Summary
As judged by the American College of Radiology Appropriateness Criteria, renal Doppler ultrasonography is the most appropriate imaging test in the evaluation of AKI and has the highest level of recommendation. Unfortunately, nephrologists are rarely specifically trained in ultrasonography technique and interpretation, and important clinical information obtained from renal ultrasonography may not be appreciated. In this review, the strengths and limitations of grayscale ultrasonography in the evaluation of patients with AKI will be discussed with attention to its use for (1) assessment of intrinsic causes of AKI, (2) distinguishing acute from chronic kidney diseases, and (3) detection of obstruction. The use of Doppler imaging and the resistive index in patients with AKI will be reviewed with attention to its use for (1) predicting the development of AKI, (2) predicting the prognosis of AKI, and (3) distinguishing prerenal azotemia from intrinsic AKI. Finally, pediatric considerations in the use of ultrasonography in AKI will be reviewed.
Introduction
Renal ultrasonography is typically the most appropriate and useful radiologic test in the evaluation of patients with AKI (1). In this report, we review the interpretation of Doppler ultrasonography in adult and pediatric patients with AKI, with attention to its most appropriate uses, which include (1) the assessment of intrinsic causes of AKI, (2) distinguishing acute from chronic kidney diseases, and (3) detection of obstruction.
Use of Specific Parameters Obtained from Ultrasonography and Their Interpretation
Echoes and Echogenicity
Proper interpretation of ultrasonography images depends on a basic understanding of how ultrasonography images are generated. For diagnostic ultrasonography, high-frequency sound waves are generated and received by the ultrasonography transducer, which is placed on the skin. Returning sound waves (echoes) are processed by a computer and displayed on a computer screen. A gray-scale image is produced when the ultrasonography machine operates in B-mode, or brightness mode, in which returning echoes are represented as bright dots; the brightness of the dots represents the strength of the reflected echoes. Echogenicity, therefore, refers to how bright or dark something appears in the gray-scale image; the brighter something appears, the more echogenic it is. With regard to the kidney, echogenicity generally refers to how bright or dark the kidney parenchyma appears in comparison to the liver.
Normally, something that reflects most of the sound waves back will appear the brightest (i.e., white); for example, fat and fibrous tissue are very echogenic and are therefore some of the brightest-appearing structures on ultrasonography images. The renal capsule consists of thin fibrous tissue, which is next to fat, and thus the kidney often appears to be surrounded by a very bright rim on ultrasonography when there is a difference of acoustic impedance relative to the adjacent tissues. Solid organs, such as the liver and spleen, have intermediate echogenicity, and the kidney parenchyma, consisting of the cortex and medulla, is normally isoechoic (equal in brightness) or hypoechoic (darker) compared with the normal liver (2–4) or normal spleen. Thus, liver and spleen echogenicity must be normal for comparison to be valid; in particular, fatty infiltration of the liver can increase its echogenicity, making evaluation of the echogenicity of the right kidney more difficult. The cortex and medulla of the kidney generally have the same echogenicity, although the medulla may be slightly darker (5,6); both kidneys should have similar echogenicity to each other. The renal sinus in the center of the kidney contains fibro-fatty tissue and thus appears very bright relative to the kidney parenchyma. Bland fluid (e.g., simple cysts), urine, and blood are anechoic (black); therefore, the medullary pyramids, which contain urine in parallel tubules, appear as dark pools between the cortex and renal sinus. (See Figure 1 for the normal echogenic appearance of the capsule, parenchyma, medullary pyramids, and central renal sinus.) Blood clots in the urinary space will appear echogenic compared with the surrounding fluid; relative to the renal parenchyma, an acute clot is hypoechoic, while subacute and chronic clots are echogenic (brighter than renal parenchyma).
Figure 1.
