Summary
Background and objectives
Kidney disease as a complication of inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC), has been the subject of case reports. However, no cases series examining IBD and kidney disease has been published to date. This study aimed to evaluate a large series of kidney biopsy specimens from patients with IBD to better define the spectrum and relative frequencies of IBD-associated kidney pathology.
Design, setting, participants, & measurements
A retrospective review of native kidney biopsy specimens obtained from March 2001 to June 2012 identified 83 patients with IBD. Standard processing of all biopsy specimens included light microscopy, immunofluorescence, and electron microscopy.
Results
There were 45 cases of CD and 38 cases of UC represented. The most common indication for kidney biopsy was acute or chronic kidney failure (63% [52 of 83]) and nephrotic-range proteinuria (16% [13 of 83]). IgA nephropathy was the most common diagnosis (24% [20 of 83]), followed by interstitial nephritis (19% [16 of 83]), arterionephrosclerosis (12% [10 of 83]), acute tubular injury (8% [7 of 83]), proliferative GN (7% [6 of 83]), and minimal-change disease (5% [4 of 83]). When compared, the frequency of IgA nephropathy in IBD was significantly higher than in all other native renal biopsy specimens from the same time period (24% [20 of 83] versus 8% [2734 of 33,630]; P<0.001). Of the 16 cases of interstitial nephritis, 9 (56%) had current or recent past exposure to aminosalicylates, including all cases of granulomatous interstitial nephritis.
Conclusions
IBD is associated with a spectrum of kidney diseases most commonly affecting the glomerular and tubulointerstitial compartments. IgA nephropathy is the most frequent kidney biopsy diagnosis in IBD and has a significantly higher diagnostic prevalence compared with all non-IBD kidney biopsy specimens. This may reflect a common pathogenic mechanism. Although many cases of tubulointerstitial nephritis are related to aminosalicylate exposure, the possibility of a direct relationship with IBD cannot be ruled out.
Introduction
Inflammatory bowel disease (IBD) is a condition characterized by chronic inflammation of the gastrointestinal tract. The two most common types are Crohn disease (CD) and ulcerative colitis (UC). The inciting agent and exact underlying mechanism of IBD are not entirely known; however, convincing evidence suggests that it is mediated by abnormal T cell function in genetically susceptible individuals (1,2). Extraintestinal manifestations of IBD are not uncommon and probably reflect systemic inflammation, autoimmune susceptibility, metabolic and nutritional derangement, or drug-related toxicity (3,4).
Kidney and lower genitourinary involvement has been reported in 4%–23% of patients with IBD manifested primarily as urinary calculi, fistulas, and kidney tubular damage (5,6). Parenchymal kidney disease is rare but has been well documented in the worldwide literature as case reports describing GN (7–10), minimal-change disease (11,12), secondary amyloidosis (13–15), and interstitial nephritis (16–19). To our knowledge, no case series on this topic has been published to date. Therefore, our aim was to evaluate a large series of kidney biopsy specimens from patients with IBD in order to define the spectrum and relative frequencies of IBD-associated kidney abnormalities. The findings are also interpreted in the context of a brief review of the previously published literature.
Materials and Methods
We retrospectively reviewed all native kidney biopsy specimens evaluated at Nephropath, Little Rock, Arkansas, from March 2001 to June 2012. The biopsy specimens were received from multiple medical centers across the United States and represent the range of nephrology practice settings, from small community groups to tertiary referral centers. Eighty-three of 33,713 biopsy specimens were from patients with IBD. All clinical information was obtained via patient data and medical records provided at the time kidney biopsy was requested. An additional standardized questionnaire was administered via telephone or fax for 42 cases with initially incomplete clinical information. The Schulman Associates Institutional Review Board approved this study.
Kidney Biopsy
Kidney biopsy specimens were processed as in our previous studies using standard methods described below (20).
Light Microscopy.
