Summary
Background and objectives
Previous studies have identified inflammatory features that enable the prediction of renal outcome of IgA nephropathy (IgAN); however, validation of these findings is still needed. This prospective study was performed to determine the characteristics of renal interstitial infiltration and tertiary lymphoid organ (TLO) neogenesis in a cohort of Chinese patients with IgAN.
Design, setting, participants, & measurements
Adult patients with IgAN were recruited into this study from June 2009 to June 2010. Inflammatory cells in renal biopsy tissues were detected by immunohistochemistry and immunofluorescence. Correlations between the density of interstitial inflammatory cells, grades of TLOs, and clinicopathologic features were evaluated. Of 152 eligible patients, 72 (47%) were successfully followed-up by telephone at 30 months after renal biopsy. Twelve patients were classified as the severe group and 60 patients were classified as the stable group, according to the progression of serum creatinine levels during the follow-up period. A comparison of the severity of interstitial infiltration and the frequency of TLO neogenesis between the two groups was performed.
Results
The accumulation of interstitial inflammatory cells was correlated with decreased renal function, heavy proteinuria, and severe glomerular, interstitial, and arterial lesions in patients with IgAN. TLOs, identified as nodular inflammatory infiltrates containing organized DC-SIGN+, CD4+, CD8+, and CD20+ cells, were observed in 37.5% of patients. Patients with high-grade TLOs exhibited a high percentage of mesangial hypercellularity and crescents as well as severe interstitial and arterial lesions. Patients in the severe group exhibited more severe interstitial infiltration and a higher percentage of TLO neogenesis (83.3% versus 33.3%; P=0.001) compared with patients in the stable group.
Conclusions
As contributors to an active local inflammatory response, the severity of interstitial infiltration and the frequency of TLO neogenesis are correlated with glomerular, interstitial, and arterial lesions as well as IgAN progression.
Introduction
IgA nephropathy (IgAN), which features IgA deposition in the glomerular mesangium, is the most common form of primary GN worldwide (1,2). The average renal survival rates at 5 and 10 years are 85.1% and 77.1%, respectively (3). Assessing the prognosis is challenging but extremely important. Previous studies have identified that clinical features such as severe proteinuria, arterial hypertension, and elevated serum creatinine are predictors of IgAN (4–7). Histologic features are also recognized as important prognostic factors in IgAN (7). According to the Oxford IgAN classification, mesangial hypercellularity (M), segmental glomerulosclerosis (S), endocapillary hypercellularity (E), and tubular atrophy/interstitial fibrosis (T) are histologic predictors of IgAN prognosis independent of the clinical features (8,9). The MEST scoring system has recently been confirmed in several independent populations and is proven to be a valuable tool for prognostic purposes (10,11). However, other risk factors associated with MEST variables should also be included.
Interstitial inflammatory infiltration is another prominent pathologic feature associated with IgAN. Inflammatory cells present in the renal interstitium include monocytes/macrophages, dendritic cells, and T and B lymphocytes (12–16). CD68+ macrophage accumulation and CD3+ or CD8+ lymphocyte infiltration are suggested to predict IgAN progression (12,13,15) DC-SIGN+ cells, a special subset of dendritic cells that is essential for dendritic cell–induced T cell proliferation, have also been found in the kidney of different types of human GN (14). However, there is no detailed research on DC-SIGN+ cells in IgAN. Interstitial B lymphocyte infiltration has also been reported (16). However, there are few studies of all cellular combinations in patients with IgAN.
Tertiary lymphoid organs (TLOs) are actually nodular inflammatory infiltrates containing organized dendritic cells, B and T lymphocytes, and other cellular components in chronically inflamed nonlymphoid tissues or organs (17–19).TLOs have also been identified in CKD, including IgAN (16,20,21). Heller et al. reported that proliferating and memory B cells were present within TLOs and chemokine CXCL13 might contribute to TLO neogenesis by recruiting CXCR5+ B cells to injured kidneys (16). TLOs support the production of autoantibodies and predict poor outcomes of chronic rejection (22,23). However, the precise characteristics of TLOs have not been fully defined in a cohort of patients with IgAN.
In this prospective study, interstitial CD68+, DC-SIGN+, CD4+, CD8+, and CD20+ cells and the cellular components of TLOs were examined by immunohistologic staining in renal biopsy samples of patients with IgAN. The main interest focused on the correlations between severity of inflammatory cell infiltration, frequency of TLO neogenesis, and clinicopathologic features.
Materials and Methods
Patients
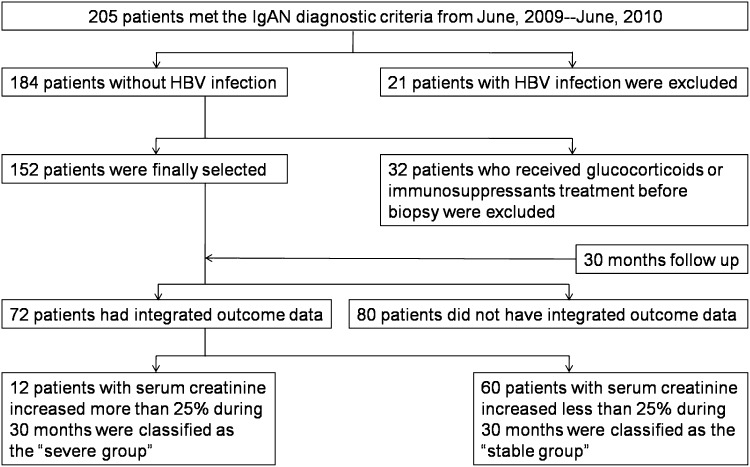
This study complied with the Declaration of Helsinki and was approved by the Committee on Research Ethics of the Huazhong University of Science and Technology, Tongji Hospital. From June 2009 to June 2010, 205 adult patients with IgAN were recruited to this study according to the scheme presented in Figure 1. All patients met the diagnostic criteria for IgAN, which were published by the Oxford classification working group (9). Patients with hepatitis B virus infection as well as individuals who had received glucocorticoids or immunosuppressant treatment before renal biopsy were excluded (Figure 1).
