhIL-2 were observed for all salt formulations compared to PBS 0.1%BSA control solution (Figure 1A–D, n=9 from three independent experiments).
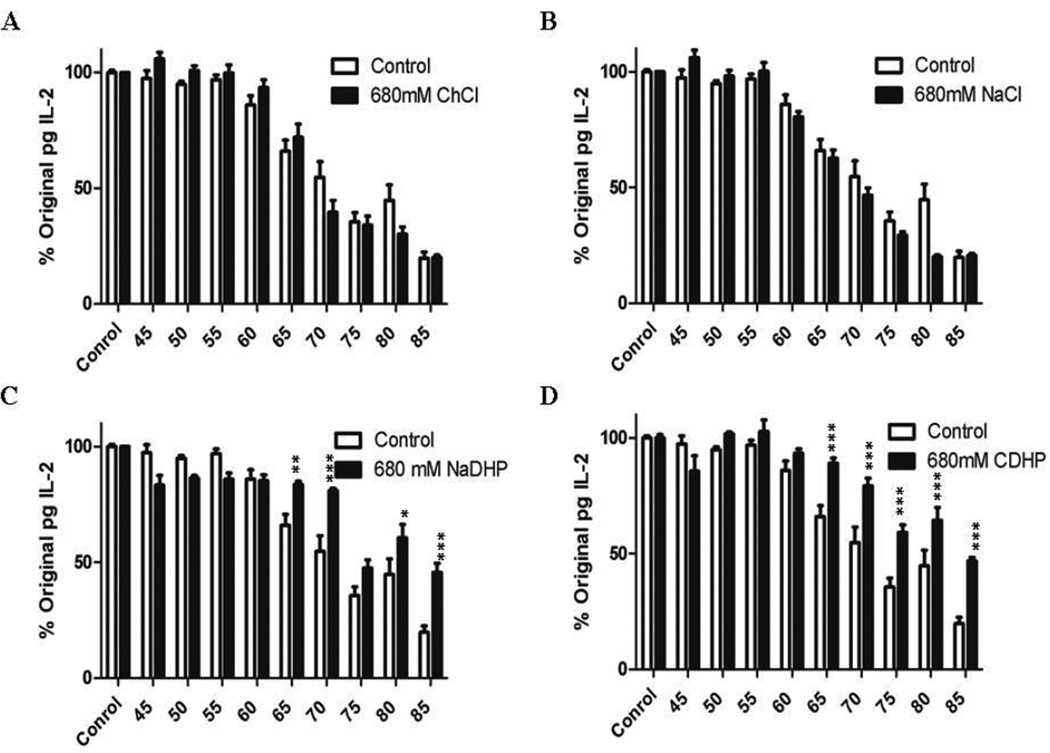
Figure 1. rhIL-2 formulated in 680mM pH7.4 CDHP and 680mM NaDHP remains structurally intact when heated.
Sandwich ELISA measurements of rhIL-2 concentrations formulated in (A) 680mM ChCl pH7.4, (B) 680mM NaCl pH7.4, (C) 680mM NaDHP pH7.4, and (D) 680mM CDHP pH 7.4 versus no CDHP control (white bars), and heated at temperatures ranging from 45°C to 85°C. Data are expressed as mean relative rhIL-2 concentrations ±SEM, normalized to the initial amount (T0) of protein. n=9; *: p<0.05; **: P<0.01.
At and above Tm (>60°C), a significant decrease in the ability of rhIL-2 to bind at two structurally intact epitopes was observed in 680mM pH7.4 ChCl and NaCl (Figure 1A–B). At 65°C only 72±5.6%, and 62.5±3.6% of the original amount of rhIL-2 remained able to bind in a sandwich ELISA in the presence of ChCl and NaCl formulations respectively (Figure 1A–B, p<0.01 versus 60°C, n=9 from three independent experiments). A similar loss in rhIL-2 binding activity was observed at 65°C in PBS 0.1% BSA control solution (66±4.7%). This effect was further magnified at increasing temperatures where ≈20% further loss occurred per 5°C increment in temperature increase in control, ChCl, and NaCl formulations. In 680mM ph7.4 NaDHP and CDHP, 83.6±1.3% and 89.1±2.1% of rhIL-2 maintained its binding ability at 65°C, respectively (Figure 1C–D, p<0.01 versus 60°C, =9 from three independent experiments). Furthermore, subsequent 5°C increases in temperature resulted in ≈10% further decreases in intact rhIL-2 detected (Figure 1C–D). Collectively these studies demonstrate that thermal stability imparted by 680mM pH7.4 CDHP and NaDHP translates into better retention of rhIL-2 structural integrity at temperatures up to 23.3°C beyond Tm (Figure 1C–D; 19.7±2.8% rhIL-2 incubated alone versus 45.7±3.9% in NaDHP and 46.8±1.6% in CDHP formulation at 85°C, n=9 from three independent experiments).
Near-isosmotic CDHP (pH≥7.2) is not cytotoxic to primary splenocyte cultures
Depending on the site and mode of in vivo delivery of a CDHP containing formulation, chronic cellular exposure levels to CDHP could vary considerably. We investigated the effect of CDHP on primary splenocyte cultures exposed to 0–80mM CDHP concentrations in complete RPMI culture medium. Under these conditions, relatively low levels of CDHP (10mM) led to a significant decrease in complete RPMI medium pH from 7.3 to 7.0 (Figure 2A, p<0.05, n=6 from two independent experiments), effects exacerbated by increasing levels of CDHP (Figure 2A). At CDHP levels >30mM medium pH was 6.7, a level that proved cytotoxic to primary splenocyte cultures. To counter the effects of CDHP on culture medium pH, sodium bicarbonate (NaHCO3) was added to adjust and maintain medium pH at or above 7.2 (Figure 2A).