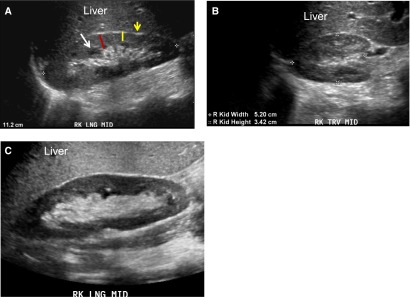
Grayscale ultrasonographic image demonstrating normal kidney size and echogenicity. (A) Grayscale longitudinal ultrasonographic image of a normal right kidney. The kidney is 11.2 cm long and surrounded by the bright rim of renal capsule (yellow arrow) around the parenchyma. The normal parenchyma (red line) forms a thick darker rim around the central bright (echogenic) sinus. Normal renal parenchyma is similar (isoechoic) or slightly darker (hypoechoic) compared with liver and includes the cortex and medulla. The thickness of the renal cortex is measured from the outer border of the medullary pyramids (yellow line) or from the arcuate arteries to the renal capsule. The medullary pyramids (white arrow) contain fluid in parallel tubules, which is anechoic (black) and appear as regularly spaced dark pools at the inner margin of the parenchyma (arrow). Because fat is echogenic, the central renal sinus appears bright. (B) Grayscale transverse ultrasonographic image of normal right kidney showing normal width (5.2 cm) and height (3.4 cm, anterior/posterior measurement) and appearance. (C) Grayscale longitudinal ultrasonographic image of normal right kidney next to a hyperechoic-appearing liver that has steatosis (because fat is echogenic, the accumulation of fat increases the liver echogenicity/brightness). LNG, longitudinal view; MID, midline; RK, right kidney; TRV, transverse.
What Is the Significance of Echogenicity in AKI and Other Kidney Diseases?
Increased echogenicity of the kidney parenchyma results from the increased presence of material that can reflect sound waves back, thus increasing its brightness on the ultrasonography image. Because fibrous tissue (e.g., glomerulosclerosis, interstitial fibrosis) increases echogenicity, CKD is typically associated with increased echogenicity. Inflammatory infiltrates may explain the increased echogenicity that occurs with acute interstitial nephritis and GN (7). Proteinaceous casts are thought to cause the increased echogenicity associated with acute tubular necrosis (ATN) (8). Calcium deposits and stones are very echogenic; thus, medullary nephrocalcinosis is characterized by increased medullary echogenicity and a relatively normal-appearing cortex (Figure 2). Several renal diseases are associated with medullary nephrocalcinosis, including medullary sponge kidney, hyperparathyroidism, renal tubular acidosis, and vitamin D toxicity (9,10). Other kidney diseases that have been associated with a hyperechoic medulla include sickle cell disease and gout (9).
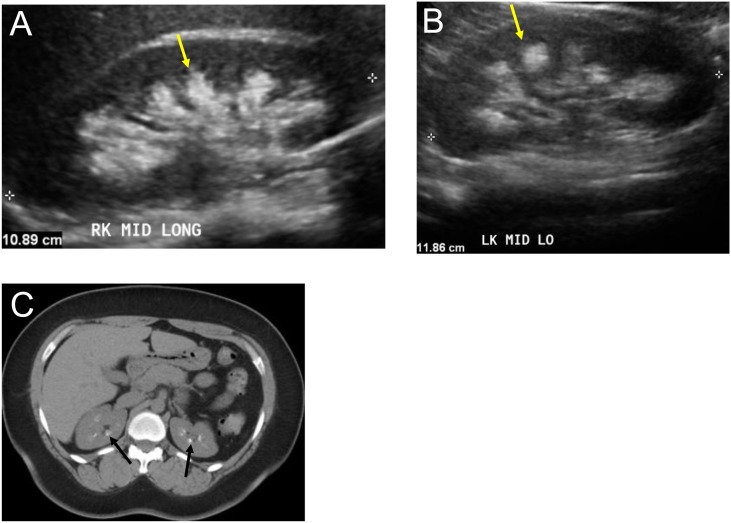
Figure 2.
Hyperechoic (bright) medulla from medullary nephrocalcinosis. Several kidney diseases may be associated with a hyperechoic medulla, including medullary nephrocalcinosis, sickle cell diseases, and gout. The midline longitudinal image of the right kidney (A) and left kidney (B) demonstrate hyperechoic (bright) medulla (yellow arrow) in a 35-year-old woman with medullary calcinosis. (C) Axial noncontrast computed tomographic image of the same patient demonstrates calcification in medullary pyramids of both kidneys (black arrow). LK, left kidney; LO, long axis; MID, midline.
Cortical necrosis is a rare cause of AKI that is classically characterized by a dark, hypoechogenic renal cortex on ultrasonography. Cortical necrosis is a consequence of severe ischemia due to vascular abnormalities such as hemolytic-uremic syndrome, complications of pregnancy (e.g., eclampsia), or sepsis, which results in necrosis of tubular cells of the cortex; the necrotic cells and resultant increase in interstitial fluid cause the decrease in cortical echogenicity (11).