Briefly, kidney biopsy specimens were fixed in buffered formalin, dehydrated in graded alcohols, and embedded in paraffin using standard techniques. Serial 3-mm–thick sections were cut and treated with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, or periodic acid-Schiff reagent.
Granulomatous interstitial nephritis was defined as an interstitial nephritis in which the inflammatory infiltrate contained at least one aggregate of epithelioid histiocytes admixed with lymphocytes with or without multinucleated giant cells.
Immunofluorescence Microscopy.
Samples were transported in Michel media, washed in buffer, and frozen in a cryostat. Sections, cut at 5 mm, were rinsed in buffer and reacted with fluorescein-tagged polyclonal rabbit antihuman antibodies to IgG, IgA, IgM, C3, C4, C1q, fibrinogen, κ or λ light chains (Dako, Carpenteria, CA; Kent Laboratories, Bellingham, WA) for 1 hour and rinsed; a coverslip was applied using aqueous mounting media.
Electron Microscopy.
The ends of the kidney biopsy specimen were removed as 1-mm cubes, dehydrated using graded alcohols, and embedded in Epon/Araldite resin. Sections 1 mm thick were cut using an ultramicrotome, stained with toluidine blue, and examined with a light microscope. Thin sections were examined in a Jeol JEM-1011 electron microscope (Jeol, Tokyo, Japan). Photomicrographs were routinely taken at magnifications of ×4000, ×12,000, and ×20,000.
Statistical Analyses.
Data analysis, including a two-sample test of proportions (Z-test), was performed using Stata 11 statistical software (Stata Corp., College Station, TX). Statistical significance was assumed at P<0.05.
Results
Clinical Characteristics
The IBD cohort included 51 men and 32 women with a mean age ± SD of 46±18 years. There were 45 cases of CD and 38 cases of UC represented. One case of UC had concomitant sclerosing cholangitis. The most common indication for kidney biopsy was acute or chronic kidney failure (63% [52 of 83]), nephrotic-range proteinuria (16% [13 of 83]), and subnephrotic proteinuria (14% [12 of 83]). Only 6 of 83 (7%) patients underwent biopsy for isolated hematuria (Table 1). The median serum creatinine at time of biopsy was 2.7 mg/dl (25th, 75th percentiles, 1.7, 4.3 mg/dl).
Table 1.
Clinical characteristics and demographic characteristics of patients with inflammatory bowel disease referred for kidney biopsy
| Characteristic | Data |
|---|---|
| Patients (n) | 83 |
| Men, n (%) | 51 (61) |
| Mean age ± SD (yr) | 46±18 |
| Ulcerative colitis, n (%) | 38 (46) |
| Crohn disease, n (%) | 45 (54) |
| Median serum creatinine (mg/dl) (25th, 75th percentiles) | 2.7 (1.7, 4.3) |
| Indication for kidney biopsy, n (%) | |
| AKI | 26 (31) |
| CKD | 9 (11) |
| Acute-on-chronic kidney disease | 17 (21) |
| Nephrotic-range proteinuria | 13 (16) |
| Subnephrotic proteinuria | 12 (14) |
| Isolated hematuria | 6 (7) |
All patients were evaluated clinically for systemic lupus erythematosus by serologic testing and physical examination. Eight patients (4 with CD and 4 with UC) had a positive antinuclear antibody titer that ranged from 1:320 to 1:640. All of these patients were negative for anti–double-stranded DNA antibodies except for one patient with UC and autoimmune hepatitis and one patient with CD with rheumatoid arthritis and psoriasis. None of the patients met American College of Rheumatology criteria for the diagnosis of systemic lupus erythematosus.