Figure 1.
Study scheme. The patients were recruited into the study according to the scheme shown. HBV, hepatitis B virus; IgAN, IgA nephropathy.
Histologic Evaluation
Paraffin sections for light microscopy were stained with hematoxylin and eosin, periodic acid–Schiff, Masson’s trichrome, and periodic acid–silver methenamine methods. Direct immunofluorescence for IgA, IgG, IgM, C3, C1q, and folate receptor α was performed in frozen sections. Histopathologic evaluation was independently performed according to the Oxford classification (8,9) by M.H. and Y.L. who were not aware of the clinical data.
Immunohistochemistry and Immunofluorescence
Detection of CD68 (Long Island Biotech, Shanghai, China), DC-SIGN (Santa Cruz Biotechnology, Dallas, TX), CD4, CD8, CD20, CD21, CD138, D2–40 (Maxim-bio, Fuzhou, China), CXCL12, CXCR4, CXCL13 (Bioss-bio, Beijing, China), CXCR5, CCL21, and CCR7 (Abcam, Cambridge, UK) was performed on paraffin sections using a streptavidin-peroxidase kit (ZSGB-bio, Beijing, China) according to the manufacturer’s instructions. Antibody reactions were visualized by using diaminobenzidine (DAKO, Tokyo, Japan). For immunofluorescence double staining, primary antibodies against CD3, CD45RO, CD20 (Maxim-bio), CD27 (Abcam), IgG, and IgM (ZSGB-bio) and Alexa Fluor 488 or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used. Colocalization was analyzed by confocal laser scanning microscopy.
Quantitative Analyses of Inflammatory Cells and Definition of TLOs
The numbers of each subset of interstitial inflammatory cells were counted under five equivalent high-power cortical fields (HPFs) (×400) and were expressed as the average number of cells per square millimeter (24).
To evaluate the presence of TLOs, we initially defined nodular inflammatory infiltrates (including small cellular aggregates and larger follicular-like structures) in the renal interstitium on periodic acid–Schiff-stained sections as TLO candidates, which were then examined in serial sections stained with hematoxylin and eosin, Masson’s trichrome, and periodic acid–silver methenamine. TLOs were finally identified after immunohistochemical staining confirmed that the structures contained organized DC-SIGN+, CD4+, CD8+, and CD20+ cells. The frequency of TLO neogenesis in patients with IgAN was analyzed by a simple grading system comprising three grades: without TLOs (without TLOs under 10 equivalent HPFs), grade 1 (with 1 tertiary lymphoid organ under 10 equivalent HPFs), and grade 2 (with ≥2 TLOs under 10 equivalent HPFs).
Statistical Analyses
Statistical analyses were performed using SPSS 15.0 software (SPSS, Inc., Chicago, IL). Nonparametric statistics were used to analyze between-group differences and relationships. Correlations were assessed using the Spearman’s rank correlation test for two continuous variables and the Mann–Whitney U test when median comparisons were made. Rate comparisons were performed by chi-squared tests. All statistical tests were two sided.
Results
Patients’ Baseline Features
According to the selection scheme, 152 patients were enrolled. A total of 72 patients were followed-up by telephone 30 months after renal biopsy. The clinical characteristics of these patients are presented in Table 1.
Table 1.
Baseline clinical characteristics of the whole cohort and the cohort with longitudinal data
| Clinical Characteristic | Whole Cohort | Cohort with Longitudinal Data |
|---|---|---|
| Patients (n) | 152 | 72 |
| Sex (women/men) | 69/83 | 35/37 |
| Age (yr) | 35.0 (26.0–44.0) | 35.0 (28.0–44.8) |
| Serum creatinine (mg/dl) | 0.9 (0.7–1.2) | 0.9 (0.7–1.1) |
| Serum uric acid (mg/dl) | 5.8 (4.7–6.9) | 5.6 (4.6–7.0) |
| Proteinuria (g/24 h) | 1.1 (0.5–1.8) | 1.0 (0.5–1.6) |
| Erythrocyturiaa | 95.4 | 94.4 |
| Systolic BP (mmHg) | 125 (117–138) | 126 (117–139) |
| Diastolic BP (mmHg) | 80 (74–90) | 80 (74–90) |
| Renin-angiotensin system blockade before renal biopsy | 6.6 | 5.6 |
Data are presented as the median (25th–75th percentiles) or as a percentage unless otherwise indicated.
To assess the presence of erythrocyturia, 10 ml of clean fresh urine from the middle piece was centrifuged for 5 minutes at 1500 rpm; 0.2 ml of sediment was taken and observed under microscopy. Erythrocyturia was defined as ≥3 red blood cells per high-power cortical field.