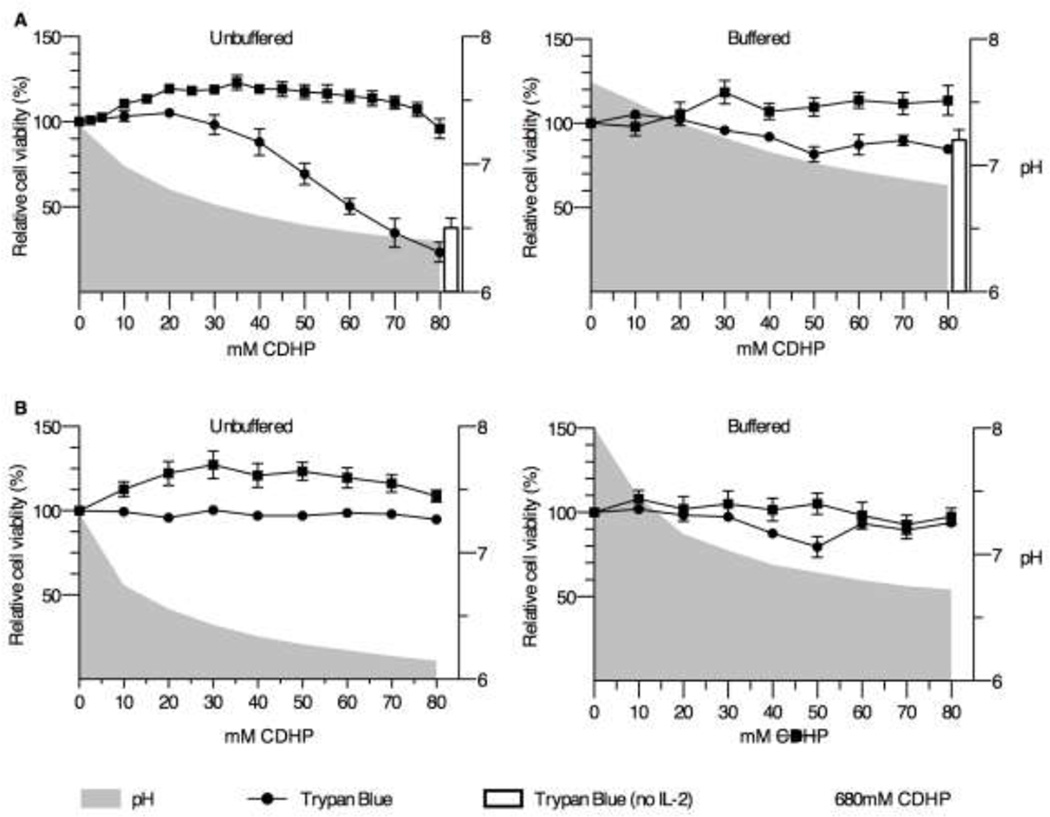
Figure 2. near-isosmotic CDHP is not cytotoxic when buffered in vitro.
Primary splenocyte culture (A) and B16F10 cell line (B) viability, after 24 hours of chronic exposure to 0–80mM CDHP (left panel), or CDHP in NaHCO3 (pH≥7.2) (right panel), as measured by trypan blue exclusion (circle) and resazurin reduction (square) assays. The white bars indicate splenocyte viability culture in IL-2-free media containing 80mM CDHP. Data are expressed as mean relative cell viability, normalized to no CDHP control samples ±SEM, and compared to culture media pH (grey area). n=6 for splenocytes and n=9 for B16F10 cells.
To determine whether cytotoxicity was due to changes in pH, as opposed to CDHP per se, splenocyte viability was determined by trypan blue exclusion following chronic in vitro exposure to 0–80mM CDHP, or CDHP supplemented with NaHCO3 (pH≥7.2). In the absence of pH buffering, relative splenocyte viability significantly declined at 40mM CDHP (Figure 2A; 88±7.8% relative viability at 40mM CDHP, p<0.05 versus no CDHP; n=6 from two independent experiments). At the upper level of 80mM CDHP, relative cell viability decreased to 37.7±8.2%. For splenocytes to remain viable in culture for at least 24h, 100 IU rhIL-2 was added to the culture medium, cell viability decreasing by ≈50% in the absence of rhIL-2 (data not shown). A control experiment with 80mM CDHP was performed using rhIL-2-free medium and showed no significant difference between relative cell viability in the presence (37.7%±8.2%) versus absence of rhIL-2 (23.4±5.9%) (Figure 2A). Increasing the buffer capacity with NaHCO3 to maintain physiologic pH levels resulted in relative splenocyte viability approaching 100% after 24h incubation for all concentrations of CDHP used ≤80mM (Figure 2A, right panel).
Of note, the resazurin reduction method of assessing cell viability did not reflect those data obtained for trypan blue exclusion. That is, the resazurin reduction assay, a cell metabolism based assay, demonstrated an increase in relative resazurin reduction at lower CDHP concentrations (10–40mM), followed by a return to baseline at increasing CDHP concentrations (50–80mM), thus exhibiting a hormetic effect. No significant change in relative resazurin reduction was measured for all concentrations of CDHP formulated with NaHCO3, up to 80mM (Figure 2A).
Exposure to buffered, near-isosmotic CDHP is not cytotoxic in B16F10 melanoma cells in vitro
Compared with primary cell cultures, immortalized cancerous cell lines represent a more robust model requiring simpler culture media to sustain growth. To further investigate CDHP biocompatibility in vitro, B16F10 melanoma cells were exposed to near-isosmotic CDHP concentrations (10–80mM) in complete DMEM medium for 24h. Compared to complete RPMI, complete DMEM exhibited weaker inherent buffering capacity, 10mM CDHP being sufficient to lower pH to 6.7, an effect corrected with the addition of NaHCO3 (pH≥7.2) (Figure 2B, n=9 from three independent experiments). Using the trypan blue exclusion assay, no significant changes in B16F10 cell viability were measured when cells were exposed to any of the concentrations of CDHP, or CDHP in NaHCO3 (pH≥7.2) (Figure 2B).
In a similar manner to primary splenocyte cultures, relative resazurin reduction activity did not correlate with trypan blue exclusion data. That is, relative resazurin reduction by B16F10 increased by 12.2±4.5% when incubated with 10mM CDHP, an increase that peaked at 30mM CDHP (27.2±7.1%) before plateauing at 40–70mM CDHP, and returning to baseline at 80mM CDHP (Figure 2B). Resazurin reduction activity did not significantly change for any concentrations of CDHP in NaHCO3 (pH≥7.2) employed (10–80mM) (Figure 2B, n=9 from three independent experiments).