Kidney Biopsy Abnormalities
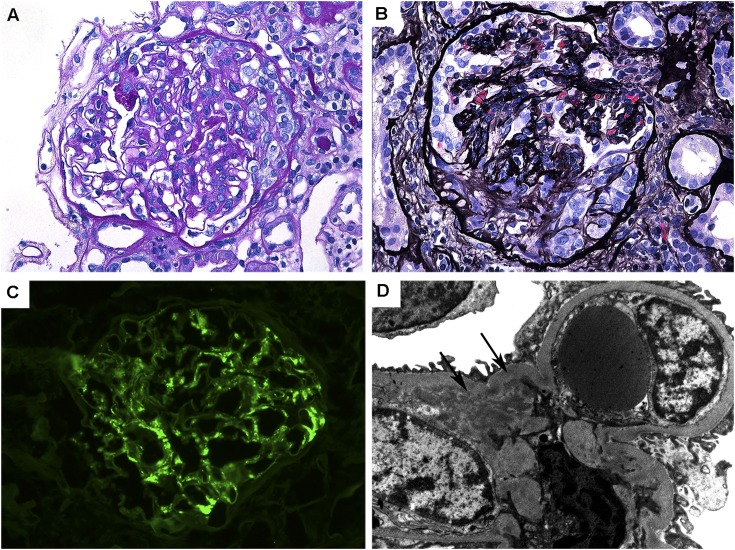
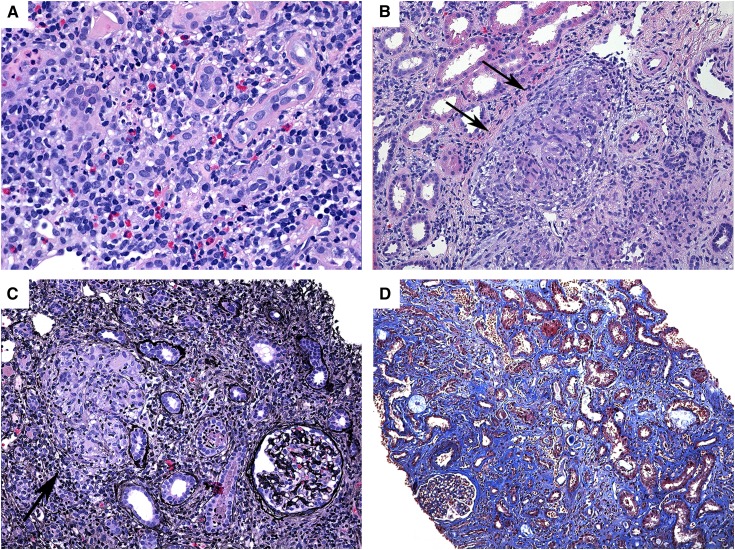
On kidney biopsy, IgA nephropathy (IgAN) was the most common diagnosis (Figure 1), present in 20 of 83 cases (24%), followed by interstitial nephritis in 16 of 83 cases (19%) (Figure 2). The next most common diagnoses were arterionephrosclerosis (12% [10 of 83]), acute tubular injury (8% [7 of 83]), proliferative GN (7% [6 of 83]), and minimal-change disease (5% [4 of 83]). Twelve additional primary findings were represented in the remaining 20 cases (Table 2). One case of secondary amyloidosis was diagnosed in a patient with CD.
Figure 1.
Pathologic features of IgA nephropathy associated with inflammatory bowel disease. (A) Glomerulus with mild segmental mesangial matrix expansion and mesangial hypercellularity (periodic acid-Schiff, original magnification ×400). (B) Glomerulus with a small cellular crescent and compression of the underlying glomerular tuft (Jones methenamine silver, original magnification ×400). (C) Glomerulus with global mesangial staining with antisera for IgA (fluorescein, original magnification ×400). (D) Electron microscopy of mesangial region containing small electron-dense deposits (arrows) (original magnification ×12,000).
Figure 2.
Pathologic features of interstitial nephritis associated with inflammatory bowel disease. (A) Acute interstitial nephritis with dense interstitial inflammation composed predominantly of lymphocytes and eosinophils (hematoxylin and eosin, original magnification ×200). (B and C) Granulomatous interstitial nephritis with interstitial infiltration by mononuclear cells and noncaseating granulomas with multinucleated giant cells (arrows) (hematoxylin and eosin, Jones methenamine silver, both original magnification ×200). (D) Chronic interstitial nephritis with diffuse interstitial fibrosis and tubular atrophy. The glomerulus is preserved (Masson trichrome, original magnification ×100).
