Abstract
Introduction
It is imperative that laboratories receive uncontaminated urine samples to avoid giving false-positive results and reduce antimicrobial use.
Aim
The aim of the study was to investigate a novel urine collection device (Peezy) in a renal outpatient clinic to determine whether it reduced contamination of urine samples.
Methods
The novel device was used in 420 renal transplant recipients and the results were compared with 424 matched historical controls, who used the standard method of urine collection. High epithelial cell counts on microscopy and mixed urine cultures were used to identify contaminated samples.
Results
Peezy increased the rates of both epithelial cells and mixed growths in the urine samples when compared with the historical controls.
Conclusions
Further randomised studies in other more generalisable populations need to be performed.
Keywords: Diagnosis, Urine, Infections, Transplantation
Introduction
Urinary tract infections (UTIs) are an extremely common cause of antibiotic prescriptions both in primary and secondary care and affect up to 15% of the female population each year.1 UTIs account for 1–3% of all consultations and are the second commonest reason for an antibiotic script to be given in general practice after respiratory tract infections.2
Accurate diagnosis depends on a positive urine culture correlated with symptoms in the patient.3 Urine samples are often contaminated by commensal organisms in the distal urethra whatever the collection method and this can lead to false-positive results. A background contamination rate of 30% in young symptomatic women is not unusual and women are more likely to provide contaminated specimens.4
UTIs are the commonest infection in renal transplant recipients. Studies have estimated the incidence to be between 30% and 60%5 6 and they account for approximately 40–50% of all infectious complications.7 In transplant patients, it is especially important to make the correct diagnosis, as symptoms are often lacking. When a mixed urine culture is detected in transplant patients, the clinician may be inclined to treat rather than repeat the sample. However if this was a contaminated sample, this would lead to inappropriate antibiotic use, which selects for more resistant and difficult-to-treat organisms in future.8 There is already evidence of high levels of resistance in transplant populations to the commonly used oral antibiotics, such as co-amoxiclav, trimethoprim and ciprofloxacin.9
The standard method of urine collection is a mid-stream urine (MSU).
Early studies suggested that this may be as effective as catheterisation,10 but other studies have not backed this up.4 11 In the antenatal population, one study indicated that contamination was reduced by using a novel collection device, which relied on urodynamic principles; this excluded the initial low-flow portion of the urinary stream and collects the mid-stream volume without interrupting the flow.12 Reduction of contamination should improve patient care by reducing repeat samples and inappropriate antibiotic prescriptions.
This study investigated a novel urine collection device (Peezy, Funnelly Enough Ltd, London, UK, figure 1) to determine whether this device would reduce the rates of contamination in female renal transplant patients.
Figure 1.
The Peezy device.
Methods
Between June 2010 and February 2011, consecutive female patients, attending renal outpatient clinics as part of their routine follow-up, consented verbally to give a urine sample using the Peezy device. Patients were excluded if they were men, catheterised, unable to hold the device or unable to understand the written and/or verbal instructions. Transplant patients have urine sent for analysis at each clinic appointment routinely in the first 12 weeks after surgery. After this period, urine is sent for analysis where the dipstick is suggestive of infection.
The Peezy device (figure 1) incorporates a sponge to collect the first pass of urine. The device is simply placed under the perineum and the patient allows the urine to flow. The urine passes through the device and the cellulose sponge expands as the first 10–15 mL of urine is passed. The urine is then forced into the universal container. Any overflow from this passes into the toilet. The container can then be unscrewed from the device. This device has been shown in laboratory tests using dye to accurately collect the mid-stream portion of the urine. It has also been shown that it does not contaminate sterile saline, nor affects the growth of common urinary pathogens or have an effect on urine dipstick testing.
One thousand devices were distributed over this time period. Written instructions were provided and posters were present in the unit. Trained outpatient nurses assisted with explanations. Patients were instructed to screw the universal gently onto the funnel device and hold it against the perineum where marked on the funnel, then ‘let the urine flow’. Once completed, the patient unscrewed the upright universal container from the device, discarded the funnel, put a lid on the universal container and returned it to the clinic staff.