Although clinically relevant kidney diseases may be present without changes in echogenicity, if increased parenchymal echogenicity is noted (echogenicity is greater than a normal liver) it is usually abnormal (except in neonates). For example, increased echogenicity was reported to have a 96% specificity (and 67% positive predictive value) for the presence of parenchymal kidney disease (3).
Use of Increased Echogenicity to Distinguish AKI from CKD.
Increased echogenicity may occur in AKI or CKD and cannot be used to reliably distinguish between the two. It is important to recognize, however, that in patients with CKD, it is unusual for kidney echogenicity to be less than that of the liver. In one study, for example, only 11% of patients with CKD (serum creatinine, 8 mg/dl) had kidney echogenicity that was less than liver echogenicity (2); of note, 30% of patients with AKI had kidney echogenicity that was less than liver echogenicity. Furthermore, when ultrasonography characteristics were directly compared with renal histopathology, the combination of small kidneys and increased cortical echogenicity correlated very well with chronic, untreatable kidney disease (12). It should be noted, however, that diabetic kidney disease is one cause of CKD that may be associated with normal kidney echogenecity.
Use of Echogenicity to Distinguish among Different Causes of AKI.
Many studies have attempted to determine whether echogenicity might be useful to distinguish among different forms of AKI. For example, it was originally reported that ischemic ATN might be characterized by normal or reduced echogenicity (presumably from edema), while nephrotoxic ATN was characterized by increased echogenicity (13). Additional studies did not support this conclusion, and it is now clear that increased echogenicity may also occur with ischemic ATN (2) (see Figure 3A). Increased echogenicity has been observed in several forms of AKI, including acute interstitial nephritis (possibly from increased inflammatory infiltrate), GN (possibly from vascular changes), multiple myeloma (Figure 4), HIV nephropathy, and obstruction (6,14). Decreased echogenicity may occur with renal infarcts and renal lymphoma (14). Overall, changes in kidney echogenicity alone are unlikely to be useful for distinguishing among the different causes of AKI; however, in conjunction with kidney length and symptoms, certain disease may be more likely than others, as summarized in Table 1.
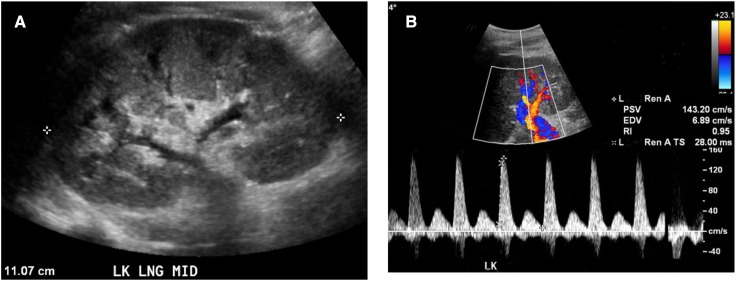
Figure 3.
Grayscale ultrasonographic image and resistive index (RI) in severe ischemic acute tubular necrosis. (A) Grayscale ultrasonographic image demonstrates normal renal length (11.7 cm), mild increased parenchymal echogenicity, and increased parenchymal thickness, and (B) Doppler imaging demonstrates very high RI (0.95) in a 21-year-old woman with severe ischemic acute tubular necrosis who required hemodialysis for 3 weeks. The patient eventually recovered normal kidney function. (Note: RI was determined in the renal artery near the segmental branch.) EVD, end diastolic velocity; L, left; PSV, peak systolic velocity; RenA, renal artery; RenA TS, renal artery time slope (acceleration time).
Figure 4.
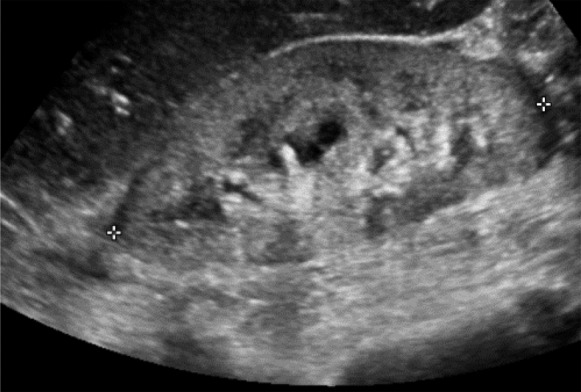
AKI in a patient with multiple myeloma. Grayscale longitudinal ultrasonographic image of the right kidney demonstrates abnormal bright renal parenchyma in a 39-year-old woman with multiple myeloma and AKI.