Table 2.
Primary kidney biopsy findings in patients with inflammatory bowel disease
| Diagnosis | Patients (n/N) |
|---|---|
| IgA nephropathy | 20/83 |
| Interstitial nephritis (acute and chronic) | 16/83 |
| Arterionephrosclerosis | 10/83 |
| Acute tubular injury | 7/83 |
| Proliferative GN | 6/83 |
| Minimal-change disease | 4/83 |
| Fibrillary glomerulopathy | 3/83 |
| FSGS | 3/83 |
| Diabetic nephropathy | 2/83 |
| Membranous glomerulopathy | 2/83 |
| Normal | 2/83 |
| AL amyloid | 2/83 |
| AA amyloid | 1/83 |
| C1q nephropathy | 1/83 |
| Cholesterol emboli | 1/83 |
| Pauci-immune GN | 1/83 |
| Thin-basement-membrane nephropathy | 1/83 |
| Thrombotic microangiopathy | 1/83 |
In cases of acute interstitial nephritis, the inflammatory infiltrate showed no evidence of microabscess formation, neutrophilic tubulitis, or white blood cell casts that would suggest acute pyelonephritis, and none of the patients had a clinical history or manifestations of ascending urinary tract infection. Only one case of chronic interstitial nephritis showed intratubular calcium oxalate deposition in a patient with a >20-year history of CD.
Treatment
In terms of IBD-related therapy, 28 patients had known current or past exposure to aminosalicylates; 5-aminosalicylic acid (5-ASA; i.e., mesalamine) was the most common drug. This included 21 patients with UC and 7 patients with CD. One of these patients had a history of “hypersensitivity nephritis” clinically diagnosed 1 year before kidney biopsy. Eight additional patients were being treated with the TNF-α inhibitor infliximab. Twenty patients had undergone a bowel resection.
Analyses
The frequency of IgAN in IBD was significantly higher than in all other native kidney biopsy specimens evaluated at our institution during the same time period (24% [20 of 83] versus 8% [2734 of 33,630]; P<0.001). This IgAN subgroup included 13 patients with CD and 7 with UC. Four of these cases had a concurrent vasculitic rash, two of which were confirmed as leukocytoclastic vasculitis on skin biopsy. One additional case had a previous clinical diagnosis of Henoch-Schönlein purpura.
Of the 16 cases with interstitial nephritis, 7 were classified as acute, 5 as granulomatous, and 4 as chronic. All of the cases of granulomatous interstitial nephritis had a history of current or recent past exposure to aminosalicylates. Known exposure to this class of drugs was present in 3 cases of acute and 1 case of chronic interstitial nephritis. Three of 4 patients with minimal-change disease were also currently taking aminosalicylates.
Discussion
We report our experience of kidney biopsy findings in patients with IBD. To our knowledge, this is the largest clinicopathologic series to date on this topic. Our series shows that IBD is associated with a spectrum of kidney diseases, with IgAN and interstitial nephritis being the most common (43% [36 of 83]) diagnosis on kidney biopsy. Given the relative frequency of subclinical IgAN in otherwise healthy populations, it was possible that the high frequency of IgAN found in patients with IBD was due to chance alone (21,22). We therefore compared the biopsy findings in this cohort with our native kidney biopsy specimens from patients without IBD. The prevalence of IgAN was significantly higher in patients with IBD than in patients without IBD. This included only one patient with known recent history of infection in the form of lower-extremity cellulitis and one patient with a history of cirrhosis and clinical suspicion for Henoch-Schönlein purpura.
