Abstract
C. elegans, with its invariant cell lineage, provides a powerful model system in which to study signaling-dependent asymmetric cell division. The C. elegans β-catenin-related protein, WRM-1, specifies endoderm at the 4-cell stage during the first cell signaling-induced asymmetric cell division of embryogenesis. During this interaction, Wnt signaling and the cell cycle regulator CDK-1 act together to induce the asymmetric cortical release of WRM-1 at prophase of the EMS cell cycle. Genetic studies suggest that release of WRM-1 unmasks a cortical site that drives EMS spindle rotation onto the polarized axis of the cell, simultaneously making WRM-1 available for nuclear translocation, and downstream signaling to specify endoderm. These studies suggest a general paradigm for how cortical factors like WRM-1 can function at the cell cortex to mask potentially confounding polarity cues, and when released with appropriate cell cycle timing, can also function downstream to define cell fate.
Introduction
WRM-1, pronounced, “worm one,” is a multi-functional protein, involved in numerous cell-signaling events during both embryonic and post-embryonic development. It was the first of four β-catenin homologs identified in C. elegans where it was shown to play a central role in a Wnt signaling event required to specify the fate and polarity of an early blastomere called EMS.1 Originally, WRM-1 was believed to function only in regulating transcriptional events downstream of Wnt signaling, while other transcription-independent signals were believed to control the orientation of EMS cell division. In this review we will discuss the diverse roles that WRM-1 plays in polarity signaling, including recent work that has revealed a role for WRM-1 in regulating the EMS cell division axis.2
An Inductive Asymmetric Cell Division in the Four-Cell Stage Embryo
During C. elegans development, the intestinal lineage is specified through a signal-dependent asymmetric cell division. In the four-cell embryo, a cell–cell interaction between EMS and its posterior sister cell, P2, induces EMS to reorient its mitotic spindle 90° from an initially left-right (l-r) to a final anterior-posterior (a-p) orientation. After division, the anterior descendant of EMS called MS produces primarily mesoderm, while the posterior descendant called E produces the entire endoderm.3-6 In the absence of P2/EMS signaling, endoderm is not specified, and EMS fails to rotate its mitotic spindle, dividing instead with a default l-r orientation and producing two equivalent cells with MS-like cell fates. Over the last decade, a large body of work has begun to uncover a remarkably complex multi-branched and interacting group of signal-transduction pathways, hereafter referred to as P2/EMS signaling, which together specify endoderm and control EMS polarity.7-12
WRM-1/β-catenin Functions at the Crux of P2/EMS Signaling
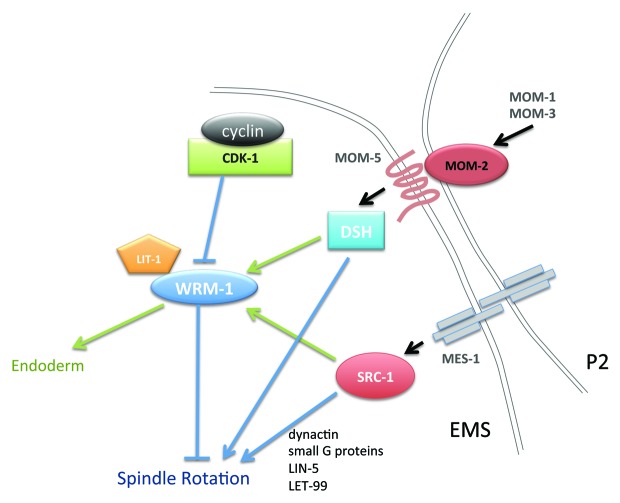
Genetic studies revealed that P2/EMS signaling involves at least two parallel pathways (Fig. 1) including: (1) a conserved Wnt signaling pathway comprised of MOM-2/Wnt, MOM-5/Frizzled, and DSH-1, 2, and 3/disheveled; and (2) a tyrosine kinase signaling pathway comprised of SRC-1/Src (a non-receptor tyrosine kinase) and MES-1 (a receptor tyrosine kinase-like protein).1,7,11 Inactivation of either the Wnt- or Src-pathway was shown to cause low penetrance defects in endoderm-specification and EMS spindle-rotation, whereas simultaneous inactivation of both pathways was shown to cause highly penetrant defects.10-12
Figure 1. Genetic model of P2/EMS signaling. Schematic representation of signaling inputs controlling EMS cell fate (green lines) and division orientation (blue lines).
