In the field of veterinary medicine, there are few reports regarding the incidence of respiratory diseases, including cancer of the nose and lung among pet animals with passive tobacco smoke exposure (PTSE). However, demonstrating the evidence of relationship between PTSE levels and clinical symptoms in dogs is considered difficult (Hawkins and others 2010).
The nicotine component of tobacco smoke is ultimately eliminated from the body (Benowitz and others 1994). Therefore, in human medicine, urinary and serum cotinine levels have been used as biomarkers of PTSE (Matsumoto and others 2010). In dogs, nicotine is metabolised to cotinine in the same way as in humans (Brazell and others 1984), and household smoking levels, as assessed by questionnaire, have been significantly associated with urinary cotinine levels in healthy dogs (Bertone-Johnson and others 2008).
In this study, we examined the effects of PTSE on pulmonary function using barometric whole-body plethysmography (BWBP) and discuss our findings in dogs with chronic cough.
Between January 2008 and March 2012, 43 dogs were referred to the Animal Medical Center of Nihon University, Kanagawa, Japan, for chronic cough. These dogs were classified into two groups, exposed and unexposed to tobacco smoke, based on serum cotinine levels and a cut-off value of 0.21 ng/mL (Sasaki and others 2011). Blood samples were collected from the jugular vein; serum was isolated by centrifugation and stored at −20°C until the time of cotinine measurement. Serum cotinine levels were measured using a Cotinine for Passive Smoking ELISA Kit (Cosmic Corporation, Tokyo, Japan).
BWBP was performed by placing the dogs into a clear rectangular plethysmograph chamber (Buxco Electronics Inc., Wilmington, North Carolina, USA) of 80 cm (h)×108 cm (l)×80 cm (w) for large dogs and 43 cm (h)×55 cm (l)×47 cm (w) for small dogs. The chamber was ventilated with continuous bias airflow (Buxco BFL0404) at 40 L/minute for the large chamber and 20 L/minute for the small chamber. A MAX2275 card incorporating the calibration signal and an external gain were used. The plethysmograph was connected to a preamplifier; commercial software was used for analysis (Biosystem XA V.2.10.5; Buxco). The temperature of the room where the experiment was performed was held constant at approximately 22°C. After the dogs acclimatised to the chamber for five minutes, data were recorded for five minutes. The following variables were recorded and computed: respiratory rate (RR), estimated tidal volume (TV), estimated minute ventilation, expiratory (Te); inspiratory (Ti); and relaxation times (RT, the time point when 65 per cent of TV expired), peak inspiratory and expiratory flow (PIF and PEF), PEF/PIF and pause (PAU, formula: (Te-RT)/RT) and enhanced pause (PENH, formula: (PEF/PIF)×PAU). The two groups of exposed and unexposed dogs were compared using the unpaired t test or Mann–Whitney rank sum test to determine significant differences. The differences within diseases were analysed by Kruskal–Wallis one-way analysis of variance on ranks. P<0.05 was considered statistically significant. All the aforementioned examinations were performed with the informed consent of the dogs' owners.
Based on serum cotinine levels, 29 dogs (67 per cent) were estimated to have experienced PTSE. We excluded 12 dogs that were either brachycephalic breeds or diagnosed as having bronchomalacia for analysing pulmonary function, because their airway flow may be limited (Bernaerts and others 2010). However, all dogs (n=7) with bronchomalacia were classified as having PTSE. The respiratory diseases of the remaining dogs included interstitial lung disease (three unexposed and six exposed dogs, respectively), bronchiectasis (two and six dogs, respectively ), single subpleural pulmonary nodule of under 5 cm diameter (three and four dogs, respectively), chronic bronchitis (two and three dogs, respectively) and bronchopneumonia (two and zero dogs, respectively), as determined by computed tomography (CT) imaging and bronchoscopy, and there were no any upper airway diseases in all cases. Table 1 shows the body weight, age and BWBP variables in exposed and unexposed dogs. There were no significant differences between the groups in terms of body weight, age and BWBP variables without PEF, PEF/PIF, PAU and PENH. The indexes of airway limitation were significantly higher in exposed dogs than in unexposed dogs (Fig 1). However, there were no statistical differences across five diseases for PEF, PEF/PIF, PAU and PENH.
TABLE 1:
Body weight, age and BWBP variables (median with minimum and maximum values) in dogs with respiratory diseases exposed and unexposed to tobacco smoke
| Variable | Exposed | Unexposed | P value |
|---|---|---|---|
| Body weight, kg | 11.1 (3.1–42.0) | 8.4 (3.5–38.9) | 1.000 |
| Age, years | 9 (4–13) | 8 (6–13) | 0.609 |
| RR, cycles/min | 128 (13–320) | 67 (18–216) | 0.383 |
| TV/BW, mL/kg | 7.4 (1.8–60.3) | 5.9 (2.1–18.0) | 0.478 |
| MV/BW, mL/kg | 789 (149–6374) | 626 (255–1188) | 0.100 |
| Te/Ti | 1.36 (0.80–2.04) | 1.36 (1.00–3.44) | 0.529 |
| RT, s | 0.13 (0.05–0.69) | 0.29 (0.10–1.22) | 0.133 |
| PEF/BW, mL/s/kg | 50 (14–490) | 28 (12–61) | 0.005 |
| PIF/BW, mL/s/kg | 52 (10–352) | 34 (18–64) | 0.100 |
BWBP, barometric whole-body plethysmography; MV, minute ventilation; PEF, peak expiratory flow; PIF, peak inspiratory flow; RR, respiratory rate; RT, relaxation times; TV, tidal volume.