Hubert et al. (23) reported the first cases of IBD-associated IgAN in 1984. They described both clinical and pathologic remission of kidney disease concomitant with the treatment of symptoms of intestinal disease. Nineteen subsequent case reports in the literature have described IgAN in IBD (9,22,24–34). A majority of these patients had IgAN during onset or exacerbation of IBD, as well as clinical remission of kidney disease in conjunction with successful treatment of bowel inflammation. Elevated serum IgA levels were not consistently measured but were reported to be elevated in several patients. Repeat kidney biopsy confirming histologic remission of GN was rare. However, when a biopsy was repeated, it showed resolution of both mesangial proliferation and IgA deposits (23).
Secondary forms of IgAN have been described, most commonly in the setting of liver disease. However, an increasing number of reports in the literature have associated mucosal inflammation or infection with IgAN (21,24). This is perhaps not surprising given the important immunologic role IgA plays in the defense against environmental and microbial antigenic exposure occurring at mucosal sites, in particular the gastrointestinal tract. Secondary IgAN in IBD is therefore likely to represent a complex interplay of mucosal inflammation, loss of antigenic exclusion, and tolerance, chronic immune stimulation, and dysregulated IgA production and transport (21). Given that intestinal mucosal immune responses are highly dependent on co-stimulation, the role of T cell dysfunction in this process has also been implicated (35). In their study of transgenic mice, Wang et al. (35) showed that T cell–mediated mucosal immunity was critical in intestinal inflammation and in the pathogenesis of IgAN. Localized gastrointestinal immunosuppression (i.e., enteric budesonide) as a potential treatment of primary IgAN likewise alludes to a pathogenic role of gut immune responses in the development of GN (36). Genetic susceptibility has also been investigated, and an association with HLA-DR1 has been described in both IgAN and IBD (29). Taken together, these findings support a pathogenic link between immune mechanisms operating in IBD and IgAN rather than the idea that IBD only exacerbates primary IgAN as has been suggested (22).
Drug-induced nephrotoxicity has been well described in IBD, particularly in patients treated with 5-ASA and its derivatives (e.g., sulfasalazine, mesalamine). The most common kidney histologic finding reported in association with 5-ASA is interstitial nephritis (4,37,38). The pathogenesis of this is unknown, and studies have been unable to identify a clear relationship between duration and dose of 5-ASA and the development of kidney disease (37,38). It is therefore thought to probably represent an idiosyncratic, delayed-type hypersensitivity that is independent of dose and duration of exposure (39). Unfortunately, the most frequent form of 5-ASA–related interstitial nephritis is that of severe, chronic, and progressive kidney injury, which often escapes early clinical detection (37). A high index of clinical suspicion is therefore warranted, and monitoring of kidney function during 5-ASA therapy is suggested (37,40).
Our finding that all patients with granulomatous interstitial nephritis had recent exposure to aminosalicylates is more consistent with a cell-mediated hypersensitivity reaction than a true extraintestinal manifestation of IBD. However, kidney tubular damage, in the form of proteinuria and enzymuria, has been frequently observed in IBD and is more strongly correlated with disease activity than therapy (41–43). There have also been several case reports of interstitial nephritis in therapy-naive patients to include the development of kidney failure before or concurrently with the diagnosis of bowel inflammation (44–49). This appears to be more common in CD than in UC. Overall, these findings raise the possibility of interstitial nephritis as a genuine extraintestinal manifestation of IBD, perhaps secondary to systemic immune dysregulation and cytokine activation (44,48).
This study has several limitations. First, the retrospective, observational study design limits any findings to hypothesis generation rather than validation, and patient and kidney outcomes were not examined. Second, the duration and activity of IBD as well as the dose and duration of therapeutic exposure were often not known, precluding further analysis of these variables. Because the nephrotoxicity of aminosalicylates is thought to be idiosyncratic and not dose or duration dependent, our finding of a possible association between granulomatous interstitial nephritis and this class of drugs remains plausible in patients with a history of recent exposure. Finally, correlation between kidney biopsy findings and IBD subtype was not performed. One must be cautious in doing this given the potential pitfalls of IBD diagnosis, where endoscopic and pathologic features can overlap between CD and UC (50).