CDHP-dependent changes in resazurin reduction activity are due to metabolic changes, not proliferation
Discrepancies between trypan blue and resazurin assay data suggest the higher resazurin reduction activity by B16F10 cells incubated with CDHP may be due to metabolic changes rather than increased proliferation. To test this, FBS depletion experiments were performed to inhibit cell proliferation while increasing mitochondrial stress/activity.23,24 For these studies B16F10 cells were cultured for 24h in complete DMEM containing either 0.1% or 10% (v/v) FBS, in the absence or presence of 40mM CDHP. Using this approach, decreasing culture medium FBS content to 0.1% (v/v) led to a significant increase in resazurin reduction activity in the absence of CDHP (Figure 3; resofurin fluorescence intensity increased 1.73±0.05 fold, 0.1% versus 10% (v/v) FBS; n=9 from three independent experiments). The addition of 40mM CDHP to the culture medium significantly increased resazurin reduction/resofurin fluorescence intensity in both 0.1 and 10% (v/v) FBS culture conditions compared to pair matched 0mM CDHP analyses (Figure 3, p<0.05 0mM versus 40mM CDHP). However, there was no significant difference in resazurin reduction/resofurin fluorescence intensity between cells maintained in 0.1% versus 10% (v/v) FBS when both were treated with 40mM CDHP (Figure 3).
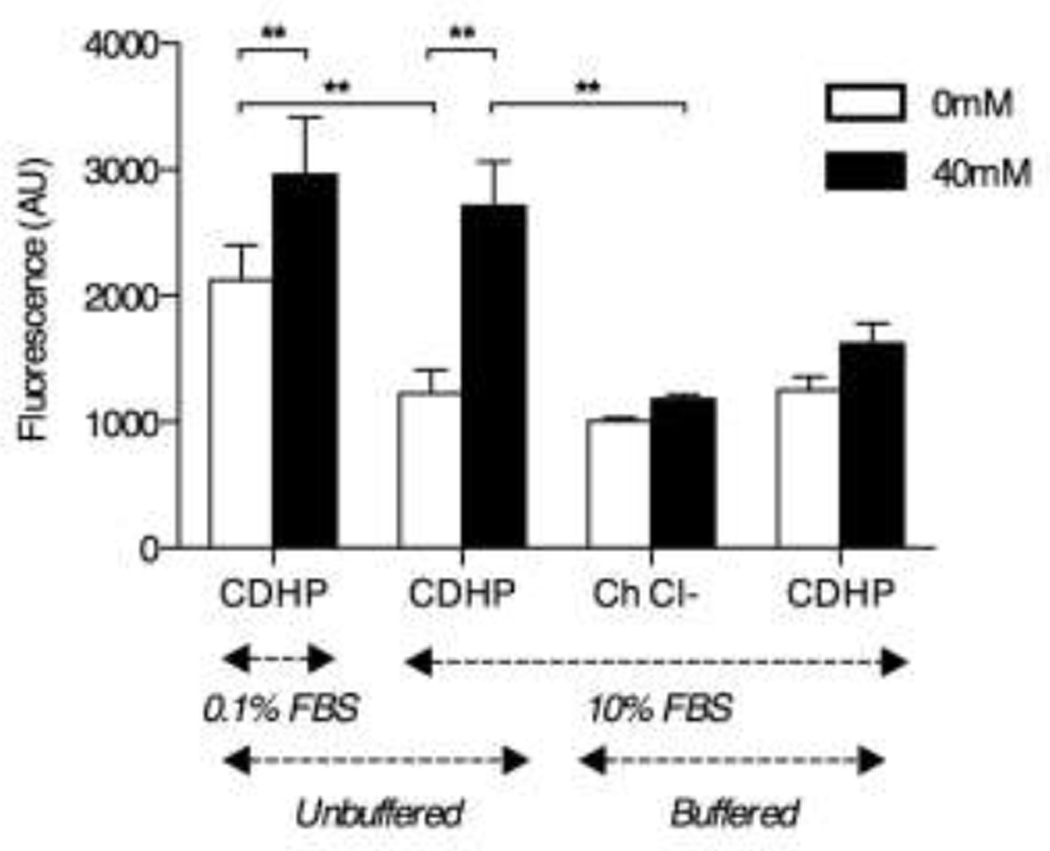
Figure 3. CDHP acidic nature stimulates cell metabolism.
Mitochondrial resazurin reduction activity by B16F10 cell incubated 24 hours with 0mM (white bars) or 40mM CDHP (black bars), in function of media FBS content (0.1% vs. 10% (v/v), salt substitution (CDHP vs. Choline Chloride) and pH control (CDHP vs. CDHP in NaHCO3 (pH≥E;7.2)). Data are expressed as mean absolute resofurin fluorescence ±SEM. n=9; **: p<0.01.
In an attempt to further address the possible effects of CDHP on mitochondrial activity we assessed the specific effect of choline on B16F10 cell metabolism, choline having previously been reported to stimulate cell growth.25 Culturing B16F10 cells in the presence of 40mM choline chloride (ChCl) failed to significantly affect resazurin reduction/resofurin fluorescence intensity as compared to untreated cells (Figure 3, n=9 from three independent experiments). Furthermore, resazurin reduction/resofurin fluorescence intensity was significantly lower in cells treated with ChCl as compared to cells treated with 40mM CDHP (Figure 3, p<0.05). Interestingly while ChCl was pH neutral, previous experiments established that CDHP acidifies cell culture medium (Figure 2B). To confirm the impact of decreasing pH on cell metabolism, B16F10 cells were next cultured in 40mM buffered CDHP in NaHCO3 (pH≥7.2). This approach demonstrated that pH correction was sufficient to decrease resazurin reduction activity to levels not significantly different to that measured in untreated cells or those treated with 40mM choline chloride (Figure 3, n=9 from three independent experiments).
