Highlights
-
•
The conjugative transfer mechanism of broad-host-range, Enterococcus sex pheromone and Clostridium plasmids is reviewed.
-
•
Comparisons with Gram-negative type IV secretion systems are presented.
-
•
The current understanding of the unique Streptomyces double stranded DNA transfer mechanism is reviewed.
Abbreviations: T4SS, type IV secretion system; T4S, type IV secretion; G+, Gram-positive; G−, Gram-negative; ss, single stranded; ds, double stranded; PG, peptidoglycan; TMH, trans-membrane helix; TMD, trans-membrane domain; aa, amino acid(s)
Keywords: Conjugative transfer, Plasmid, Type IV secretion, Antibiotic resistance, Enterococcus
Abstract
Bacterial conjugation presents the most important means to spread antibiotic resistance and virulence factors among closely and distantly related bacteria. Conjugative plasmids are the mobile genetic elements mainly responsible for this task. All the genetic information required for the horizontal transmission is encoded on the conjugative plasmids themselves. Two distinct concepts for horizontal plasmid transfer in Gram-positive bacteria exist, the most prominent one transports single stranded plasmid DNA via a multi-protein complex, termed type IV secretion system, across the Gram-positive cell envelope. Type IV secretion systems have been found in virtually all unicellular Gram-positive bacteria, whereas multicellular Streptomycetes seem to have developed a specialized system more closely related to the machinery involved in bacterial cell division and sporulation, which transports double stranded DNA from donor to recipient cells. This review intends to summarize the state of the art of prototype systems belonging to the two distinct concepts; it focuses on protein key players identified so far and gives future directions for research in this emerging field of promiscuous interbacterial transport.
1. Introduction
Horizontal gene transfer is an important driver in evolution, enabling bacteria to acquire new characteristics (Babic et al., 2011; Frost et al., 2005; Ochman et al., 2000; Thomas and Nielsen, 2005; Wozniak and Waldor, 2010). During conjugative transfer, DNA translocation across membranes of two cells forming a mating pair is mediated by two types of mobile genetic elements: conjugative plasmids and integrating conjugative elements (ICEs). The majority of conjugative plasmids and ICEs apply a sophisticated protein secretion apparatus, the so-called type IV secretion system (T4SS) to transfer DNA to a recipient cell (Bordeleau et al., 2012). While both types of mobile genetic elements have been found in virtually all bacterial species (Babic et al., 2011; Burrus et al., 2002; Burrus and Waldor, 2004; Heuer and Smalla, 2012; Liu et al., 2013; Popowska and Krawczyk-Balska, 2013; Roberts and Mullany, 2009; Thoma and Muth, 2012; Wozniak and Waldor, 2010), this minireview will mainly focus on conjugative plasmids and their mechanism of interbacterial DNA transfer. The multiprotein T4SS complexes have been reviewed recently in a series of elegant articles focusing either on the mechanistic and functional features (Alvarez-Martinez and Christie, 2009; Bhatty et al., 2013; Zechner et al., 2012) or on the more structural characteristics of these transport complexes (Chandran et al., 2009; Fronzes et al., 2009; Wallden et al., 2010). Furthermore, Guglielmini and coworkers reviewed the evolution of conjugation and T4SSs (Guglielmini et al., 2013). Beside the T4SSs, another type of conjugative DNA translocation machinery exists and appears to be unique to conjugative plasmids and ICEs of the Actinomycetales order, a group of high GC content Gram-positive (G+) bacteria (Bordeleau et al., 2012). This conjugative system is reminiscent of the machinery that enables segregation of chromosomal DNA during bacterial cell division and sporulation and relies on a single FtsK-homolog protein to translocate double stranded (ds) DNA to the recipient cell (Bordeleau et al., 2012; Sepulveda et al., 2011; Vogelmann et al., 2011). Key factors of both translocation systems have been identified and the first models for conjugative plasmid transfer in G+ bacteria via both distinct modes have been presented (Abajy et al., 2007; Bhatty et al., 2013; Grohmann et al., 2003; Li et al., 2013; Sepulveda et al., 2011; Steen et al., 2009).
Interestingly, on basis of the data of several Streptococcus genomes which comprise only subsets of the T4SS building blocks employed by the well-characterized systems (Zhang et al., 2012) the Christie lab presented an interesting new concept on the minimized T4SSs. These systems have evolved from ancestral conjugation systems, but appear to exhibit alternative or additional functions such as effector translocators (Bhatty et al., 2013).
The purpose of this review is to summarize the current state of knowledge of conjugative plasmid transfer in G+ bacteria explaining the distinct concepts as far as understood to date on basis of three prominent model organisms, the enterococci, the Clostridia and the Streptomycetes.
2. Two different conjugative transfer concepts
In G+ bacteria two distinct conjugative concepts have evolved, most likely dependent on the cell structure “on a macro level”, meaning the tendency to form larger cell aggregates reminiscent of multicellular eukaryotic organisms (Grohmann et al., 2003). The vast majority of G+ bacteria seem to conjugate via passage of single stranded (ss) DNA through T4SSs. Nevertheless, these G+ T4SSs appear to be more simply organized than the better characterized G− counterparts. However, multicellular G+ bacteria like Streptomyces seem to employ a completely different mechanism which is more reminiscent of the machinery involved in bacterial cell division or spore formation. Moreover, once a Streptomyces cell has acquired a plasmid molecule it is easily transferred to the neighboring cells via a process called spreading (Brolle et al., 1993; Grohmann et al., 2003; Tiffert et al., 2007).
2.1. ssDNA transfer in unicellular Gram-positive bacteria
Considerable progress on the regulation of conjugative DNA transfer has been obtained through studies on the integrating conjugative element ICEBs1 from Bacillus subtilis, by the Grossman group.
ICEBs1 gene expression was shown to be derepressed in vivo during the RecA-dependent SOS response or when the ICEBs1-encoded cell–cell signaling regulator RapI is present and active (Auchtung et al., 2005). Derepression also requires the antirepressor ImmA, a protease which cleaves ImmR, the ICEBs1 immunity repressor (Bose and Grossman, 2011). ImmA-mediated ImmR cleavage is enhanced by an increase in the specific activity of ImmA (Bose and Grossman, 2011). Induction of ICEBs1 gene expression leads to excision from the chromosome in >90% of the cells, autonomous rolling-circle replication of the ICE, and mating in the presence of appropriate recipients (Auchtung et al., 2005; Lee et al., 2007, 2010; Menard and Grossman, 2013). Recently a transcriptional regulator, Rok, which binds A + T-rich DNA, was shown to repress excision of ICEBs1 from the B. subtilis chromosome (Smits and Grossman, 2010).
The Grossman group has postulated a new mechanism for ICEBs1-mediated plasmid mobilization. ICEBs1 was demonstrated to mobilize plasmids lacking dedicated mobilization functions, namely oriT and relaxase (Lee et al., 2012). B. subtilis cells carrying ICEBs1 transferred three different plasmids, formerly categorized as nonmobilizable, to recipient bacteria at high frequencies (Lee et al., 2012). Plasmid mobilization required ICEBs1 transfer proteins, including the putative coupling protein. In contrast, it did not require the conjugative relaxase or cotransfer of ICEBs1, indicating that the putative coupling protein likely interacts with the plasmid replicative relaxase and directly targets the plasmid DNA to the ICEBs1 conjugation apparatus (Lee et al., 2012).
Conjugative transfer of pLS20, originally isolated from B. subtilis (natto) (Tanaka and Koshikawa, 1977), was shown to be most efficient during the early phase of logarithmic growth (Itaya et al., 2006). Bauer and colleagues investigated the subcellular localization of T4SS proteins encoded by pLS20 (Bauer et al., 2011). VirB1, VirB4, VirB11, VirD2 and VirD4 homologs assembled at a single pole, but also at other sites along the cell membrane, in Bacillus cells from the lag phase of growth. VirB4 and VirD4 interacted at the cell pole and, however, less frequently, at other sites along the membrane (Bauer et al., 2011). VirB1 and VirB11 also colocalized at the cell pole. The plasmid itself was also largely membrane associated and was frequently found at the cell pole, indicating that transfer takes place at the pole, which is the preferred site for the assembly of the active T4SS apparatus. VirD2, VirB4, and VirD4 started to localize to the pole or the membrane in stationary-phase cells. VirB1 and VirB11 were observed as foci in cells resuspended in fresh medium but no longer in cells from the exponential growth phase (Bauer et al., 2011). The data suggest an unusual assembly/disassembly timing for the pLS20 conjugation apparatus and suggest that specific localization of T4SS proteins in lag-phase cells and delocalization during growth are the reason why pLS20 conjugation occurs only at a significant rate during early exponential growth phase (Bauer et al., 2011).
The broad-host-range plasmid pAW63 from Bacillus thuringiensis is the model plasmid studied by the Mahillon group. Potential T4SS components including VirB4, VirB11, and VirD4 homologs were detected on the ∼72-kb plasmid genome (van der Auwera et al., 2005). Van der Auwera and Mahillon investigated the transcriptome of pAW63 using a custom DNA microarray. Gene expression profiles suggested that in the absence of mating partners, a partial “standby mode” was in effect, with little synthesis of most of the putative structural T4SS elements thought to be involved in mating pair formation and DNA transfer, while components of a proposed quorum sensing mechanism were actively expressed (van der Auwera and Mahillon, 2008).
Recently, first mechanistic insights on conjugative plasmid transfer in Rhodococcus were published. Yang and coworkers showed that two megaplasmids, pREA400 and pREA250, from Rhodococcus erythropolis were conjugative. Gene disruption of the putative relaxase gene, traA, in plasmid pREA400 completely abolished transfer to R. erythropolis recipients (Yang et al., 2007). Tripathi and colleagues studied conjugative transfer of an 81-kb plasmid which is essential for virulence and intramacrophage growth of the facultative intracellular pathogen, Rhodococcus equi (Tripathi et al., 2012). The virulence plasmid was transferred to plasmid-free R. equi recipients at high frequencies. Plasmid transfer required cell-to-cell contact and traA, a putative relaxase gene located in the proposed conjugative transfer region of the plasmid (Tripathi et al., 2012). Thus, conjugative DNA translocation in Rhodococcus spp. likely proceeds via a T4S mechanism.
Conjugative transfer in mycobacteria seems to be diverse; distinct mechanisms appear to have evolved in different species. Rabello and colleagues demonstrated conjugative transfer of the 100-kb linear plasmid, pMA100, from Mycobacterium avium to Mycobacterium kansasii as well as to Mycobacterium bovis (Rabello et al., 2012). They postulated that pMA100 contains a protein covalently bound to the 5′ ends, a characteristic of linear plasmids with inverted repeats at their ends such as the linear plasmid pCLP from Mycobacterium celatum (Picardeau and Vincent, 1998). Interestingly, no transconjugants were obtained in mating experiments performed with Mycobacterium smegmatis as the recipient. In contrast to M. avium, M. kansasii and M. bovis, which served as recipients for pMA100, M. smegmatis are rapid-growing mycobacteria. The authors argued that conjugative mechanisms could differ between slow- and rapid-growing mycobacteria (Rabello et al., 2012).
