Abstract
Aim
This study models the cost-effectiveness of brief advice (BA) in primary care for physical activity (PA) addressing the limitations in the current limited economic literature through the use of a time-based modelling approach.
Methods
A Markov model was used to compare the lifetime costs and outcomes of a cohort of 100 000 people exposed to BA versus usual care. Health outcomes were expressed in terms of quality-adjusted life years (QALYs). Costs were assessed from a health provider perspective (£2010/11 prices). Data to populate the model were derived from systematic literature reviews and the literature searches of economic evaluations that were conducted for national guidelines. Deterministic and probability sensitivity analyses explored the uncertainty in parameter estimates including short-term mental health gains associated with PA.
Results
Compared with usual care, BA is more expensive, incurring additional costs of £806 809 but it is more effective leading to 466 QALYs gained in the total cohort, a QALY gain of 0.0047/person. The incremental cost per QALY of BA is £1730 (including mental health gains) and thus can be considered cost-effective at a threshold of £20 000/QALY. Most changes in assumptions resulted in the incremental cost-effectiveness ratio (ICER) falling at or below £12 000/QALY gained. However, when short-term mental health gains were excluded the ICER was £27 000/QALY gained. The probabilistic sensitivity analysis showed that, at a threshold of £20 000/QALY, there was a 99.9% chance that BA would be cost-effective.
Conclusions
BA is a cost-effective way to improve PA among adults, provided short-term mental health gains are considered. Further research is required to provide more accurate evidence on factors contributing to the cost-effectiveness of BA.
Keywords: Health promotion through physical activity
Introduction
The positive association between inactive lifestyle and morbidity and mortality is well documented.1 2 However, only 3 of 10 individuals in England undertake a level of activity that is sufficient to meet the recommended levels of physical activity (PA), as defined by the national guidance.3 In England, the National Institute for Health and Care Excellence (NICE) has recommended brief advice (BA) in primary care as an effective way of increasing PA in adults.4–6 The US Preventive Services Task Force recommends that clinicians counsel adults to engage in PA for prevention of cardiovascular disease (CVD).7 The European Union's PA guidelines recognise the important role the primary care physicians can play in encouraging patients to engage in PA.8 BA includes verbal advice, discussion, negotiation or encouragement, with or without written or other support or follow-up. It could be opportunistic and can typically take from less than a minute to up to 20 min. To date, BA has not been adequately implemented, which might be partially attributed to the rather inadequate effectiveness evidence base.9
Orrow et al10 recently conducted a systematic review and meta-analysis of the effectiveness of PA promotion based in primary care. They concluded that encouraging PA in primary care settings leads to improved levels of PA and that intensive interventions do not necessarily lead to better outcomes than brief interventions. The scope of this review was, however, relatively narrow as they focused only on RCTs with a minimum follow-up of 12 months. Therefore, to assist the update of guidance in this area, the NICE recently commissioned a systematic review and meta-analysis of BA. This review undertaken by Campbell et al11 had a broader perspective as they included all available evidence, RCTs and non-RCTs without limitations on follow-up time. Campbell et al11 found that at 1 year BA is correlated with a higher probability of being physically active, although this result did not quite reach the 5% significance level (relative risk (RR): 1.42; 95% CI 0.98 to 2.06). The addition of further elements to support BA yielded no statistically significant benefit.
In this paper, this current effectiveness evidence is used to conduct cost-effectiveness analysis in an area where there is little economic evidence to inform resource allocation.12 A recent systematic literature review13 identified only three economic evaluations of BA (one model-based,5 one trial-based14 and one audit-based analysis15). Pringle et al14 did not report on the cost-effectiveness of BA per se, but their results for a similar intervention (motivational interviews) indicated that the cost per person improving moderate PA was between £2659 and £2789 and the estimated cost per QALY was £47–£229. Boehler et al15 estimated an incremental cost of £887 to increase self-reported PA levels to 150 min of moderate intensity activity per week (3 months postintervention) when disease register screening was compared with opportunistic patient recruitment. Matrix5 estimated that BA compared with usual care led to a cost per Quality Adjusted Life Years (QALY) of £159 for advice by a family physician during consultation and £425 for advice plus a booklet (mailed 2 weeks after).
