Abstract
Ultrasound is commonly used in nephrology for diagnostic studies of the kidneys and lower urinary tract and to guide percutaneous procedures, such as insertion of hemodialysis catheters and kidney biopsy. Nephrologists must, therefore, have a thorough understanding of renal anatomy and the sonographic appearance of normal kidneys and lower urinary tract, and they must be able to recognize common abnormalities. Proper interpretation requires correlation with the clinical scenario. With the advent of affordable, portable scanners, sonography has become a procedure that can be performed by nephrologists, and both training and certification in renal ultrasonography are available.
Introduction
Sonography is an essential tool in nephrology for not only the diagnosis and management of kidney disease, but also for the guidance of invasive procedures. For this reason, it is essential for nephrologists to have a thorough understanding of sonography and its uses in nephrology. Technological advances over the past 15 years have resulted in high-quality scanners that are both portable and affordable, which has greatly expanded the use of point-of-care sonography by clinicians. Although nephrologists have been lagging in this area, an increasing number are incorporating sonography into their practice, and training programs are finally starting to meet this need. This review will cover the salient points of renal ultrasonography and its application to the evaluation of kidney disease and the performance of invasive procedures. For more complete coverage, several articles and books directed toward nephrologists can be found (1–4).
Sonographic Evaluation of the Kidneys and Urinary Tract
Because of their location, architecture, and limited spectrum of pathology, the kidneys are ideally suited for evaluation by ultrasound. In addition, it is safe, readily available, easily performed at the bedside or in the office, and free of radiation. For these reasons, sonography is the preferred imaging modality and often the only one required. Evaluation includes assessment of the size and shape, the echogenicity, the urinary space (including the lower urinary tract), the presence of masses, and the vasculature. Very few findings are specific, and interpretation, therefore, requires clinical correlation, another reason for the participation of nephrologists in this procedure.
Size and Shape
Size is a key parameter that should be measured carefully, since it is the basis for important clinical decisions (Figure 1). Given its poor precision (5), this measurement should be performed several times. Since the poor precision stems mostly from under-measurement, the maximum length is the value that should be reported. Measurement of other dimensions is even more imprecise and is of no utility. The same applies to the estimation of kidney volume, which correlates poorly with more accurate techniques (5). Kidney length in adults should usually be between 10–12 cm but varies with body size and, unfortunately, there are no nomograms for normal kidneys based on large population studies. Nomograms are available for children (2,6). Cortical thickness should be estimated in addition to length and is measured from the base of the medullary pyramid to the edge of the kidney. It generally should be between 7 and 10 mm (7–9) but varies within a kidney, being thicker at the poles.When the medullae are not visible, one has to rely on parenchymal thickness, which should be 1.5–2.0 cm, but varies within the kidney. Accentuation of the lobulation is often a sign of cortical thinning. Enlargement of the kidney because of inflammation or infiltration is often accompanied by a decrease in the aspect ratio, resulting in a more globular shape.
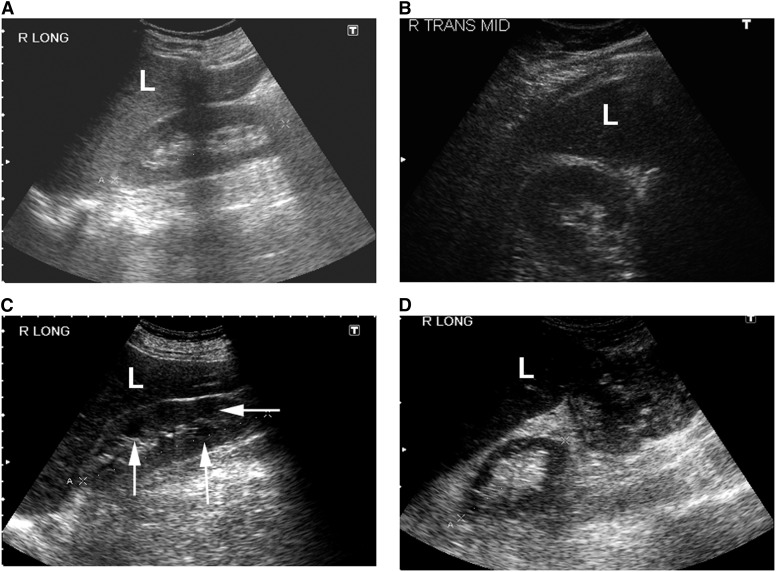
Figure 1.
Sonographic appearance of the renal parenchyma. (A) Normal right kidney (longitudinal view). (B) Normal right kidney (transverse view). (C) Echogenic right kidney with prominent medullary pyramids (arrows). (D) Atrophic right kidney (longitudinal view) with thin parenchyma and containing mostly sinus fat. L, liver.