The Derbyshire group presented a putative mechanism for conjugative transfer of chromosomal DNA in rapid-growing M. smegmatis (Parsons et al., 1998). DNA transfer proceeds via ssDNA, it is unidirectional and distinct donor and recipient strains are involved, as is well established for classical conjugative transfer. However, there are clear genetic and mechanistic differences from the prototypical transfer systems encoded on bacterial plasmids (Wang and Derbyshire, 2004): The M. smegmatis chromosome contains multiple cis-acting sites from which transfer can be initiated. This contrasts with the single cis-acting site, oriT, from which plasmid transfer is initiated (Wang et al., 2003, 2005). Analysis of chromosomal DNA of mycobacterial transconjugants revealed that they can acquire different segments of the donor chromosome (i.e., non-contiguous segments of DNA can be transferred), and transconjugants can be readily converted to donors following chromosomal transfer, unlike Hfr transfer in Escherichia coli (Coros et al., 2008). Moreover, Coros and coworkers provided experimental evidence for regulation of conjugative transfer in M. smegmatis by the secretory apparatus ESX-1, a protein secretion apparatus required for virulence in Mycobacterium tuberculosis. They demonstrated that ESX-1 negatively regulates DNA transfer in M. smegmatis donor cells but is essential in recipients (Coros et al., 2008).
The most extensively studied conjugative T4SSs originate from the nosocomial pathogens, Enterococcus faecalis and Enterococcus faecium as well as from clostridia which comprise many anaerobic pathogenic species such as Clostridium perfringens. Insights on mechanistic details on the G+ T4S process are discussed on basis of model plasmids from these microbes.
2.1.1. Conjugative plasmid transfer in Enterococcus
Enterococcal conjugative plasmids establish contact between donor and recipient cells via a sophisticated, tightly controlled system utilizing small peptides, the so-called sex pheromones, which are secreted by potential plasmid recipients. pAD1 and pCF10 are the best characterized sex pheromone-responsive plasmids which are encountered in many E. faecalis strains (Clewell, 2007; Dunny, 2007; Francia et al., 2004; Kozlowicz et al., 2006). For pCF10, conjugative plasmid transfer has been studied in some detail by the Christie and Dunny groups. Upon sensing of peptide pheromone, E. faecalis efficiently transfers plasmid pCF10 through a T4SS to recipient cells (Chen et al., 2008). To date, the 67.6-kb plasmid has been found only in enterococci but it was shown to mobilize transfer of oriT-containing plasmids to Lactococcus lactis and Streptococcus agalactiae (Chen et al., 2008; Staddon et al., 2006). In addition, pCF10 encodes tetracycline resistance and a surface adhesin denominated aggregation substance, which is important both for conjugation and virulence (Chen et al., 2008).
2.1.2. Conjugative plasmid transfer in Clostridia
To date, five toxin plasmids originating from the anaerobic pathogen C. perfringens have been shown experimentally to be conjugative, but virtually all of the large toxin plasmids of C. perfringens carry a tcp (transfer clostridial plasmid) locus, which is very closely related to the tcp conjugative transfer region of pCW3 and therefore is most likely conjugative (Li et al., 2013). The first toxin plasmid shown to be self-transmissible was pMRS4969, a genetically marked derivative of the CPE plasmid pCPF4969 (Brynestad et al., 2001). Plate matings into suitable recipient strains of C. perfringens demonstrated high transfer frequencies in the range of 2.0–4.6 × 10−4 transconjugants per donor cell for plasmid pMRS4969 (Li et al., 2013).
2.1.3. Conjugative transfer of broad-host-range plasmids
Transfer of broad-host-range plasmids is not limited to closely related species. Physical contact between the varying mating partners is thought to be established by plasmid-encoded adhesins (Alvarez-Martinez and Christie, 2009; Bhatty et al., 2013).
One of the most extensively studied broad-host-range plasmids from G+ origin is pIP501, originally isolated from S. agalactiae (Horodniceanu et al., 1979), which is studied in our groups. For the Inc18 plasmid pIP501, self-transfer to virtually all G+ bacteria and additionally to E. coli was shown (Kurenbach et al., 2003). The TraA relaxase from pIP501 has been characterized in some biochemical detail (Kopec et al., 2005; Kurenbach et al., 2002, 2006). In addition to its function as site-specific DNA cleavage enzyme it has been shown to negatively regulate the expression of all T4SS proteins encoded in the trapIP501 operon thereby autoregulating its own expression (Fig. 1). Interestingly, in contrast to most other conjugative T4SSs (de la Cruz et al., 2010; Ragonese et al., 2007; Zechner et al., 2012), up to now no accessory factors required for TraA-mediated oriTpIP501 cleavage have been identified (Kurenbach et al., 2002).
Fig. 1.
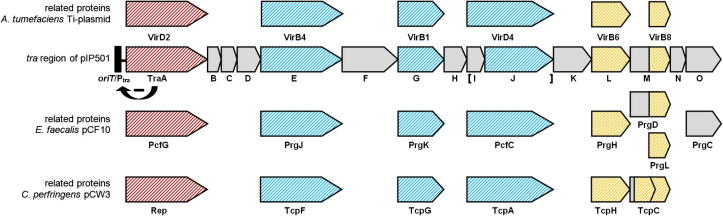
Genetic organization of the pIP501 tra operon. Proteins with sequence similarities to the corresponding A. tumefaciens Ti-plasmid VirB/D4, E. faecalis pCF10 and C. perfringens pCW3 T4SS proteins are colored in blue; the potential two-protein fusion coupling protein (consisting of TraIpIP501 and TraJpIP501) is indicated with brackets; relations based on structure (TraM C-terminal domain – VirB8-like Goessweiner-Mohr et al., 2013) and domain prediction (TraL – VirB6-like) based similarities are marked in yellow; the gene encoding the putative relaxase is colored in red. Ptra, tra operon promoter. The bent arrow with the (-) indicates the TraA-mediated regulation of the expression of all proteins encoded in the trapIP501 operon. The genes of the pIP501 tra operon are drawn to scale.
2.1.4. Gram-positive type IV secretion systems
Members of all major protein families of G− T4SSs have also been detected in the G+ bacteria (Abajy et al., 2007; Alvarez-Martinez and Christie, 2009). T4SSs dedicated to conjugative DNA transfer encode members of an additional family of DNA processing enzymes not present in T4SSs participating in protein effector transport. These relaxases are required for preparation of the ss plasmid DNA molecule which is transferred via the mating pair formation complex (Mpf) constituted by the other T4SS proteins to the recipient cell. The DNA processing reaction in G+ bacteria is mechanistically identical to that in G− bacteria (recently summarized by Zechner et al., 2012).
Protein families present in G− and G+ T4SSs are (i) the energizing component (motor protein) family, (ii) the PG hydrolase family, (iii) the T4SS scaffold/putative core component family and (iv) the surface factor/adhesin family.
2.1.4.1. Protein families involved in ssDNA transfer
2.1.4.1.1. Energizing component (motor protein) family
The pIP501 tra region encodes two putative ATPases (TraE, TraJ) containing the NTP-binding motifs typically found in proteins with ATPase activity. The TraE protein is composed of 653 aa with a total molecular weight of 75.8 kDa. It shows similarities to VirB4 (COG3451) at position 121–570 (GenBank AAA99470, e-value 1.56 e−13) and Walker A and Walker B motifs are predicted at position 251–258 and 510–516, respectively. TraJ consists of 551 aa with a total molecular weight of 63.1 kDa. Similarities to VirD4 (COG3505) are predicted between position 34–442 (GenBank CAD44390, e-value 3.54 e−51) as well as Walker A and Walker B motifs at position 39–46 and 291–295, respectively. For both proteins, ATP binding and hydrolysis activity have been detected (TraE; Celik, E.-K., Abajy, M.Y. et al., unpublished data; TraJ, Celik, E.-K., Guridi, A. et al., unpublished data).
Yeast-two-hybrid and pull-down experiments showed that TraE strongly interacts with itself (Abajy et al., 2007), confirming the formation of oligomers which were predicted based on its relation to T4SS ATPases of G− and G+ origin alike (Dang et al., 1999; Durand et al., 2011; Li et al., 2012; Pena et al., 2012; Wallden et al., 2012). Furthermore, the protein was found to interact with the PG hydrolase TraG and the dsDNA binding, cytosolic protein TraN (Goessweiner-Mohr, N. et al., unpublished data). Recent interaction studies suggest two further candidate proteins for a close interaction with TraE: TraH and TraJ (Goessweiner-Mohr, N. et al., unpublished data). These interactions mark the protein as an important factor in the T4S process, likely driving crucial processes in the course of the conjugative transfer by providing the necessary energy, e.g., for the buildup of the transfer channel and/or the active transport of the substrate.
For TraJ, we postulate that it acts as a T4SS coupling protein in the pIP501 plasmid transfer process, likely aided by TraI which would allow TraJ to locate to the cell membrane through protein–protein interaction (Goessweiner-Mohr, N. et al., unpublished data). If this assumption holds true, TraI and TraJ will comprise a novel two-partner T4SS coupling protein as postulated by Alvarez-Martinez and Christie (2009). Surprisingly, no self-interaction was detected for TraJ (Abajy et al., 2007). It is likely that a stable oligomerization, as reported for related T4SS coupling proteins (Gomis-Rüth et al., 2001, 2004; Haft et al., 2007; Steen et al., 2009; Vecino et al., 2011), requires the presence of TraI. TraJ was found to interact with the relaxase TraA (Abajy et al., 2007), further confirming its putative role as a coupling protein, which links the relaxosomal protein–DNA complex to the actual transfer channel. Moreover, TraJ showed affinity for the double-domain, membrane-spanning protein TraF, for the PG hydrolase TraG (Abajy et al., 2007) and recently binding has been demonstrated to the membrane protein TraH, as well as the cytosolic DNA-binding protein TraN (Goessweiner-Mohr, N. et al., unpublished data).
In agreement with pIP501, the sex-pheromone responsive plasmid pCF10 encodes two proteins with putative ATPase activity, PcfC, a membrane-bound putative ATPase related to the coupling proteins of G− T4SSs (Chen et al., 2008) and PrgJ, a VirB4-like ATPase (Li et al., 2012). A PcfC Walker A NTP binding site mutant fractionated with the E. faecalis membrane and also formed foci, whereas an amino-terminal deletion variant of PfcC lacking the putative TMD distributed uniformly throughout the cytoplasm. PcfC wild type and mutant proteins were shown to bind only the processed form of the pCF10 plasmid in vivo (Chen et al., 2008). Reminiscent of findings in the Agrobacterium tumefaciens T4SS, formation of the pCF10-PcfC formaldehyde cross-link required production of the Dtr factors (the PcfG relaxase and its accessory factor, PcfF) but not the Mpf channel components or ATP hydrolysis by PcfC (Bhatty et al., 2013; Chen et al., 2008). Chen and coworkers postulated that the PcfC coupling protein initiates DNA substrate transfer through the pCF10 T4S channel in an NTP-dependent mode (Chen et al., 2008).
PrgJ is a member of the VirB4 family of ATPases that are found in virtually all T4SSs. Purified PrgJ dimers were demonstrated to bind and hydrolyze ATP (Li et al., 2012). A PrgJ NTP binding site mutation slightly diminished ATP binding but abolished ATP hydrolysis in vitro and blocked pCF10 transfer in vivo. PrgJ wild type and the mutant protein interacted with the coupling protein PcfC and with relaxase PcfG, as well as with the accessory factor PcfF (Li et al., 2012). Moreover, PrgJ and its mutant protein bound ss and dsDNA substrates without sequence specificity in vitro, and in vivo by a mechanism dependent on an intact pCF10 oriT sequence and co-synthesis of the coupling protein (PcfC), the relaxase accessory factor (PcfF) and the relaxase PcfG. Li and coworkers presented a model in which the PcfC coupling protein coordinates with the PrgJ ATPase to drive early steps of pCF10 transfer. In this model, PrgJ catalyzes DNA substrate transfer to the membrane translocase thereby pointing to a novel function of VirB4-type ATPases in mediating early steps of T4S (Li et al., 2012).