Different Subsets of Interstitial Inflammatory Cells
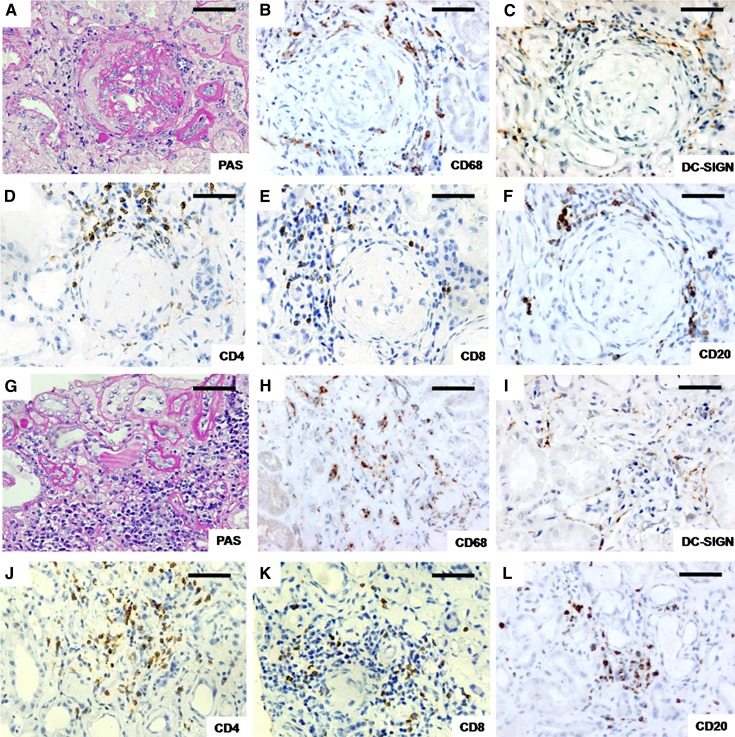
In our renal biopsy specimens of IgAN, CD68+, DC-SIGN+, CD4+, CD8+, and CD20+ cells were detected in the serial sections and were found in 100%, 96.7%, 100%, 100%, and 96.7% of patients, respectively. CD68+, DC-SIGN+, CD4+, CD8+, and CD20+ cells were found around the Bowman’s capsule and between tubules (Figure 2). CD68+ and DC-SIGN+ cells were scattered throughout the renal interstitium. Interstitial CD4+, CD8+, and CD20+ cells could be analogously assigned to three patterns of distribution: a diffuse pattern, small cellular aggregates, and larger follicular-like structures (25).
Figure 2.
Different subsets of inflammatory cells in the renal interstitium. (A–F) Inflammatory cells around Bowman’s capsule of glomeruli. (G–L) Inflammatory cells between tubules. A and G were stained with PAS. Cells stained by immunohistochemistry were as follows: CD68+ (a macrophage marker) (B and H), DC-SIGN+ (marked a subset of dendritic cells) (C and I), CD4+ (a T helper cell marker) (D and J), CD8+ (a cytotoxic T cell marker) (E and K), and CD20+ (a B lymphocyte marker) (F and L). Antibody reactions (dark brown) were visualized by DAB. DAB, diaminobenzidine; PAS, periodic acid–Schiff. Bar, 200 μm.
Association of Interstitial Inflammatory Cells with Clinicopathologic Features
Correlations between interstitial infiltration and clinical features were analyzed by Spearman’s analysis (Table 2). The density of CD68+, DC-SIGN+, CD4+, CD8+, and CD20+ cells was significantly associated with serum creatinine and proteinuria levels. Among the different subsets, the density of DC-SIGN+ cells were the most strongly correlated with serum creatinine levels (r=0.42, P<0.001), and the density of CD68+ cells were the most strongly correlated with proteinuria levels (r=0.44, P<0.001). The density of DC-SIGN+ cells and CD20+ cells had good correlation with serum uric acid levels (r=0.24, P<0.01; and r=0.27, P<0.01, respectively).The density of CD4+ and CD8+ cells was associated with the severity of erythrocyturia (r=0.24, P<0.01; and r=0.27, P<0.01, respectively) (Table 2).
Table 2.
Correlation of interstitial infiltration with clinical, demographic, and cellular parameters
| Parameter | CD68 | DC-SIGN | CD4 | CD8 | CD20 |
|---|---|---|---|---|---|
| Male sex | −0.07 | 0.10 | 0.01 | 0.02 | 0.23a |
| Age (yr) | 0.14 | 0.19a | 0.02 | 0.08 | 0.09 |
| Serum creatinine (mg/dl) | 0.34b | 0.42b | 0.26c | 0.30c | 0.38b |
| Serum uric acid (mg/dl) | 0.08 | 0.24c | 0.14 | 0.15 | 0.27c |
| Proteinuria (g/24 h) | 0.44b | 0.22a | 0.35b | 0.36b | 0.31b |
| Erythrocyturia (+)d | 0.19 | 0.12 | 0.24c | 0.27c | 0.21a |
| Systolic BP (mmHg) | 0.22a | 0.18a | 0.18a | 0.12 | 0.19a |
| Diastolic BP (mmHg) | 0.13 | 0.15 | 0.13 | 0.10 | 0.09 |
| Mesangial hypercellularity | 0.23a | 0.27c | 0.23c | 0.20a | 0.12 |
| Segmental glomerulosclerosis | 0.17 | 0.19a | 0.25c | 0.33b | 0.32c |
| Endocapillary hypercellularity | 0.20a | 0.01 | 0.01 | 0.04 | 0.03 |
| Tubular atrophy/interstitial fibrosis | 0.62b | 0.56b | 0.56b | 0.60b | 0.55b |
| Global glomerulosclerosise | 0.15 | 0.15 | 0.08 | 0.17a | 0.17 |
| Crescentsf | 0.18 | 0.22a | 0.25c | 0.18a | 0.13 |
| Arterial wall thickening | 0.37b | 0.36b | 0.40b | 0.42b | 0.39b |
| Arterial hyalinosisf | 0.29c | 0.22c | 0.21a | 0.24c | 0.35b |
| CD68+ | — | 0.43b | 0.53b | 0.49b | 0.54b |
| DC-SIGN+ | 0.43b | — | 0.62b | 0.60b | 0.62b |
| CD4+ | 0.53b | 0.62b | — | 0.79b | 0.70b |
| CD8+ | 0.49b | 0.60b | 0.79b | — | 0.69b |
| CD20+ | 0.54b | 0.62b | 0.70b | 0.69b | — |
Data are presented as Spearman’s correlation coefficients. Values of the respective cell types are presented as the number of cells per millimeter squared. RBC, red blood cell; HPF, high-power cortical field.