The study specimens were identified in the microbiology laboratory by a yellow label, which was put on during the kit manufacture. The urines underwent routine microscopy and culture according to the local standard operating procedure. Microscopy was carried out using 60 μL urine and examined in a microtitre plate with an inverted light microscope. Then, a 2.5 μL loop was used to culture the urine onto Cysteine lactose electrolyte deficient (CLED) media, which was then incubated aerobically at 35°C overnight. These procedures were carried out by trained healthcare scientists, who were unblended. A tag was added to the laboratory information system to mark that the sample was included in the study.
Contaminated urine was defined as either squamous (epithelial) cells visible in urine microscopy or a mixed growth on urine culture. Mixed growth was defined as those with ≥2 different types of organisms at >105/mL or ≥2 different types of organisms at 104–105/mL or ≥1 different type of organism at <104/mL.13 Squamous epithelial cells from the skin surface or from the outer urethra can appear in urine. They can act as a surrogate marker for contamination, indicating that skin flora from around the perineum may have contaminated the sample. Therefore, data on epithelial cell counts on microscopy were also collected.
The study samples were then compared with historical consecutive controls from female transplant patients who had given a urine specimen in the same clinic using the standard method (passing the MSU into a sterile jug) between January 2010 and March 2010. The processing methods in the microbiology department were identical across both periods.
Individuals who used the Peezy device were also requested to complete a questionnaire. Questions about ease of use and urine spillage were included. Patients were also asked how likely they would be to use the device again and how it compared with their usual method of giving a MSU. The questionnaire was adapted after an initial pilot, such that questions were added to determine whether patients found it easier to use after more than one attempt.
Demographic details, including age, reason for renal failure and underlying renal function, were collected.
This study was performed as a service evaluation to assess the results and feasibility in this patient cohort. There was no randomisation or double collecting of samples. The Peezy device was chosen as it was available in the National Health Service (NHS) prescription service. It was practical to use and had a sound method for producing a MSU.
Statistical analysis
Sample size was calculated as a total of 870 patients (n=435 of Peezy patients compared with 435 historical controls) based on an estimated reduction from 10% to 5% in mixed growths from prior studies.
Statistical analysis was performed using STATA V.11.0. Means and medians were calculated, and Student t test and Mann-Whitney U test were used to compare variables as appropriate. Categorical/grouped variables were analysed using χ2 tests.
Results
Within the time frame, 424 historical patients (control group) and 420 Peezy users (study group) were included in the analysis. Table 1 outlines age, estimated glomerular filtration rate (eGFR) and underlying diagnosis in each group. The patient's eGFR was calculated using the serum creatinine, age and sex according to the Modification of Diet in Renal Disease (MDRD) formula.
Table 1.
Baseline characteristics of control and study groups
| Peezy group n=420 | Controls n=424 | p Value | |
|---|---|---|---|
| Age Mean (95% CI)* |
47.7 (46.3 to 49.1) | 46.4 (45.1 to 47.7) | 0.17 |
| eGFR Median (IQR)† |
51 (35–71) | 46 (29–66) | 0.002 |
| Underlying renal diagnosis n (%)‡ | <0.001 | ||
| Hereditary | 75 (17.6) | 54 (12.7) | |
| Autoimmune | 129 (30.2) | 142 (33.5) | |
| Vascular | 36 (8.5) | 19 (4.5) | |
| Infection | 51 (12.0) | 40 (9.4) | |
| Functional | 2 (0.5) | 12 (3.1) | |
| Metabolic | 60 (14.1) | 46 (10.9) | |
| Unknown | 75 (14.1) | 110 (25.9) |
*t Test.
†Mann–Whitney test.
‡χ2 Test.
eGFR, estimated glomerular filtration rate.