Exposure to buffered CDHP does not impact rhIL-2 structural integrity
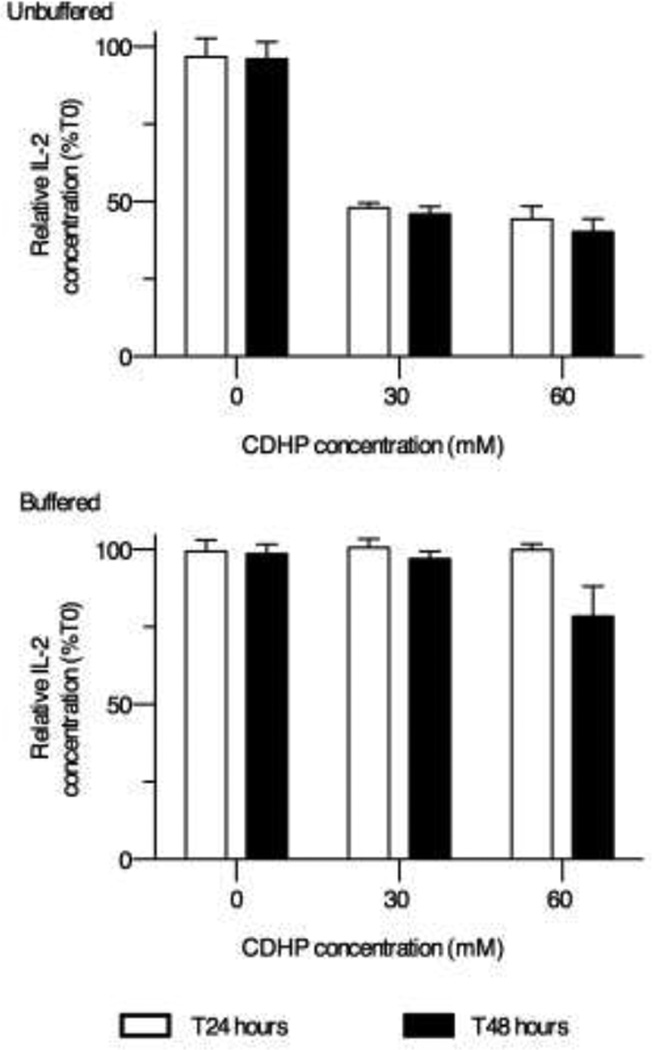
The rhIL-2 used in the previously described experiments, and for the treatment of late stage melanoma, differs from native IL-2 in that it is not glycosylated (Cys125 being replaced by Ser), and it does not possess an N-terminus Ala. While improving protein half-life following injection, these modifications also make the protein more sensitive to acidic environments.13 To determine the effects of prolonged exposure of rhIL-2 to CDHP on structural integrity, rhIL-2 was incubated for 24h or 48h with 30 or 60mM CDHP (with and without NaHCO3) at 37°C under sterile conditions. At the end of this period, culture media samples were flash frozen and rhIL-2 concentration measured by sandwich ELISA. Both the 30mM and 60mM CDHP concentrations significantly decreased the amount of binding-active rhIL-2 remaining after incubation, although no significant difference was detected between the 24h or the 48h time points (Figure 4, n=6 from two independent experiments). However, this effect on rhIL-2 integrity was completely abrogated when NaHCO3 was added to maintain pH≥7.2 (Figure 4).
Figure 4. rhIL-2 remains structurally intact after a lasting exposure to near-isosmotic buffered CDHP.
Sandwich ELISA measurements of rhIL-2 concentrations incubated 24 hours (white bars) and 48 hours (black bars) in 30–60mM CDHP (upper panel), or CDHP in NaHCO3 (pH≥7.2) (lower panel). Data are expressed as mean relative rhIL-2 concentrations ±SEM, normalized to the initial amount (T0) of protein (n=6).
Exposure to buffered CDHP alters rhIL-2 biological activity in vitro
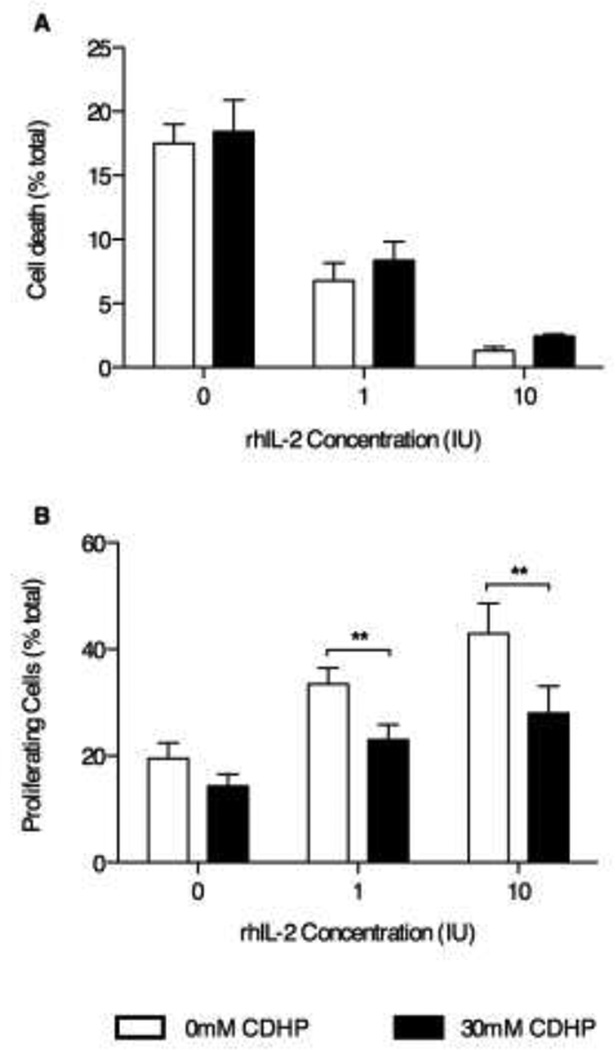
Although structural integrity is typically necessary for biological functionality, it is not always sufficient. Thus, we sought to determine whether CDHP affects rhIL-2 biological activity using the CTLL-2 cell line, a murine cytotoxic T cell line that requires IL-2 to survive and proliferate.22 In the absence of rhIL-2, no significant differences in cell death were measured by PI-staining-flow cytometry between control samples (no CDHP) or samples incubated in 30mM CDHP with NaHCO3 (pH≥7.2) (Figure 5A, 18.4±2.5% and 15.5±1.5% cell death respectively, n=9 from three independent experiments). As expected, increasing the amount of rhIL-2 in cell culture medium significantly reduced CTLL-2 cell death in a dose dependent-manner (Figure 5A). Inclusion of 30mM CDHP (pH≥7.2) did not significantly affect the ability of rhIL-2 to protect against CTLL-2 cell death (Figure 5A).
Figure 5. rhIL-2 activity decreases when formulated in buffered 30mM CDHP.
Flow cytometry analysis of CTLL-2 cell viability (A) and proliferation (B), respectively measured by propidium iodide and carboxyfluorescein succinimidyl ester stainings, after 24 hours stimulation with 1–10IU rhIL-2 in presence (black bars) or absence (white bars) of 30mM CDHP in NaHCO3 (pH≥7.2). Data are expressed as mean percentile of dead or proliferating cells ±SEM, among total cells in each samples. n=9 for both assays.
Using CFSE staining as a marker of cell proliferation, CTLL-2 proliferation was measured by flow cytometry in samples cultured in the absence, or presence, of rhIL-2 (0, 1, or 10 IU/mL) and/or 30mM buffered CDHP in NaHCO3 (pH≥7.2). Using this approach, CDHP (pH≥7.2) did not significantly affect cell proliferation (Figure 5B, n=9 from three independent experiments). Increasing the amount of rhIL-2 in the culture medium significantly increased cell proliferation in the absence, or presence, of buffered CDHP in NaHCO3 (pH≥7.2) (Figure 5B). However, proliferation appeared to be adversely affected by the presence of 30mM CDHP (pH≥7.2) for both the 1 and 10 IU/mL rhIL-2 treatments (Figure 5B, 43±5.7% versus 28±5.1% respectively, p<0.05).