Echogenicity
Echogenicity of a structure (acoustic interface) refers to the amount of sound it reflects back to the probe, which is dependent on the amplitude of incident sound, how much of the sound is absorbed, how much is reflected, and the angle of reflection. These same properties also dictate tissue echogenicity, which is the collective back-scatter from numerous microscopic interfaces and is determined by the micro-architecture. Increased echogenicity lacks specificity and in histologic studies has correlated with interstitial fibrosis, tubular atrophy, inflammation, and glomerulosclerosis (10,11). Decreased echogenicity usually results from edema. Cortical echogenicity can only be evaluated qualitatively and should be less than the liver or spleen (12). The medulla should have less echogenicity than the cortex, but the ability to discern this is dependent on scanning parameters and overlying structures. Although the lack of cortico-medullary differentiation is frequently mentioned in ultrasound reports, the inability to see the medullae is common and not abnormal. Rather, prominence of the medullae is usually abnormal, generally indicating increased echogenicity of the cortex (Figure 1C).
Urinary Space
The center of the kidney should be echogenic because of the presence of sinus fat, and the calyces are not visible unless dilated (Figure 2). Hydronephrosis appears as branching, interconnected areas of decreased echogenicity that show sonographic evidence of fluid (see below). This is usually easily distinguishable from dilated renal veins but can be confirmed with Doppler ultrasound if necessary. In cases of hydronephrosis, attempts should be made to visualize the ureter, which normally is not visible. The urinary bladder should be imaged in all cases of hydronephrosis. Volume is estimated from cross-sectional dimensions on transverse imaging and the longitudinal dimension in the sagittal plane using the formula for the volume of an ellipsoid (0.52×the three orthogonal dimensions). The distal ureters can be visible at the base of the bladder, and dilatation indicates a problem at the bladder.
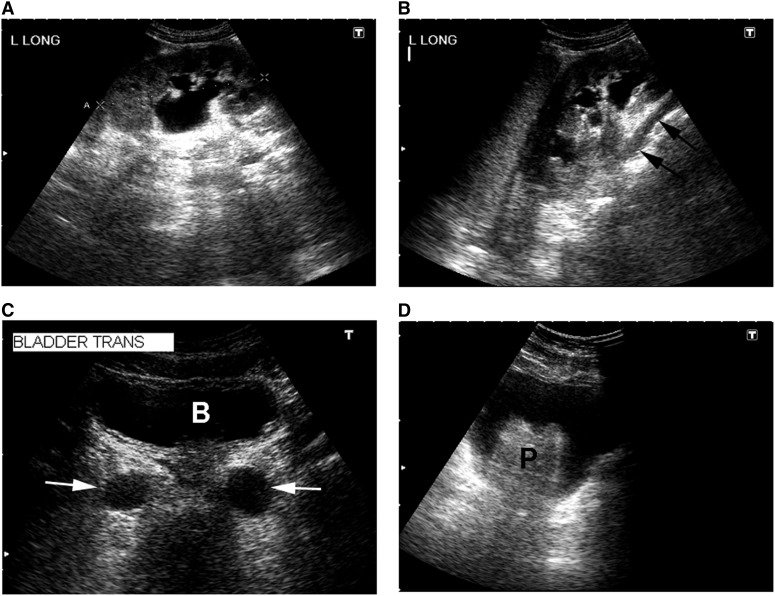
Figure 2.
Sonographic appearance of the upper and lower urinary tract. (A) Hydronephrosis of a left kidney (longitudinal view) with dilated renal pelvis and major and minor calyces. (B) Another longitudinal view of the same kidney showing a dilated ureter (arrows) tracking underneath the lower pole. (C) Transverse view of the urinary bladder (B) showing dilated distal ureters (arrows). (D) Transverse view of the urinary bladder with an enlarged prostate gland (P).
Masses
Masses are usually apparent as distortions in the renal contour or architecture, although this appearance may not be apparent for masses with echogenicity that matches the surrounding tissue (Figure 3). Most are benign simple cysts, which are round and surrounded by a smooth, thin wall. The fluid is characterized by the complete lack of echogenicity (i.e., black) and enhancement of the distal wall and deeper structures because of the lack of sound attenuation in fluid. Difficulties arise with complex cysts, which exhibit internal echoes, have some thickening or irregularity of the wall, or contain septations. The vast majority of these are minimally complex and do not require additional imaging (13) beyond follow-up sonography. More complex cysts (prominent thickening or irregularity of the wall) should prompt additional imaging. Cysts are common and are often associated with CKD (14,15), with acquired cystic disease being the extreme form. Autosomal dominant polycystic kidney disease (ADPKD) is the other common multicystic disease, and it is characterized by severe renal enlargement. Echogenic masses are usually neoplasms but can also represent hemorrhagic cysts, and they often require additional imaging. Stones typically appear as echogenic foci within the collecting system that cast acoustic shadows, but either finding may be absent. A twinkling artifact (nonvascular color signal) on Doppler imaging has been proposed as an additional method to detect stones, but it has poor sensitivity and specificity (16).