The tcp locus of C. perfringens plasmid pCW3 encodes three putative ATPases, the probable FtsK-like coupling protein, TcpA (Parsons et al., 2007), TcpB (a possible gene duplication of TcpA) and TcpF, a Tn916 ORF16-like potential ATPase (Li et al., 2013). TcpA and TcpF were shown to be essential for pCW3 conjugation (Li et al., 2013). Both, TcpA and TcpB are FtsK/SpoIIIE homologs, which is interesting in the light that FtsK domains are found in coupling proteins of G− T4SSs. Mutation and complementation analysis demonstrated that tcpA was essential for pCW3 conjugative transfer and that tcpB was not required (Parsons et al., 2007). Moreover, complementation of a pCW3ΔtcpA mutant with tcpA orthologs from other C. perfringens plasmids, some of them highly differing in their amino acid sequence (54.2–86.6% identity with TcpApCW3) provided evidence that all of the known conjugative plasmids from C. perfringens use a similar transfer mechanism. Functional analysis of the TcpA protein proved the essential role in conjugative transfer of its Walker A and Walker B ATP-binding motifs and its FtsK-like RAAG motif (Parsons et al., 2007). The Rood lab postulated that TcpA is the essential coupling protein encoded by pCW3 and as such represents a key component of the DNA transfer process in C. perfringens (Parsons et al., 2007).
The TcpF protein contains a conserved ATPase domain, is related to ORF16 from the conjugative tetracycline resistance transposon Tn916, and is likely to provide at least some energy required to drive plasmid transfer. It is essential for conjugative transfer, since mutagenesis of tcpF abolishes pCW3 transfer, which can be restored by complementation (Bannam et al., 2006; Li et al., 2013).
2.1.4.1.2. The peptidoglycan hydrolase family
The hydrolases associated with G+ T4SSs differ from prototypical VirB1 from A. tumefaciens, which is secreted to the periplasm and has a single hydrolase domain. The G+ PG hydrolases instead contain an N-terminal TM motif and thus are likely anchored in the membrane (Alvarez-Martinez and Christie, 2009; Bhatty et al., 2013).
An extensive comparison of PG hydrolases of G− and G+ origin led to a new classification based on the secondary structure composition of the respective proteins (Goessweiner-Mohr, N. et al., unpublished data). PG hydrolases originating from G− T4SSs are significantly smaller than their G+ counterparts and contain a single SLT domain. In contrast, hydrolases of G+ origin can be sorted with respect to the size of the proteins. All of the G+ proteins contain an N-terminal TMH, as well as two PG degrading domains (SLT, CHAP). A further group of PG hydrolases was only found in two G+ T4SSs, with the respective proteins being significantly larger and containing a large uncharacterized N-terminal part.
The pCF10 PG hydrolase, PrgK belongs to the latter group of G+ PG hydrolases (Goessweiner-Mohr, N. et al., unpublished data). PrgK is a large, 871 aa protein with an N-proximal TMD and three predicted hydrolase domains of the LytM, gp13/SLT, and CHAP (NlpC/P60) families (Bhatty et al., 2013). Recent studies of the Christie lab have demonstrated that the latter two domains are catalytically active and that only one, but not both of these domains are required for the assembly of a functional T4SS. Deletions or catalytic site mutations of both gp13/SLT and CHAP domains completely abolished pCF10 transfer (Bhatty et al., 2013). As demonstrated for the pIP501 PG hydrolase TraG (Arends et al., 2013), the pCF10-encoded hydrolase is absolutely required for T4SS function (Bhatty et al., 2013).
Bhatty and coworkers suggested that the G+ VirB1 homologs form a crucial part of the T4SS membrane complex: They postulated that the amino-terminal TMD of the PG hydrolase forms part of the membrane translocon and the carboxy-terminal hydrolase domain and the VirB8-like domains (PrgL in the case of pCF10) extend across the cell wall and form a channel or fiber through or along which the secretion substrates pass (Bhatty et al., 2013).
TraG from pIP501 is a 40.4 kDa PG cleavage protein with a total of 369 aa, which has been shown to be indispensable for pIP501 transfer in E. faecalis (Arends et al., 2013). It is the first PG hydrolyzing protein for which indispensability in the T4S process was demonstrated. For TraG, a modular architecture is anticipated: at the N-terminus of the protein a TMH is predicted (position 20–36, GenBank: CAD44387, HMMTOP Tusnady and Simon, 2001), postulated to be required for its proper location in the cell membrane, followed by a specific lytic transglycosylase (SLT) domain at position 80–165 (e-value 3.2 e−4). At the C-terminus (position 243–369, e-value 1.74 e−31) a cysteine, histidine-dependent amidohydrolases/peptidases (CHAP) domain is anticipated. Furthermore, TraG contains N-acetyl-D-glucosamine binding sites within the SLT domain at positions 87, 99, 122, and 144 likely being required for a proper linkage of the protein to its PG substrate. TraG-mediated PG cleavage has been shown in vitro for PG isolated both from E. faecalis and E. coli, thereby underpinning the broad host range of pIP501 transfer (Arends et al., 2013). Moreover, the TraG domains were expressed separately, and both domains, SLT as well as CHAP, degraded PG isolated from the G+ host. We postulate that the SLT domain acts as a lytic transglycosylase as it was efficiently inhibited by the addition of the lytic transglycosylase inhibitors, Bulgecin A and hexa-N-acetylchitohexaose, respectively (Arends et al., 2013). Furthermore, an exchange of the conserved glutamate residue in the putative catalytic center of the SLT domain to glycine (E87G) resulted in almost complete inactivity, which is consistent with this part of TraG being a lytic transglycosylase (Arends et al., 2013).
Besides self-interaction (TraG is postulated to be a dimer), it shows protein–protein interactions with six other pIP501 T4SS proteins, namely with TraB, TraE, TraH, TraI, TraJ and TraN (Abajy et al., 2007, Goessweiner-Mohr, N. et al., unpublished data).
We postulate that TraG locally punches holes in the PG. Through its interactions with other T4SS proteins, particularly with the putative motor protein, TraE, the VirB6 homolog, TraL (Alvarez-Martinez and Christie, 2009; Bhatty et al., 2013), and the putative two-protein fusion coupling protein TraI/TraJ, TraG might recruit these to their proper location in the T4SS complex, speculatively enabling positioning of the T4SS complex at discrete foci on the cell surface (Arends, K. and Grohmann, E., unpublished data). The putative key role of TraG in the DNA transfer process and its numerous interactions with other T4SS components are depicted in the interaction model of the pIP501 transfer components (Fig. 2).
Fig. 2.
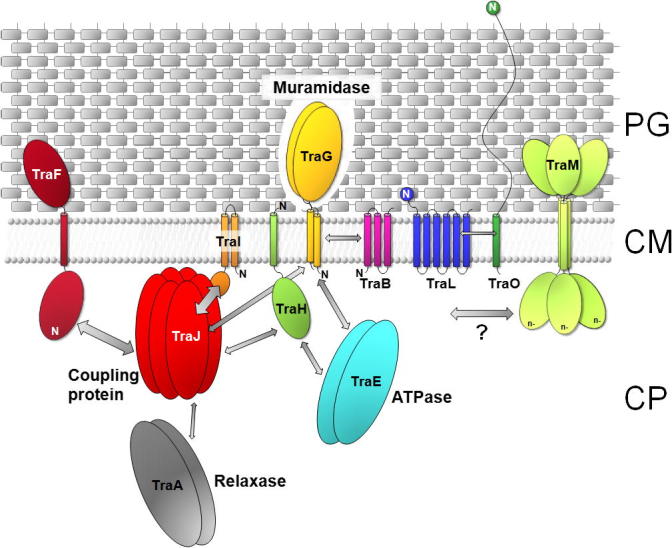
Model of localization and interactions of the pIP501 encoded T4SS proteins. Decreased shading of PG symbolizes TraG-mediated local opening of PG. The localization and orientation of the T4SS proteins is based on in silico predictions and localization studies (Arends et al., 2013; Goessweiner-Mohr et al., 2013). The N-terminus of the transfer proteins is marked (N). Arrows indicate protein–protein interactions determined by yeast-two-hybrid studies and validated by pull-down assays (Abajy et al., 2007), as well as interactions found by using the thermofluor method (Goessweiner-Mohr, N. et al., unpublished data). The VirB8-like T4S protein TraM is marked with a question mark as no protein–protein interactions have been detected so far. The thickness of the arrows marks the strength of the detected interactions. The putative function of key members of the pIP501 tra operon in the DNA secretion process is indicated. PG, peptidoglycan; CM, cytoplasmic membrane; CP, cytoplasm.
Conjugative plasmids from C. perfringens all encode two putative PG hydrolases, TcpG and TcpI, within the conserved tcp locus (Bantwal et al., 2012). Bantwal and coworkers demonstrated that a functional tcpG gene, but not the tcpI gene, was required for efficient transfer of pCW3. Furthermore, it was also shown that each of the two predicted catalytic domains of TcpG (an amino-terminal muramidase-like FlgJ glucosaminidase domain and a carboxy-terminal NlpC/P60 endopeptidase domain) was functional in C. perfringens and that the predicted catalytic glutamic acid, aspartic acid and cysteine residues present in these functional domains were necessary for optimal TcpG function (Bantwal et al., 2012).
In terms of its domain composition and number of aa, TcpG is a model for PG hydrolases of G+ origin (Goessweiner-Mohr, N. et al., unpublished data). The distinct domain composition is shared by proteins from diverse C. perfringens plasmids, as well as transposons and ICEs of enterococcal, streptococcal and Bacillus species. Partially purified TcpG protein was shown to have PG hydrolase-like activity on cognate PG from C. perfringens. The Rood lab suggested that TcpG is a functional PG hydrolase that is required for efficient conjugative transfer of pCW3, presumably by facilitating the penetration of the pCW3 translocation complex through the cell wall (Bantwal et al., 2012).
2.1.4.1.3. The T4SS scaffold/putative core complex component family
Only two of the conserved components of the G+ T4SSs are predicted to localize to the exterior face of the cytoplasmic membrane, the VirB1-like PG hydrolase and the carboxy-terminal domain of the VirB8-like subunits (Bhatty et al., 2013). Together with the polytopic VirB6-like protein, they could form the major constituents of the T4SS channel spanning the G+ cell envelope. Based on findings originated from G- T4SSs the VirB6 homolog could be part of the cytoplasmic translocon/ATPase complex (Bhatty et al., 2013).
For TraL, the putative pIP501 VirB6-homolog, only interactions with the cytoplasmic protein TraN and the putative surface adhesin, TraO, have been demonstrated experimentally so far (Abajy et al., 2007). Nevertheless, due to the fact that VirB6-like proteins are conserved among G− and G+ T4SSs alike, they are supposed to play an important role in the scaffolding or even the makeup of the inner membrane transfer channel, as suggested for G− transfer systems (Jakubowski et al., 2005; Judd et al., 2005a,b; Villamil Giraldo et al., 2012).