Interestingly, although WRM-1 was absolutely required for endoderm induction, it was found to be dispensable for the rotation of the EMS spindle onto the a-p axis.10,11 These findings suggested that Wnt and Src signals transmitted from the cell surface converge on WRM-1 to specify endoderm, while other WRM-1-independent activities, which were later shown to involve a G protein α subunit GPA-16, GoLoco proteins GPR-1/2, a coiled-coil protein LIN-5, a DEP-domain protein LET-99, and dynactin function downstream of signaling to regulate the rotation of the EMS spindle13-17 (Fig. 1). It is not yet known how Wnt and Src signaling converge on WRM-1 regulation. WRM-1 contains many residues that can be phosphorylated in vitro by a variety of kinases, including Src (our unpublished data), and phosphotyrosine staining is dramatically enriched at the boundary of P2 and EMS in the four-cell stage embryo.11 The molecular mechanism, and biological relevance of these phosphorylations however remain to be resolved.
The Role of WRM-1 in Endoderm Induction
Genetic and cell biological studies revealed that WRM-1 regulates endoderm induction through the HMG-domain transcription factor POP-1, a homolog of the TCF (T-cell factor) transcription factor.1,8,9 POP-1/TCF can function as a transcriptional repressor or a transcriptional activator depending on the cellular context or binding partners.18,19 WRM-1 and POP-1 exhibit reciprocal, asymmetric nuclear localization in the daughters of EMS. POP-1 is high in the anterior sister MS and low in the posterior sister E,20,21 while WRM-1 is low in MS and high in E.22 wrm-1 and pop-1 mutants also exhibit opposite endoderm induction phenotypes; in wrm-1 mutant both EMS daughters adopt a cell fate equivalent to MS resulting in a lack of endoderm and an excess of mesoderm, whereas in pop-1 or pop-1; wrm-1 double mutants both EMS daughters adopt a cell fate equivalent to E, resulting in excess endoderm at the expense of mesoderm.1 Combined with the fact that POP-1 nuclear asymmetry is lost in a wrm-1 mutant, these observations suggest that, in the MS blastomere, POP-1 acts as an inhibitor of the E cell fate, while in the E blastomere, WRM-1 negatively regulates POP-1 by lowering its nuclear level, thereby promoting the E cell fate. WRM-1 functions as an activating subunit of the LIT-1, Nemo-related MAP kinase, which phosphorylates POP-1, and promotes POP-1 nuclear export via the 14–3-3 protein, PAR-5.8,23 Wnt signaling also increases the level of nuclear LIT-1 in E, which further promotes nuclear export of POP-1.23
How does POP-1 nuclear asymmetry translate into different cell fates between MS and E? In MS, where the nuclear level of POP-1 is high, POP-1 functions together with transcriptional co-repressor to repress Wnt target genes.18 In E, nuclear POP-1 associates with a second β-catenin homolog, SYS-1, to activate the transcription of the Wnt target genes.24,25 The interactions between POP-1 and SYS-1 and between POP-1 and WRM-1 are mutually exclusive.26 Furthermore, when associated with SYS-1, POP-1 is neither phosphorylated by LIT-1 nor exported from the nucleus. SYS-1 expression is upregulated in E via Wnt-dependent stabilization of SYS-1.27,28 Thus, the activation of Wnt-responsive gene expression is thought to result from nuclear retention of the POP-1/SYS-1 co-activator complex and WRM-1/LIT-1-dependent nuclear export of a POP-1 repressor complex.29,30
WRM-1 Asymmetry in the Nucleus and on the Cortex
In the early embryos, WRM-1 is localized uniformly to all nuclei and cell–cell interfaces.2,22 How then is the WRM-1 nuclear asymmetry established in the daughters of EMS? When the EMS cell enters mitosis, WRM-1 is displaced from the posterior half of the cortex of the dividing EMS cell, and accumulates at a higher level in the nascent posterior nucleus of the future E cell.2,22 Interestingly, the nuclear and cortical asymmetries of WRM-1 are completely dependent on Wnt signaling. Src signaling, however, is only required for the nuclear asymmetry of WRM-1, as the cortical asymmetry of WRM-1 was only weakly affected in the absence of Src-signaling. These findings suggested that these two asymmetries are regulated independently.