Table 1.
Differential diagnosis of kidney diseases based on kidney size, echogenicity, and symptoms
| Differential Diagnosis of Kidney Diseases | ||
| Normal echogenicity with normal or increased kidney size | Normal or increased echogenicity with increased kidney size and reduced kidney function | |
| Early diabetic nephropathy | ||
| GN | ||
| Normal echogenicity with increased kidney size and normal kidney function | Vasculitis | |
| Tubulointerstitial disease (e.g., acute interstitial nephritis) | ||
| Steroid use | ||
| Obesity | Amyloid | |
| Tall stature | Multiple myeloma | |
| Acromegaly | HIV nephropathy | |
| Diabetes | Pre-eclampsia | |
| Asymptomatic, unilateral increase in kidney size | Increased medullary echogenicity with normal cortex | |
| Hypertrophy from decreased function of other kidney | Medullary nephrocalcinosis | |
| Renal duplication anomaly | Tamm Horsfall protein in tubules | |
| Lymphoma/leukemia (usually affects both kidneys) | Urate nephropathy | |
| Sickle cell disease | ||
| Symptomatic, unilateral increase in kidney size | Sjögren syndrome | |
| Medullary sponge kidney | ||
| Pyelonephritis | ||
| Acute vascular insult (e.g., renal vein thrombosis) | Decreased cortical echogenicity (i.e., dark cortex) | |
| Urinary tract obstruction | ||
| Acute cortical necrosis | ||
| Edema | ||
Kidney diseases may have a particular appearance based on kidney length, echogenicity, and symptoms as listed in the table above. Although baseline kidney size is often not known, if a change in kidney size >1 cm is detected relative to a prior study, it is unlikely to be due to interobserver variability (6).
Kidney Size
Although renal volume is the most accurate measure of renal size, kidney length is the most clinically useful measurement of kidney size because it is simple to obtain and minimally affected by interobserver variability (15).
Kidney length is useful in the assessment of patient with AKI and may help distinguish AKI from CKD. Kidney length to distinguish between AKI and CKD has been prospectively evaluated; in a study of 127 patients with creatinine>3.0 mg/dl, right kidney length was 11.2±1.4 cm among those with AKI and significantly shorter in patients with CKD at 9.0±1.5 cm (2). Thus, small kidneys suggest the diagnosis of CKD; however, normal or increased kidney length may occur in either AKI or CKD. Large kidneys in AKI may result from infiltrative diseases (e.g., multiple myeloma, amyloidosis, lymphoma), acute parenchymal disorders due to edema and inflammation (e.g., acute GN [Figure 5, A and C], acute interstitial nephritis), and renal vein thrombosis due to obstruction of blood flow and subsequent edema.
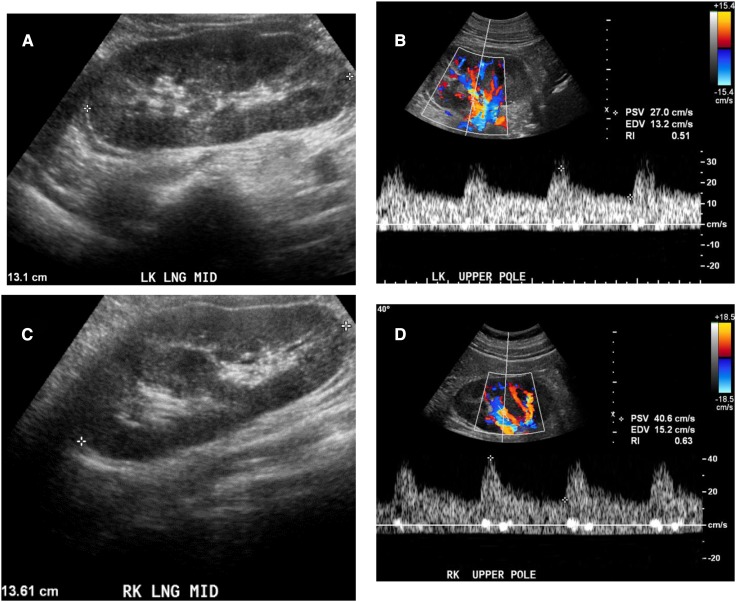
Figure 5.