P<0.05.
P<0.001.
P<0.01.
The severity of erythrocyturia was characterized as follows: 3 ≤ RBC ≤ 5/HPF was defined as 1+; 5 <RBC ≤ 20/HPF as 2+; 20 < RBC ≤ 50 RBC/HPF as 3+; and >50 RBC/HPF as 4+. Pathologic features were scored using the Oxford classification (8,9).
The severity of global glomerulosclerosis and arterial wall thickening was graded in three groups: normal, mild, and marked.
Crescents and arterial hyalinosis were scored as either present or absent.
We also evaluated the relationship between interstitial infiltration and pathologic features. The density of CD68+, DC-SIGN+, CD4+, CD8+, and CD20+ cells was significantly associated with the severity of tubular atrophy/interstitial fibrosis, arterial wall thickening, and presence of arterial hyalinosis (Table 2). Among the different subsets, the density of CD68+ cells most strongly correlated with the severity of tubular atrophy/interstitial fibrosis (r=0.62, P<0.001). The density of CD4+ cells most strongly correlated with the presence of crescents (r=0.25, P<0.001). The density of CD8+ cells most strongly correlated with the severity of arterial wall thickening (r=0.42, P<0.001). The density of CD20+ cells most strongly correlated with the presence of arterial hyalinosis (r=0.35, P<0.001) (Table 2). The density of DC-SIGN+ and CD4+ cells was significantly correlated with the presence of mesangial hypercellularity (r=0.27, P<0.01; and r=0.23, P<0.01, respectively) (Table 2). The association of interstitial CD4+, CD8+, and CD20+ cell density with the presence of segmental glomerulosclerosis was also significant (r=0.33, P<0.001; r=0.32, P<0.001; and r=0.25, P<0.01, respectively) (Table 2). In addition, the density of all cellular combinations was also significantly correlated (Table 2).
TLOs in the Kidney of Patients with IgAN
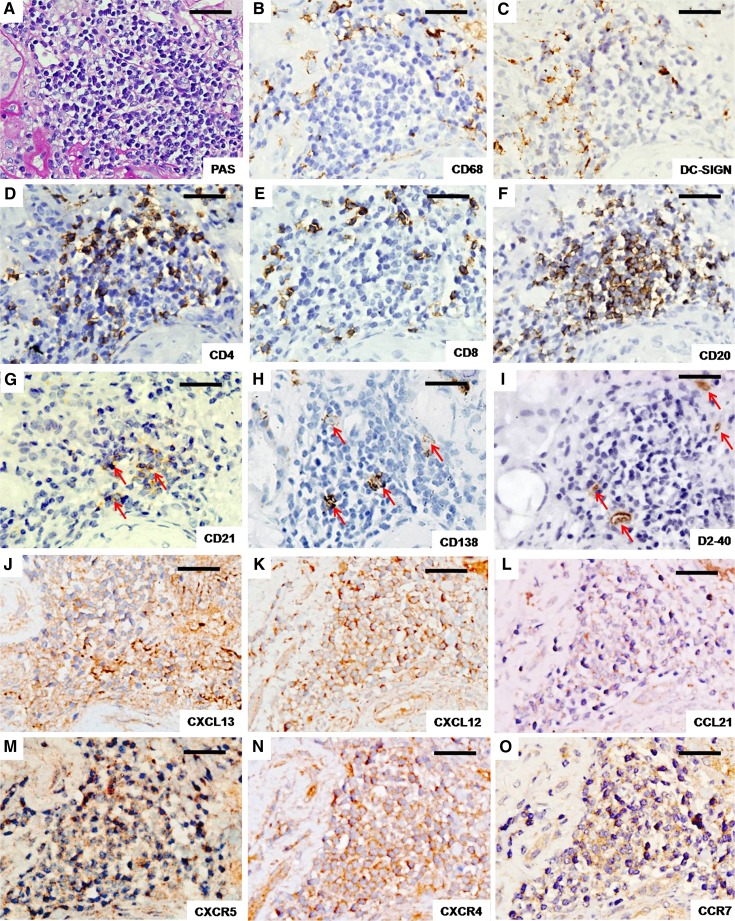
Small cellular aggregates and larger follicular-like structures defined as TLOs were found in 37.5% of patients. TLOs were frequently found adjacent to the injured glomerulus and arteries or beneath the renal capsule (21). We found that the CD20+ B cells (Figure 3F) and CD21+ follicular dendritic cells (Figure 3G) were distributed in the center of the TLOs, the periphery of which contained clustered CD4+ cells, CD8+ cells, scattered DC-SIGN+ cells, and CD138+ plasma cells (Figure 3, A–I). CD68+ cells surrounded the TLOs, but were rarely in the TLOs (Figure 3B). D2–40+ lymphatic vessels surrounded or were dispersed within the TLOs (Figure 3I).