An extensive, secondary structure prediction based comparison of VirB6-like proteins of G− and G+ origin showed significant differences between, but also within G− and G+ T4SSs (Goessweiner-Mohr, N. et al., unpublished data). The G+ VirB6-like proteins feature a relatively high variability of their structural motifs and can be grouped according to their length, with proteins showing a length of about 300 aa and other candidates being about four times larger, respectively (Goessweiner-Mohr, N. et al., unpublished data). PrgHpCF10 and TraLpIP501 belong to the group of smaller proteins and resemble the classical VirB6 composition (six predicted TM motifs of similar size) of A. tumefaciens (Alvarez-Martinez and Christie, 2009), whereas TcpHpCW3 belongs to the group of larger VirB6-likes. Similar to the G+ VirB6-like proteins, the respective proteins of G− origin can also be graded, depending on the overall number of aa and the observed secondary structure motifs, as well as the number of the predicted TM motifs (Goessweiner-Mohr, N. et al., unpublished data). The classical VirB6 proteins show only a limited variability and consist of about 295–350 aa. Other proteins possess up to three times the length of the classical VirB6 T4S component (e.g., TraG of the E. coli conjugative plasmids R1 or R100). A last group of G− VirB6-like proteins comprises much shorter candidates (e.g., TrbP of the Bordetella pertussis conjugative plasmid pBP136 or PilU of the E. coli plasmid R64).
The VirB6-like proteins contain up to eight TM motifs with diverse extra- and intracellular domains as linkers between distinct TMHs. While members of the shorter VirB6 protein type only show short linker regions between the individual TMH motifs, large cytoplasmic domains can be found among other VirB6-likes. The exact function of these domains is still not known. Most likely they enable VirB6-like proteins to establish protein–protein interactions or to form stable oligomers, necessary in the buildup of an inner-membrane transfer channel and the subsequent transport of the plasmid DNA (Alvarez-Martinez and Christie, 2009; Cascales and Christie, 2004; Jakubowski et al., 2004).
For the second putative major constituent of the T4SS channel, the VirB8-like proteins, Bhatty and coworkers postulated that they – PrgL in the case of pCF10 – could extend across the cell wall together with the carboxy-terminal domain of the PG hydrolase and generate a channel or fiber for the secretion of the substrate (Bhatty et al., 2013). Interestingly, pCF10 encodes a second VirB8-like protein, which resembles TraMpIP501 regarding its domain composition, a VirB8 class GAMMA protein (Goessweiner-Mohr et al., 2013). The presence of a second VirB8-like protein in the pCF10 T4SS points towards two distinct roles for these proteins, reflected in their diverse structural compositions. Alternatively, one of the proteins might be a remnant of a basic ancestral T4SS, a prototypic conjugation machinery from which the respective G− and G+ T4SSs might have branched off earlier in evolution.
The pIP501 T4SS comprises, in addition to the TraG PG hydrolase, two putative channel components, the VirB8-like protein TraM (Goessweiner-Mohr et al., 2013) and the putative VirB6 homolog TraL (Alvarez-Martinez and Christie, 2009; Bhatty et al., 2013). Even though the highly different domain composition of TraM (Fig. 3) points towards a divergent role in the T4S process (Goessweiner-Mohr et al., 2013), a possible scaffolding role in the secretion complex has been suggested for the protein, based on its surprising structural similarities to the G− VirB8 proteins (Bailey et al., 2006; Terradot et al., 2005) and to TcpC from C. perfringens (Porter et al., 2012). However, we have no definite proof for the key role of TraM in the secretion process as no protein–protein interactions have been detected so far for this protein (Abajy et al., 2007; Goessweiner-Mohr, N. et al., unpublished data). This could be partly due to the fact that all TraM interaction studies have been performed with the soluble variant of TraM, the C-terminal domain TraMΔ, as the full length protein could not be expressed in suitable amounts thus far. It is likely that TraM interactions with other T4SS proteins require the amino-terminal TMD; studies to prove this hypothesis are currently being performed in our laboratories.
Fig. 3.
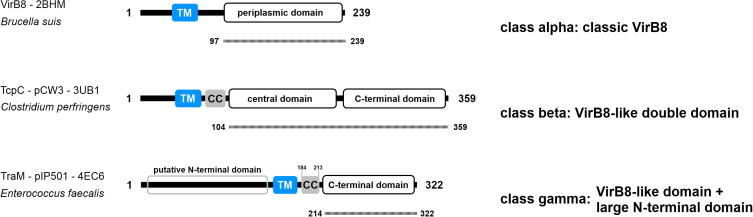
The three VirB8-like protein classes. Class ALPHA proteins (prototype: VirB8 of Brucella suis) contain the classical VirB8 fold with an N-terminal TM motif (blue) and a periplasmic VirB8-like domain. Class BETA proteins (prototype: TcpC of C. perfringens plasmid pCW3) possess an N-terminal TM motif, followed by a potential coiled-coil (CC (grey)) motif and two VirB8-like domains. Class GAMMA proteins (prototype: TraM of E. faecalis plasmid pIP501) contain a large putative N-terminal domain, a central TM motif followed by a potential CC motif and a VirB8-like domain at the C-terminus. The aa sequence present in the available structures is indicated by a dotted line below the individual representations (modified from Goessweiner-Mohr et al., 2013).
The tcp region of pCW3 encodes two potential T4SS scaffold proteins, or in other words two components which could form part of the core complex of the Clostridium DNA transfer machinery, the TcpC protein and the TcpH protein (Li et al., 2013). The 41 kDa TcpC protein is required for efficient conjugative transfer of pCW3, localizes to the cell membrane independently of other conjugative proteins, and its membrane localization was demonstrated to be important for its function, oligomerization and interaction with other conjugative proteins (Porter et al., 2012). The crystal structure of the C-terminal domain of TcpC (TcpC99–359) was determined to 1.8 Å resolution. TcpC99–359 contains two NTF2-like domains separated by a short linker (Porter et al., 2012). Based on its domain composition, TcpC was classified as the model for class BETA VirB8-like proteins. This class comprises transfer proteins originating from C. perfringens conjugative plasmids, as well as transposons and ICEs of clostridial, enterococcal, streptococcal and Bacillus species (Goessweiner-Mohr et al., 2013). Interestingly, the list of TcpC-like proteins contains the same T4SS hits found for the TcpG-like G+ PG hydrolases. As the aa sequences of the TcpC-like proteins of these systems are nearly identical and the same holds true for the TcpG-like proteins, the findings suggest that these T4SS components and their corresponding T4SSs might have coevolved.
Both TcpC domains were shown to be important in the T4S process, as two-hybrid studies revealed that the TcpC C-terminal domain is crucial for protein–protein interactions and the TcpC N-terminal region is required for efficient conjugation, oligomerization and protein–protein interactions (Porter et al., 2012). The Rood lab postulated that by forming oligomeric complexes, TcpC contributes to the stability and integrity of the T4SS apparatus, facilitating efficient pCW3 transfer (Porter et al., 2012).
TcpH is a large 832-aa protein that has similarities to ORF15 from Tn916 and has been demonstrated to be essential for pCW3 transfer (Bannam et al., 2006; Li et al., 2013). It contains an amino-terminal region with eight putative TMDs, including a VirB6-like region, and a putative cytoplasmic carboxy-terminal domain. It is located in the cell envelope of C. perfringens (Li et al., 2013; Teng et al., 2008). Teng and coworkers have shown that the amino-terminal domain, a conserved VQQPW motif, and TMDs 5–8, which include the VirB6-like domain, are essential for TcpH function (Teng et al., 2008). TcpH was shown to interact with itself, the TcpA coupling protein and the VirB8-like TcpC (Teng et al., 2008). The amino-terminal domain, but not the VQQPW motif, is required for these interactions (Teng et al., 2008). The Rood lab proposed that TcpH is the major structural protein of the pCW3 conjugation apparatus (Li et al., 2013). Taking the data on the pCW3 tcp locus together, they suggested a model for the pCW3 conjugation machinery (Li et al., 2013; Porter et al., 2012; Steen et al., 2009).
In this model, the VirB8-like TcpC protein is the scaffolding protein that helps to form a complex at the cell envelope. This complex includes TcpC, the coupling protein TcpA, the PG hydrolase TcpG, and the VirB6-like TcpH, which is proposed to form the cell wall pore through which the DNA is transported into the recipient cell (Li et al., 2013).
However, neither through sequence nor through structural feature-based comparisons relatives for the actual G− channel proteins VirB7, 9, and 10 were detected (Bhatty et al., 2013). Thus, it can be argued that while the periplasmic/outer-membrane channel is missing in G+ T4SSs, the inner-membrane part (VirD4, VirB6 and even VirB8) is still conserved.
2.1.4.1.4. Surface factor/adhesin family
The G− T4SSs generate conjugative pili, but to date no G+ system has been demonstrated to elaborate similar structures. Instead, the G+ systems enable target cell attachment at least partly through the generation of surface adhesins (Bhatty et al., 2013). The best-characterized adhesin is the aggregation substance, PrgB encoded by plasmid pCF10 (Hirt et al., 2005; Olmsted et al., 1991). PrgB is a large approximately 137-kDa protein with homologs found primarily in related pheromone-inducible plasmid transfer systems of enterococci. It possesses an amino-terminal signal sequence, a carboxy-terminal LPxTG cell wall anchor motif, cell adhesion RGD motifs, and a glucan-binding or aggregation domain similar to those of the Streptococcus glucan-binding protein C and surface protein antigen (Spa)-family proteins (Bhatty et al., 2013; Waters and Dunny, 2001; Waters et al., 2003). A prgB mutant was shown to transfer pCF10 at significant frequencies in solid-surface matings, suggesting that other surface adhesins or the secretion channel itself can also promote target cell contacts (Bhatty et al., 2013). Besides PrgB, the pCF10 tra region encodes two other cell wall-anchored surface proteins, PrgA and PrgC (Alvarez-Martinez and Christie, 2009). PrgA is involved in surface exclusion, but it might also participate in the formation of mating junctions (Olmsted et al., 1993).
Based on our current knowledge, the only pIP501 T4SS protein which could act as a surface adhesin is the protein TraO (29.9 kDa, GenBank CAD44395). The protein is composed of 282 aa and a signal peptide is predicted with a putative cleavage site at position 24–25 (SignalP 4.1). An LPxTG cell wall anchor motif (Navarre and Schneewind, 1999) is located at the C-terminal part of the protein (aa 252–256) followed by mainly hydrophobic and positively charged aa and a putative TMH at position 261–278 (HMMTOP). A Pro-rich domain is found at the N-terminus of the protein with a highly repetitive region [(Asp-Pro-Val)7-(Glu-Pro-Thr)37] at position 54–184. The C-terminal part of TraO is therefore most likely anchored to the cell wall via a covalent bond between Thr of the LPxTG motif and the PG mediated by the transpeptidase sortase A, and the N-terminal part of TraO may be exposed to the surface of the enterococcal cell wall (Dawson et al., 2010; Hendrickx et al., 2009). TraO is related to the putative cell wall-anchored surface protein PrgC from pCF10 (Alvarez-Martinez and Christie, 2009). Similar to TraO, PrgC also contains highly repetitive sequence motifs of a 3-residue periodicity comprised of Pro-uncharged-Glu/Asp residues (Alvarez-Martinez and Christie, 2009; Bhatty et al., 2013). Such repeat regions are characteristics of other G+ surface adhesins, such as the IgA-binding proteins of S. agalactiae, and Staphylococcus aureus and streptococcal fibronectin binding proteins (Bhatty et al., 2013; Krishnan and Narayana, 2011). These features make a role of PrgCpCF10 and TraOpIP501 in establishing the contact with bacterial recipient cells likely (Bhatty et al., 2013). Based on preliminary data from the Grohmann lab, deletion of the TraO LPxTG cell wall anchor motif appeared to abolish pIP501 transfer in E. faecalis.