There are a number of concerns about the rigour of the evidence base that point to the need for further evaluation. First, there is a significant uncertainty around the cost-effectiveness estimates partly owing to notably weak effectiveness data. The effectiveness data were obtained from single studies that largely had high attrition rates. In addition, parameter uncertainty was inadequately explored with the studies mostly using deterministic sensitivity analysis. Second, although the literature recognises that individual's PA behaviour is unstable, the economic models have not explicitly accounted for this, but rather assumed constancy of behaviour once people settle in the active state. This approach potentially biases the estimated cost-effectiveness of interventions.
This paper contributes to knowledge by addressing the limitations in the current limited economic literature through the use of the most up-to-date meta-analysed effectiveness data, and a time-based modelling approach that accounts for variations in PA behaviour.
Methods
Modelling approach
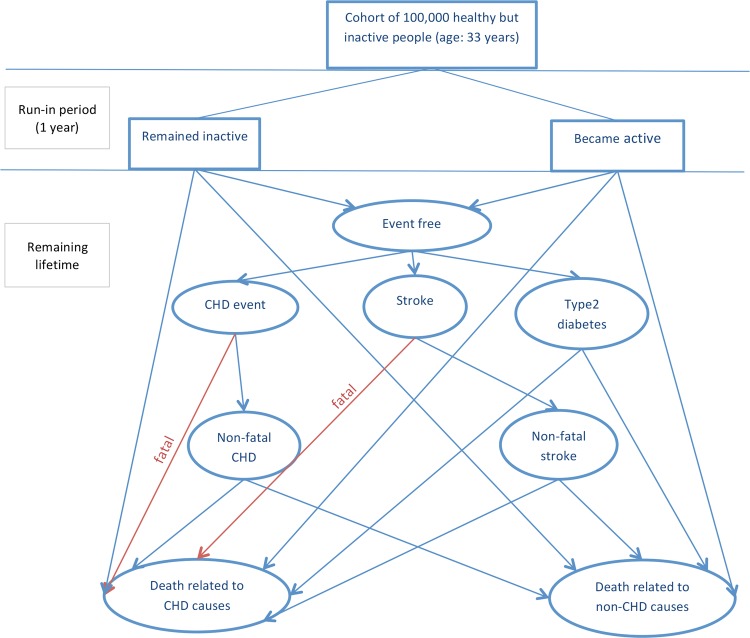
A Markov model was developed to follow a cohort of physically inactive but healthy adults over their remaining lifetime. The starting age of the cohort (33 years) was chosen to reflect the average age of participants in the trials of BA;11 however, results were also estimated for cohorts of different ages in sensitivity analysis. Figure 1 illustrates the model structure. The model was run twice, once to estimate the cost and health effects with the cohort being offered a BA (the intervention arm), and once following a usual care with no active intervention (the control arm).
Figure 1.
Illustration of pathways within the model. CHD, coronary heart disease.
For the intervention arm, BA was delivered at the start of the first model cycle (year 0). Then over an initial ‘run-in’ period of 1 year, members of the cohort either remained ‘inactive’ or became ‘active’, with the proportion becoming active in each arm reflecting the effectiveness evidence. The definition of activity used in the model was: undertaking a minimum of 150 min of at least moderate intensive PA or at least 75 min of vigorous intensive PA per week. This definition was chosen for consistency with PA for health guidance, and the literature on the effectiveness and risks for disease conditions.11 16–18 The run-in period of 1 year was to allow the members of the cohort to settle in more sustainable states of activity.
The model incorporated health benefits from PA via reduced risk for coronary heart disease (CHD), stroke and type 2 diabetes (T2D), as they have a high prevalence and PA interventions exist which protect against these conditions. While many other conditions are thought to be associated with PA, these three conditions were chosen because there is relatively more robust quantifiable evidence on the relationship between PA and their incidence.19 Members of the cohort who were physically active at the end of the run-in period were assumed to have a better life expectancy and quality of life, due to lower risks of developing CHD, stroke and T2D. However, the model assumed that nobody would develop CHD, stroke or T2D during the run-in period, although they could die from other causes during this time. This was a conservative assumption, as it introduced a delay in the onset of protective health benefits after people become physically active.