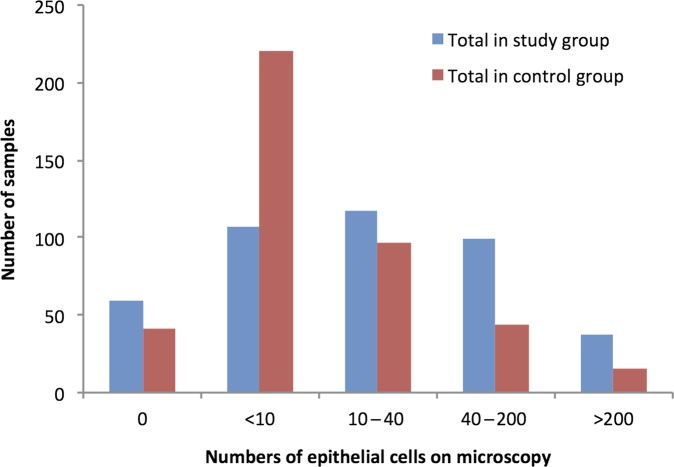
The results of the laboratory analysis are outlined in table 2. There were an additional 3.7% mixed growth cultures in the Peezy group compared with historical controls (p=0.1). However, both white blood cells and epithelial cells were present in significantly higher concentrations in those samples taken with the Peezy device. The epithelial cell data are outlined in figure 2.
Table 2.
Urine results in control and study groups
| Peezy group n=420 N (%) |
Controls n=424 N (%) |
p Value* | |
|---|---|---|---|
| Urine results | 0.1 | ||
| Negative | 349 (83.0) | 356 (84.0) | |
| Mixed growths | 39 (9.3) | 21 (5.6) | |
| Pure significant growths | 32 (7.6) | 47 (11.1) | |
| WBC/mm3 | <0.001 | ||
| <10 | 205 (49.0) | 299 (71.7) | |
| 10–40 | 104 (24.9) | 54 (13.0) | |
| 40–200 | 71 (17.0) | 40 (9.6) | |
| >200 | 38 (9.1) | 24 (5.7) | |
| Epithelial cells/mm3 | 0.008 | ||
| Low (<200) | 379 (90.5) | 397 (95.2) | |
| High (>200) | 40 (9.5) | 20 (4.8) |
*χ2 Test.
WBC, white cell count.
Figure 2.
Numbers of epithelial cells on microscopy in control and study groups.
A multivariate analysis was performed to determine whether the differences in groups contributed to the test results. Elevated concentrations of epithelial cells were twice as likely in the Peezy group compared with the controls (OR 2.1 (95% CI 1.2 to 3.7)) when controlled for significant variables in the univariate analysis (eGFR and underlying diagnosis).
Thirty-five ‘pilot’ questionnaires were returned. Forty-six per cent reported that they found the Peezy more difficult than their normal collection method. Looking at the adapted questionnaire, only 31 were returned for evaluation. Thirty-five per cent reported that they had problems, including spillage, using the Peezy device. In those patients who used Peezy more than once, 75% reported finding it just the same with 13% finding it easier and the rest finding it more difficult.
Discussion
The Peezy device, designed to reduce contamination rates of MSU, did not demonstrate this in our study. The reason for this is unclear, with both white cell counts and epithelial cells being significantly higher in the Peezy group and also a clinically (although not statistically) significant increase in mixed cultures.
Previous studies have demonstrated higher contamination rates of MSU than we detected in our population, which is likely to have influenced our results.4 10 The historical controls used in our study detected only 5.6% had a mixed culture or contaminated urine compared with a multicentre study using an alternative device, which demonstrated that the device reduced contamination from 14% to 9%.12 This may reflect that this specialist population have acquired skills in performing MSU; renal patients may have a better understanding of the importance and principles of providing a MSU than patients attending an antenatal clinic and using a sterile jug as standard.
The main difference between the alternative device that has been studied and the Peezy is method of removal of the first pass urine.12 The first pass was collected in the sponge in the Peezy and we postulated that this may have diverted more cells and bacteria from the first part of the flow into the universal container. Some patients voiced concern that the way one held the device may have mixed the urine in the collection container with the urine in the funnel and/or sponge.
During the study, over 1000 Peezy kits were distributed to the outpatient clinic but only 424 urine specimens labelled with the yellow sticker were entered into the study database in microbiology. We explored reasons for this: some Peezy containers may have been used for cytology or biochemistry; once a patient is greater than 12 weeks post-transplant, samples are only sent for culture if the dipstick is positive, so samples taken with the device would have been discarded. The clinic nurses also reported that some patients were given a Peezy device but were then unable to produce a sample to the device was discarded. However, the mismatch in devices provided and samples reaching the laboratory should not have biased the results, as the policy of when to send urines for culture did not change between the historical control period and study period. It is also possible that some samples with yellow tops were missed from the study database but again this should not have influenced the results.