For Clostridium plasmids only scarce information on putative surface factors is available. pCW3 encodes a sortase and Cna, a surface-anchored protein with multiple repeats of a collagen-binding domain. Cna might mediate contacts with bacterial and/or eukaryotic cells, the former possibly enabling the high-frequency transfer rates of this plasmid (Bhatty et al., 2013).
2.2. dsDNA transfer in multicellular Gram-positive bacteria
2.2.1. Conjugative transfer in Streptomyces
Antibiotic-producing soil bacteria of the genus Streptomyces represent a vast natural reservoir of antibiotic resistance genes for the spread of resistance within the soil community. Streptomyces plasmids encode a unique conjugative DNA transfer system clearly distinguished from the classical conjugative transfer systems involving a ssDNA molecule and a T4SS. Only a single plasmid-encoded protein, TraB, is required to translocate a dsDNA molecule into the recipient in Streptomyces matings (Thoma and Muth, 2012). Recently a potentially distinct mode for the transfer of linear ds plasmid DNA versus transfer of related circular ds plasmid DNA in Streptomyces has been suggested (Wang and Pettis, 2010). The potential key players of the classical Streptomyces conjugative transfer mechanism and some indications for a second mechanism involved in transfer of linear plasmids are presented.
2.2.1.1. The classical Streptomyces mode
Streptomycetes feature a unique conjugative DNA transfer system relying on dsDNA translocation, which is carried out by a single plasmid encoded protein, TraB, related to FtsK (Dubarry et al., 2010), mediating chromosome segregation during bacterial cell division (Sepulveda et al., 2011). The first experimental evidence on the distinct mechanism of the Streptomyces conjugative transfer system came from the work of Possoz et al. (2001) demonstrating that conjugation of the Streptomyces ambofaciens plasmid pSAM2 was sensitive to the presence of the SalI restriction/modification system in the recipient, indicating that the transferred incoming DNA must be ds (Possoz et al., 2001). Probably due to the toxic effects of the transfer determinants, plasmid transfer in Streptomyces becomes visible via the formation of so-called pock structures with a diameter of 1–3 mm. Pocks are formed when donor spores germinate on a lawn of a plasmid-free recipient. The pocks represent temporally retarded growth inhibition zones and indicate the area, where the recipient mycelium has acquired a plasmid by conjugation (Thoma and Muth, 2012). Formation of pock structures has been interpreted as the result of intramycelial plasmid spreading via the septal cross-walls of the recipient mycelium (Grohmann et al., 2003; Hopwood and Kieser, 1993; Thoma and Muth, 2012).
In intramycelial plasmid spreading additional plasmid-encoded factors, termed Spd proteins, appear to be involved (Thoma and Muth, 2012). These two protein families, TraB and Spd, and their characteristics will be briefly reviewed.
2.2.1.1.1. TraB is sufficient for dsDNA transfer
Alignments of TraB homologs from various Streptomyces plasmids revealed a highly diverse family of proteins with only very limited sequence similarity (Thoma and Muth, 2012). However, secondary structure predictions showed an identical domain architecture for all TraB homologs resembling that of FtsK: an amino-terminal membrane association domain that is followed by a translocase/ATPase domain with Walker A and B motifs and a carboxy-terminal winged helix-turn-helix fold (Vogelmann et al., 2011). ATPase activity and membrane association have been experimentally proved for TraB proteins of various plasmids (Kosono et al., 1996; Pettis and Cohen, 1996; Reuther et al., 2006a; Thoma and Muth, 2012). Inactivation of the ATP binding site of TraB from the Streptomyces nigrifaciens plasmid pSN22 confirmed that the ATPase activity is essential for conjugative transfer (Kosono et al., 1996).
The TraB protein of the Streptomyces venezuelae plasmid pSVH1 was expressed in Streptomyces lividans and purified (Reuther et al., 2006a). Chemical cross-linking revealed higher oligomeric structures that were also observed when the membrane association domain of TraB was deleted. Ring-shaped TraB particles were detected by electron microscopy. The images revealed symmetric hexamers of approximately 12 nm in diameter containing a central pore (Thoma and Muth, 2012). This structure was in agreement with a predicted TraB-DNA-translocase structure obtained by homology modeling, using the Pseudomonas aeruginosa FtsK translocase domain crystal structure as a template (Vogelmann et al., 2011). Both structures contained a central pore of 3.0 and 3.1 nm, respectively, which is of sufficient width to accommodate a dsDNA molecule (Thoma and Muth, 2012).
Conjugative transfer of DNA by direct cell to cell contact implies that the DNA has to pass the cell envelopes of donor and recipient. In the case of Streptomyces, this means: two cytoplasmic membranes and two PG layers (Thoma and Muth, 2012). PG-binding by TraB was demonstrated by Vogelmann et al. (2011). For the membrane passage, a pore structure has been postulated for TraB (Thoma and Muth, 2012). Studies using planar lipid bilayers demonstrated that TraB spontaneously inserted into the membrane at various voltages and formed pores with an opening time of about 47–81 ms (positive voltage applied) and 105–200 ms, respectively, when a negative voltage was applied (Vogelmann et al., 2011).
Only a small DNA region, termed clt locus, consisting of 8-bp repeats, is required in cis on the plasmid for TraB-mediated DNA transfer (Thoma and Muth, 2012). Gel retardation assays showed a specific interaction of TraB with a plasmid region at the 3′ end of traB, which represents the clt region of pSVH1 (Reuther et al., 2006a). The pSVH1 clt region contains nine imperfectly conserved copies of the GACCCGGA motif. A detailed analysis of this region even detected binding of TraB to a synthetic 20-bp fragment containing only two copies, confirming the GACCCGGA motif as the TraB recognition sequence (Vogelmann et al., 2011). Analysis of other Streptomyces plasmids also detected specific 8-bp repeats in the (predicted) clt regions (Franco et al., 2003; Thoma and Muth, 2012; Vogelmann et al., 2011). Except for two plasmids, the clt locus localizes in all Streptomyces plasmids to the 3′ end of traB, forming a transfer module of only 2.5 kb in size and consisting of traB and its binding site clt next to it (Thoma and Muth, 2012). Gel retardation assays applying covalently closed circular (ccc) DNA as substrate revealed that TraB binds noncovalently to plasmid DNA and that the plasmid DNA molecule was not processed by TraB binding (Reuther et al., 2006a). A model for dsDNA transfer in Streptomyces is shown in Fig. 4 (from Sepulveda et al., 2011).
Fig. 4.
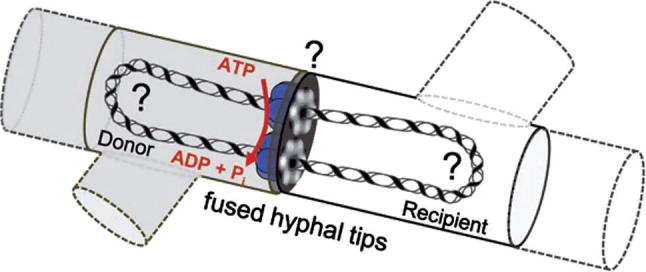
Model of conjugative DNA translocation in mycelial streptomycetes. After establishing contact between donor (grey) and recipient mycelium (white, dashed lines indicate neighboring mycelial compartments) and partial fusion of the hyphal tips, TraB hexamers (blue) assemble at clt by specifically recognizing 8-bp TraB recognition sequence repeats. The TraB hexamers form pore structures to the recipient and direct the translocation of a ds plasmid molecule. DNA transfer is energized by ATP hydrolysis. Up to now it is unclear (?) whether the circular plasmid DNA has to be processed into a linear molecule and which enzymes might be involved (with permission from Sepulveda et al., 2011).
2.2.1.1.2. Intramycelial DNA spread requires plasmid-encoded Spd proteins
The formation of pock structures during Streptomyces conjugation has been interpreted as the results of intramycelial plasmid spreading following the primary DNA transfer event from a donor into the recipient (Grohmann et al., 2003; Hopwood and Kieser, 1993; Thoma and Muth, 2012). Whereas plasmid transfer from a donor into the recipient requires only TraB, plasmid spreading involves five to seven plasmid-encoded Spd proteins in addition to TraB. This likely reflects the challenge to cross the septal cross-walls. The Spd proteins show no significant similarity to any functionally characterized protein complicating prediction of their function (Thoma and Muth, 2012). Inactivation of a single spd gene reduces the size of the pock structures (Kataoka et al., 1994; Kieser et al., 1982; Reuther et al., 2006b; Servin-Gonzalez et al., 1995; Thoma and Muth, 2012). Furthermore, the genetic organization of the spd genes with overlapping stop and start codons and protein–protein interaction studies indicate that the Spd proteins form a multiprotein complex together with TraB (Thoma and Muth, 2012; Tiffert et al., 2007). How this putative multiprotein complex functions in intramycelial plasmid spreading remains to be elucidated.
2.2.1.2. A potential novel linear DNA transfer mechanism in Streptomyces
Wang and Pettis (2010) compared the ability of the Streptomyces plasmid pIJ101 transfer apparatus to promote conjugative transfer of circular versus linear versions of the same replicon (Wang and Pettis, 2010). While the pIJ101 tra locus containing traB and clt readily transferred the circular form of the plasmid, the linear version was transferred orders of magnitude less efficiently and all plasmids isolated from the transconjugants were circular, regardless of their original configuration in the donor (Wang and Pettis, 2010). Moreover, relatively rare circularization of linear plasmids was observed in the donor cells, which is in agreement with the notion that circularization is a prerequisite for transfer mediated by TraBpIJ101. Interestingly, a linear version of the same replicon transferred efficiently from donor strains harboring the conjugative linear plasmid SLP2 (Wang and Pettis, 2010).
Relatively little is known about conjugation of Streptomyces linear plasmids. Some linear plasmids are known to encode a SpoIIIE/FtsK homolog, which in the case of SLP2 enables efficient conjugative transfer of not only linear SLP2 but also its circularized derivatives (Xu et al., 2006). For the transfer of linear plasmids in their natural configuration, an end-first model has been postulated where transfer is initiated from a terminal protein-capped end (Chen, 1996). Whether linear plasmids also contain clt loci is unknown, however, additional functions potentially unique to the transfer of linear molecules have been identified (Bentley et al., 2004; Huang et al., 2003; Xu et al., 2006). These functions include ttrA, a putative helicase gene present in a single copy near the end of the SLP2 genome (Bey et al., 2000; Huang et al., 2003). Huang and coworkers demonstrated that the ttrA gene of SLP2 was important for SLP2 transfer (Huang et al., 2003).
Summing up these data, Wang and coworkers suggested that functions that were sufficient for efficient transfer of circular DNA in Streptomyces were insufficient for effective transfer of linear DNA, strengthening the notion that the conjugation mechanisms of linear versus circular DNA in Streptomyces are inherently different (Wang and Pettis, 2010).