From the beginning of year 1 (cycle 2), each member of the cohort had to be in one, and only one, of six health states: (1) event free (no CHD, stroke, or T2D), (2) non-fatal CHD, (3) non-fatal stroke, (4) T2D, (5) death related to CVD and (6) death from non-CVD causes. Those who survived the run-in period started in the event-free state (1), and then faced defined annual risks of moving to the other states. RRs for developing CHD, stroke and T2D were estimated from epidemiological studies, which measured baseline PA (exposure) and related this to subsequent onset of CHD, stroke or T2D (outcomes) over a defined follow-up period (10 years). There is evidence that PA habits can be quite changeable.20 Note that although changes in PA after the run-in period were not explicitly modelled, the impact of changing habits is incorporated in the cohort RR estimates. The studies used16–18 followed up the same people (who were either active or inactive at baseline) for a number of years, during which some of the inactive people might have become active or vice versa, diluting the observed relationships between activity and outcomes.
The model assumed that a given proportion of CHD events and of strokes would be immediately fatal. People who survived one of these CVD events had an increased subsequent risk of CVD and non-CVD mortality. Similarly, the onset of T2D increased CVD and non-CVD mortality. For simplicity, individuals were assumed to experience only one type of disease event (CHD, stroke or T2D). The non-fatal CHD, non-fatal stroke and T2D health states should therefore be regarded as containing mixed populations, including patients with comorbidities and established disease as well as incident cases with a single diagnosis.
Estimates of lifetime costs and QALYs were obtained from the model by weighting the time spent by the cohort in the various health states by the annual costs of treatment and utility values associated with each state. The cost of delivering BA was also added in the first year for the intervention arm. In addition, individuals who became active in the first year (in both arms) were attributed a utility gain to reflect the short-term psychological benefits of PA.21 22 Economic evaluation of PA interventions, to date, has hardly accounted for the short-term psychological benefits (ie, mental simulation during exercise, or improved social interactions resulting from group participation)22 and rather has focused on the long-term effects of sustained PA on the incidence of chronic conditions. The details of the derivation of this utility estimate are available from Pavey et al.22
The model adopted a healthcare (National Health Service, England) perspective, applied discounting to treatment costs and health outcomes at the rates of 3.5% per annum23 and costs were adjusted using inflation indices.24 The costs are expressed in £2010–2011 prices.
Model inputs
Online supplementary table S1 shows the data used to populate the model. Data were obtained from a systematic literature review and meta-analysis of clinical and economic evidence on BA (for the effectiveness and cost of BA); economic evaluations that were conducted for the existing NICE guidelines for CVD and diabetes (for cost and utility estimates for disease conditions) and the national/international guideline reports that set out the science-based guidance on PA and health, for example, the US Physical Activity Guidelines Advisory Committee Report25 (for health impacts of PA). The clinical evidence (in rates) was converted to probabilities following the approach in Briggs et al26 as appropriate.
The RRs of CHD, stroke and T2D for physically active compared with inactive people were based on cohorts that were followed up for 19 (CHD, stroke) and 12 years (diabetes).16–18 As noted earlier, these RRs accounted for variations in PA during the follow-up periods. However, assuming that these estimates would hold beyond the follow-up periods might be unrealistic. It was therefore assumed, conservatively, that these RR estimates held for an initial 10-year period, after which no benefit would persist, that is, the RRs for developing CHD, stroke and T2D in the first 10 years of the model were based on Hu et al,16–18 but from year 11 onwards they were assumed to be equal to 1. This assumption was tested in the sensitivity analysis.
The probabilities for developing the disease conditions used in the model were derived using the following steps. First, the probabilities of developing these conditions among inactive people were estimated by adjusting the UK general population age-specific incidence rates28–30 using the attributable risk fraction.34 To adjust these estimates appropriately, the second step estimated the probability of developing the conditions among active individuals using the RR estimates identified from Hu et al.16–18
The probability that a primary stroke or CHD event was fatal28 was assumed to be independent of PA. Although this is a simplification, as these probabilities could depend on the level of PA, lack of data precluded adjusting for such a possibility.