Grayscale ultrasonographic image and resistive index in glomerular diseases. Patients with glomerular disease are classically described as having increased kidney size, with normal or increased parenchymal echogenicity, and normal resistive index. (A) Grayscale ultrasonographic image of LK demonstrates increased kidney size (13.1 cm) and normal parenchymal echogenicity and (B) Doppler image demonstrates resistive index of 0.51 in a 50-year-old man with biopsy-proven minimal-change disease. (C) Grayscale ultrasonographic image of RK demonstrating increased kidney size (13.6 cm) and normal parenchymal echogenicity, and (D) normal resistive index (0.63) in 57-year-old man with rapidly progressive GN and biopsy-certain necrotizing and crescentic GN from a pauci-immune GN; creatinine was 4.3 mg/dl at time of biopsy.
Because kidney length is especially important in distinguishing AKI from CKD, a clear understanding of “normal” kidney length is important. Studies of kidney length have revealed the following: (1) normal kidney length is approximately 11 cm, with a normal range of 10–12 cm; (2) the left kidney is longer than the right by 0.3 cm; (3) women have smaller kidneys than men by 0.5 cm; (4) kidney sizes<10 cm are unusual in people younger than age 60 years, (5) kidney length decreases with age beginning at age 60 (see Table 2) (16,17). Thus, 9.5-cm kidneys probably represent CKD in a 30-year-old man but may be expected in an 80-year-old woman. It remains unknown whether kidney length “normally” decreases with aging or whether the decrease in kidney length represents the development of CKD. Kidney length also varies with height; when adjusted for height, the relative differences in kidney sizes between men and women are eliminated. In one study (18), kidney size varied by weight and height according to the following formula:
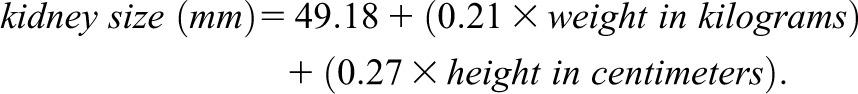 |
Table 2.
Normal kidney length (in cm) in adults based on age (in years)
| Kidney Length | Age | ||||
| 30 yr | 40 yr | 50 yr | 60 yr | 70 yr | |
| Left median kidney length | 11.5 | 11.3 | 11.3 | 11.1 | 10.5 |
| 10th percentile | 10.4 | 10.3 | 10.2 | 10.0 | 9.4 |
| 90th percentile | 12.8 | 12.3 | 12.5 | 12.2 | 12.0 |
| Right median kidney length | 11.1 | 11.2 | 11.0 | 10.8 | 10.4 |
| 10th percentile | 10.1 | 10.0 | 10.0 | 9.5 | 9.1 |
| 90th percentile | 12.4 | 12.3 | 12.2 | 12.0 | 11.8 |
Kidney length was determined in a random population of study of 665 adult volunteers without ultrasonographic evidence of kidney disease; median left and right kidney size with 10th and 90th percentiles were reported according to age (16).
In addition to kidney length, assessment of the kidney parenchyma thickness is also useful to distinguish AKI from CKD. The kidney parenchyma includes the cortex (containing glomeruli) and the medulla; the cortex is normally about 1 cm (17,19), and the entire parenchyma is normally about 1.5 cm (16). Thinning of the renal parenchyma or cortex commonly occurs in CKD and is a useful measure to identify CKD if it is present (Figure 6); normal cortical thickness may occur in acute or chronic kidney disease. In one study, mean parenchymal thickness was 10.7±0.4 mm in CKD versus 13.8±0.3 mm in AKI and 13.6±1 mm in healthy controls (2). Measurement of the cortical thickness can be affected by technique because the cortex may be thicker at the poles or in the region of a column of Bertin.
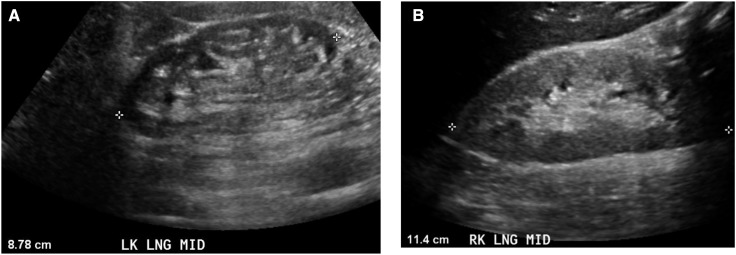
Figure 6.