DISCUSSION
The aim of the current study was to investigate the applicability of CDHP as a stabilizing excipient for therapeutic rhIL-2. In aqueous solution proteins exist in an equilibrium state between native (folded) and denatured (unfolded) conformations. The Tm is the temperature at which the ratio between folded:unfolded protein states in solution is 1:1, thus representing an indicator of protein stability. In general, the higher the Tm, the more thermally stable the protein.26 Previous studies, using model proteins, such as lysozyme and cytochrome C, formulated in high CDHP concentrations (>80%wt), report dramatic increases in Tm values for these proteins.2,4 While these high concentration levels may be appropriate for some therapeutic protein storage applications, such formulations are not easily injected without dilution. The current study thus investigated the use of these salts at lower concentrations, which might be directly injectable, or appropiate for continuous drug release devices.
In our previous studies we reported that the Tm of rhIL-2 increased by 12.5°C when formulated in 680mM CDHP pH 7.4.9 This increased thermal stability of rhIL-2 was associated with kinetic instability since rhIL-2 readily aggregated upon unfolding in CDHP formulations (both 185 and 680mM CDHP pH 7.4), a trend similar to that previously reported for rhIL-2 formulated in 5% (w/v) glucose and 10mM sodium acetate at pH 4.0.27 In the current work we demonstrated that at high CDHP concentrations and high temperature (up to 23.3°C above Tm) an overall increase in the binding ability of rhIL-2 was observed, indicating that CDHP exhibits potential as a stabilizing excipient against thermal stresses. This is particularly relevant for cytokines such as rhIL-2, because unlike previously studied model proteins (i.e. lysozyme, cytochrome c), rhIL-2 does not exhibit a reversible 2-state unfolding pathway.9 Once it has thermally denatured and the unfolded protein aggregated, the change is permanent. The protein rhIL-2 will not convert to its native structural conformation upon cooling without a chaperone molecule. Similar increases in thermal stability of rhIL-2 in the presence of 680mM CDHP and NaDHP suggests that in the case of rhIL2 the anion is the main contributor to the stabilizing effect. While previous studies have shown that salts containing a kosmotropic anion and chaotropic anion are usually optimal for stabilizing proteins7, 28, in the case of rhIL-2 for the salts studied here the stabilizing effect is clearly dominated by the kosmotropicity of the anion. As observed with other salts containing kosmotropic anions, high salt concentrations can stabilize proteins against thermal denaturation via non-specific interactions.29–34 Kosmotropic anions are thought to stabilize the native structure of proteins through a ‘salting-out’ effect, decreasing protein solubility, while increasing protein stability.35,36 By excluding water from the surface of the protein, kosmotropic anions encourage water-water interactions, thus shielding the protein from potential denaturing perturbations of bulk water. While phosphate is ranked as a kosmotropic ion, the chloride anion ranks nearly in the middle of the Hoffmeister series between kosmotropic and chaotropic ions.37 This suggests that Cl− has a rather neutral role in affecting protein solubility and stability.35 Thus, the salt formulations containing a strong kosmotropic anion (CDHP, NaDHP) in this study are better positioned to prevent thermal denaturation than those with a weakly kosmotropic or weakly chaotropic anion (such as NaCl or ChCl).
Organic salts have been observed to increase the surface tension of solvents in direct proportion to their concentration in solution. Thermal stability, as measured by an increase in Tm, has been shown to directly correlate with the surface tension of organic salt solutions for a range of proteins, with variations between proteins attributed to differences in their physico-chemical properties.32 The increased solvent surface tension preferentially stabilizes the folded protein conformation over the denatured one, which has a much higher surface area than the folded state.38 We have observed a concentration dependent effect on rhIL2 thermal stability, suggesting that surface tension effects may also be playing a role in the stabilizing effect of CDHP. It should be noted that because the phosphate containing salts require the addition of some sodium hydroxide to increase the pH, the ionic strength of these solutions are likely marginally higher than the chloride containing solutions, but the similarity in the osmolality of these solutions would suggest that this difference would be modest. Ionic strength can certainly play a part in protein stability39, but in the absence of detailed studies on the speciation of ions in solution it is difficult to uncouple ionic strength as a stabilization effect in the current study. It is likely that the charge-shielding effects of high ionic strength40, together with stabilizing increases in surface tension are both contributing to some degree to increases in thermal stability.However, the dramatic difference in the thermal stability observed between solutions containing different anions would suggest that the anion kosmotropicity is the dominant stabilizing effect.
Unlike previous studies using model proteins4,7, data from the studies herein provide evidence of protein activity changes at CDHP concentrations as low as 30mM, which led to decreased rhIL-2-induced CTLL-2 cell proliferation in vitro. As opposed to lysozyme, interleukin-2 has no “inherent” biological activity and does not catalyze enzymatic reactions. Rather, IL-2 binds to a high-affinity receptor consisting of three subunits: IL-2Ra (p55), IL-2Rβ (p75) and γc (p64), expressed by specific immune cell subsets, which mediate IL-2 activity.41 To survive and proliferate, CTLL-2 cells require IL-2 to interact with the IL-2 receptor and, as a result, CTLL-2 cells are commonly used to assess IL-2 activity/stability.22 Therefore the diminished ability of rhIL-2 formulated in 20mM CDHP to trigger CTLL-2 cell proliferation without affecting survival, could be due to modifications in the tertiary structure of the protein. Interestingly though, ELISA data demonstrated that rhIL-2 formulated in buffered CDHP in NaHCO3 (pH≥7.2) shows no signs of structural integrity disruption. To be detected by a sandwich ELISA, two conformational motifs have to be recognized by two separated monoclonal antibodies, thus providing indirect evidence of tertiary protein structure integrity. In addition, previous CD scan analysis performed on rhIL-2 formulated in CDHP (680mM, pH 7.4) demonstrated no evidence of disruption of IL-2 tertiary structure.9 Alternatively, the loss of rhIL-2 functionality, when formulated with CDHP, could be explained by an increase in protein stability. While the parameters that define thermal stability (improved hydrogen bonding, hydrophobic packing, Van der Waals interactions) may interact to create a more conformationally robust protein, they can equally restrict conformational mobility to limit protein functionality.42 The three IL-2R subunits are not preformed as stable heterotrimers. Interleukin-2 itself drives the assembly of the high affinity trimetric receptor. It is first captured by IL-2Rα through a large hydrophobic binding surface surrounded by a polar periphery that results in a relatively weak interaction (Kd 10−8M).43 The IL-2Rα-IL-2 binary complex leads to conformational change in IL-2 that promotes association with IL-2Rβ through a distinct polar interaction between IL-2 and IL-2Rβ. The extracellular domain of IL-2Rα complex does not directly interact with IL-2Rβ, instead the binary complex IL-2Rα-IL-2 presents cis IL-2 to IL-2Rβ forming a ternary structure with intermediate stability (Kd 10−9M). The IL-2Rα-IL-2Rβ-IL-2 complex then recruits γc through a weak interaction with IL-2 and a stronger interaction with IL-2Rβ to produce a stable quaternary high affinity IL-2R (Kd 10−11M).43 While our study focused on rhIL-2 structural integrity, we cannot rule out that CDHP also intervenes in the assembly of the high affinity, three-unit IL-2R, impacting rhIL-2 signaling in CTLL-2 cell chronically exposed to CDHP.