Recent work indicates that the cortical asymmetry of WRM-1 promotes its nuclear asymmetry through a microtubule-mediated mechanism.31,32 WRM-1, which is initially symmetrically localized, becomes asymmetrically localized to the anterior cortex of the dividing EMS blastomere. APR-1, a worm homolog of APC (adenomatous polyposis coli protein), is also recruited to the anterior cortex of EMS. APC is a component of the β-catenin destruction complex in other systems, and is known to bind and stabilize the plus ends of microtubules.33,34 In EMS, APR-1 does not appear to promote WRM-1 degradation, but rather stabilizes the microtubules in the anterior region of the cell, significantly increasing the number of astral microtubules in the anterior cytoplasm relative to the posterior cytoplasm of the dividing EMS blastomere. This anterior enrichment of astral microtubules could promote transport of WRM-1 toward the anterior cortex moving WRM-1 away from the anterior nucleus of the dividing EMS blastomere. According to this model, signaling events that control microtubule dynamics could thus enforce both the nuclear and cortical asymmetries of WRM-1 protein.32
CDK-1 Regulates WRM-1 Asymmetry by Phosphorylation
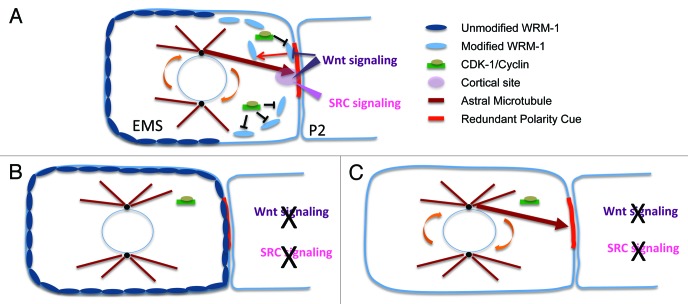
Another layer of regulation of WRM-1 localization was recently reported.2 Close inspection of the dynamics of WRM-1 asymmetry revealed that the loss of WRM-1 from the posterior cortex of EMS coincides with the onset of mitosis, suggesting a role for the major cell cycle regulator CDK-1 (cyclin-dependent kinase-1), in the process. Several lines of evidence support this idea. First, WRM-1 can be specifically phosphorylated in vitro on conserved N-terminal CDK consensus sites by CDK-1 kinase immunoprecipitated from worm extracts, as well as by purified vertebrate CDK1, suggesting that WRM-1 is a direct substrate of CDK-1. Second, WRM-1 persists at the posterior cortex of EMS, a phenotype identical to that observed in upstream Wnt-signaling mutants, in a hypomorphic temperature-sensitive (ts) cdk-1 mutant, and in transgenic strains expressing a GFP::WRM-1 fusion protein with non-phosphorylatable amino-acid substitutions in the CDK-1 consensus sites. Furthermore, in both instances, persistent localization of WRM-1 on the posterior cortex of EMS is correlated with a complete loss of asymmetric accumulation of WRM-1 in the nucleus of E relative to the nucleus of MS, consistent with a mechanism in which the nuclear and cortical WRM-1 asymmetries are coupled.31,32 These findings support the idea that phosphorylation of WRM-1 by CDK-1 serves to link the disappearance of WRM-1 from the posterior EMS cortex to the onset of mitosis. Whether CDK-1 selectively phosphorylates WRM-1 residing on the posterior cortex is not known. However, it is interesting to speculate that Wnt-induced cortical changes form a membrane-associated signalsome like that described in other systems35 that might modify WRM-1 to render it accessible and recognizable by CDK-1. Conversely, it’s possible that modification by CDK-1 at the appropriate time in the cell cycle is necessary to render the WRM-1 complex sensitive to cortical perturbations induced by signaling. In either case, the requirement for CDK-1 phosphorylation ensures that WRM-1 release from the cortex is coupled to the division of EMS, such that the cortical asymmetries induced by signaling are inherited differentially by anterior and posterior descendants (Fig. 2).
Figure 2. WRM-1 masks a redundant intrinsic signal for EMS spindle rotation. Schematic representation showing WRM-1 localization (dark and light blue ovals) at prophase just prior to EMS spindle rotation in (A) wild-type, and in a P2/EMS signaling mutant (BandC), with, (B) and without (C) WRM-1 activity. In (A), the concerted action of Wnt (purple arrowhead) and Src (pink arrowhead) establishes a cortical site (pink sphere). An astral microtubule (brown arrow) is shown capturing this cortical site prior to force generation and spindle rotation. Modifications (black bars) induced by Wnt signaling and CDK-1/Cyclin (green rectangle with light green oval) promote release of WRM-1 (light blue ovals) from the posterior cortex unmasking the cortical site. In the absence of both Wnt and Src signaling (B), WRM-1 is not released from the cortex and spindle rotation fails to occur. Removal of WRM-1 in (C) rescues spindle rotation by exposing a redundant polarity cue (thick red line), which may represent the remnant of a polarity signal established at fertilization.