Grayscale ultrasonographic image in CKD. (A) Grayscale longitudinal ultrasonographic image of a 67-year-old man with stage 4 CKD (creatinine, 4.0 mg/dl) and long-standing hypertension. Kidney size is small (8.78 cm) and renal parenchyma is thin. The finding of small kidneys or a thin parenchyma is diagnostic of CKD. (B) Grayscale longitudinal ultrasonographic image of RK demonstrates kidney size of 11.4 cm, abnormal bright (diffusely increased echogenicity) renal parenchyma, although parenchymal thickness is normal, in a 32-year-old man with CKD (creatinine 3.3 mg/dl) due to severe poorly controlled hypertension (BP, 230/130 mmHg at time of examination).
Doppler Ultrasonography and Resistive Index
Doppler imaging is used to assess the velocity of blood flow. Blood velocity is determined by computer interpretation of the magnitude of the frequency shift of the returning ultrasonography echoes of moving red blood cells and the Doppler equation. Resistive index (RI) is probably the most commonly used variable in Doppler imaging to evaluate blood flow in vessels. RI is very reproducible (20) when performed by a trained imager because the value is calculated as a ratio of two velocities obtained from the same transducer angle (in contrast to the importance of angle to assess renal artery stenosis in the main renal artery). In the kidney, RI is determined by assessing systolic and diastolic blood velocity in the segmental arteries and applying the following formula:
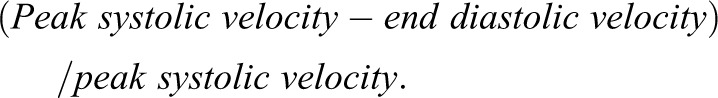 |
Because RI is a calculation of the relationship between systole and diastole, it is an indicator of the resistance to flow within the kidney. For example, if the resistive index is 0.9, then there is a large difference (i.e., a 90% drop) between systolic and diastolic blood flow that reflects an increased resistance to blood flow during diastole. If the RI is low, then renal vascular resistance is low. Normal RI is approximately 0.6 (ranging between 0.56 and 0.66), and in a study of 120 kidneys, only 3% had a mean RI>0.70 (20) (Figure 7A). Increased RI can occur in both CKD and AKI, and, in general, higher levels of RI are associated with worse renal parenchymal damage and worse outcome. In patients with CKD, for example, an RI>0.70 was independently associated with worsening kidney function in patients with CKD at both 2 and 4 years of follow-up (21,22).
Figure 7.
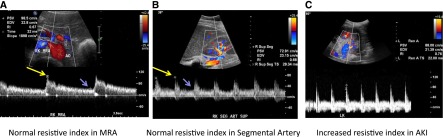
Doppler imaging and resistive index. (A) Normal color and spectral Doppler ultrasonogram of the right main renal artery (MRA) demonstrates normal peak systolic velocity (98.5 cm/sec), resistive index (0.67), and acceleration time (32 milliseconds). (B) Normal color and spectral Doppler ultrasonogram of the right superior segmental renal artery (SEG ART SUP) demonstrates normal resistive index (0.68). (C) Spectral Doppler image of left inferior segmental renal artery demonstrates an abnormally high resistive index of 0.76 in a 39-year-old woman with multiple myeloma and AKI. Resistive index is determined by the following formula: (peak systolic velocity − end diastolic velocity)/(peak systolic velocity); in A and B, peak systolic velocity is indicated by the long yellow arrows, and end diastolic velocity is indicated by the short purple arrows. AO, aorta.
Several factors can influence RI independent of renal disease, including heart rate, vessel wall compliance, and systemic vascular resistance. Bradycardia increases the RI because there is more time for the diastolic flow to decrease; on the other hand, tachycardia does not allow the diastolic flow to fully decrease, thus lowering the RI (23). In patients with widespread atherosclerosis (24) or reduced vascular compliance in general (as in elderly patients [20]), the renal RI may be increased even with normal kidney function. The influence of these factors needs to be considered to properly interpret renal RI; indeed, the potential utility of RI in AKI (discussed below) and kidney disease in general rests on a clear understanding of the renal and nonrenal factors that affect RI (25).