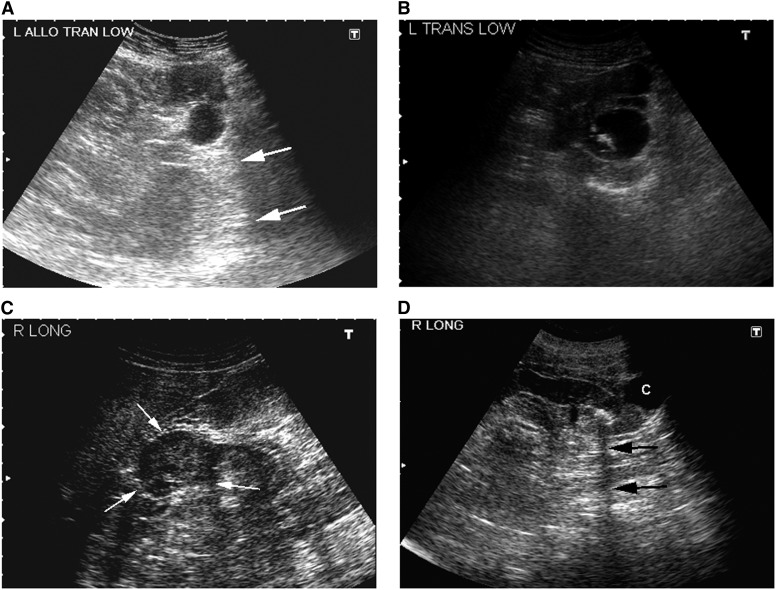
Figure 3.
Sonographic appearance of renal masses. (A) Simple cyst in the lower pole of a renal allograft (transverse view). Note the round, smooth, and thin wall and absence of internal echoes. The distal enhancement (arrows) indicates that it is fluid-filled. (B) Complex cyst in the left mid-kidney containing echogenic material (transverse view). (C) Solid mass in the right mid-kidney (longitudinal view). Note the echogenicity within the mass and the lack of distal enhancement. (D) Stone in the lower pole of a left kidney (longitudinal view) casting an acoustic shadow (arrows). There is also a simple cyst (C).
Vasculature
Although blood vessels can be seen with B-mode ultrasound, they are usually imaged with Doppler ultrasound. A detailed description of renal vascular ultrasound is beyond the scope of this review and can be found in a recent review (4). Imaging usually consists of direct visualization of the renal arteries or veins with measurement of blood velocity and of analysis of velocity waveforms (such as resistive index) in segmental arteries. Examination of the renal artery is technically difficult due to poor insonation angles (should be less than 70o), overlying bowel, and the frequent occurrence of multiple arteries, and requires significant expertise. It has a high failure rate (up to 30%) even in expert hands (4). While sensitivity and specificity can be high, they vary considerably between published studies (17) indicating that the results are center-specific and probably reliable only in highly experienced centers. While intra-renal studies are much easier and angle-independent, interpretation of the results is problematic. Although resistive index is commonly used as an indication of renal vascular resistance, it is also influenced by a number of extra-renal factors, including heart rate and peripheral vascular resistance, and is not specific for renal disease (4,18). Resistive index has been reported to provide prognostic information (19,20), but this has been questioned and may be based on extra-renal, rather than renal, factors (21).
Diagnostic Applications of Sonography in Kidney Disease
Sonography is useful in many aspects of the diagnosis and management of kidney disease. Because the findings are rarely specific, clinical correlation is essential to the interpretation, an important reason why nephrologists should be performing these studies.
CKD
Sonography should be performed in all patients with CKD primarily to recognize advanced, irreversible kidney disease that would obviate any additional workup, including biopsy (11). Signs include small size, thin cortex, and cysts, but one must be cautious in making the diagnosis based solely on size in the 9- to 10-cm range when the kidneys are otherwise normal. Although cortical echogenicity is often increased in CKD, normal echogenicity is not unusual, and echogenicity can increase in reversible disease. Thus, echogenicity alone is not a reliable indicator of CKD. Sonography can also identify specific causes, such as urinary obstruction, polycystic kidney disease, reflux nephropathy, and interstitial nephritis.
Acute Renal Failure
While sonography can be useful in acute renal failure, its use should be limited to those patients in whom the cause is not apparent or in whom urinary obstruction is possible (22,23). In addition to ruling out urinary obstruction, sonography is useful in diagnosing unrecognized CKD. The kidneys often appear normal in acute tubular necrosis (ATN) but can be enlarged and/or echogenic. Assessment of kidney size in ATN is difficult because the baseline size is rarely known and there is often underlying CKD. In a study of 26 patients with ATN, only 6 had a renal length greater than the normal range (24). The aspect ratio was increased in 12 patients, indicative of swelling, but this measurement is unreliable and dependent on the plane in which the kidney is scanned. An increase in kidney size can also occur in other causes of acute renal failure. Echogenicity is nonspecific and can increase in other causes of acute renal failure, including glomerulonephritis and interstitial nephritis, and can be influenced by fluid status (12). Increased resistive index is a nonspecific finding that lacks sensitivity in diagnosing ATN (25).
Cystic Kidney Disease
Cystic kidney disease is either genetic or acquired. ADPKD is the most common genetic type and characterized by renal enlargement in addition to multiple cysts. Hepatic cysts are common but absent in other conditions that can mimic ADPKD, such as tuberous sclerosis and von Hippl–Lindau disease. Ultrasound is sufficient to make the diagnosis, and although the measurement of length is imprecise, it suffices for prognosis (26). Criteria for the diagnosis of ADPKD in subjects at risk have been developed based on the prevalence of sporadic cysts (27). Acquired cystic disease is associated with advanced CKD and easily distinguished from ADPKD on the basis of kidney size. Sonography can also be useful in identifying infected or hemorrhagic cysts.