Functional Asymmetry of WRM-1 in Endoderm Induction
Surprisingly, the physical asymmetry of WRM-1 is not absolutely essential for its function. Two different mutant contexts were identified in which all visible asymmetries in WRM-1 localization were abolished and yet embryos exhibited wild-type EMS polarity and viability. These included the WRM-1 CDK-1-phosphoacceptor site transgenic strains which fully rescued the viability of a wrm-1 null mutation,2 and the conditional cdk-1(ne2257) mutant which was fully viable at 20 °C, despite (in both cases) symmetric localization of both cortical and nuclear WRM-1.2 These unexpected findings suggests that in addition to the physical asymmetries in WRM-1 localization, qualitative, or functional, asymmetries must also exist. Genetic analysis suggests that the Src pathway is responsible in part for these qualitative asymmetries. Embryos with symmetric WRM-1 localization exhibited strongly enhanced EMS polarity defects when SRC-1 signaling was depleted.2 Thus, SRC-1 activity might directly phosphorylate WRM-1 or components of the WRM-1 signaling complex, leading to functional asymmetries that can compensate in part for the loss of Wnt-signaling dependent physical asymmetries in WRM-1 localization. It is also possible that the MAP-kinase arm of P2/EMS signaling contributes to functional asymmetries in WRM-1 activity. More studies are needed to determine the nature of the qualitative differences such as differences in the phosphorylation of WRM-1 or its co-factors and how these differences alter the function of WRM-1 in regulating EMS polarity. In contrast to the situation described for EMS above, cortical WRM-1 was shown to interfere with asymmetric cell division and cell fate specification in post-embryonic T cell,36 suggesting that WRM-1 function may be regulated differently in different cell types.
Additional Potential Modes of WRM-1 Regulation by Phosphorylation
In addition to CDK-1 consensus sites, the N-terminal region of WRM-1 protein spanning amino acid 1–180 has a number of conserved serine/threonine and tyrosine residues that can be phosphorylated. It has been previously shown that WRM-1 complexed with LIT-1 is phosphorylated by LIT-1 in vitro.2,8 An N-terminal region of WRM-1 between amino acid 140–180, containing multiple serine or threonine residues, can indeed be phosphorylated by LIT-1 in vitro (our unpublished observations). Furthermore, in the lit-1 mutant background or in mom-4 (a MAPKKK that activates LIT-137) mutant background, WRM-1 protein fails to be localized to all the cell-cell interface of the early embryo.2 These observations suggest that WRM-1 phosphorylation by LIT-1 is important for the membrane recruitment of WRM-1.
In addition to CDK-1 and LIT-1, the N-terminal region of WRM-1 can also be phosphorylated by GSK-3 and Src kinases in vitro2 (our unpublished observation). The GSK-3 site is intriguingly linked to one of the consensus CDK-1 sites, and at least in vitro is phosphorylated by GSK3 in a manner dependent on priming phosphorylation by CDK-1.2 However, introduction of an un-phosphorylatable mutation in this putative GSK-3 site appeared to have no effect on WRM-1 localization or on WRM-1 function, at least in early embryos.2 It remains to be seen whether the sequential phosphorylations of WRM-1 by CDK-1 and GSK-3 has any biological function in other context during the development. Vertebrate Src kinase also robustly phosphorylates the N-terminal fragment of WRM-1 in vitro on the conserved tyrosine residues (our unpublished observation), consistent with the idea that Src signaling results in tyrosine phosphorylation of WRM-1. However, again, the physiological significance of Src phosphorylation of WRM-1 has not been shown in vivo.
Role of WRM-1 in EMS Spindle Rotation: WRM-1 as a Masking Factor of Intrinsic Polarity
What then is the biological significance of removing WRM-1 from the EMS posterior cortex? In wild-type embryos, astral microtubules emanating from one of the centrosomes appear to capture a region in the posterior cortex of EMS. This event correlates with the generation of a pulling force that rotates the centrosome-nuclear complex 90° onto the a-p axis of the embryo.38 Since the disappearance of WRM-1 at the posterior cortex of EMS occurs just prior to the initiation of spindle rotation,2 cortical WRM-1 could mask an inherent polarity cue present at the posterior cortex (Fig. 2). For example, WRM-1 could inhibit spindle rotation in a manner analogous to the cortical PAR-3/6 complex, which is required for proper division polarity in 1-cell and 2-cell stage embryos.