To derive the probability of CVD-related (ie, CHD and stroke) and non-CVD-related mortality, RRs of CVD-related and non-CVD-related mortality27 28 among people with CHD, stroke and T2D were used to adjust age-specific probabilities for ‘healthy people’. The age-specific probabilities were derived using age-specific UK interim life tables from Government Actuaries Department that were adjusted by age-specific UK annual incidence of mortality prepared by the Office of National Statistics. The RR estimates for diabetic patients were based on a cohort of Framingham Heart Study (aged 45–74) that were followed for up to 25 years.28 For stroke patients, data were obtained from Bronum-Hansen et al27 that followed a cohort of 25+-year-olds for 10 years (after their first non-fatal stroke). These same data28 were applied to patients with CHD due to data constraints.
Sensitivity analyses
Deterministic and probabilistic sensitivity analyses (PSA) were used to explore the uncertainty around parameter estimates. One-way deterministic sensitivity analysis was used to examine the uncertainty around effectiveness parameter, health impacts of PA, the starting age of cohort, the discount rate and costs. Changes in starting age of cohort were to provide an indication of the impact of exposing older people to BA. The variations in cost were to demonstrate how differing recruitments to BA (ie, opportunistic vs disease register) or staff changes affect the efficiency of BA given that different definitions for BA can be provided by various types of health professionals. The choice of parameter ranges reflected alternative values from the literature and potential policy targets that were noted in discussions with the NICE officials.
Uncertainties around all parameters in the model (except baseline mortality—from census data and national database that are less likely to have errors) were addressed simultaneously using PSA. The details of the distributions and data used in the PSA are provided in table 1. A total of 10 000 Monte Carlo simulations were used for the PSA to generate stable estimates.
Table 1.
Base-case cost-effectiveness results comparing BA with usual care
| BA | Usual care | Incremental | |
|---|---|---|---|
| Mean (95% interval*) | |||
| Costs (lifetime)† | £155 m (140.5, 170.5) | £154.2 m (138.7, 169.7) | £806k (610, 998) |
| QALYs (lifetime) | 1.828 m (1.507, 2.186) | 1.827 m (1.506, 2.185) | 466 (101, 1109) |
*Based on 10 000 Monte Carlo simulations.
†In 2011 prices; m=million, k=thousand.
BA, brief advice; QALYs, quality-adjusted life-years.
Model verification and validation
Good practice guidance for verification and validation35 36 was followed. The computer model was reviewed and tested by experienced modellers not connected to this study to ensure that it behaved in accordance with the conceptual model. In addition, the models were cross-validated with real-world observations. For example, model predictions of the incidence of cardiovascular events were compared with observed event rates from clinical trials (not used in the construction of the model).
Results
Base case analysis
On the basis of a cohort of 100 000 people offered BA compared with usual care, an estimated 6994 additional people would become active (at the end of year one) at a total cost of £950 000 (£136/additionally active person). BA also averted an estimated 2.4 CHD, 1.8 stroke and 3.1 diabetes events, as well as 1 death in 10 years. Table 1 shows that compared with usual care, BA is more expensive as it is estimated to generate additional costs of £806 809, but it is also more effective leading to 466 QALYs gained in the total cohort (mean QALY gain of 0.0047/person). The incremental cost effectiveness ratio (ICER) for BA compared with usual care was £1730/QALY gained, which can be considered cost-effective at a threshold of £2000/QALY and well below the £20 000/QALY threshold as indicated by the NICE public health reference case.23
Sensitivity analyses
Online supplementary table S3 indicates that variations in parameter estimates largely resulted in some occasions ICERs that were not ‘decisionally’ significant according to the current thresholds for cost-effectiveness in England. For example, plausible changes to the assumed effectiveness of BA led to ICER estimates that remained well below the lower NICE threshold of £20 000/QALY gained. The ICER was, however, sensitive to the inclusion of short-term psychological benefits associated with PA. The longer the length and the higher the value of gain, the lower the ICER is. Moving from 0.07 to 0.01 still resulted in an ICER of less than £9000/QALY. However, excluding any short-term psychological benefits from exercise itself led to an ICER of £27 000/QALY gained, which is of borderline cost-effectiveness. Conversely, improved cost-effectiveness estimates were observed for relatively older cohorts. For example, using a start-up age of 54 years (and beyond) for the cohort resulted in BA dominating usual care.
Whether the BA (compared with usual care) is considered cost-effective depends on the maximum amount decision makers are willing to spend to obtain an additional QALY. The cost-effectiveness acceptability curve (see figure 2) based on 10 000 Monte Carlo simulations shows that at a threshold of £2000/QALY there is an estimated 0.52 probability that BA is cost-effective. This increases to 0.91 when a threshold of £5000 is considered and the probability further rises to about 0.99 given a threshold of £20 000.