Pain and Hematuria
CT scanning is usually indicated for the workup of pain and hematuria, but in some cases the diagnosis can be made by ultrasound and it is not an unreasonable initial study. Stones are usually visible, but up to 50% can be missed (28), particularly when small or within the ureter. Thus, computed tomography scanning is more appropriate for acute renal colic. Sonography is not indicated in uncomplicated pyelonephritis, which usually appears as hypoechoic enlargement of a single lobule caused by edema. Renal infarction may have a similar appearance but with absent blood flow on Doppler imaging, although the latter may be difficult to demonstrate.
Screening for Carcinoma
Certain individuals are at increased risk of renal malignancies, most notably those individuals with prior tumors and patients with ESRD, even with functioning transplants. With the exception of ESRD, screening should ideally be performed with the more sensitive modalities of computed tomography or magnetic resonance imaging. Whether or not and how to screen in ESRD remains controversial based largely on the limited lifespan, and it may be appropriate only before and after transplantation, although it is not currently recommended (29). There are no large prospective studies comparing ultrasound with other modalities for this purpose. Although sonography may be less sensitive, it is less expensive and does not involve radiation exposure. Because kidneys with acquired cystic disease are at particular risk (30), a reasonable strategy may be the initial use of sonography to screen for cysts.
Transplant Nephrology
Sonography is indicated in most cases of acute renal failure because of the single functioning kidney and the frequency of urologic complications. The routine perioperative use of ureteral stents has reduced ureteral obstruction, but bladder dysfunction remains common. Because of proximity to the probe and other factors, the urinary space is often visible in the absence of obstruction, and some experience is necessary in determining when it is clinically significant. Sonography is not useful in diagnosing acute rejection unless it is severe, in which case the allograft is swollen and echogenic. However, this finding can also be seen in acute tubular necrosis and nephritis. Doppler ultrasound is useful in diagnosing vascular causes of transplant failure, but these are rare and occur almost exclusively in the immediate postoperative period. It can be useful in the diagnosis of transplant artery stenosis as a cause of hypertension. The measurement of resistive indices is of no utility in differentiating causes of transplant failure and should not routinely be performed (25).
Procedural Applications of Ultrasound in Nephrology
Because of its ease of use and availability at the bedside, sonography has revolutionized the performance of percutaneous procedures. The enhanced success rates and reduced complication rates with ultrasound guidance have made it the standard of care for many of these procedures, including those procedures performed by nephrologists. However, like any tool, success depends on proper use and an understanding of the limitations. There are two basic approaches. The entry site, angle, and depth can be determined with ultrasound, after which the needle is placed without direct ultrasound guidance (ultrasound marking), or ultrasound can be used during the needle insertion (real-time guidance).
Marking versus Real-Time Guidance
Advantages and disadvantages exist to each approach, and in experienced hands, the success and complication rates are probably similar. No prospective, randomized comparisons have been performed. Procedures performed without real-time guidance depend on careful marking of the entry site and, for deeper targets, adherence to a perpendicular angle during the ultrasound marking and needle insertion. The principal advantage is having both hands available for the actual procedure. The major disadvantage is that sonographic depth is often less than the true depth, which can result in the needle not being placed deep enough. Also, matching insonation and insertion angles is sometimes difficult, and the target may move.
While real-time guidance would seemingly be superior, needles are difficult to detect by ultrasound. The straight, smooth surface reflects sound at the angle of incidence and will only return sound to the probe when maintained perpendicular to the beam, which is difficult to achieve (Figure 4A). Although the ends of needles are often roughened so that sound reflects at multiple angles, the increase in visibility is marginal. Thus, localization of the needle usually depends on movement of adjacent tissue. However, this only indicates the presence of the needle, it does not necessarily localize the end, which could be outside the sound beam and, usually, deeper (Figure 4B). Thus, the probe must also be moved to be certain of where the needle ends and to ensure that the needle is not being advanced too far.
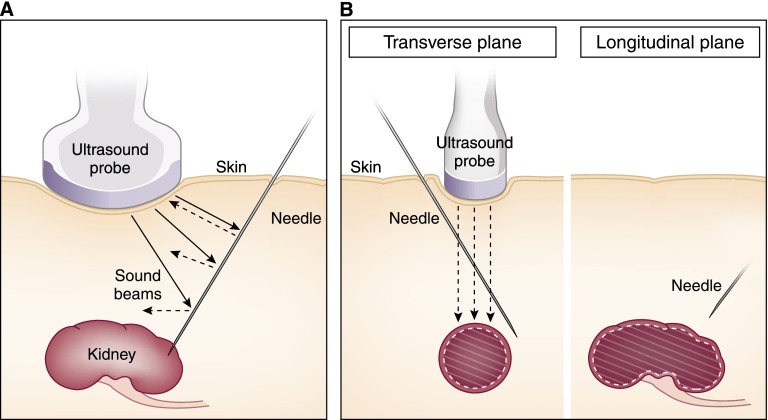
Figure 4.
Ultrasound principles of real-time visualization. (A) Needles are specular reflectors from which sounds reflect at the angle of incidence. Only the sound that strikes at a perpendicular angle returns to the probe. Other sounds may not reach the probe and those portions of the needle will not be detected. (B) Needles (left) not maintained within the width of the sound beam will appear shallower than (right) their true depth on the sonogram.