Our findings also raise several unanswered questions related to kidney monitoring and outcome in patients with IBD. First, do patients with IBD-associated IgAN progress to ESRD more often than those with primary IgAN? Although a long-term direct comparison between the two is needed to answer this question, the current literature would suggest that kidney outcome likely depends on the course of the intestinal disease in IBD-associated IgAN. In addition, what kidney function markers correlate best with IBD activity, and how often should kidney function be monitored in patients with IBD, particularly those receiving aminosalicylate therapy? Although there are several approaches for screening and monitoring with serum creatinine, the optimal markers and frequency of testing remain to be established (37). Finally, are there other clinical and patient variables that would allow better identification of patients with IBD at higher risk of developing drug-related nephrotoxicity?
In summary, IBD is associated with a spectrum of kidney complications likely related to chronic inflammation or drug therapy in these patients. The most common diagnosis found on kidney biopsy is IgAN, and its significantly higher prevalence in this population suggests a shared pathophysiology between intestinal and kidney disease. Interstitial nephritis is the second most common diagnosis, which is often, although not invariably, associated with aminosalicylate therapy. Overall, a high degree of clinical suspicion is needed for the early diagnosis and prevention of these IBD-related kidney complications.
Disclosures
None.
Acknowledgments
This work was presented in part at the United States & Canadian Academy of Pathology Annual Meeting, held March 5, 2013, in Baltimore (Ambruzs et al. Mod Pathol 26: 384, 2013).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Marsal J, Agace WW: Targeting T-cell migration in inflammatory bowel disease. J Intern Med 272: 411–429, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB: Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3: 390–407, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, Gasbarrini G, Gasbarrini A: Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol 11: 7227–7236, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oikonomou K, Kapsoritakis A, Eleftheriadis T, Stefanidis I, Potamianos S: Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis 17: 1034–1045, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Shield DE, Lytton B, Weiss RM, Schiff M, Jr: Urologic complications of inflammatory bowel disease. J Urol 115: 701–706, 1976 [DOI] [PubMed] [Google Scholar]
- 6.Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT: Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol 93: 504–514, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Ridder RM, Kreth HW, Kiss E, Gröne HJ, Gordjani N: Membranous nephropathy associated with familial chronic ulcerative colitis in a 12-year-old girl. Pediatr Nephrol 20: 1349–1351, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Shaer AJ, Stewart LR, Cheek DE, Hurray D, Self SE: IgA antiglomerular basement membrane nephritis associated with Crohn’s disease: A case report and review of glomerulonephritis in inflammatory bowel disease. Am J Kidney Dis 41: 1097–1109, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Peeters AJ, van den Wall Bake AW, Daha MR, Breeveld FC: Inflammatory bowel disease and ankylosing spondylitis associated with cutaneous vasculitis, glomerulonephritis, and circulating IgA immune complexes. Ann Rheum Dis 49: 638–640, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellwege HH, Bläker F, Gebbers JO: [Hypocomplementemic membranoproliferative glomerulonephritis in a child with ulcerative colitis (author’s transl)]. Monatsschr Kinderheilkd 124: 706–711, 1976 [PubMed] [Google Scholar]