In the C. elegans one cell embryo (called P0), cortical events following sperm entry establish an initial “intrinsic” anterior-posterior (a-p) embryonic polarity. A highly orchestrated cascade of cell intrinsic events ensue that are thought to utilize this initial polarity cue to direct asymmetries in cytoplasmic localization and division orientation during the next few divisions.39-42 For example, the first division is always positioned such that the anterior sister (AB) is larger than its posterior sister (P1) and inherits distinct cytoplasmic contents. During the second division, AB divides equally and orthogonal to the a-p axis while P1 rotates its spindle and divides once again to produce anterior and posterior daughters (EMS and P2, respectively) that, once again, differ in size and cytoplasmic contents. The conserved PAR proteins play a central role in this process and appear to do so by masking and unmasking the intrinsic a-p polarity cue established at fertilization. The conserved PAR-3/6 complex is required for the masking function. Cells with uniform PAR-3/6 localization, including AB and its daughters, exhibit symmetric divisions without spindle reorientation. In contrast, in P0, P1, and P2, the PAR-3/6 complex becomes restricted to one end of the cell, while PAR-2 accumulates at the opposite cortex. This appears to allow astral microtubules associated with the nascent spindle complex to capture a cortical site on the region of cortex depleted of PAR-3/6. These microtubules then appear to shorten causing the spindle to rotate until it aligns along the polarized axis of the cell. Interestingly, in the absence of all the PAR proteins, the default behavior of early embryonic cells is to undergo the spindle rotation process typical of the P lineage, supporting the idea that intrinsic cues that direct spindle rotation are masked and unmasked through the concerted actions of PAR-3/6 and PAR-2.
The EMS cell does not exhibit PAR protein cortical asymmetries and is the first cell to undergo a cell interaction-dependent reorientation of its cell division axis.43,44 Interestingly, the P2 cell does exhibit PAR asymmetries but they are reversed such that the PAR3/6 complex localizes at the posterior and PAR-2 localizes at the anterior adjacent to EMS.43 The reversal of P2 polarity depends on MES-1/SRC-1 signaling, but does not depend on Wnt signaling. MES-1/SRC-1 signaling is bidirectional; resulting in the accumulation of cortical phosphotyrosine staining in both EMS and P2 at their region of contact.11 MES-1/SRC-1 signaling appears to override an intrinsic polarity cue in the P2 cell. In MES-1/SRC-1-pathway, mutants P2 divides with an orientation opposite to wild-type and similar to that of P0 and P1.45 It seems plausible that this same intrinsic polarity also exists in the EMS cell. If so, the ability to override this intrinsic cue could be important for EMS in order to allow it to more precisely position its division axis in response to signals from P2. Indeed, a set of genetic experiments suggested that cortical WRM-1 functions to mask a P2/EMS signaling-independent cortical cue.2 Loss of P2/EMS signaling in Src and Wnt-pathway double mutant backgrounds causes WRM-1 to persist on the posterior cortex of EMS and causes a highly penetrant EMS division orientation defect. This orientation defect is strongly suppressed by removing wrm-1 activity, suggesting that persistence of WRM-1 is masking an intrinsic polarity cue present on the posterior cortex of EMS (Fig. 2). It is tempting to speculate that by serving a dual function in masking polarity cues when associated with the cortex and in translocating to the nucleus in response to cell cycle and cell contact-mediated signals, WRM-1 can ensure that interacting cells ignore irrelevant or preexisting cues and respond to inductive cell contacts with proper spatial and temporal coordination (Fig. 3).
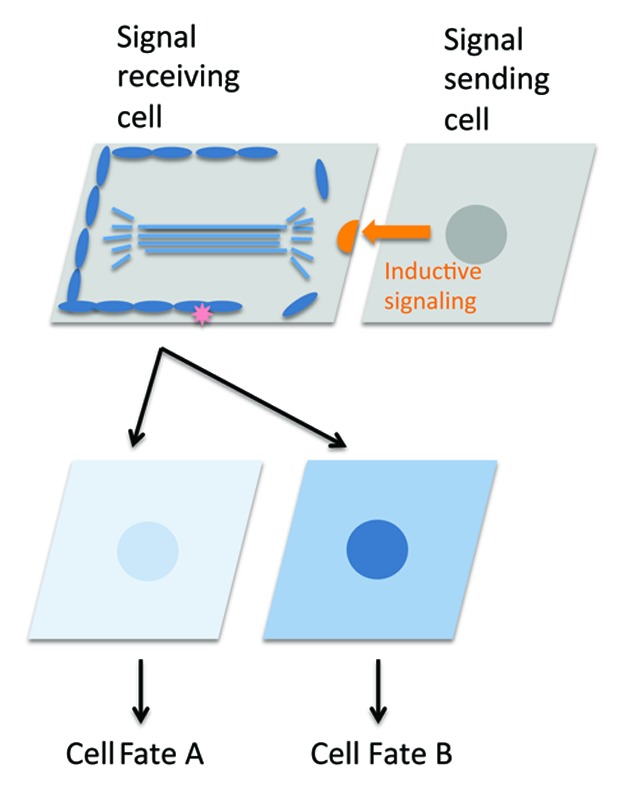
Figure 3. A general model for cortical unmasking during polarity signaling. Schematic showing a polarizing interaction between neighboring cells. A cortical factor uniformly localized prior to division (blue ovals) masks a pre-existing polarity cue (pink asterisk). Inductive signaling (orange arrow) releases the masking factor proximal to the signaling cell establishing a new cortical cue (orange half circle) that drives spindle orientation in the responding cell. The masking factor that is released from the cortex (or other signaling intermediates) becomes asymmetrically enriched in the daughter cells (represented by different shades of blue) to drive asymmetric cell fate specification.