Hemodialysis Catheters
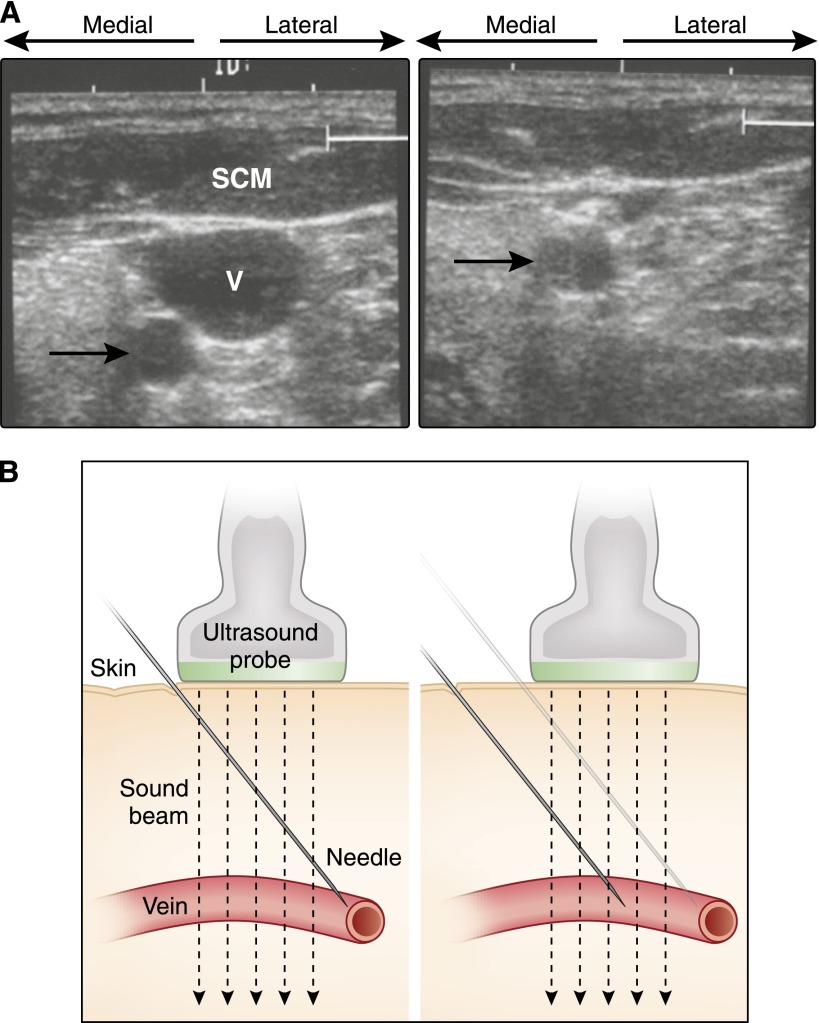
Although ultrasound guidance is not essential for catheterization of central veins, it is now considered the standard of care, markedly increasing success rates and lowering complication rates (31,32). The site should be imaged before the procedure to ensure normal anatomy and adequate visualization of the target (33,34). The internal jugular vein is lateral to the carotid artery while the femoral vein is medial to the femoral artery. Sites of previous catheterizations or attempts should be examined carefully for venous thrombosis or arterial pseudoaneurysms, and the internal jugular vein should be imaged down to the subclavian vein to confirm patency. The vein should be easily compressible (Figure 5A), and, in the case of the internal jugular vein, venous pulsations should be easily visible. If only one vessel is visible, it is far more likely to be the artery. The insertion path should not include the artery, which, even when distal to the vein, can be easily entered even during real-time guidance. It is critical that the insertion angle and probe placement be adjusted so that the needle enters the vein within the sound beam (Figure 5B). Care must be used not to compress the vein during real-time guidance. It can be avoided by guiding the needle to the surface of the vein and then puncturing the vein without real-time guidance.
Figure 5.
Sonographic guidance of central venous cannulation. (A) Transverse view of the anterior neck. The internal jugular vein (V) is underneath the sternocleidomastoid muscle (SCM). The carotid artery (arrow) is posterior and medial to the vein, and it is positioned outside the path of a needle into the center of the vein. Right panel shows the collapse of the vein, but not the artery, with compression. (B) The needle should be offset from the probe and at an angle such that (right) it enters the vein within the sound beam.
Percutaneous Renal Biopsy
It is unfortunate that few nephrologists outside of academic centers perform renal biopsies, despite the fact that nephrologists can perform ultrasound-guided biopsies safely and with high yield (35). In fact, the yield of glomeruli in this study was higher than in other published studies, indicating that biopsies performed by nephrologists result in higher quality specimens. The great majority of biopsies not performed by nephrologists are performed by CT-guidance, which results in unnecessary radiation exposure and expense since the success and complication rates are similar for CT and ultrasound guidance (35,36). Sonography should be performed before scheduling a biopsy to ensure that the kidneys can be adequately visualized and rule out anatomic abnormalities (such as lower pole cysts) or advanced CKD that would preclude biopsy. Even if the kidneys can be visualized, a depth greater than 10 cm can be problematic due to the length of the biopsy needles. Since obesity is more ventral than dorsal, ultrasound guidance is feasible in many obese patients. Ultrasound guidance should not be attempted when the kidneys are not well visualized. There are no randomized studies comparing the outcomes with ultrasound marking and real-time guidance, but they are probably similar in experienced hands (36). The choice of technique should be based on individual or center preference, experience, and outcomes as well as patient characteristics.