- 11.Firwana BM, Hasan R, Chalhoub W, Ferwana M, Kang JY, Aron J, Lieber J: Nephrotic syndrome after treatment of Crohn’s disease with mesalamine: Case report and literature review. Avicenna J Med 2: 9–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molnár T, Farkas K, Nagy F, Iványi B, Wittmann T: Sulfasalazine-induced nephrotic syndrome in a patient with ulcerative colitis. Inflamm Bowel Dis 16: 552–553, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Guardiola-Arévalo A, Alcántara-Torres M, Valle-Muñoz J, Lorente-Poyatos RH, Romero-Gutiérrez M, Rodríguez-Merlo R, Pérez-Martínez A, Carrobles-Jiménez JM: Amyloidosis and Crohn’s disease. Rev Esp Enferm Dig 103: 268–274, 2011 [PubMed] [Google Scholar]
- 14.Wester AL, Vatn MH, Fausa O: Secondary amyloidosis in inflammatory bowel disease: a study of 18 patients admitted to Rikshospitalet University Hospital, Oslo, from 1962 to 1998. Inflamm Bowel Dis 7: 295–300, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Greenstein AJ, Sachar DB, Panday AK, Dikman SH, Meyers S, Heimann T, Gumaste V, Werther JL, Janowitz HD: Amyloidosis and inflammatory bowel disease. A 50-year experience with 25 patients. Medicine (Baltimore) 71: 261–270, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Tadic M, Grgurevic I, Scukanec-Spoljar M, Bozic B, Marusic S, Horvatic I, Galesic K: Acute interstitial nephritis due to mesalazine. Nephrology (Carlton) 10: 103–105, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Margetts PJ, Churchill DN, Alexopoulou I: Interstitial nephritis in patients with inflammatory bowel disease treated with mesalamine. J Clin Gastroenterol 32: 176–178, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Agharazii M, Marcotte J, Boucher D, Noël R, Lebel M: Chronic interstitial nephritis due to 5-aminosalicylic acid. Am J Nephrol 19: 373–376, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Witte T, Olbricht CJ, Koch KM: Interstitial nephritis associated with 5-aminosalicylic acid. Nephron 67: 481–482, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Walker PD: The renal biopsy. Arch Pathol Lab Med 133: 181–188, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Pouria S, Barratt J: Secondary IgA nephropathy. Semin Nephrol 28: 27–37, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Filiopoulos V, Trompouki S, Hadjiyannakos D, Paraskevakou H, Kamperoglou D, Vlassopoulos D: IgA nephropathy in association with Crohn’s disease: A case report and brief review of the literature. Ren Fail 32: 523–527, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Hubert D, Beaufils M, Meyrier A: [Immunoglobulin A glomerular nephropathy associated with inflammatory colitis. Apropos of 2 cases]. Presse Med 13: 1083–1085, 1984 [PubMed] [Google Scholar]
- 24.Pipili C, Michopoulos S, Sotiropoulou M, Mpakirtzi T, Grapsa E: Is there any association between IgA nephropathy, Crohn’s disease and Helicobacter pylori infection? Ren Fail 34: 506–509, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Ku E, Ananthapanyasut W, Campese VM: IgA nephropathy in a patient with ulcerative colitis, Graves’ disease and positive myeloperoxidase ANCA. Clin Nephrol 77: 146–150, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Choi JY, Yu CH, Jung HY, Jung MK, Kim YJ, Cho JH, Kim CD, Kim YL, Park SH: A case of rapidly progressive IgA nephropathy in a patient with exacerbation of Crohn’s disease. BMC Nephrol 13: 84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onime A, Agaba EI, Sun Y, Parsons RB, Servilla KS, Massie LW, Tzamaloukas AH: Immunoglobulin A nephropathy complicating ulcerative colitis. Int Urol Nephrol 38: 349–353, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Forshaw MJ, Guirguis O, Hennigan TW: IgA nephropathy in association with Crohn’s disease. Int J Colorectal Dis 20: 463–465, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Takemura T, Okada M, Yagi K, Kuwajima H, Yanagida H: An adolescent with IgA nephropathy and Crohn disease: Pathogenetic implications. Pediatr Nephrol 17: 863–866, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Trimarchi HM, Iotti A, Iotti R, Freixas EA, Peters R: Immunoglobulin A nephropathy and ulcerative colitis. A focus on their pathogenesis. Am J Nephrol 21: 400–405, 2001 [DOI] [PubMed] [Google Scholar]