The final limitation of this study is that it was not a randomised trial. By matching the historical controls and the study patients, this should have minimised bias but there were some significant differences in the two patient populations, where renal function and underlying diagnosis were concerned, which we adjusted for in the multivariate analysis. However, we cannot know for certain that another unintended sampling bias may have been introduced at the clinic.
With only a 16% return rate from the questionnaire, we cannot make an assessment on patient considered feedback. However, there were complaints of urine being spilt and the instructions not being clear despite the staff in the unit explaining the method and posters and information leaflets being freely available. This is contrary to the more positive feedback of the device used in the antenatal population and likely relates to the renal patient expertise with this test.
Conclusion
Using the Peezy in the female renal transplant population did not decrease rates of contamination in urine samples. We cannot endorse the widespread use of this device without further studies, ideally randomised, in non-renal populations.
Take-home messages.
It is imperative that laboratories receive uncontaminated urine samples to be able to diagnose infection accurately.
The study investigated the use of a new urine collection device to determine whether it decreased contamination rates.
In the female renal transplant patient population, the device did not decrease contamination rates.
What this study adds to the literature.
To the best of our knowledge, this is the only study that has investigated the use of this novel urine collection device; therefore, it makes an important contribution to the current literature on the best method for the collection of an uncontaminated urine sample.
Footnotes
Contributors: All of the named authors contributed to the design of the study, data collection and analysis. SEC drafted this article, which was then commented by the other authors.
Funding: The Peezy kits were provided by Funnelly Enough Ltd and they also provided onsite training for nurses in the renal clinics.
Competing interests: None.
Ethics approval: This study was performed as a service evaluation to assess the results and feasibility in this patient cohort.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The authors would be happy for unpublished data from the study to be seen by the reviewers or editors as necessary.
References
- 1.Car J. Urinary tract infections in women: diagnosis and management in primary care. BMJ 2006;332:94–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen I, Hayward AC. Antibacterial prescribing in primary care. J Antimicrob Chemother 2007;60(Suppl 1):i43–7 [DOI] [PubMed] [Google Scholar]
- 3.Diagnosis of UTI—quick reference guide for primary care. Health Protection Agency 2011 24 January 2013. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947404720
- 4.Lifshitz E, Kramer L. Outpatient urine culture: does collection technique matter? Arch Intern Med 2000;160:2537–40 [DOI] [PubMed] [Google Scholar]
- 5.Chuang P, Parikh CR, Langone A. Urinary tract infections after renal transplantation: a retrospective review at two US transplant centers. Clin Transplant 2005;19:230–5 [DOI] [PubMed] [Google Scholar]
- 6.Sorto R, Irizar SS, Delgadillo G, et al. Risk factors for urinary tract infections during the first year after kidney transplantation. Transplant Proc 2010;42:280–1 [DOI] [PubMed] [Google Scholar]
- 7.Saemann M, Horl WH. Urinary tract infection in renal transplant recipients. Eur J Clin Invest 2008;38(Suppl 2):58–65 [DOI] [PubMed] [Google Scholar]
- 8.Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- 9.Brudney D, Harber M, Seneviratne L, et al. A retrospective review of urinary tract infections in our 2010 renal transplant patients: predictors of infection and sensitivities data, in American Transplant Congree 2012:Boston.
- 10.Walter FG, Knopp RK. Urine sampling in ambulatory women: midstream clean-catch versus catheterization. Ann Emerg Med 1989;18:166–72 [DOI] [PubMed] [Google Scholar]
- 11.Morris RW, Watts MR, Reeves DS. Perineal cleansing before midstream urine, a necessary ritual. Lancet 1979;2:158–9 [DOI] [PubMed] [Google Scholar]
- 12.Jackson SR, Dryden M, Gillett P, et al. A novel midstream urine-collection device reduces contamination rates in urine cultures amongst women. BJU Int 2005;96:360–4 [DOI] [PubMed] [Google Scholar]
- 13.Michael L. Wilson and Loretta Gaido Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis 2004;38:1150–8 [DOI] [PubMed] [Google Scholar]