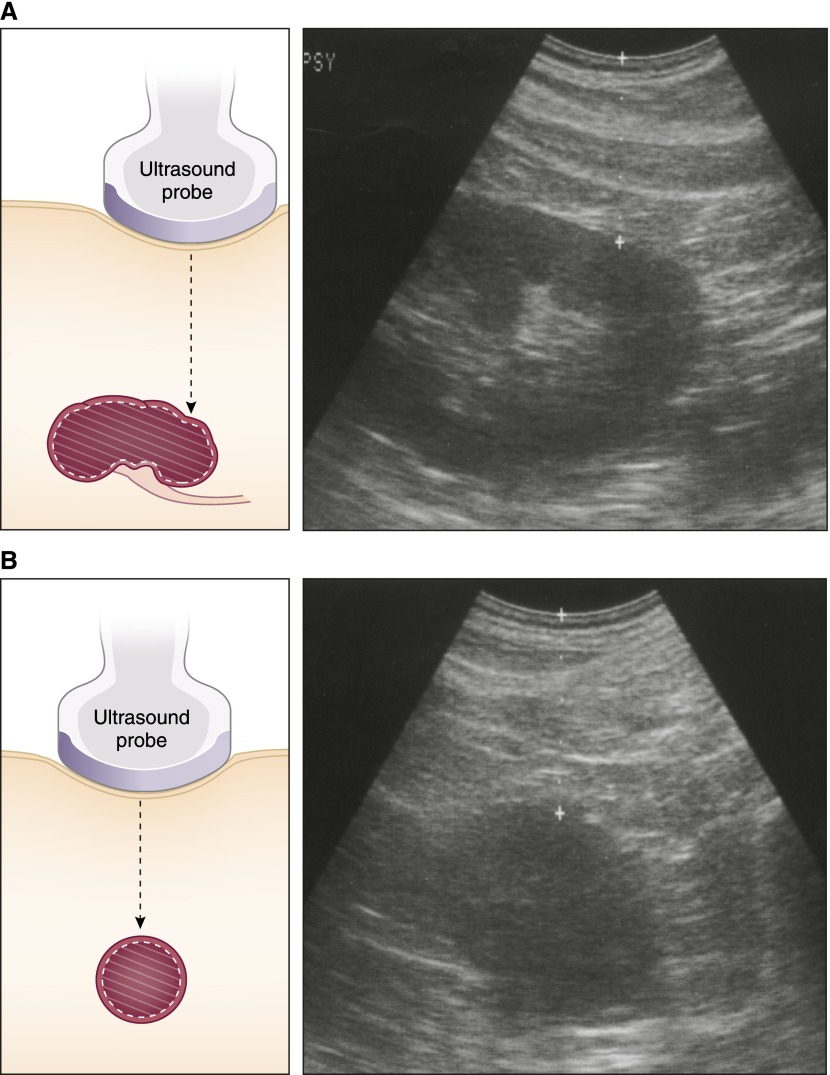
The angle of insertion and timing with respirations are critically important for ultrasound marking. For native kidneys, the patient is prone with back as flat as possible to assist in maintaining a perpendicular angle. Timing of the biopsy is dictated by the presence of overlying ribs and movement of the kidney with respirations. End expiration is preferable, because it is more consistent; also, the kidney tends to move deeper with inspiration. The lower pole of the kidney is localized in both the longitudinal and transverse planes with attention to maintaining a perpendicular angle (Figure 6), and the depth is noted. The sonographic depth is usually 1.5–2 cm less than the actual biopsy depth because of pressure from the ultrasound probe. In our institution, the true depth is determined with a spinal needle that is also used to deliver deep anesthesia. Initial passes may not be successful, usually because the needle is not deep enough or the patient’s position or respiratory movement has changed.
Figure 6.
Ultrasound marking for percutaneous renal biopsy. The lower pole is positioned directly under the center of the probe in the (A) longitudinal and (B) transverse planes. Note the absence of sinus fat in the transverse view, indicating that only renal parenchyma will be in the path of the needle.
Real-time guidance avoids the issues of angle and timing, thus increasing the accuracy. However, this can also result in a false sense of security for inexperienced operators. Attention must be focused on the location of the end of the biopsy needle, which, if not within the sound beam, can appear shallower than it is. Reliable localization requires movement of both the needle and the probe. The tendency to go too deep is compounded by the fact that a second hand is not available to hold the biopsy device at the skin and prevent the further advancement that can easily occur during activation. This advancement can be avoided by abandoning the probe during the activation.
The low complication rate of ultrasound-guided biopsies has been well documented (36) and this procedure is extremely safe in knowledgeable and experienced hands. However, major complications do occur, but are rarely reported in the literature. The author is aware of several deaths and a number of serious complications from native renal biopsies, even ones performed under real-time guidance.