3. Conclusions and perspectives
Since the identification of a novel T4SS, the type-IVC secretion system in Streptococcus in 2012 by Zhang and coworkers, the DNA translocation research in G+ bacteria has obtained a further fascinating perspective in the light that these systems appear to be ubiquitously present in the genomes of important human pathogens belonging to the genus Streptococcus (Zhang et al., 2012). Zhang and coworkers further identified that VirB1, VirB4, VirB6 and VirD4 are the minimal key components of this system. Using genome comparisons and evolutionary relationship analysis, they proposed that the type-IVC secretion system is movable via transposon factors, such as Tn916, and can mediate the conjugative transfer of DNA, enhance bacterial pathogenicity, and could cause large-scale outbreaks of infections in humans (Zhang et al., 2012). Bhatty and coworkers picked up the idea and developed it further to the concept of the minimized T4SSs (Bhatty et al., 2013). The Christie lab found other T4SS genes and surface protein genes adjacent to the virB4-like T4SS signature genes in several other G+ species, namely L. lactis, E. faecalis, E. faecium, S. aureus, C. perfringens, Listeria monocytogenes and Actinomyces naeslundii. These T4SSs were localized on plasmids, ICEs and pathogenicity islands of the respective strains. Adjacent to the virB4 genes in the G+ genomes they typically detected intact T4SS genes encoding proteins similar to VirB1, VirB3, VirB6, and VirD4 (Bhatty et al., 2013).
In a secondary structure prediction based approach, we were able to identify numerous previously undetected proteins, which share the VirB8 fold (Goessweiner-Mohr et al., 2013). Performing a search among uncharacterized T4SSs of G+ origin listed in Bhatty et al. (2013) we were able to identify potential hits for VirB8-like proteins (Table 1). Furthermore, the domain comparison revealed striking differences in the composition of the VirB8-like proteins (Fig. 3). These structural and accordingly functional differences suggest the gradual adaptation of ancestral T4SSs to the requirements of new hosts.
Table 1.
Potential VirB8-like proteins of uncharacterized G+ T4SSs.
| Strain | Localization | Accession number | VirB8-like class |
|---|---|---|---|
| E. faecalis V583 | PAIa | AAM75222.1 | BETA |
| AAM75215.1 | Potential GAMMA | ||
| S. aureus T0131 | Chromosomeb | AEB89692.1 | BETA |
| L. monocytogenes 08-5578 | Chromosomeb | ADB68621.1 | BETA |
| E. faecium E1679 | Chromosomeb | WP_002302456.1 | BETA |
PAI – within pathogenicity island.
Chromosome – not further characterized location on the genome.
The minimized T4SS machines were found to be commonly associated with surface attachment/virulence factors as well as phage immunity systems (Bhatty et al., 2013). This fits well with the observation made by Li et al. (2011), they provided experimental evidence that a minimized T4SS from the Streptococcus suis 89 K pathogenicity island evolved virulence functions resembling those of the effector translocators of G− systems (Bhatty et al., 2013; Li et al., 2011).
Thus, the field is open for further decades of fascinating creative studies on the molecular biology, structural aspects and medical relevance of the emerging G+ T4S machines.
Acknowledgments
This work was supported by grants “Concordia microbial dynamics” and “Biofilms” from BMWI/DLR to E.G. and by a grant from the Austrian Science Foundation (FWF project P19794-B12) to W.K. The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under BioStruct-X (grant agreement No. 283570).
Contributor Information
Nikolaus Goessweiner-Mohr, Email: n.goessweiner.mohr@gmail.com.
Karsten Arends, Email: ArendsK@rki.de.
Walter Keller, Email: walter.keller@uni-graz.at.
Elisabeth Grohmann, Email: elisabeth.grohmann@uniklinik-freiburg.de.
References
- Abajy M.Y., Kopec J., Schiwon K., Burzynski M., Doring M., Bohn C., Grohmann E. A type IV-secretion-like system is required for conjugative DNA transport of broad-host-range plasmid pIP501 in Gram-positive bacteria. J. Bacteriol. 2007;189(6):2487–2496. doi: 10.1128/JB.01491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Martinez C.E., Christie P.J. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 2009;73(4):775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends K., Celik E.-K., Probst I., Goessweiner-Mohr N., Fercher C., Grumet L., Soellue C., Abajy M.Y., Sakin T., Broszat M., Schiwon K., Koraimann G., Keller W., Grohmann E. TraG encoded by the pIP501 type IV secretion system is a two domain peptidoglycan degrading enzyme essential for conjugative transfer. J. Bacteriol. 2013;195(19):4436–4444. doi: 10.1128/JB.02263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchtung J.M., Lee C.A., Monson R.E., Lehman A.P., Grossman A.D. Regulation of a Bacillus subtilis mobile genetic element by intercellular signaling and the global DNA damage response. Proc. Natl. Acad. Sci. USA. 2005;102(35):12554–12559. doi: 10.1073/pnas.0505835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic A., Berkmen M.B., Lee C.A., Grossman A.D. Efficient gene transfer in bacterial cell chains. mBio. 2011;2(2):e00027-11. doi: 10.1128/mBio.00027-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S., Ward D., Middleton R., Grossmann J.G., Zambryski P.C. Agrobacterium tumefaciens VirB8 structure reveals potential protein–protein interaction sites. Proc. Natl. Acad. Sci. USA. 2006;103(8):2582–2587. doi: 10.1073/pnas.0511216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannam T.L., Teng W.L., Bulach D., Lyras D., Rood J.I. Functional identification of conjugation and replication regions of the tetracycline resistance plasmid pCW3 from Clostridium perfringens. J. Bacteriol. 2006;188(13):4942–4951. doi: 10.1128/JB.00298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantwal R., Bannam T.L., Porter C.J., Quinsey N.S., Lyras D., Adams V., Rood J.I. The peptidoglycan hydrolase TcpG is required for efficient conjugative transfer of pCW3 in Clostridium perfringens. Plasmid. 2012;67(2):139–147. doi: 10.1016/j.plasmid.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Bauer T., Rosch T., Itaya M., Graumann P.L. Localization pattern of conjugation machinery in a Gram-positive bacterium. J. Bacteriol. 2011;193(22):6244–6256. doi: 10.1128/JB.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley S.D., Brown S., Murphy L.D., Harris D.E., Quail M.A., Parkhill J., Barrell B.G., McCormick J.R., Santamaria R.I., Losick R., Yamasaki M., Kinashi H., Chen C.W., Chandra G., Jakimowicz D., Kieser H.M., Kieser T., Chater K.F. SCP1, a 356,023 bp linear plasmid adapted to the ecology and developmental biology of its host, Streptomyces coelicolor A3(2) Mol. Microbiol. 2004;51(6):1615–1628. doi: 10.1111/j.1365-2958.2003.03949.x. [DOI] [PubMed] [Google Scholar]
- Bey S.-J., Tsou M.-F., Huang C.-H., Yang C.-C., Chen C.W. The homologous terminal sequence of the Streptomyces lividans chromosome and SLP2 plasmid. Microbiology. 2000;146(4):911–922. doi: 10.1099/00221287-146-4-911. [DOI] [PubMed] [Google Scholar]
- Bhatty M., Laverde Gomez J.A., Christie P.J. The expanding bacterial type IV secretion lexicon. Res. Microbiol. 2013;164(6):620–639. doi: 10.1016/j.resmic.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau E., Ghinet M.G., Burrus V. Diversity of integrating conjugative elements in actinobacteria: coexistence of two mechanistically different DNA-translocation systems. Mob. Genet. Elements. 2012;2(2):119–124. doi: 10.4161/mge.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose B., Grossman A.D. Regulation of horizontal gene transfer in Bacillus subtilis by activation of a conserved site-specific protease. J. Bacteriol. 2011;193(1):22–29. doi: 10.1128/JB.01143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brolle D.-F., Pape H., Hopwood D.A., Kieser T. Analysis of the transfer region of the Streptomyces plasmid SCP2∗. Mol. Microbiol. 1993;10(1):157–170. doi: 10.1111/j.1365-2958.1993.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Brynestad S., Sarker M.R., McClane B.A., Granum P.E., Rood J.I. Enterotoxin plasmid from Clostridium perfringens is conjugative. Infect. Immun. 2001;69(5):3483–3487. doi: 10.1128/IAI.69.5.3483-3487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrus V., Pavlovic G., Decaris B., Guédon G. Conjugative transposons: the tip of the iceberg. Mol. Microbiol. 2002;46(3):601–610. doi: 10.1046/j.1365-2958.2002.03191.x. [DOI] [PubMed] [Google Scholar]
- Burrus V., Waldor M.K. Shaping bacterial genomes with integrative and conjugative elements. Res. Microbiol. 2004;155(5):376–386. doi: 10.1016/j.resmic.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Cascales E., Christie P.J. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science. 2004;304(5674):1170–1173. doi: 10.1126/science.1095211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V., Fronzes R., Duquerroy S., Cronin N., Navaza J., Waksman G. Structure of the outer membrane complex of a type IV secretion system. Nature. 2009;462(7276):1011–1015. doi: 10.1038/nature08588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.W. Complications and implications of linear bacterial chromosomes. Trends Genet. 1996;12(5):192–196. doi: 10.1016/0168-9525(96)30014-0. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang X., Manias D., Yeo H.-J., Dunny G.M., Christie P.J. Enterococcus faecalis PcfC, a spatially localized substrate receptor for type IV secretion of the pCF10 transfer intermediate. J. Bacteriol. 2008;190(10):3632–3645. doi: 10.1128/JB.01999-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D.B. Properties of Enterococcus faecalis plasmid pAD1, a member of a widely disseminated family of pheromone-responding, conjugative, virulence elements encoding cytolysin. Plasmid. 2007;58(3):205–227. doi: 10.1016/j.plasmid.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Coros A., Callahan B., Battaglioli E., Derbyshire K.M. The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol. Microbiol. 2008;69(4):794–808. doi: 10.1111/j.1365-2958.2008.06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang T.A., Zhou X.R., Graf B., Christie P.J. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter. Mol. Microbiol. 1999;32(6):1239–1253. doi: 10.1046/j.1365-2958.1999.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P., Clancy K.W., Melvin J.A., McCafferty D.G. Sortase transpeptidases: insights into mechanism, substrate specificity, and inhibition. Biopolymers. 2010;94(4):385–396. doi: 10.1002/bip.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz F., Frost L.S., Meyer R.J., Zechner E.L. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol. Rev. 2010;34(1):18–40. doi: 10.1111/j.1574-6976.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- Dubarry N., Possoz C., Barre F.-X. Multiple regions along the Escherichia coli FtsK protein are implicated in cell division. Mol. Microbiol. 2010;78(5):1088–1100. doi: 10.1111/j.1365-2958.2010.07412.x. [DOI] [PubMed] [Google Scholar]