Wnt Signaling and Asymmetric Cell Division in Mammalian Stem Cells
Wnt signal-regulated asymmetric cell division may work similarly in mammalian embryonic stem cells. In a recent study,46 active Wnt proteins were immobilized on beads and presented to cultured ES cell in a defined orientation (Fig. 4). Interestingly, this artificial “polarized” Wnt signal induced asymmetric distribution of Wnt-signaling components in the responding ES cells. Cells proximal to the beads oriented their axis of cell division toward the source of the Wnt signal, accumulated a higher level of β-catenin in the daughter cell proximal to the source of the Wnt signal, and divided into two daughter cells with different cell fates: in this case, the cell distal to the source of Wnt signal acquired a differentiated state, while the cell proximal to the source of Wnt signal remained pluripotent. It will be interesting to learn whether other aspects of P2/EMS signaling, such as redundancy between Wnt and Src signaling pathways, or the involvement of the cell cycle machinery (CDK1), are also conserved in mammals.
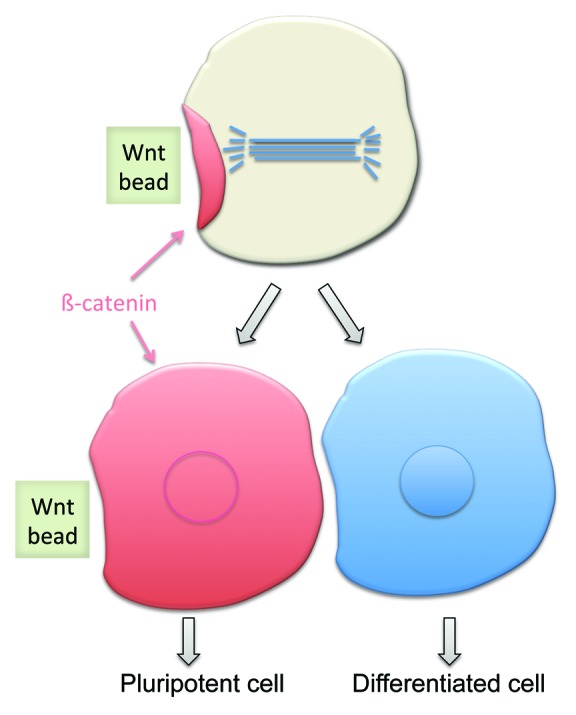
Figure 4. Artificial, external Wnt cue can orient cell division axis and induce asymmetric cell fate in mammalian embryonic stem cell. Beads loaded with active Wnt proteins positioned next to a cell induces: (1) orientation of the cell division axis of that cell toward the Wnt source; (2) asymmetric accumulation of β-catenin in the cell proximal to the Wnt source; and (3) two different cell fates in the daughter cells.