Figure 3.
Inflammatory cells, lymphatic vessels, chemokines, and their receptors within TLOs. (A) Representative PAS-stained renal TLOs. Cells stained by immunohistochemistry were as follows: CD68 (B), DC-SIGN (C), CD4 (D), CD8 (E), CD20 (F), CD21 (a follicular dendritic cell marker) (G), CD138 (a plasma cell marker) (H), D2–40 (labeled lymphatic vessels) (I), CXCL13 (J), CXCL12 (K), CCL21 (L), CXCR5 (M), CXCR4 (N), and CCR7 (O). Antibody reactions (dark brown) were visualized using DAB. CXCL13/CXCR5, CXCL12/CXCR4, and CCL21/CCR7 indicate the chemokines and corresponding receptors that are most likely involved in TLO neogenesis (17). Organized inflammatory cells in TLOs are shown in A–H. Arrows in G suggest follicular dendritic cells in the center of the TLOs, arrows in H indicate CD138+ plasma cells in TLOs, and arrows in I indicate D2–40+ lymphatic vessels that surround or disperse within the TLOs. All panels depict serial sections from the same patient. DAB, diaminobenzidine; PAS, periodic acid-Schiff; TLO, tertiary lymphoid organ. Bar, 100 μm.
Local production of chemokines is a critical event in TLO neogenesis (17). CXCL13 was reported to recruit CXCR5+ B cells, CXCL12 was reported to recruit CXCR4+ T cells, and CCL21was reported to recruit CCR7+ dendritic cells and T cells, respectively (17,19). As shown in Figure 3, chemokines CXCL13, CXCL12, and CCL21 and the corresponding receptors CXCR5, CXCR4, and CCR7 were also detected within the TLOs.
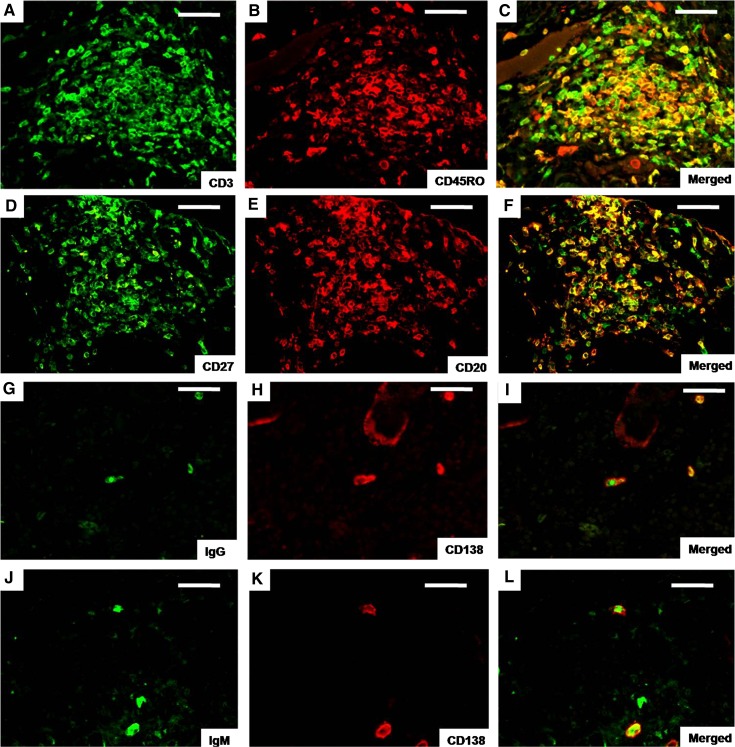
CD45RO is a marker of memory T cells and CD27 is expressed on memory B cells (26,27). In this study, many of the CD3+ cells within the TLOs were colocalized with CD45RO, indicating that these T lymphocytes were activated memory T cells (Figure 4, A–C). In addition, the majority of CD20+ cells expressed CD27, suggesting that these B lymphocytes were mature memory B cells (Figure 3, D–F). IgG+ CD138+ cells (Figure 3, G–I) and IgM+ CD138+ cells (Figure 3, J–L) were also discovered within the TLOs, suggesting that the plasma cells within the TLOs were capable of producing Ig.
Figure 4.
T and B lymphocytes and plasma cells within tertiary lymphoid organs. Double immunofluorescence was performed for CD3 (green) (A), CD45RO (red) (B), double-positive (yellow) (C), CD27 (green) (D), CD20 (red) (E), double-positive (yellow) (F), IgG (green) (G), CD138 (red) (H), double-positive (yellow) (I), IgM (green) (J), CD138 (red) (K), and double-positive (yellow) (L) cells. CD45RO and CD27 are markers of memory T cells and memory B cells, respectively. G–L suggest that plasma cells have the ability to produce antibodies. Bar, 100 μm.
The frequency of TLO neogenesis was also assessed with clinical parameters at the time of the renal biopsy. As shown in Table 3, patients with high-grade TLOs showed a elevated level of serum creatinine, heavy proteinuria, high BP, a high percentage of mesangial hypercellularity (grade 0, 54.8%; grade 1, 72.7%; grade 2, 84.6%; P=0.003), segmental glomerulosclerosis (grade 0, 38.7%; grade 1, 45.5%; grade 2, 61.5%; P=0.04), crescents (grade 0, 8.6%; grade 1, 21.2%; grade 2, 50.0%; P=0.01), arterial hyalinosis (grade 0, 29.0%; grade 1, 42.4%; grade 2, 57.7.%, P=0.01), severe tubular atrophy/interstitial fibrosis (grade 0, 14.0%; grade 1, 27.6%; grade 2, 70.0%; P<0.001), and marked arterial wall thickening (grade 0, 20.5%; grade 1, 36.4%; grade 2, 46.2%; P<0.001).