- Dunny G.M. The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell–cell signalling, gene transfer, complexity and evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007;362(1483):1185–1193. doi: 10.1098/rstb.2007.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E., Waksman G., Receveur-Brechot V. Structural insights into the membrane-extracted dimeric form of the ATPase TraB from the Escherichia coli pKM101 conjugation system. BMC Struct. Biol. 2011;11(1):4. doi: 10.1186/1472-6807-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia M.V., Varsaki A., Garcillán-Barcia M.P., Latorre A., Drainas C., de La Cruz F. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 2004;28(1):79–100. doi: 10.1016/j.femsre.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Franco B., Gonzalez-Ceron G., Servin-Gonzalez L. Direct repeat sequences are essential for function of the cis-acting locus of transfer (clt) of Streptomyces phaeochromogenes plasmid pJV1. Plasmid. 2003;50(3):242–247. doi: 10.1016/s0147-619x(03)00063-5. [DOI] [PubMed] [Google Scholar]
- Fronzes R., Christie P.J., Waksman G. The structural biology of type IV secretion systems. Nat. Rev. Microbiol. 2009;7(10):703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L.S., Leplae R., Summers A.O., Toussaint A. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 2005;3(9):722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- Goessweiner-Mohr N., Grumet L., Arends K., Pavkov-Keller T., Gruber C.C., Gruber K., Birner-Gruenberger R., Kropec-Huebner A., Huebner J., Grohmann E., Keller W. The 2.5 Å structure of the Enterococcus conjugation protein TraM resembles VirB8 type IV secretion proteins. J. Biol. Chem. 2013;288(3):2018–2028. doi: 10.1074/jbc.M112.428847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth F.X., Moncalían G., de la Cruz F., Coll M. Conjugative plasmid protein TrwB, an integral membrane type IV secretion system coupling protein. J. Biol. Chem. 2001;277(9):7556–7566. doi: 10.1074/jbc.M110462200. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth F.X., Solà M., de la Cruz F., Coll M. Coupling factors in macromolecular type-IV secretion machineries. Curr. Pharm. Des. 2004;10(13):1551–1565. doi: 10.2174/1381612043384817. [DOI] [PubMed] [Google Scholar]
- Grohmann E., Muth G., Espinosa M. Conjugative plasmid transfer in Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003;67(2):277–301. doi: 10.1128/MMBR.67.2.277-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmini J., de La Cruz F., Rocha E.P.C. Evolution of conjugation and type IV secretion systems. Mol. Biol. Evol. 2013;30(2):315–331. doi: 10.1093/molbev/mss221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft R.J.F., Gachelet E.G., Nguyen T., Toussaint L., Chivian D., Traxler B. In vivo oligomerization of the F conjugative coupling protein TraD. J. Bacteriol. 2007;189(18):6626–6634. doi: 10.1128/JB.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx A.P., Willems R.J., Bonten M.J., van Schaik W. LPxTG surface proteins of enterococci. Trends Microbiol. 2009;17(9):423–430. doi: 10.1016/j.tim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Heuer H., Smalla K. Plasmids foster diversification and adaptation of bacterial populations in soil. FEMS Microbiol. Rev. 2012;36(6):1083–1104. doi: 10.1111/j.1574-6976.2012.00337.x. [DOI] [PubMed] [Google Scholar]
- Hirt H., Manias D.A., Bryan E.M., Klein J.R., Marklund J.K., Staddon J.H., Paustian M.L., Kapur V., Dunny G.M. Characterization of the pheromone response of the Enterococcus faecalis conjugative plasmid pCF10: complete sequence and comparative analysis of the transcriptional and phenotypic responses of pCF10-containing cells to pheromone induction. J. Bacteriol. 2005;187(3):1044–1054. doi: 10.1128/JB.187.3.1044-1054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D.A., Kieser T. Conjugative plasmids of Streptomyces. In: Clewell D.B., editor. Bacterial Conjugation. Plenum Press; New York: 1993. pp. 293–311. [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bouanchaud D., Chabbert Y. Conjugative R plasmids in Streptococcus agalactiae (group B) Plasmid. 1979;2(2):197–206. doi: 10.1016/0147-619x(79)90038-6. [DOI] [PubMed] [Google Scholar]
- Huang C.-H., Chen C.-Y., Tsai H.-H., Chen C., Lin Y.-S., Chen C.W. Linear plasmid SLP2 of Streptomyces lividans is a composite replicon. Mol. Microbiol. 2003;47(6):1563–1576. doi: 10.1046/j.1365-2958.2003.03403.x. [DOI] [PubMed] [Google Scholar]
- Itaya M., Sakaya N., Matsunaga S., Fujita K., Kaneko S. Conjugational transfer kinetics of pLS20 between Bacillus subtilis in liquid medium. Biosci. Biotechnol. Biochem. 2006;70(3):740–742. doi: 10.1271/bbb.70.740. [DOI] [PubMed] [Google Scholar]
- Jakubowski S.J., Cascales E., Krishnamoorthy V., Christie P.J. Agrobacterium tumefaciens VirB9, an outer-membrane-associated component of a type IV secretion system, regulates substrate selection and T-Pilus biogenesis. J. Bacteriol. 2005;187(10):3486–3495. doi: 10.1128/JB.187.10.3486-3495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski S.J., Krishnamoorthy V., Cascales E., Christie P.J. Agrobacterium tumefaciens VirB6 domains direct the ordered export of a DNA substrate through a type IV secretion system. J. Mol. Biol. 2004;341(4):961–977. doi: 10.1016/j.jmb.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd P.K., Kumar R.B., Das A. Spatial location and requirements for the assembly of the Agrobacterium tumefaciens type IV secretion apparatus. Proc. Natl. Acad. Sci. USA. 2005;102(32):11498–11503. doi: 10.1073/pnas.0505290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd P.K., Mahli D., Das A. Molecular characterization of the Agrobacterium tumefaciens DNA transfer protein VirB6. Microbiology. 2005;151(11):3483–3492. doi: 10.1099/mic.0.28337-0. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Kiyose Y.M., Michisuji Y., Horiguchi T., Seki T., Yoshida T. Complete nucleotide sequence of the Streptomyces nigrifaciens plasmid, pSN22: genetic organization and correlation with genetic properties. Plasmid. 1994;32(1):55–69. doi: 10.1006/plas.1994.1044. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D.A., Wright H.M., Thompson C.J. PIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol. Gen. Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Kopec J., Bergmann A., Fritz G., Grohmann E., Keller W. TraA and its N-terminal relaxase domain of the Gram-positive plasmid pIP501 show specific oriT binding and behave as dimers in solution. Biochem. J. 2005;387(2):401–409. doi: 10.1042/BJ20041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosono S., Kataoka M., Seki T., Yoshida T. The TraB protein, which mediates the intermycelial transfer of the Streptomyces plasmid pSN22, has functional NTP-binding motifs and is localized to the cytoplasmic membrane. Mol. Microbiol. 1996;19(2):397–405. doi: 10.1046/j.1365-2958.1996.379909.x. [DOI] [PubMed] [Google Scholar]
- Kozlowicz B.K., Shi K., Gu Z.-Y., Ohlendorf D.H., Earhart C.A., Dunny G.M. Molecular basis for control of conjugation by bacterial pheromone and inhibitor peptides. Mol. Microbiol. 2006;62(4):958–969. doi: 10.1111/j.1365-2958.2006.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Narayana S.V. Crystallography of Gram-positive bacterial adhesins. Adv. Exp. Med. Biol. 2011;715:175–195. doi: 10.1007/978-94-007-0940-9_11. [DOI] [PubMed] [Google Scholar]
- Kurenbach B., Bohn C., Prabhu J., Abudukerim M., Szewzyk U., Grohmann E. Intergeneric transfer of the Enterococcus faecalis plasmid pIP501 to Escherichia coli and Streptomyces lividans and sequence analysis of its tra region. Plasmid. 2003;50(1):86–93. doi: 10.1016/s0147-619x(03)00044-1. [DOI] [PubMed] [Google Scholar]
- Kurenbach B., Grothe D., Farias M.E., Szewzyk U., Grohmann E. The tra region of the conjugative plasmid pIP501 is organized in an operon with the first gene encoding the relaxase. J. Bacteriol. 2002;184(6):1801–1805. doi: 10.1128/JB.184.6.1801-1805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenbach B., Kopéc J., Mägdefrau M., Andreas K., Keller W., Bohn C., Abajy M.Y., Grohmann E. The TraA relaxase autoregulates the putative type IV secretion-like system encoded by the broad-host-range Streptococcus agalactiae plasmid pIP501. Microbiology. 2006;152(3):637–645. doi: 10.1099/mic.0.28468-0. [DOI] [PubMed] [Google Scholar]
- Lee C.A., Auchtung J.M., Monson R.E., Grossman A.D. Identification and characterization of int (integrase), xis (excisionase) and chromosomal attachment sites of the integrative and conjugative element ICEBs1 of Bacillus subtilis. Mol. Microbiol. 2007;66(6):1356–1369. doi: 10.1111/j.1365-2958.2007.06000.x. [DOI] [PubMed] [Google Scholar]
- Lee C.A., Babic A., Grossman A.D. Autonomous plasmid-like replication of a conjugative transposon. Mol. Microbiol. 2010;75(2):268–279. doi: 10.1111/j.1365-2958.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.A., Thomas J., Grossman A.D. The Bacillus subtilis conjugative transposon ICEBs1 mobilizes plasmids lacking dedicated mobilization functions. J. Bacteriol. 2012;194(12):3165–3172. doi: 10.1128/JB.00301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Alvarez-Martinez C., Chen Y., Choi K.-J., Yeo H.-J., Christie P.J. Enterococcus faecalis PrgJ, a VirB4-Like ATPase, mediates pCF10 conjugative transfer through substrate binding. J. Bacteriol. 2012;194(15):4041–4051. doi: 10.1128/JB.00648-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Adams V., Bannam T.L., Miyamoto K., Garcia J.P., Uzal F.A., Rood J.I., McClane B.A. Toxin plasmids of Clostridium perfringens. Microbiol. Mol. Biol. Rev. 2013;77(2):208–233. doi: 10.1128/MMBR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Shen X., Yan J., Han H., Zheng B., Liu D., Cheng H., Zhao Y., Rao X., Wang C., Tang J., Hu F., Gao G.F. GI-type T4SS-mediated horizontal transfer of the 89K pathogenicity island in epidemic Streptococcus suis serotype 2. Mol. Microbiol. 2011;79(6):1670–1683. doi: 10.1111/j.1365-2958.2011.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.A., Kwong S.M., Jensen S.O., Brzoska A.J., Firth N. Biology of the staphylococcal conjugative multiresistance plasmid pSK41. Plasmid. 2013;70(1):42–51. doi: 10.1016/j.plasmid.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Menard K.L., Grossman A.D. Selective pressures to maintain attachment site specificity of integrative and conjugative elements. PLoS Genet. 2013;9(7):e1003623. doi: 10.1371/journal.pgen.1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre W.W., Schneewind O. Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999;63(1):74–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Lawrence J.G., Groisman E.A. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405(6784):299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- Olmsted S.B., Erlandsen S.L., Dunny G.M., Wells C.L. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encoded by the pheromone-inducible conjugative plasmid pCF10. J. Bacteriol. 1993;175(19):6229–6237. doi: 10.1128/jb.175.19.6229-6237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted S.B., Kao S.-M., van Putte L.J., Gallo J.C., Dunny G.M. Role of the pheromone-inducible surface protein AsclO in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J. Bacteriol. 1991;173(23):7665–7672. doi: 10.1128/jb.173.23.7665-7672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J.A., Bannam T.L., Devenish R.J., Rood J.I. TcpA, an FtsK/SpoIIIE homolog, is essential for transfer of the conjugative plasmid pCW3 in Clostridium perfringens. J. Bacteriol. 2007;189(21):7782–7790. doi: 10.1128/JB.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L.M., Jankowski C.S., Derbyshire K.M. Conjugal transfer of chromosomal DNA in Mycobacterium smegmatis. Mol. Microbiol. 1998;28(3):571–582. doi: 10.1046/j.1365-2958.1998.00818.x. [DOI] [PubMed] [Google Scholar]