Future Challenges
Genetic, cell biological, and biochemical studies have advanced our understanding of the clockwork like asymmetric divisions of the early C. elegans embryo. Perhaps the most striking feature that has emerged from these studies is the built-in redundancy of the signaling mechanisms. Perhaps this redundancy provides robustness and stability essential for faithful execution of embryonic patterning under the myriad stresses encountered in nature. Despite progress, many genetic and cell biological observations remain to be reconciled with existing models. For example, there is much to learn about how, at a molecular level, the different aspects of P2/EMS signaling fit together to orchestrate such an exquisitely defined biological outcome. In particular, how are multiple signaling inputs integrated at the cortex, and how does the integrated signal translate to the physical and/or functional asymmetry of signal transducing proteins such as WRM-1? Studies in this versatile model organism are poised to address how intricate networks of cellular signaling pathways control the orientation of cell division and cell fate specification at single cell resolution, in vivo.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ahmed Elewa and Dr Darryl Conte for critical reading of the manuscript and helpful comments.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/26276
References
- 1.Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–16. doi: 10.1016/S0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Ishidate T, Sharma R, Soto MC, Conte D, Jr., Mello CC, Shirayama M. Wnt and CDK-1 regulate cortical release of WRM-1/β-catenin to control cell division orientation in early Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A. 2013;110:E918–27. doi: 10.1073/pnas.1300769110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein B. Induction of gut in Caenorhabditis elegans embryos. Nature. 1992;357:255–7. doi: 10.1038/357255a0. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein B. Establishment of gut fate in the E lineage of C. elegans: the roles of lineage-dependent mechanisms and cell interactions. Development. 1993;118:1267–77. doi: 10.1242/dev.118.4.1267. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein B. An analysis of the response to gut induction in the C. elegans embryo. Development. 1995;121:1227–36. doi: 10.1242/dev.121.4.1227. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein B. Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J Cell Biol. 1995;129:1071–80. doi: 10.1083/jcb.129.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/S0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- 8.Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999;97:717–26. doi: 10.1016/S0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- 9.Meneghini MD, Ishitani T, Carter JC, Hisamoto N, Ninomiya-Tsuji J, Thorpe CJ, Hamill DR, Matsumoto K, Bowerman B. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature. 1999;399:793–7. doi: 10.1038/21666. [DOI] [PubMed] [Google Scholar]
- 10.Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999;13:2028–38. doi: 10.1101/gad.13.15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bei Y, Hogan J, Berkowitz LA, Soto M, Rocheleau CE, Pang KM, Collins J, Mello CC. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev Cell. 2002;3:113–25. doi: 10.1016/S1534-5807(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 12.Walston T, Tuskey C, Edgar L, Hawkins N, Ellis G, Bowerman B, Wood W, Hardin J. Multiple Wnt signaling pathways converge to orient the mitotic spindle in early C. elegans embryos. Dev Cell. 2004;7:831–41. doi: 10.1016/j.devcel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Tsou MF, Hayashi A, Rose LS. LET-99 opposes Galpha/GPR signaling to generate asymmetry for spindle positioning in response to PAR and MES-1/SRC-1 signaling. Development. 2003;130:5717–30. doi: 10.1242/dev.00790. [DOI] [PubMed] [Google Scholar]
- 14.Werts AD, Roh-Johnson M, Goldstein B. Dynamic localization of C. elegans TPR-GoLoco proteins mediates mitotic spindle orientation by extrinsic signaling. Development. 2011;138:4411–22. doi: 10.1242/dev.070979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan DG, Fisk RM, Xu H, van den Heuvel S. A complex of LIN-5 and GPR proteins regulates G protein signaling and spindle function in C elegans. Genes Dev. 2003;17:1225–39. doi: 10.1101/gad.1081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotta M, Dong Y, Peterson YK, Lanier SM, Ahringer J. Asymmetrically distributed C. elegans homologs of AGS3/PINS control spindle position in the early embryo. Curr Biol. 2003;13:1029–37. doi: 10.1016/S0960-9822(03)00371-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Skop AR, White JG. Src and Wnt signaling regulate dynactin accumulation to the P2-EMS cell border in C. elegans embryos. J Cell Sci. 2008;121:155–61. doi: 10.1242/jcs.015966. [DOI] [PubMed] [Google Scholar]