Ultrasound guidance is similar for biopsy of transplanted kidneys, except that the patient is in the supine position. One should avoid entering the peritoneum, which can be immediately adjacent to the allograft and often overlie it; it is recognizable by the peristalsis. Although it is unavoidable in intraperitoneal allografts, bowel loops should clearly be avoided. The allograft is often very shallow and easily accessed, but it can be very deep in obese patients. Due to the difficulty in matching needle and insonation angles on the abdomen and the possibility of overlying bowel, real-time guidance is recommended in these patients and patients with intraperitoneal allografts.
Sonography also has a role in postbiopsy management. Routine imaging immediately after a biopsy is not unreasonable but generally not helpful, because small perirenal hematomas and pseudoaneurysms can be seen in otherwise stable patients. In a recent study of ultrasound-guided biopsy in native kidneys, small hematomas were noted in 13% of patients. However, the absence of these findings several hours after a biopsy indicates a very low rate of subsequent complications and may allow for early discharge of patients (37). Although sonography should be performed in cases of pain, hypotension, or laboratory evidence of blood loss, other imaging is often required. Perirenal and subcapsular hematomas are easily seen, but retroperitoneal hematomas may be difficult to discern; also, their extent cannot be reliably determined. Bleeding within the urinary space may only be apparent as calyceal and ureteral dilatation.
Other Applications of Ultrasound in Nephrology
Space considerations prevent a detailed discussion of additional applications of sonography relevant to the practice of nephrology and potentially performable by nephrologists. Foremost among these is in hemodialysis vascular access, particularly arteriovenous fistulae, for both planning and evaluation of dysfunction. Others include insertion of peritoneal catheters (38), diagnosis of tunnel infections in peritoneal dialysis catheters (39), assessment of intravascular volume status through examination of the inferior vena cava (40), and detection of lung water (41).
Ultrasound as a Nephrology Procedure
Sonography is essential to the practice of nephrology; it is inexpensive and relatively easy to learn, making it an ideal tool to be incorporated into the practice of nephrology. With appropriate training, nephrologists can be competent at both performing and interpreting sonograms (42), and can provide the clinical correlation that is usually required for interpretation. However, nephrology has the dubious distinction of being one of the few specialties or subspecialties that has not embraced ultrasound and incorporated it into its training and practice. Although this void has been filled to some extent by the American Society of Diagnostic and Interventional Nephrology in the form of training guidelines and certification (43), few fellowship programs offer training that meets these guidelines (44). However, comprehensive training is available (45) along with certification (46), and an increasing number of nephrology practices are incorporating this procedure. It is hoped that renal ultrasonography will eventually become an integral component of nephrology training.
Disclosures
W.C.O. has consultancy agreements with Baxter Healthcare, Astellas Pharmaceuticals, Synageva BioPharma, Novo Nordisk, and BioMarin Pharmaceutical; received research funding from Baxter Healthcare, Genzyme, Amgen, and Synageva BioPharma; received honoraria from Baxter Healthcare, Astellas Pharmaceuticals, and Amgen; has patents and inventions with Baxter Healthcare; is a scientific advisor to, or has a membership with, Amgen, Kidney International, and American Journal of Kidney Disease; and has other interests/relationships with Emory University Office of Continuing Medical Education.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.O'Neill W: Atlas of Renal Ultrasonography, Philadelphia, Saunders, 2000 [Google Scholar]
- 2.O’Neill WC: Sonographic evaluation of renal failure. Am J Kidney Dis 35: 1021–1038, 2000 [DOI] [PubMed] [Google Scholar]
- 3.O’Neill WC, Baumgarten DA: Ultrasonography in renal transplantation. Am J Kidney Dis 39: 663–678, 2002 [DOI] [PubMed] [Google Scholar]
- 4.O’Neill WC, Bardelli M, Yevzlin AS: Imaging for renovascular disease. Semin Nephrol 31: 272–282, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bakker J, Olree M, Kaatee R, de Lange EE, Moons KGM, Beutler JJ, Beek FJ: Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology 211: 623–628, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H: Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol 15: 38–43, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Khademi M: Angiographic measurement of renal compartments. Corticomedullary ratio in normal, diseased states and sickle cell anemia. Radiology 113: 51–58, 1974 [DOI] [PubMed] [Google Scholar]
- 8.Abrams HL: Quantitative derivates of renal radiologic studies. An overview. Invest Radiol 7: 240–279, 1972 [DOI] [PubMed] [Google Scholar]
- 9.Raj DSC, Hoisala R, Somiah S, Sheeba SD, Yeung M: Quantitation of change in the medullary compartment in renal allograft by ultrasound. J Clin Ultrasound 25: 265–269, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Page JE, Morgan SH, Eastwood JB, Smith SA, Webb DJ, Dilly SA, Chow J, Pottier A, Joseph AEA: Ultrasound findings in renal parenchymal disease: comparison with histological appearances. Clin Radiol 49: 867–870, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA, O’Neill WC: Correlation of renal histopathology with sonographic findings. Kidney International 67: 1515–1520, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Manley JA, O’Neill WC: How echogenic is echogenic? Quantitative acoustics of the renal cortex. Am J Kidney Dis 37: 706–711, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Bosniak MA: Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR Am J Roentgenol 169: 819–821, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Al-Said