Table 3.
Clinical and demographic characteristics in patients with different grades of tertiary lymphoid organs at the time of biopsy
| Characteristic | Tertiary Lymphoid Organ Grade | P Value | ||
|---|---|---|---|---|
| 0 (n=93) | 1 (n=33) | 2 (n=26) | ||
| Male sex | 51.6 | 57.6 | 61.5 | 0.33 |
| Age≥50 (yr) | 7.5 | 21.2 | 15.4 | 0.11 |
| Serum creatinine≥1.2 (mg/dl) | 20.5 | 36.4 | 46.2 | 0.01 |
| Serum uric acid≥7.0 (mg/dl) | 16.5 | 34.5 | 26.0 | 0.16 |
| Proteinuria≥2.0 (g/24 h) | 31.2 | 50.0 | 64.7 | 0.003 |
| Erythrocyturia≥3+a | 50.5 | 42.4 | 61.5 | 0.54 |
| BP grade≥2b | 5.4 | 15.6 | 19.2 | 0.01 |
| Oxford classificationc | ||||
| M1 | 54.8 | 72.7 | 84.6 | 0.003 |
| S1 | 38.7 | 45.5 | 61.5 | 0.04 |
| E1 | 10.8 | 6.1 | 7.7 | 0.51 |
| T1 and T2 | 14.0 | 27.6 | 70.6 | <0.001 |
| Marked global glomerulosclerosisd | 28.1 | 38.1 | 50.0 | 0.11 |
| Presence of crescentse | 12.9 | 28.6 | 35.3 | 0.01 |
| Marked arterial wall thickening | 8.6 | 21.2 | 50.0 | <0.001 |
| Presence of arterial hyalinosis | 29.0 | 42.4 | 57.7 | 0.01 |
Data are presented as percentages. Comparisons between groups were performed by chi-squared tests. RBC, red blood cell; HPF, high-power cortical field; SBP systolic BP; DBP, diastolic BP.
The severity of erythrocyturia was defined as follows: 3 ≤ RBC ≤ 5/HPF was defined as 1+; 5 < RBC ≤ 20/HPF as 2+; 20 < RBC ≤ 50 RBC/HPF as 3+; and >50 RBC/HPF was defined as 4+.
BP was graded in three groups: 0, SBP < 140 mmHg and DBP < 90 mmHg; 1, 160 mmHg > SBP ≥ 140 mmHg or 100 mmHg > DBP ≥ 90 mmHg; and 2, SBP ≥ 160 mmHg or DBP ≥ 100 mmHg.
Pathologic features were scored using the Oxford classification (8,9). M1 was defined by the presence of >3 cells in the most cellular mesangial area, but not adjacent to the vascular stalk, on >50% of the glomeruli. S1 was defined by the presence of tuft adhesion and segmental sclerosis but not global sclerosis. E1 was defined by the presence of an increased number of cells within the capillary lumina causing narrowing. The percentage of a cortical area damaged by interstitial fibrosis or tubular atrophy was defined as T0 (≤25%), T1 (26%–50%), or T2 (>50%).
The severity of global glomerulosclerosis and arterial wall thickening was divided into three groups: normal, mild, and marked.
Crescents and arterial hyalinosis were scored as present or absent.
Outcome after 30-Month Follow-Up
Two patient subgroups were identified according to the progression of serum creatinine levels during the 30-month follow-up after renal biopsy (Table 4). Twelve patients were classified as the severe group and their serum creatinine increased by >25% above the baseline values at 30 months. Sixty patients who had decreasing or stable serum creatinine levels within the normal range were classified as the stable group. Patients in the severe group had a high level of heavy proteinuria, serum creatinine, and serum uric acid, a high percentage of mesangial hypercellularity (91.7% versus 55.0%; P=0.02) and arterial hyalinosis (66.7% versus 25.0%; P=0.02), severe global glomerulosclerosis (33.3% versus 10.0%; P=0.02), tubular atrophy/interstitial fibrosis (91.7% versus 16.7%; P<0.001), and arterial wall thickening (66.7% versus 13.3%; P<0.001) at the time of biopsy (Table 4). Patients in the severe group also exhibited severe interstitial infiltration of CD68+, DC-SIGN+, CD4+, CD8+, and CD20+cells as well as a high percentage of TLO neogenesis at the time of biopsy compared with patients in the stable group (83.3% versus 33.3%; P=0.001) (Table 4).
Table 4.