- 18.Calvo D, Victor M, Gay F, Sui G, Luke MP, Dufourcq P, Wen G, Maduro M, Rothman J, Shi YA. A POP-1 repressor complex restricts inappropriate cell type-specific gene transcription during Caenorhabditis elegans embryogenesis. EMBO J. 2001;20:7197–208. doi: 10.1093/emboj/20.24.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shetty P, Lo MC, Robertson SM, Lin R. C. elegans TCF protein, POP-1, converts from repressor to activator as a result of Wnt-induced lowering of nuclear levels. Dev Biol. 2005;285:584–92. doi: 10.1016/j.ydbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Lin R, Thompson S, Priess JR. pop-1 encodes an HMG box protein required for the specification of a mesoderm precursor in early C. elegans embryos. Cell. 1995;83:599–609. doi: 10.1016/0092-8674(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 21.Lin R, Hill RJ, Priess JR. POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell. 1998;92:229–39. doi: 10.1016/S0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Kim S, Ishidate T, Bei Y, Pang KM, Shirayama M, Trzepacz C, Brownell DR, Mello CC. Wnt signaling drives WRM-1/beta-catenin asymmetries in early C. elegans embryos. Genes Dev. 2005;19:1749–54. doi: 10.1101/gad.1323705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo MC, Gay F, Odom R, Shi Y, Lin R. Phosphorylation by the beta-catenin/MAPK complex promotes 14-3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell. 2004;117:95–106. doi: 10.1016/S0092-8674(04)00203-X. [DOI] [PubMed] [Google Scholar]
- 24.Siegfried KR, Kidd AR, 3rd, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;166:171–86. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd AR, 3rd, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A beta-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–72. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Yang XD, Huang S, Lo MC, Mizumoto K, Sawa H, Xu W, Robertson S, Lin R. Distinct and mutually inhibitory binding by two divergent β-catenins coordinates TCF levels and activity in C. elegans. Development. 2011;138:4255–65. doi: 10.1242/dev.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Shetty P, Robertson SM, Lin R. Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator beta-catenin SYS-1. Development. 2007;134:2685–95. doi: 10.1242/dev.008268. [DOI] [PubMed] [Google Scholar]
- 28.Phillips BT, Kidd AR, 3rd, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:3231–6. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips BT, Kimble J. A new look at TCF and β-catenin through the lens of a divergent C. elegans Wnt pathway. Dev Cell. 2009;17:27–34. doi: 10.1016/j.devcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson SM, Lin R. Our evolving view of Wnt signaling in C. elegans. . Worm. 2011;1:82–9 . doi: 10.4161/worm.19156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeshita H, Sawa H. Asymmetric cortical and nuclear localizations of WRM-1/beta-catenin during asymmetric cell division in C. elegans. Genes Dev. 2005;19:1743–8. doi: 10.1101/gad.1322805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugioka K, Mizumoto K, Sawa H. Wnt regulates spindle asymmetry to generate asymmetric nuclear β-catenin in C. elegans. Cell. 2011;146:942–54. doi: 10.1016/j.cell.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 33.Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–8. doi: 10.1016/S0960-9822(00)00600-X. [DOI] [PubMed] [Google Scholar]
- 34.Srayko M, Kaya A, Stamford J, Hyman AA. Identification and characterization of factors required for microtubule growth and nucleation in the early C. elegans embryo. Dev Cell. 2005;9:223–36. doi: 10.1016/j.devcel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizumoto K, Sawa H. Cortical beta-catenin and APC regulate asymmetric nuclear beta-catenin localization during asymmetric cell division in C. elegans. Dev Cell. 2007;12:287–99. doi: 10.1016/j.devcel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Shin TH, Yasuda J, Rocheleau CE, Lin R, Soto M, Bei Y, Davis RJ, Mello CC. MOM-4, a MAP kinase kinase kinase-related protein, activates WRM-1/LIT-1 kinase to transduce anterior/posterior polarity signals in C. elegans. Mol Cell. 1999;4:275–80. doi: 10.1016/S1097-2765(00)80375-5. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein B. When cells tell their neighbors which direction to divide. Dev Dyn. 2000;218:23–9. doi: 10.1002/(SICI)1097-0177(200005)218:1<23::AID-DVDY3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Priess JR. Establishment of initial asymmetry in early Caenorhabditis elegans embryos. Curr Opin Genet Dev. 1994;4:563–8. doi: 10.1016/0959-437X(94)90073-C. [DOI] [PubMed] [Google Scholar]
- 40.Rose LS, Kemphues KJ. Early patterning of the C. elegans embryo. Annu Rev Genet. 1998;32:521–45. doi: 10.1146/annurev.genet.32.1.521. [DOI] [PubMed] [Google Scholar]
- 41.Cowan CR, Hyman AA. Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol. 2004;20:427–53. doi: 10.1146/annurev.cellbio.19.111301.113823. [DOI] [PubMed] [Google Scholar]
- 42.Kemphues K. PARsing embryonic polarity. Cell. 2000;101:345–8. doi: 10.1016/S0092-8674(00)80844-2. [DOI] [PubMed] [Google Scholar]
- 43.Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell. 1995;83:743–52. doi: 10.1016/0092-8674(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 44.Boyd L, Guo S, Levitan D, Stinchcomb DT, Kemphues KJ. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development. 1996;122:3075–84. doi: 10.1242/dev.122.10.3075. [DOI] [PubMed] [Google Scholar]
- 45.Berkowitz LA, Strome S. MES-1, a protein required for unequal divisions of the germline in early C. elegans embryos, resembles receptor tyrosine kinases and is localized to the boundary between the germline and gut cells. Development. 2000;127:4419–31. doi: 10.1242/dev.127.20.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–8. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]