- Pena A., Matilla I., Martin-Benito J., Valpuesta J.M., Carrascosa J.L., de La Cruz F., Cabezon E., Arechaga I. The hexameric structure of a conjugative VirB4 protein ATPase provides new insights for a functional and phylogenetic relationship with DNA translocases. J. Biol. Chem. 2012;287(47):39925–39932. doi: 10.1074/jbc.M112.413849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettis G.S., Cohen S.N. Plasmid transfer and expression of the transfer (tra) gene product of plasmid pIJ101 are temporally regulated during the Streptomyces lividans life cycle. Mol. Microbiol. 1996;19(5):1127–1135. doi: 10.1046/j.1365-2958.1996.493986.x. [DOI] [PubMed] [Google Scholar]
- Picardeau M., Vincent V. Mycobacterial linear plasmids have an invertron-like structure related to other linear replicons in actinomycetes. Microbiology. 1998;144(7):1981–1988. doi: 10.1099/00221287-144-7-1981. [DOI] [PubMed] [Google Scholar]
- Popowska M., Krawczyk-Balska A. Broad-host-range IncP-1 plasmids and their resistance potential. Front. Microbiol. 2013;4 doi: 10.3389/fmicb.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C.J., Bantwal R., Bannam T.L., Rosado C.J., Pearce M.C., Adams V., Lyras D., Whisstock J.C., Rood J.I. The conjugation protein TcpC from Clostridium perfringens is structurally related to the type IV secretion system protein VirB8 from Gram-negative bacteria. Mol. Microbiol. 2012;83(2):275–288. doi: 10.1111/j.1365-2958.2011.07930.x. [DOI] [PubMed] [Google Scholar]
- Possoz C., Ribard C., Gagnat J., Pernodet J.-L., Guérineau M. The integrative element pSAM2 from Streptomyces: kinetics and mode of conjugal transfer. Mol. Microbiol. 2001;42(1):159–166. doi: 10.1046/j.1365-2958.2001.02618.x. [DOI] [PubMed] [Google Scholar]
- Rabello M.C.d.S., Matsumoto C.K., Almeida L.G.P., Menendez M.C., Oliveira R.S., Silva R.M., Garcia M.J., Leão S.C. First description of natural and experimental conjugation between mycobacteria mediated by a linear plasmid. PLoS One. 2012;7(1):e29884. doi: 10.1371/journal.pone.0029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragonese H., Haisch D., Villareal E., Choi J.-H., Matson S.W. The F plasmid-encoded TraM protein stimulates relaxosome-mediated cleavage at oriT through an interaction with TraI. Mol. Microbiol. 2007;63(4):1173–1184. doi: 10.1111/j.1365-2958.2006.05576.x. [DOI] [PubMed] [Google Scholar]
- Reuther J., Gekeler C., Tiffert Y., Wohlleben W., Muth G. Unique conjugation mechanism in mycelial streptomycetes: a DNA-binding ATPase translocates unprocessed plasmid DNA at the hyphal tip. Mol. Microbiol. 2006;61(2):436–446. doi: 10.1111/j.1365-2958.2006.05258.x. [DOI] [PubMed] [Google Scholar]
- Reuther J., Wohlleben W., Muth G. Modular architecture of the conjugative plasmid pSVH1 from Streptomyces venezuelae. Plasmid. 2006;55(3):201–209. doi: 10.1016/j.plasmid.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Roberts A.P., Mullany P. A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol. 2009;17(6):251–258. doi: 10.1016/j.tim.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Sepulveda E., Vogelmann J., Muth G. A septal chromosome segregator protein evolved into a conjugative DNA-translocator protein. Mob. Genet. Elements. 2011;1(3):225–229. doi: 10.4161/mge.1.3.18066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin-Gonzalez L., Sampieri A., Cabello J., Galvan L., Juarez V., Castro C. Sequence and functional analysis of the Streptomyces phaeochromogenes plasmid pJV1 reveals a modular organization of Streptomyces plasmids that replicate by rolling circle. Microbiology. 1995;141(10):2499–2510. doi: 10.1099/13500872-141-10-2499. [DOI] [PubMed] [Google Scholar]
- Smits W.K., Grossman A.D. The transcriptional regulator Rok binds A+T-rich DNA and is involved in repression of a mobile genetic element in Bacillus subtilis. PLoS Genet. 2010;6(11):e1001207. doi: 10.1371/journal.pgen.1001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon J.H., Bryan E.M., Manias D.A., Chen Y., Dunny G.M. Genetic characterization of the conjugative DNA processing system of enterococcal plasmid pCF10. Plasmid. 2006;56(2):102–111. doi: 10.1016/j.plasmid.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen J.A., Bannam T.L., Teng W.L., Devenish R.J., Rood J.I. The putative coupling protein TcpA interacts with other pCW3-encoded proteins to form an essential part of the conjugation complex. J. Bacteriol. 2009;191(9):2926–2933. doi: 10.1128/JB.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Koshikawa T. Isolation and characterization of four types of plasmids from Bacillus subtilis (natto) J. Bacteriol. 1977;131(2):699–701. doi: 10.1128/jb.131.2.699-701.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng W.L., Bannam T.L., Parsons J.A., Rood J.I. Functional characterization and localization of the TcpH conjugation protein from Clostridium perfringens. J. Bacteriol. 2008;190(14):5075–5086. doi: 10.1128/JB.00386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradot L., Bayliss R., Oomen C., Leonard G.A., Baron C., Waksman G. Structures of two core subunits of the bacterial type IV secretion system, VirB8 from Brucella suis and ComB10 from Helicobacter pylori. Proc. Natl. Acad. Sci. USA. 2005;102(12):4596–4601. doi: 10.1073/pnas.0408927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma L., Muth G. Conjugative DNA transfer in Streptomyces. FEMS Microbiol. Lett. 2012;337(2):81–88. doi: 10.1111/1574-6968.12031. [DOI] [PubMed] [Google Scholar]
- Thomas C.M., Nielsen K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005;3(9):711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- Tiffert Y., Gotz B., Reuther J., Wohlleben W., Muth G. Conjugative DNA transfer in Streptomyces: SpdB2 involved in the intramycelial spreading of plasmid pSVH1 is an oligomeric integral membrane protein that binds to dsDNA. Microbiology. 2007;153(9):2976–2983. doi: 10.1099/mic.0.2006/005413-0. [DOI] [PubMed] [Google Scholar]
- Tripathi V.N., Harding W.C., Willingham-Lane J.M., Hondalus M.K. Conjugal transfer of a virulence plasmid in the opportunistic intracellular actinomycete Rhodococcus equi. J. Bacteriol. 2012;194(24):6790–6801. doi: 10.1128/JB.01210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusnady G.E., Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17(9):849–850. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- van der Auwera G.A., Andrup L., Mahillon J. Conjugative plasmid pAW63 brings new insights into the genesis of the Bacillus anthracis virulence plasmid pXO2 and of the Bacillus thuringiensis plasmid pBT9727. BMC Genomics. 2005;6(1):103. doi: 10.1186/1471-2164-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Auwera G.A., Mahillon J. Transcriptional analysis of the conjugative plasmid pAW63 from Bacillus thuringiensis. Plasmid. 2008;60(3):190–199. doi: 10.1016/j.plasmid.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Vecino A.J., de La Arada I., Segura R.L., Goñi F.M., de la Cruz F., Arrondo J.L., Alkorta I. Membrane insertion stabilizes the structure of TrwB, the R388 conjugative plasmid coupling protein. Biochim. Biophys. Acta, Biomembr. 2011;1808(4):1032–1039. doi: 10.1016/j.bbamem.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Villamil Giraldo A.M., Sivanesan D., Carle A., Paschos A., Smith M.A., Plesa M., Coulton J., Baron C. Type IV secretion system core component VirB8 from Brucella binds to the globular domain of VirB5 and to a periplasmic domain of VirB6. Biochemistry. 2012;51(18):3881–3890. doi: 10.1021/bi300298v. [DOI] [PubMed] [Google Scholar]
- Vogelmann J., Ammelburg M., Finger C., Guezguez J., Linke D., Flötenmeyer M., Stierhof Y.-D., Wohlleben W., Muth G. Conjugal plasmid transfer in Streptomyces resembles bacterial chromosome segregation by FtsK/SpoIIIE. EMBO J. 2011;30(11):2246–2254. doi: 10.1038/emboj.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallden K., Rivera-Calzada A., Waksman G. Microreview: type IV secretion systems: versatility and diversity in function. Cell. Microbiol. 2010;12(9):1203–1212. doi: 10.1111/j.1462-5822.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallden K., Williams R., Yan J., Lian P.W., Wang L., Thalassinos K., Orlova E.V., Waksman G. Structure of the VirB4 ATPase, alone and bound to the core complex of a type IV secretion system. Proc. Natl. Acad. Sci. USA. 2012;109(28):11348–11353. doi: 10.1073/pnas.1201428109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Derbyshire K.M. Plasmid DNA transfer in Mycobacterium smegmatis involves novel DNA rearrangements in the recipient, which can be exploited for molecular genetic studies. Mol. Microbiol. 2004;53(4):1233–1241. doi: 10.1111/j.1365-2958.2004.04201.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Karnati P.K., Takacs C.M., Kowalski J.C., Derbyshire K.M. Chromosomal DNA transfer in Mycobacterium smegmatis is mechanistically different from classical Hfr chromosomal DNA transfer. Mol. Microbiol. 2005;58(1):280–288. doi: 10.1111/j.1365-2958.2005.04824.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Parsons L.M., Derbyshire K.M. Unconventional conjugal DNA transfer in mycobacteria. Nat. Genet. 2003;34(1):80–84. doi: 10.1038/ng1139. [DOI] [PubMed] [Google Scholar]
- Wang J., Pettis G.S. The tra locus of streptomycete plasmid pIJ101 mediates efficient transfer of a circular but not a linear version of the same replicon. Microbiology. 2010;156(9):2723–2733. doi: 10.1099/mic.0.036467-0. [DOI] [PubMed] [Google Scholar]
- Waters C.M., Dunny G.M. Analysis of functional domains of the Enterococcus faecalis pheromone-induced surface protein aggregation substance. J. Bacteriol. 2001;183(19):5659–5667. doi: 10.1128/JB.183.19.5659-5667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters C.M., Wells C.L., Dunny G.M. The aggregation domain of aggregation substance, not the RGD motifs, is critical for efficient internalization by HT-29 enterocytes. Infect. Immun. 2003;71(10):5682–5689. doi: 10.1128/IAI.71.10.5682-5689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak R.A.F., Waldor M.K. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 2010;8(8):552–563. doi: 10.1038/nrmicro2382. [DOI] [PubMed] [Google Scholar]
- Xu M.-X., Zhu Y.-M., Shen M.-J., Jiang W.-H., Zhao G.-P., Qin Z.-J. Characterization of the essential gene components for conjugal transfer of Streptomyces lividans linear plasmid SLP2. Prog. Biochem. Biophys. 2006;33(10):986–993. [Google Scholar]
- Yang J.C., Lessard P.A., Sengupta N., Windsor S.D., O’Brien X.M., Bramucci M., Tomb J.-F., Nagarajan V., Sinskey A.J. TraA is required for megaplasmid conjugation in Rhodococcus erythropolis AN12. Plasmid. 2007;57(1):55–70. doi: 10.1016/j.plasmid.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Zechner E.L., Lang S., Schildbach J.F. Assembly and mechanisms of bacterial type IV secretion machines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1592):1073–1087. doi: 10.1098/rstb.2011.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Rong C., Chen C., Gao G.F., Schlievert P.M. Type-IVC secretion system: a novel subclass of type IV secretion system (T4SS) common existing in Gram-positive genus Streptococcus. PLoS One. 2012;7(10):e46390. doi: 10.1371/journal.pone.0046390. [DOI] [PMC free article] [PubMed] [Google Scholar]