Figure 2.
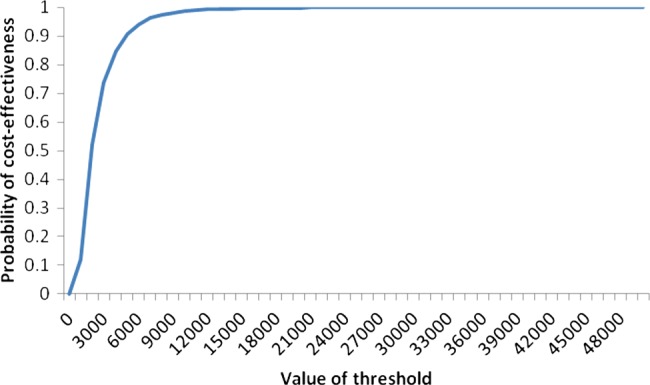
Cost-effectiveness acceptability curve showing the probability of cost-effectiveness for brief advice at different threshold levels.
Discussion
This study evaluates the cost-effectiveness of BA using a Markov modelling approach, and addresses key limitations in the literature regarding efficiency. The base case analysis resulted in an estimated cost-effectiveness ratio of £1730/QALY gained from BA compared with usual care. This is significantly below the usual cost-effectiveness threshold for England which ranges from £20 000 to £30 000/QALY. The lifetime QALY gain per person as a result of BA is estimated at approximately 0.005. If each QALY gain is valued at £20 000 then BA could generate benefits that in monetary terms is about £93/person which exceeds the cost of the intervention (£9.50/person). These findings are consistent with the existing limited literature of BA that suggests that it is cost-effective at £20 000/QALY.
The base case results were generally robust to probabilistic and deterministic sensitivity analysis with the latter showing that at £20 000 threshold, there is a 99.9% chance that BA will be cost-effective. The ICERs improve when the start-age of the cohort increases or recruitment to BA was changed from the opportunistic centres to disease register. This suggests that the payoffs may be higher if BA is targeted at older individuals (particularly beyond 50 years as indicated in the deterministic sensitivity analysis).
However, a number of uncertainties remain. As the ICER was sensitive to inclusion and size of direct quality of life benefits (short-term psychological benefits) of PA, it is essential to assess the limitations of the evidence base related to these benefits. First, the analysis used an estimate based on cross-sectional data, and as such a causal relationship between utility gains and PA cannot be claimed. While participation in PA could lead to mental health benefits,21 concerns exist over whether the utility estimate used in our study represents a mental health gain or the long-term benefits (reduced risk for ill-health conditions) of PA. Nevertheless, as other disease conditions related to PA were adjusted for in the analysis, the utility gain might be argued to more closely approximate mental health benefits.37 Also, sensitivity analysis showed that assumptions around the magnitude and duration of the mental health gains were not decisionally important, as BA remained cost-effective at the upper threshold value of £30 000/QALY when this benefit was excluded. Nevertheless, further evidence on mental health or ‘well-being’ benefits of PA (estimated using, eg, longitudinal data) would still be valuable.
The model accounted for the long-term impact of PA on selected morbidities. However, there are other morbidities that may be affected by PA that were excluded from the analysis. Their exclusion might have underestimated the cost-effectiveness of BA. Another possible bias may arise from our omission of secondary transitions between the disease conditions, which may have reduced the estimated negative effects of physical inactivity. Conversely, the exclusion of adverse effects of PA (eg, injuries) may have led to overestimated cost-effectiveness. Although fear of injury could influence delivery of BA or participation in PA, mostly in the elderly, the evidence suggests such injuries might be rare38 and not likely to significantly affect the results at a population level.
This study uses previous research as a point of departure, and builds on this through a number of improvements including: (1) time-based modelling (2) more extensive exploration of uncertainty around input parameters and assumptions, (3) more conservative assumptions around changes in PA over time that underestimated benefits of PA and (4) use of meta-analysed effectiveness data. Although this study did not produce ‘decisionally’ different results, there are a number of benefits associated with the new approach. First, it demonstrated that assumptions around the maintenance of PA levels beyond BA determine how cost-effective the intervention is. Second, short-term mental health benefit was found to be an important outcome in modelling the cost-effectiveness of BA. Third, the cost-effectiveness of BA was shown to vary by age, with improved cost-effectiveness in older cohorts. Nevertheless, the limitations of this analysis point to the need for new data and for more accurate evidence on factors contributing to the cost-effectiveness of BA to increase PA.