Differences in clinical, demographic, cellular, and tertiary lymphoid organ parameters between patients with good and poor outcomes
| Parameter | Severe Group | Stable Group | P Value |
|---|---|---|---|
| Patients (n) | 12 | 60 | |
| Male sex (%) | 58.3 | 50 | 0.60 |
| Age (yr) | 37.5 (27.5–50.8) | 35.0 (27.0–44.0) | 0.25 |
| Serum creatinine (mg/dl) | 1.5 (1.1–2.5) | 0.9 (0.6–1.1) | <0.001 |
| Serum uric acid (mg/dl) | 6.8 (5.8–8.2) | 5.5 (4.5–6.9) | 0.01 |
| Proteinuria (g/24 h) | 2.1 (1.2–4.1) | 1.0 (0.5–1.6) | 0.01 |
| Erythrocyturia≥3+a | 41.7 | 53.3 | 0.46 |
| BP (mmHg) | |||
| Systolic | 126.0 (109.0–157.0) | 125.5 (117.5–138.8) | 0.97 |
| Diastolic | 81.5 (72.3–94.0) | 80.0 (72.3–89.8) | 0.82 |
| Immunosuppressionb | 91.7 | 87.8 | 0.58 |
| Oxford classificationc | |||
| M1 | 91.7 | 55.0 | 0.02 |
| S1 | 66.7 | 38.3 | 0.07 |
| E1 | 8.3 | 6.7 | 0.84 |
| T2 | 91.7 | 16.7 | <0.001 |
| Marked global glomerulosclerosisd | 33.3 | 10.0 | 0.03 |
| Presence of crescentse | 25.0 | 13.3 | 0.31 |
| Marked arterial wall thickening | 66.7 | 13.3 | <0.001 |
| Presence of arterial hyalinosis | 66.7 | 25.0 | 0.05 |
| CD68+ | 216.7 (136.9–371.7) | 79.2 (39.5–146.6) | 0.001 |
| DC-SIGN+ | 126.3 (32.7–143.1) | 29.2 (12.5–67.1) | 0.002 |
| CD4+ | 395.8 (221.5–633.3) | 142.3 (75.8–275.8) | <0.001 |
| CD8+ | 164.0 (98.6–250.0) | 73.5 (41.8–135.6) | 0.001 |
| CD20+ | 180.4 (55.7–329.3) | 43.8 (11.9–83.3) | 0.01 |
| Presence of TLOs | 83.3 | 33.3 | 0.001 |
Data are presented as a percentage or as the median (25th–75th percentile). Comparisons between the groups were performed by chi-squared tests or nonparametric tests. Values of the respective cell types are presented as the number of cells per millimeter squared.
The severity of erythrocyturia was defined as follows: 3≤RBCs≤ 5/HPF was defined as 1+; 5<RBCs ≤ 20/HPF as 2+; 20<RBCs ≤ 50 red blood cells/HPF as 3+; and >50 RBCs/HPF as 4+.
Follow-up data.
Pathologic features were scored using Oxford classification (8,9). M1 was defined by the presence of >3 cells in the most cellular mesangial area, but not adjacent to the vascular stalk, on >50% of the glomeruli. S1 was defined by the presence of tuft adhesion and segmental sclerosis but not global sclerosis. E1 was defined by the presence of an increased number of cells within the capillary lumina causing narrowing. The percentage of cortical area damaged by interstitial fibrosis or tubular atrophy was defined as T0 (≤25%), T1 (26%–50%), or T2 (>50%).
The severity of global glomerulosclerosis and arterial wall thickening was divided into three groups: normal, mild, and marked.
Crescents and arterial hyalinosis were scored as present or absent.
Discussion
Interstitial infiltration, one common but special feature in CKD, was recently confirmed to play an initiative role in regulating renal lesions (28). In this prospective study, we investigated the density of CD68+, DC-SIGN+, CD4+, CD8+, and CD20+ cells and the grades of TLOs and evaluated their association with clinicopathologic features in renal biopsy tissues of patients with IgAN.
Among the pathologic variables, the density of interstitial inflammatory cells showed a strong correlation with the severity of interstitial and arterial lesions (Table 2). We also observed that the density of inflammatory cells was significantly correlated with the glomerular lesions (Table 2). In a mouse model, renal interstitial dendritic cells were found to contribute to GN. They captured glomerular antigens and presented them to T helper cells, and the latter recruited and activated cytotoxic T cells thereby drived renal lesions (28). In other words, different cellular subsets cooperate with each other locally to accelerate renal inflammation and injury of the entire kidney. On the basis of the strong correlations of different subsets of inflammatory cells in this study (Table 2), we believed that interstitial inflammatory cells contributed to IgAN progression.
The severity of interstitial infiltration has been used for prognostic purposes (12,13,15). Myllymäki et al. reported that severe interstitial CD3+ infiltration indicated poor outcomes of IgAN, according to a retrospective study (12). In our prospective study, we found that patients in the severe group exhibited more interstitial CD68+, DC-SIGN+, CD4+, CD8+, and CD20+ cell infiltration compared with patients in the stable group (Table 4). Because CD3 is a common T cell marker of both T helper cells (CD4+ T cells) and cytotoxic T cells (CD8+ T cells), our results were consistent with those of Myllymäki et al (12).
TLOs provide a special space in which the “cooperation” of inflammatory cells occurs in an organized manner in situ in nonlymphoid tissues or organs. TLOs, similar to secondary lymphoid organs, generate effector and memory T cells that lead to allograft rejection and are considered as a fast track for autoimmunity (23,29).
Heller et al. reported that TLOs in IgAN contained B cells, T cells, and lymphatic vessels (16). In our IgAN renal sections, we further observed the presence of DC-SIGN+ dendritic cells, follicular dendritic cells, and plasma cells in TLOs (Figure 3). We also found that part of T and B lymphocytes within TLOs showed a memory phenotype and plasma cells within the TLOs secreted IgG or IgM (Figure 4). TLOs contained all of the essential elements for a local immune response and thus might play a role in IgAN progression (23,29,30). Kelly et al. revealed that renal TLO neogenesis was associated with matrix accumulation and loss of renal function in a mouse model (31). In our study, we found that high-grade TLOs were significantly associated with an elevated level of serum creatinine and severe glomerular, interstitial, and arterial lesions. In addition, patients in the severe group exhibited a high percentage of TLO neogenesis compared with patients in the stable group (Table 3).