- 31.McCallum D, Smith L, Harley F, Yiu V: IgA nephropathy and thin basement membrane disease in association with Crohn disease. Pediatr Nephrol 11: 637–640, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Hirsch DJ, Jindal KK, Trillo A, Cohen AD: Acute renal failure in Crohn’s disease due to IgA nephropathy. Am J Kidney Dis 20: 189–190, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Iida H, Asaka M, Izumino K, Takata M, Sasayama S, Tanaka M: IgA nephropathy complicated by ulcerative colitis. Nephron 53: 285–286, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Kammerer J, Genin I, Michel P, Gassmann-Cosme H: [Glomerulonephritis caused by mesangial deposits of immunoglobulins A associated with Crohn disease]. Gastroenterol Clin Biol 18: 293, 1994 [PubMed] [Google Scholar]
- 35.Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX: Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest 113: 826–835, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smerud HK, Bárány P, Lindström K, Fernström A, Sandell A, Påhlsson P, Fellström B: New treatment for IgA nephropathy: Enteric budesonide targeted to the ileocecal region ameliorates proteinuria. Nephrol Dial Transplant 26: 3237–3242, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Gisbert JP, González-Lama Y, Maté J: 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm Bowel Dis 13: 629–638, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Oikonomou KA, Kapsoritakis AN, Stefanidis I, Potamianos SP: Drug-induced nephrotoxicity in inflammatory bowel disease. Nephron Clin Pract 119: c89–c94, discussion c96, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Arend LJ, Springate JE: Interstitial nephritis from mesalazine: Case report and literature review. Pediatr Nephrol, 19: 550-553 discussion c596, 2004 [DOI] [PubMed]
- 40.Corrigan G, Stevens PE: Review article: Interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Aliment Pharmacol Ther 14: 1–6, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Fraser JS, Muller AF, Smith DJ, Newman DJ, Lamb EJ: Renal tubular injury is present in acute inflammatory bowel disease prior to the introduction of drug therapy. Aliment Pharmacol Ther 15: 1131–1137, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Herrlinger KR, Noftz MK, Fellermann K, Schmidt K, Steinhoff J, Stange EF: Minimal renal dysfunction in inflammatory bowel disease is related to disease activity but not to 5-ASA use. Aliment Pharmacol Ther 15: 363–369, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Kreisel W, Wolf LM, Grotz W, Grieshaber M: Renal tubular damage: an extraintestinal manifestation of chronic inflammatory bowel disease. Eur J Gastroenterol Hepatol 8: 461–468, 1996 [PubMed] [Google Scholar]
- 44.Tokuyama H, Wakino S, Konishi K, Hashiguchi A, Hayashi K, Itoh H: Acute interstitial nephritis associated with ulcerative colitis. Clin Exp Nephrol 14: 483–486, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Waters AM, Zachos M, Herzenberg AM, Harvey E, Rosenblum ND: Tubulointerstitial nephritis as an extraintestinal manifestation of Crohn’s disease. Nat Clin Pract Nephrol 4: 693–697, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Zeier M, Schmidt R, Andrassy K, Waldherr R, Ritz E: Idiopathic interstitial nephritis complicating ulcerative colitis. Nephrol Dial Transplant 5: 901, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Izzedine H, Simon J, Piette AM, Lucsko M, Baumelou A, Charitanski D, Kernaonet E, Baglin AC, Deray G, Beaufils H: Primary chronic interstitial nephritis in Crohn’s disease. Gastroenterology 123: 1436–1440, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Shahrani Muhammad HS, Peters C, Casserly LF, Dorman AM, Watts M: Relapsing tubulointerstitial nephritis in an adolescent with inflammatory bowel disease without aminosalicylate exposure. Clin Nephrol 73: 250–252, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Semjén D, Fábos Z, Pakodi F, Vincze A, Szabó I, Degrell P, Csete M, Tornóczky T: Renal involvement in Crohn’s disease: Granulomatous inflammation in the form of mass lesion. Eur J Gastroenterol Hepatol 23: 1267–1269, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Yantiss RK, Odze RD: Pitfalls in the interpretation of nonneoplastic mucosal biopsies in inflammatory bowel disease. Am J Gastroenterol 102: 890–904, 2007 [DOI] [PubMed] [Google Scholar]