FIG 1:
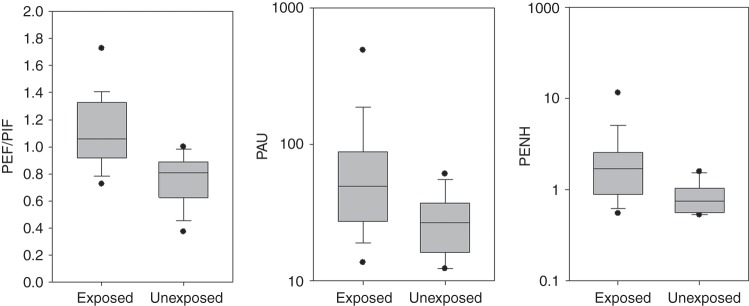
Ratio of peak expiratory to inspiratory flows (PEF/PIF), pause (PAU) and enhanced pause (PENH) in dogs with respiratory diseases exposed and unexposed to tobacco smoke. Solid horizontal bars represent median values and boxes represent the 25th–75th percentiles. Whiskers represent the 5th–95th percentiles and outliers are indicated by black dots. There are significant differences among the two groups in PEF/PIF (P<0.001), PAU (P=0.043) and PENH (P=0.002)
Our study showed slightly increases in airway limitation in dogs due to PTSE. This small change of airway limitation on BWBP may be invisible, as any clinical signs such as a respiratory effort (except chronic cough) would not be detected on visual inspection. In fact, there were no remarkable differences between two groups on the severity of diseases in this study, although we were not able to ascertain the feeling of breathlessness of any of the dogs, of course. BWBP is usually performed under sedation in dogs and was thought less sensitive to assess upper airway function in dogs (Bernaerts and others 2010). In this study, however, BWBP was a useful method to investigate canine pulmonary function because the respiratory effort may be maintained without sedation.
The airway limitation seen in this study is not indicative of the acute effect of PTSE on pulmonary function, because all dogs were isolated from clients for physical examination and inhaled clean air in our hospital at least four hours. An increase in serum nicotine level may induce mild and persistent bronchial contraction because nicotine activates parasympathetic functions via acetylcholine receptors. In humans with chronic bronchitis, tobacco smoke increases sputum secretion. (Comandini and others 2009, Camiciottoli others 2013). Histopathological changes of the airway in dogs with exposure to mainstream tobacco smoke have been reviewed previously (Coggins 2001). Therefore, persistent increases in airway limitation also suggest that chemical and physical insults by PTSE contribute to the development of increased sputum secretion and histopathological changes. In this clinical study, we were not able to examine the sole effect of PTSE without airway disease because it was difficult to prove that dogs with PTSE have no other respiratory diseases, and our hospital did not allow us to perform CT examinations and bronchoscopy on dogs with no respiratory symptoms. This is the main limitation of our study.
In summary, for dogs with respiratory disease, PTSE is conceivably a factor exacerbating and influencing chronic cough.
Footnotes
Funding: This study was supported by a Nihon University Individual Research Grant for (2009) and a grant from the Smoking Research Foundation in Japan (2011–2013).
References
- Benowitz N. L., Jacob P. III, Fong I., Gupta S. (1994) Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. Journal of Pharmacology and Experimental Therapeutics 268, 296–303 [PubMed] [Google Scholar]
- Bernaerts F., Talavera J., Leemans J., Hamaide A., Claeys S., Kirschvink N., Clercx C. (2010) Description of original endoscopic findings and respiratory functional assessment using barometric whole-body plethysmography in dogs suffering from brachycephalic airway obstruction syndrome. The Veterinary Journal 183, 95–102 [DOI] [PubMed] [Google Scholar]
- Bertone-johnson E. R., Procter-gray E., Gollenberg A. L., Ryan M. B., Barber L. G. (2008) Environmental tobacco smoke and canine urinary cotinine level. Environmental Research 106, 361–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazell R. S., Stiff A. C., Henderson G. M., Jenkins R. A., Romig P. L., Auerbach O. (1984) Plasma nicotine and cotinine in tobacco smoke exposed beagle dogs. Toxicology and Applied Pharmacology 73, 152–158 [DOI] [PubMed] [Google Scholar]
- Camiciottoli G., Bigazzi F., Paoletti M., Cestelli L., Lavorini F., Pistolesi M. (2013) Pulmonary function and sputum characteristics predict computed tomography phenotype and severity of COPD. European Respiratory Journal 42, 626–635 [DOI] [PubMed] [Google Scholar]
- Coggins C. R. (2001) A review of chronic inhalation studies with mainstream cigarette smoke, in hamsters, dogs, and nonhuman primates. Toxicologic Pathology 29, 550–557 [DOI] [PubMed] [Google Scholar]
- Comandini A., Rogliani P., Nunziata A., Cazzola M., Curradi G., Saltini C. (2009) Biomarkers of lung damage associated with tobacco smoke in induced sputum. Respiratory Medicine 103, 1592–1613 [DOI] [PubMed] [Google Scholar]
- Hawkins E. C., Clay L. D., Bradley J. M., Davidian M. (2010) Demographic and historical findings, including exposure to environmental tobacco smoke, in dogs with chronic cough. Journal of Veterinary Internal Medicine 24, 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Ino T., Ohta M., Otani T., Hanada S., Sakuraoka A., Matsumoto A., Ichiba M., Hara M. (2010) Enzyme-linked immunosorbent assay of nicotine metabolites. Environmental Health and Preventive Medicine 15, 211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Braimoh T. S., Yila T. A., Yoshioka E., Kishi R. (2011) Self-reported tobacco smoke exposure and plasma cotinine levels during pregnancy–a validation study in Northern Japan. Science of the Total Environment 412–413, 114–118 [DOI] [PubMed] [Google Scholar]