These data are of particular interest given that rhIL-2 is a relatively unstable protein compared to lysozyme (an antibacterial glycoside hydrolase secreted in saliva and mucus) or cytochrome C (a heme protein component of the mitochondrial electron transport chain), which are robust proteins reported to retain functionality when exposed to acidic conditions or reactive oxygen species.44,45 By contrast, IL-2 is a cytokine that is physiologically secreted into the buffered and thermally stable milieu of the circulation. These fundamental differences in the physiological roles of cytochrome C, lysozyme, and rhIL-2 may explain the higher functional sensitivity of rhIL-2 despite similarities in molecular weight [14.7kDa (cytochrome C), 12kDa (lysozyme), and 15.5kDa (rhIL-2)]. Maintaining the pH at near physiologic levels was important for maintaining the structural integrity of rhIL-2, however when IL-2-dependent cells were treated with pH controlled CDHP based formulations, activity levels were diminished.
In order to differentiate effects of CDHP on cells compared to protein, and to better understand potential biocompatibility problems with CDHP formulations, chronic exposure tests were performed on several cells systems. The authors have previously reported an EC50 value (effective concentration that causes cell death in 50% of the population after 48h of exposure) of 20mM for CDHP.46 The goal of the prior studies was to achieve a rank ordering of relative toxicity of choline salts upon chronic exposure (48h) to a specific cell type (macrophages). In the current work, we also examined relative toxicity to other cell types (primary splenocytes and B16F10 cells) and examined these toxic effects in greater detail. By modulating pH, cells tolerated chronic exposure (24–48 hours) to CDHP at levels ≤80mM level, a concentration resulting in a moderately hypertonic solution. While these results cannot be directly extrapolated to predict in vivo behavior, or response to higher-level acute exposure levels, the results suggest that CDHP appears to be as benign as other dissociating physiological salts under typical culture conditions. Extending toxicity testing to higher levels of chronic exposure would have resulted in excessive osmotic stress to cells, and would not have revealed any further direct toxicity information about CDHP. These data suggest that if appropriately defined solution osmolarities can be achieved, there is no immediate cellular toxicity. Ideally an intravenously administered medication should have an osmolality near physiological levels (300mOsm/L) and no more than 600mOsm/L to prevent inflammation, although rate of infusion must also be considered.47 For example in an in vivo experiment by Kuwahara, et al., venous epithelial cells tolerated osmolarities of 820mOsm/kg for 8h, 690mOsm/kg for 12h, and 550mOsm/kg for 24h of a nutritional solution.48 Similarly, an intramuscularly administered volume of 0.5ml of 300–1100mOsm/kg solution has been reported to be well tolerated.49 Thus the concentrations that we studied reveal the potential for realizing a beneficial protein stabilization effect at concentrations that are theoretically injectable, or which could be employed in controlled releases devices.
Utilization of different cells enabled a more comprehensive understanding of the specific effects of CDHP on biological systems. Cell lines such as the B16F10 melanoma or J774 macrophages 4 are transformed, immortalized cells capable of surviving relatively adverse conditions such as acidic pHs, limited nutrient availability, and pro-apoptotic signals. Using the B16F10 cell line we report a lack of cytotoxicity for CDHP (0–80mM) despite dramatic decreases in culture medium pH (pH<7.0). To further address CDHP biocompatibility, we performed parallel cytotoxicity assays using primary cell cultures of freshly isolated splenocytes. This model presents a more physiologically relevant system (compared to immortalized tumor cells), splenocytes being a mixed population of spleen leucocytes (lymphoid and myeloid origin) that are non-polarized cells that propagate in culture without attaching (to solid surfaces). Using this splenocyte model system, significant cytotoxic activity was detected at levels of CDHP as low as 40mM, when pH was not tightly regulated, but this effect could be countered by increasing the buffer capacity of the media with NaHCO3 supplementation, thus maintaining pH near physiologic levels. Interestingly, cytotoxicity was only detectable in cultured splenocytes by performing direct cell counts (trypan blue exclusion assays), as data from metabolism-based assays (resazurin reduction) did not reflect that of cell count data. Indeed, the metabolic resazurin reduction assays actually demonstrated what, at first analysis, appeared to be increased relative cell viability in the presence of CDHP (10–40mM). These measurements highlight a fundamental feature of metabolism-based methods of measuring cell viability (e.g. resazurin, MTT); early cell death events can be masked by increased cellular metabolism. While this feature can lead to an overestimation of cell survival, it also provides the opportunity to quantify cell stress via changes in metabolic activity following chronic exposure to non-cytotoxic (as assessed by trypan blue exclusion) levels of CDHP, data confirmed by FBS depletion studies.23,24 Collectively these data demonstrate that at sub-cytotoxic CDHP levels (0–40mM), CDHP significantly increased cell stress, a factor that was effectively countered by increasing the buffer capacity with NaHCO3 to maintain a physiologic pH in the solutions.