This study has several limitations. The follow-up period was not long enough and the cohort with integrated outcome data was not large enough. Additional features such as the mRNA levels of related inflammatory cytokines and chemokines were not measured. Furthermore, whether the TLOs in IgAN kidneys functioned detrimentally as they did in allograft rejection are still not clear. Additional large prospective multicenter and molecular marker–based clinical trials and animal experiments are warranted to validate our results.
Taken together, our findings indicate that in addition to clinical and pathologic variables, the severity of interstitial infiltration and the frequency of TLO neogenesis also indicate the severity of renal lesions and are correlated with IgAN progression. A detailed understanding of the processes leading to TLO neogenesis and the definition of its pathophysiologic role may provide new rationales for the development of therapeutics specifically targeting this process.
Disclosures
None.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (Grants 81270770, 81170686, 81270771, 81100485, and 81370798), under the auspices of Scientific Research of Major Projects by the Ministry of Education of China (JYBZD201201, Grant 2011-313-311028).
Footnotes
G.P. and R.Z. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Galla JH: IgA nephropathy. Kidney Int 47: 377–387, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Lv J, Zhang H, Zhou Y, Li G, Zou W, Wang H: Natural history of immunoglobulin A nephropathy and predictive factors of prognosis: A long-term follow up of 204 cases in China. Nephrology (Carlton) 13: 242–246, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM: Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant 17: 1197–1203, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Li PK, Ho KK, Szeto CC, Yu L, Lai FM: Prognostic indicators of IgA nephropathy in the Chinese—clinical and pathological perspectives. Nephrol Dial Transplant 17: 64–69, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Rauta V, Finne P, Fagerudd J, Rosenlöf K, Törnroth T, Grönhagen-Riska C: Factors associated with progression of IgA nephropathy are related to renal function—a model for estimating risk of progression in mild disease. Clin Nephrol 58: 85–94, 2002 [DOI] [PubMed] [Google Scholar]
- 7.D’Amico G: Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24: 179–196, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Alamartine E, Sauron C, Laurent B, Sury A, Seffert A, Mariat C: The use of the Oxford classification of IgA nephropathy to predict renal survival. Clin J Am Soc Nephrol 6: 2384–2388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H: Validation study of oxford classification of IgA nephropathy: The significance of extracapillary proliferation. Clin J Am Soc Nephrol 6: 2806–2813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myllymäki JM, Honkanen TT, Syrjänen JT, Helin HJ, Rantala IS, Pasternack AI, Mustonen JT: Severity of tubulointerstitial inflammation and prognosis in immunoglobulin A nephropathy. Kidney Int 71: 343–348, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P: The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Segerer S, Heller F, Lindenmeyer MT, Schmid H, Cohen CD, Draganovici D, Mandelbaum J, Nelson PJ, Gröne HJ, Gröne EF, Figel AM, Nössner E, Schlöndorff D: Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int 74: 37–46, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Van Es LA, de Heer E, Vleming LJ, van der Wal A, Mallat M, Bajema I, Bruijn JA, de Fijter JW: GMP-17-positive T-lymphocytes in renal tubules predict progression in early stages of IgA nephropathy. Kidney Int 73: 1426–1433, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Heller F, Lindenmeyer MT, Cohen CD, Brandt U, Draganovici D, Fischereder M, Kretzler M, Anders HJ, Sitter T, Mosberger I, Kerjaschki D, Regele H, Schlöndorff D, Segerer S: The contribution of B cells to renal interstitial inflammation. Am J Pathol 170: 457–468, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL, Mebius RE, van der Pouw Kraan TC: Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: Identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum 56: 2492–2502, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA: Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest 116: 2622–2632, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchal-Sommé J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P: Cutting edge: Nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol 176: 5735–5739, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Segerer S, Schlöndorff D: B cells and tertiary lymphoid organs in renal inflammation. Kidney Int 73: 533–537, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Mandache E, Penescu M: Renal subcapsular tertiary lymphoid aggregates in chronic kidney diseases. Rom J Morphol Embryol 52: 1219–1225, 2011 [PubMed] [Google Scholar]
- 22.Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, Pitzalis C: Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med 6: e1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasr IW, Reel M, Oberbarnscheidt MH, Mounzer RH, Baddoura FK, Ruddle NH, Lakkis FG: Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant 7: 1071–1079, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC: Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 23: 86–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR: In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186: 1849–1860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giaretta F, Bussolino S, Beltramo S, Fop F, Rossetti M, Messina M, Cantaluppi V, Ranghino A, Basso E, Camussi G, Segoloni GP, Biancone L: Different regulatory and cytotoxic CD4+ T lymphocyte profiles in renal transplants with antibody-mediated chronic rejection or long-term good graft function. Transpl Immunol 28: 48–56, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Agematsu K: Memory B cells and CD27. Histol Histopathol 15: 573–576, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Gröne HJ, Kurts C: Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 119: 1286–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weyand CM, Kurtin PJ, Goronzy JJ: Ectopic lymphoid organogenesis: A fast track for autoimmunity. Am J Pathol 159: 787–793, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaunat O, Graff-Dubois S, Brouard S, Gautreau C, Varthaman A, Fabien N, Field AC, Louedec L, Dai J, Joly E, Morelon E, Soulillou JP, Michel JB, Nicoletti A: Immune responses elicited in tertiary lymphoid tissues display distinctive features. PLoS ONE 5: e11398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly FM, Reddy RN, Roberts BR, Gangappa S, Williams IR, Gooch JL: TGF-beta upregulation drives tertiary lymphoid organ formation and kidney dysfunction in calcineurin A-alpha heterozygous mice. Am J Physiol Renal Physiol 296: F512–F520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]