Use of RI in AKI.
RI may increase in several causes of AKI (Figures 3B and Figures 7B), including obstruction, acute rejection, hepatorenal syndrome (Figure 8B), and sepsis; the RI remains normal in prerenal azotemia and glomerular diseases (Figure 5, B and D). Studies suggest that RI may be a useful tool for predicting AKI, distinguishing ATN (or other parenchymal diseases) from prerenal azotemia, and predicting severity in AKI as summarized in Table 3. Three studies (26–28) have examined the utility of RI to predict AKI; in these studies, RIs of 0.74, 0.74, and 0.71 were found to be useful cut-points to predict AKI 1–5 days after the RI measurement in patients after cardiac bypass requiring surgery, with septic shock, or in the intensive care unit (ICU) for trauma or sepsis, respectively; patients who did not develop AKI had initial RIs of 0.68, 0.68, and 0.66, respectively. In studies of RI in prerenal azotemia versus intrinsic AKI (29–31), an RI≥0.75 correlated well with the diagnosis of ATN, while prerenal azotemia was typically associated with an RI<0.71. Finally, RI may be able to predict recovery of AKI (Figure 3B); patients with transient AKI had a median RI of 0.71, while patients with persistent AKI (lasting >3 days) had a median RI of 0.82 (11). Given the keen interest in the development of biomarkers for early AKI, the clinical difficulty in distinguishing prerenal azotemia from intrinsic AKI, and the need for markers of severity of AKI, the role of RI in these setting merits further investigation and validation in larger clinical trials.
Figure 8.
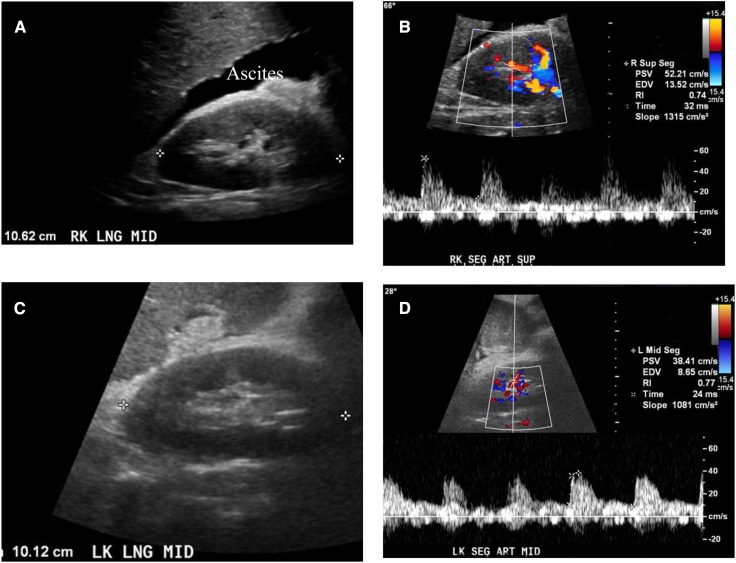
AKI due to hepatorenal syndrome. (A and C) Grayscale longitudinal ultrasonographic images of the right and left kidneys demonstrates normal renal parenchyma in a 51-year-old man with cirrhosis, ascites, end-stage liver disease due to hepatitis C, and AKI due to hepatorenal syndrome (serum creatinine, 2.5 mg/dl; low urinary sodium <10 mEq/L). (B and D) Spectral Doppler ultrasonograms of segmental renal artery in the same patient demonstrates an increased resistive index of 0.74 in the right kidney and 0.77 in the left kidney. SEG ART MID, middle segmental artery.
Table 3.