In conclusion, we have previously reported that CDHP increases the thermal stability of the therapeutic protein rhIL-2. The current study investigated the applicability of CDHP as a stabilizing excipient for therapeutic rhIL-2 formulations. The use of CDHP (pH≥7.2) allowed a better retention of rhIL-2 structural integrity at high temperature with no inherent cytotoxicity towards splenocytes and B16F10 melanoma cells up to 80mM. Formulating rhIL-2 with CDHP (pH≥7.2) reduced its biological activity in vitro but the protein retained structurally intact and functional binding sites, suggesting that a trade-off might be necessary between thermal stability and biological activity. This trade-off may be acceptable when formulations are expected to experience thermal excursions, such as might occur in the creation of new rhIL-2 delivery devices such as controlled release liposomes and nanoparticles. In the current study the anion provided the dominant stabilizing effect, thus pointing to further opportunities to tune the cation to achieve solution properties that are most desirable for rhIL2 formulation.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grant 1R21EB00740401A2 to GDE and DRM, and by an UNCC-CMC partnership grant to GDE and DMF.
BIBLIOGRAPHY
- 1.Maclean DS, Qian Q, Middaugh CR. Stabilization of proteins by low molecular weight multi-ions. J Pharm Sci. 2002;91(10):2220–2229. doi: 10.1002/jps.10219. [DOI] [PubMed] [Google Scholar]
- 2.Fujita K, Forsyth M, MacFarlane DR, Reid RW, Elliott GD. Unexpected improvement in stability and utility of cytochrome c by solution in biocompatible ionic liquids. Biotechnol Bioeng. 2006;94(6):1209–1213. doi: 10.1002/bit.20928. [DOI] [PubMed] [Google Scholar]
- 3.Byrne N, Wang LM, Belieres JP, Angell CA. Reversible folding-unfolding, aggregation protection, and multi-year stabilization, in high concentration protein solutions, using ionic liquids. Chem Commun (Camb) 2007;26:2714–2716. doi: 10.1039/b618943a. [DOI] [PubMed] [Google Scholar]
- 4.Vrikkis RM, Fraser KJ, Fujita K, Macfarlane DR, Elliott GD. Biocompatible ionic liquids: a new approach for stabilizing proteins in liquid formulation. J Biomech Eng. 2009;131(7):074514. doi: 10.1115/1.3156810. [DOI] [PubMed] [Google Scholar]
- 5.Vijayaraghavan R, Izgorodin A, Ganesh V, Surianarayanan M, MacFarlane DR. Long-term structural and chemical stability of DNA in hydrated ionic liquids. Angew Chem Int Ed Engl. 2010;49(9):1631–1633. doi: 10.1002/anie.200906610. [DOI] [PubMed] [Google Scholar]
- 6.Ru MT, Dordick JS, Reimer JA, Clark DS. Optimizing the salt-induced activation of enzymes in organic solvents: effects of lyophilization time and water content. Biotechnol Bioeng. 1999;63(2):233–241. doi: 10.1002/(sici)1097-0290(19990420)63:2<233::aid-bit12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Fujita K, MacFarlane DR, Forsyth M, Yoshizawa-Fujita M, Murata K, Nakamura N, Ohno H. Solubility and stability of cytochrome c in hydrated ionic liquids: effect of oxo acid residues and kosmotropicity. Biomacromolecules. 2007;8(7):2080–2086. doi: 10.1021/bm070041o. [DOI] [PubMed] [Google Scholar]
- 8.Constatinescu D, Herrmann C, Weingartner H. Patterns of protein unfolding and protein aggregation in ionic liquids. Phys Chem Chem Phys. 2010;12(8):1756–1763. doi: 10.1039/b921037g. [DOI] [PubMed] [Google Scholar]
- 9.Weaver KD, Vrikkis RM, Van Vorst MP, Trullinger J, Vijayaraghavan R, Foureau DM, McKillop IH, Macfarlane DR, Krueger JK, Elliott GD. Structure and function of proteins in hydrated choline dihydrogen phosphate ionic liquid. Phys Chem Chem Phys. 2011;14(2):790–801. doi: 10.1039/c1cp22965f. [DOI] [PubMed] [Google Scholar]
- 10.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14(2):105–110. [PubMed] [Google Scholar]
- 12.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173(2):1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 13.Shaker MA, Younes HM. Interleukin-2: evaluation of routes of administration and current delivery systems in cancer therapy. J Pharm Sci. 2009;98(7):2268–2298. doi: 10.1002/jps.21596. [DOI] [PubMed] [Google Scholar]
- 14.Sparano JA, Fisher RI, Sunderland M, Margolin K, Ernest ML, Sznol M, Atkins MB, Dutcher JP, Micetich KC, Weiss GR, et al. Randomized phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J Clin Oncol. 1993;11(10):1969–1977. doi: 10.1200/JCO.1993.11.10.1969. [DOI] [PubMed] [Google Scholar]
- 15.Geigert J, Solli N, Woehleke P, Vemuri S. Development and shelf-life determination of recombinant interleukin-2 (proleukin) Pharm Biotechnol. 1993;5:249–262. doi: 10.1007/978-1-4899-1236-7_8. [DOI] [PubMed] [Google Scholar]
- 16.Den Otter W, Jacobs JJ, Battermann JJ, Hordijk GJ, Krastev Z, Moiseeva EV, Stewart RJ, Ziekman PG, Koten JW. Local therapy of cancer with free IL-2. Cancer Immunol Immunother. 2008;57(7):931–950. doi: 10.1007/s00262-008-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- 18.Fujita K, MacFarlane DR, Forsyth M. Protein solubilising and stabilising ionic liquids. Chem Commun (Camb) 2005;(38):4804–4806. doi: 10.1039/b508238b. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane DR, Vijayaraghavan R, Ha HN, Izgorodin A, Weaver KD, Elliott GD. Ionic liquid buffers-pH control in ionic liquid systems. Chem Commun (Camb) 2010;46(41):7703–7705. doi: 10.1039/c0cc03089a. [DOI] [PubMed] [Google Scholar]
- 20.Foureau DM, McKillop IH, Jones CP, Amin A, White RL, Salo JC. Skin tumor responsiveness to interleukin-2 treatment and CD8 Foxp3+ T cell expansion in an immunocompetent mouse model. Cancer Immunol Immunother. 2011;60(9):1347–1356. doi: 10.1007/s00262-011-1035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mealey RH, Littke MH, Leib SR, Davis WC, McGuire TC. Failure of low- dose recombinant human IL-2 to support the survival of virus-specific CTL clones infused into severe combined immunodeficient foals: lack of correlation between in vitro activity and in vivo efficacy. Vet Immunol Immunopathol. 2008;121(1–2):8–22. doi: 10.1016/j.vetimm.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto T, He Z, Ma WY, Schmid PC, Bode AM, Yang CS, Dong Z. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 2004;64(9):3344–3349. doi: 10.1158/0008-5472.can-03-3453. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Akagi S, Takahashi S, Onishi A, Hanada H, Pinkert CA. Mitochondrial activity in response to serum starvation in bovine (Bos taurus) cell culture. Cloning Stem Cells. 2002;4(3):223–229. doi: 10.1089/15362300260339502. [DOI] [PubMed] [Google Scholar]