What is already known about this topic?
Only 3 of 10 adults in England undertake a level of activity that is sufficient to meet the recommended levels of physical activity (PA), as defined by the department of health.
Brief advice (BA) in primary care is considered as an effective way of increasing physical activity among adults.
There is little evidence on the cost-effectiveness of BA in primary care and concerns exist about the rigour of the limited evidence base.
What this study adds?
This study builds on the current limited economic literature through a number of improvements including: (1) time-based modelling, (2) more extensive exploration of uncertainty around input parameters and assumptions, (3) more conservative assumptions around changes in PA over time and (4) use of meta-analysed effectiveness data.
BA in primary care, compared with usual care, is shown to be a cost-effective way to improve physical activity among adults.
The cost-effectiveness of BA in primary care appears to vary with the start-up age of individuals.
How might it impact on clinical practice in the near future?
Supports the provision of BA in primary care for physically inactive people.
Future delivery of BA in primary care might target older adults (beyond 50 years) as it offers more value for money.
Recruitment of individuals for BA in primary care might be based on disease registers rather than opportunistic centres.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the important contribution of the National Institute for Health and Care Excellence (NICE) in conducting this work, particularly Kim Jeong, James Jagroo and Simon Ellis.
Footnotes
Contributors: NKA led the modelling with inputs from JL and JFR. JL reviewed the spreadsheet model. NKA wrote the first draft and JFR, and JL edited the drafts.
Funding: This study was funded by the NICE Centre for Public Health Excellence (grant number; R31171).
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Department of Health Healthy lives, healthy people: our strategy for public health in England. London: Department of Health, 2010 [Google Scholar]
- 2.Department of Health Start active, stay active: a report on physical activity from the four home counties’ chief medical officers. London: Department of Health, 2011 [Google Scholar]
- 3.Craig R, Mindell J, Hirani V. Health Survey for England 2008. Volume 1: physical activity and fitness. London: National Health Service Information Centre, 2009 [Google Scholar]
- 4.Matrix Rapid review of the economic evidence of physical activity interventions. London: Matrix Knowledge, 2006. [Google Scholar]
- 5.Matrix Modelling the cost-effectiveness of physical activity interventions. London: Matrix Knowledge, 2006 [Google Scholar]
- 6.National Institute for Clinical Excellence (NICE) Four commonly used methods to increase physical activity: brief interventions in primary care, exercise referral schemes, pedometers and community-based walking and cycling. Public Health Guidance No 2. London: NICE, 2006 [Google Scholar]
- 7.U.S. Preventive Services Task Force Behavioural counselling interventions to promote a healthful diet and physical activity for cardiovascular disease prevention in adults: U.S. Preventive Services Task Force Recommendation Statement. AHRQ Publication No. 11-05149-EF-2. 2012. http://www.uspreventiveservicestaskforce.org/uspstf11/physactivity/physrs.htm (accessed Nov 2012). [DOI] [PubMed]
- 8.European Union Working Group ‘Sport & Health’. European Union physical activity guidelines: recommended policy actions in support of health-enhancing physical activity. Brussels: EU, 2008 [Google Scholar]
- 9.Weiler R, Stamatakis E. Physical activity in the UK: a unique crossroad? Br J Sports Med 2010;44:912–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orrow G, Kinmonth AL, Sanderson S, et al. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ 2012;344:e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell F, Blank L, Messina J, et al. National Institute for Health and Clinical Excellence (NICE) public health intervention guidance physical activity: BA for adults in primary care. Review of effectiveness evidence. London: NICE, 2012 [Google Scholar]
- 12.National Institute for Health and Clinical Excellence (NICE) Public health guidance draft scope 1: physical activity: BA for adults in primary care. London: NICE, 2011 [Google Scholar]
- 13.Anokye NK, Jones T, Fox-Rushby J. National Institute for Health and Clinical Excellence (NICE) public health intervention guidance physical activity. Physical activity BA in primary care (partial update PH2): review of economic evidence. London: NICE, 2012 [Google Scholar]