J, O’Neill WC: Reduced kidney size in patients with simple renal cysts. Kidney Int 64: 1059–1064, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O’Neill WC: Reduced renal function in patients with simple renal cysts. Kidney Int 65: 2303–2308, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Dillman JR, Kappil M, Weadock WJ, Rubin JM, Platt JF, DiPietro MA, Bude RO: Sonographic twinkling artifact for renal calculus detection: Correlation with CT. Radiology 259: 911–916, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Vasbinder GBC, Nelemans PJ, Kessels AGH, Kroon AA, de Leeuw PW, van Engelshoven JMA: Diagnostic tests for renal artery stenosis in patients suspected of having renovascular hypertension: a meta-analysis. Ann Intern Med 135: 401–411, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Heine GH, Gerhart MK, Ulrich C, Köhler H, Girndt M: Renal Doppler resistance indices are associated with systemic atherosclerosis in kidney transplant recipients. Kidney Int 68: 878–885, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, Gebel MJ, Galanski M, Koch KM, Haller H: Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med 344: 410–417, 2001 [DOI] [PubMed] [Google Scholar]
- 20.McArthur C, Geddes CC, Baxter GM: Early measurement of pulsatility and resistive indexes: Correlation with long-term renal transplant function. Radiology 259: 278–285, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Krumme B, Hollenbeck M: Doppler sonography in renal artery stenosis—does the Resistive Index predict the success of intervention? Nephrol Dial Transplant 22: 692–696, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb RH, Weinberg EP, Rubens DJ, Monk RD, Grossman EB: Renal sonography: Can it be used more selectively in the setting of an elevated serum creatinine level? Am J Kidney Dis 29: 362–367, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Jones TB, Riddick LR, Harpen MD, Dubuisson RL, Samuels D: Ultrasonographic determination of renal mass and renal volume. J Ultrasound Med 2: 151–154, 1983 [DOI] [PubMed] [Google Scholar]
- 24.Nomura G, Kinoshita E, Yamagata Y, Koga N: Usefulness of renal ultrasonography for assessment of severity and course of acute tubular necrosis. J Clin Ultrasound 12: 135–139, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Tublin ME, Bude RO, Platt JF: Review. The resistive index in renal Doppler sonography: Where do we stand? AJR Am J Roentgenol 180: 885–892, 2003 [DOI] [PubMed] [Google Scholar]
- 26.O’Neill WC, Robbin ML, Bae KT, Grantham JJ, Chapman AB, Guay-Woodford LM, Torres VE, King BF, Wetzel LH, Thompson PA, Miller JP: Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: The Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). Am J Kidney Dis 46: 1058–1064, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulusan S, Koc Z, Tokmak N: Accuracy of sonography for detecting renal stones: comparison with CT. J Clin Ultrasound 35: 256–261, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wong G, Chapman JR, Craig JC: Cancer screening in renal transplant recipients: What is the evidence? Clin J Am Soc Nephrol 3[Suppl 2]: S87–S100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart JH, Buccianti G, Agodoa L, Gellert R, McCredie MR, Lowenfels AB, Disney AP, Wolfe RA, Boyle P, Maisonneuve P: Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: Analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 14: 197–207, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Docktor BL, Sadler DJ, Gray RR, Saliken JC, So CB: Radiologic placement of tunneled central catheters: Rates of success and of immediate complications in a large series. AJR Am J Roentgenol 173: 457–460, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Prabhu MV, Juneja D, Gopal PB, Sathyanarayanan M, Subhramanyam S, Gandhe S, Nayak KS: Ultrasound-guided femoral dialysis access placement: A single-center randomized trial. Clin J Am Soc Nephrol 5: 235–239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E: Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant 20: 1864–1867, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Forauer AR, Glockner JF: Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Radiol 11: 233–238, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Nass K, O’Neill WC: Bedside renal biopsy: Ultrasound guidance by the nephrologist. Am J Kidney Dis 34: 955–959, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Korbet SM: Percutaneous renal biopsy. Semin Nephrol 22: 254–267, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Maya ID, Allon M: Percutaneous renal biopsy: Outpatient observation without hospitalization is safe. Semin Dial 22: 458–461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maya ID: Ultrasound/fluoroscopy-assisted placement of peritoneal dialysis catheters. Sem Dial 20: 611–615, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Vychytil A, Lilaj T, Lorenz M, Horl WH, Haag-Weber M: Ultrasonography of the catheter tunnel in peritoneal dialysis patients: what are the indications? Am J Kid Dis 33: 722–727, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Brennan JM, Ronan A, Goonewardena S, Blair JEA, Hammes M, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT: Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol 1: 749–753, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A: Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 135: 1433–1439, 2009 [DOI] [PubMed] [Google Scholar]
- 42.O'Neill WC: Renal ultrasonography: a procedure for nephrologists. Am J Kidney Dis 30: 579–585, 1997 [DOI] [PubMed] [Google Scholar]
- 43.American Society of Diagnostic and Interventional Nephrology : Guidelines for training, certification, and accreditation in renal sonography. Semin Dial 15: 442–444, 2002 [PubMed] [Google Scholar]
- 44.Berns JS, O’Neill WC: Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol 3: 941–947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Available at: http://www.medicine.emory.edu/renalultrasound Accessed December 16, 2013
- 46.Available at: http://www.ASDIN.org Accessed December 16, 2013