- 25.Miquel K, Pradines A, Terce F, Selmi S, Favre G. Competitive inhibition of choline phosphotransferase by geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis and induces apoptosis in human lung adenocarcinoma A549 cells. J Biol Chem. 1998;273(40):26179–26186. doi: 10.1074/jbc.273.40.26179. [DOI] [PubMed] [Google Scholar]
- 26.Cooper A, Johnson CM, Lakey JH, Nollmann M. Heat does not come in different colours: entropy-enthalpy compensation, free energy windows, quantum confinement, pressure perturbation calorimetry, solvation and the multiple causes of heat capacity effects in biomolecular interactions. Biophys Chem. 2001;93(2–3):215–230. doi: 10.1016/s0301-4622(01)00222-8. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa T, Philo JS, Kita Y. Kinetic and thermodynamic analysis of thermal unfolding of recombinant erythropoietin. Biosci Biotechnol Biochem. 2001;65(6):1321–1327. doi: 10.1271/bbb.65.1321. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Olubajo O, Song Z, Sims AL, Person TE, Lawal RA, Holley LA. Effect of kosmotropicity of ionic liquids on the enzyme stability in aqueous solutions. Bioorganic chemistry. 2006;34(1):15–25. doi: 10.1016/j.bioorg.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Vonhippel PH, Wong KY. NEUTRAL SALTS: THE GENERALITY OF THEIR EFFECTS ON THE STABILITY OF MACROMOLECULAR CONFORMATIONS. Science (New York, N.Y.) 1964;145(3632):577–580. doi: 10.1126/science.145.3632.577. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki M, Yano H, Aoki K. Differential scanning calorimetric studies on bovine serum albumin: II Effects of neutral salts and urea. International Journal of Biological Macromolecules. 1991;13(6):322–328. doi: 10.1016/0141-8130(91)90012-j. [DOI] [PubMed] [Google Scholar]
- 31.Tadeo X, Pons M, Millet O. Influence of the Hofmeister anions on protein stability as studied by thermal denaturation and chemical shift perturbation. Biochemistry. 2007;46(3):917–923. doi: 10.1021/bi0613426. [DOI] [PubMed] [Google Scholar]
- 32.Kaushik JK, Bhat R. A mechanistic analysis of the increase in the thermal stability of proteins in aqueous carboxylic acid salt solutions. Protein science : a publication of the Protein Society. 1999;8(1):222–233. doi: 10.1110/ps.8.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komsa-Penkova R, Koynova R, Kostov G, Tenchov BG. Thermal stability of calf skin collagen type I in salt solutions. Biochimica et biophysica acta. 1996;1297(2):171–181. doi: 10.1016/s0167-4838(96)00092-1. [DOI] [PubMed] [Google Scholar]
- 34.Yamasaki M, Yano H, Aoki K. Differential scanning calorimetric studies on bovine serum albumin: II Effects of neutral salts and urea. Int J Biol Macromol. 1991;13(6):322–328. doi: 10.1016/0141-8130(91)90012-j. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Cremer PS. Interactions between macromolecules and ions: The Hofmeister series. Current opinion in chemical biology. 2006;10(6):658–663. doi: 10.1016/j.cbpa.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Arakawa T, Bhat R, Timasheff SN. Preferential interactions determine protein solubility in three-component solutions: the MgCl2 system. Biochemistry. 1990;29(7):1914–1923. doi: 10.1021/bi00459a036. [DOI] [PubMed] [Google Scholar]
- 37.Hofmeister F. Arch. Exp. Pathol. Pharmacol. 1888;24:247–260. [Google Scholar]
- 38.Tadeo X, Lopez-Mendez B, Castano D, Trigueros T, Millet O. Protein stabilization and the Hofmeister effect: the role of hydrophobic solvation. Biophysical journal. 2009;97(9):2595–2603. doi: 10.1016/j.bpj.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamasaki M, Yano H, Aoki K. Differential scanning calorimetric studies on bovine serum albumin: I. Effects of pH and ionic strength. Int J Biol Macromol. 1990;12(4):263–268. doi: 10.1016/0141-8130(90)90007-w. [DOI] [PubMed] [Google Scholar]
- 40.Miki Y, Kakuyama K, Soda K. Protein stability; optimization of electrostatic contributions by partially neutralizing surface ionic charges. Biosystems. 1997;44(1):69–77. doi: 10.1016/s0303-2647(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 41.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 42.Vogt G, Woell S, Argos P. Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol. 1997;269(4):631–643. doi: 10.1006/jmbi.1997.1042. [DOI] [PubMed] [Google Scholar]
- 43.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33(2):153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proctor VA, Cunningham FE. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit Rev Food Sci Nutr. 1988;26(4):359–395. doi: 10.1080/10408398809527473. [DOI] [PubMed] [Google Scholar]
- 45.Olteanu A, Patel CN, Dedmon MM, Kennedy S, Linhoff MW, Minder CM, Potts PR, Deshmukh M, Pielak GJ. Stability and apoptotic activity of recombinant human cytochrome c. Biochem Biophys Res Commun. 2003;312(3):733–740. doi: 10.1016/j.bbrc.2003.10.182. [DOI] [PubMed] [Google Scholar]
- 46.Weaver KD, Kim HJ, Sun J, MacFarlane DR, Elliott GD. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010;12:507–513. [Google Scholar]
- 47.Hicks RW, Becker SC. An overview of intravenous-related medication administration errors as reported to MEDMARX, a national medication error-reporting program. J Infus Nurs. 2006;29(1):20–27. doi: 10.1097/00129804-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Kuwahara T, Asanami S, Kubo S. Experimental infusion phlebitis: tolerance osmolality of peripheral venous endothelial cells. Nutrition. 1998;14(6):496–501. doi: 10.1016/s0899-9007(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 49.Nony P, Girard P, Chabaud S, Hessel L, Thebault C, Boissel JP. Impact of osmolality on burning sensations during and immediately after intramuscular injection of 0.5 ml of vaccine suspensions in healthy adults. Vaccine. 2001;19(27):3645–3651. doi: 10.1016/s0264-410x(01)00098-6. [DOI] [PubMed] [Google Scholar]