- 14.Pringle A, Marsh K, Gilson N, et al. Cost-effectiveness of interventions to improve moderate physical activity: a study in nine UK sites. Health Educ J 2010;69:211–22 [Google Scholar]
- 15.Boehler CEH, Milton KE, Bull FC, et al. The cost of changing physical activity behaviour: evidence from a ‘physical activity pathway’ in the primary care setting. BMC Public Health 2011;11:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu G, Qiao Q, Silventoinen K, et al. Occupational, commuting and leisure-time physical activity in relation to risk for type 2 diabetes in middle-aged Finnish men and women. Diabetologia 2003;46:322–9 [DOI] [PubMed] [Google Scholar]
- 17.Hu G, Sarti C, Jousilahti P, et al. Leisure time, occupational and commuting physical activity and the risk of stroke . Stroke 2005;36:1994–9 [DOI] [PubMed] [Google Scholar]
- 18.Hu G, Jousilahti P, Borodulin K, et al. Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis 2007;194:490–7 [DOI] [PubMed] [Google Scholar]
- 19.Beale S, Bending M, Trueman P. An economic analysis of environmental interventions that promote physical activity. University of York: York Health Economics Consortium, 2007 [Google Scholar]
- 20.Picavet HS, Wendel-vos GS, Vrecken HL, et al. How stable are physical activity habits among adults? The Doetinchem cohort study. Med Sci Sports Exerc 2011;43:74–9 [DOI] [PubMed] [Google Scholar]
- 21.Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry 2005;18:189–93 [DOI] [PubMed] [Google Scholar]
- 22.Pavey T, Anokye N, Taylor A, et al. The effectiveness and cost effectiveness of exercise referral schemes: a systematic review and economic evaluation. Health Technol Assess 2011;15:1–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Health and Clinical Excellence (NICE) Methods for the development of NICE public health guidance (second edition). London: NICE, 2012 [Google Scholar]
- 24.Personal Social Services Research Unit Unit costs of health & social care. Kent: University of Kent, 2011 [Google Scholar]
- 25.US Department of Health and Human Services Physical activity guidelines advisory committee report from the Physical Activity Guidelines Advisory Committee. Washington, DC: US Department of Health and Human Services, 2008 [Google Scholar]
- 26.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York: Oxford University Press Inc, 2006 [Google Scholar]
- 27.Brønnum-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke 2001;32:2131–6 [DOI] [PubMed] [Google Scholar]
- 28.Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009; 119:1728–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward S, Jones ML, Pandor A, et al. Statins for the prevention of coronary events. London: National Institute for Clinical Excellence, 2005 [Google Scholar]
- 30.National Clinical Guideline Centre Hypertension: the clinical management of primary hypertension in adults. Clinical guideline: methods, evidence and recommendations. London: National Institute for Health and Clinical Excellence, 2011 [Google Scholar]
- 31.Gonzalez ELM, Johansson S, Wallander MA, et al. Trends in the prevalence and incidence of diabetes in the UK: 1996–2005. J Epidemiol Community Health 2009;63:332–6 [DOI] [PubMed] [Google Scholar]
- 32.National Centre for Social Research, University College London—Department of Epidemiology and Public Health Health Survey for England, 2008 [computer file]. 3rd edn Colchester, Essex: UK Data Archive [distributor], 2011. SN: 6397 [Google Scholar]
- 33.Ward S, Jones ML, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11:1–160 [DOI] [PubMed] [Google Scholar]
- 34.Jamrozik K. Estimate of deaths attributable to passive smoking among UK adults: database analysis. BMJ 2005;9:7495–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philips Z, Ginnely L, Sculpher M, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8:1–172 [DOI] [PubMed] [Google Scholar]
- 36.Chilcott J, Tappenden P, Rawdin A, et al. Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review. Health Technol Assess 2010;14:1–107 [DOI] [PubMed] [Google Scholar]
- 37.Anokye NK, Trueman P, Green C, et al. The cost-effectiveness of exercise referral schemes. BMC Public Health 2011;11:954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munro J, Brazier J, Davey R, et al. Physical activity for the over-65s: could it be a cost-effective exercise for the NHS? J Public Health Med 1997;19:397–402 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.