Summary of studies examining the use of resistive index (RI) in AKI
| Study (Reference) | Renal RI/Group | Study Notes |
| Healthy volunteers (20) | 0.60±0.07/Normal | |
| Predicting AKI post-CPB (26) | 0.68±0.06/No AKI 1–5 d later | Single-center, prospective, n=65 |
| 0.77±0.08/AKI without RRT 1–5 d later | RI was determined immediately after CPB | |
| 0.84±0.03/AKI requiring RRT 1–5 d later | Presence of AKI was assessed on days 1–5 | |
| An RI>0.74 had an AUC of 0.91 to predict AKI with 85% sensitivity and 94% specificity | ||
| Predicting AKI in septic shock (27) | 0.68±0.08/No AKI 5 d later | Single-center, prospective, n=35 |
| 0.77±0.08/AKI 5 d later | RI was obtained within 24 h of ICU admission | |
| Presence of AKI was assessed on day 5 | ||
| AKI was defined as RIFLE stage 2 or greater (i.e., doubling of serum creatinine or greater) | ||
| An RI>0.74 predicted AKI with 78% sensitivity and 77% specificity | ||
| Predicting AKI in the ICU (28) (sepsis and trauma) | 0.66±0.08/No AKI 3 d later | Single-center, prospective, n=58 |
| 0.80±0.08/AKI 3 d later | RI was obtained within 12 h of ICU admission | |
| Presence of AKI was assessed on day 3 | ||
| AKI was defined as stage 2 AKIN or greater (i.e., doubling of serum creatinine or greater) | ||
| An RI of 0.71 predicted AKI with an AUC of 0.91 | ||
| Identifying prerenal azotemia (29) | 0.67±0.90/Prerenal azotemia | Single-center, n=91 |
| 0.74±0.13/Non-ATN AKI (mostly HRS) | An RI≥0.75 occurred in 91% of patients with ATN and 20% of patients with prerenal azotemia | |
| 0.85±0.60/ATN | ||
| Identifying prerenal azotemia (30) | 0.76±0.06/Prerenal azotemia | Single-center, n=50 |
| 0.82±0.07/ATN | An RI≥0.75 had 91.3% sensitivity and 85.2% specificity to distinguish ATN from prerenal azotemia | |
| Identifying prerenal azotemia/assessing severity (31) | 0.52–0.71/Prerenal azotemia | Single-center, n=40 |
| 0.77–1.0/ATN | Decreasing RI predicted renal recovery | |
| Assessing severity (57) | 0.71 (0.62–0.77)/Transient AKI | Single-center, n=51 |
| 0.82 (0.80–0.89)/Persistent AKI | Persistent AKI was defined as AKI for >3 d | |
| An RI>0.795 had 82% sensitivity and 92% specificity for persistent AKI |
Values are expressed as mean ± SD. Numbers in parentheses are references. CPB, cardiopulmonary bypass; RRT, renal replacement therapy; AUC, area under the curve; ICU, intensive care unit; RIFLE, risk, injury, failure, loss, and end-stage kidney disease; AKIN, Acute Kidney Injury Network; ATN, acute tubular necrosis; HRS, hepatorenal syndrome.
Use of Ultrasonography in Patients with AKI to Evaluate for Obstruction
Ultrasonography is the modality of choice to evaluate for the presence of obstruction, which accounts for 1%–3% of cases of AKI in the ICU (32–34), approximately 15% of non-ICU AKI cases (34), and approximately 10% of community-acquired AKI cases (35). Obstruction is due to blockage anywhere in the urinary system, which interferes with proper urine flow; thus, clinically meaningful obstruction is typically associated with hydronephrosis (dilatation of the urinary collecting system from the accumulation of urine) as shown in Figures 9 and 10. In fact, approximately 95% of patients with obstruction will have hydronephrosis on ultrasonography (36); sensitivity approaches 100% in patients with moderate to severe hydronephrosis (1). False-negative findings (obstruction without hydronephrosis) can occur in the following settings: retroperitoneal fibrosis/retroperitoneal tumors (due to the inability of the collecting system to dilate), prerenal states/volume depletion, and early obstruction (too early for dilatation to occur) (37). False-positive results (hydronephrosis without obstruction) may occur in the following settings: pregnancy, vesicoureteral reflux, after relief of obstruction, megacystis-megaureter syndrome, full bladder, urinary tract infection, and brisk diuresis (as in nephrogenic diabetes insipidus) (6,38). Thus, when hydronephrosis is identified, tests to confirm the presence of true obstruction may need to be performed (e.g., diuretic renography, Doppler imaging for ureteral jets [Figure 11]).
Figure 9.
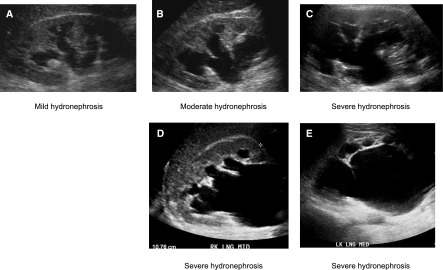
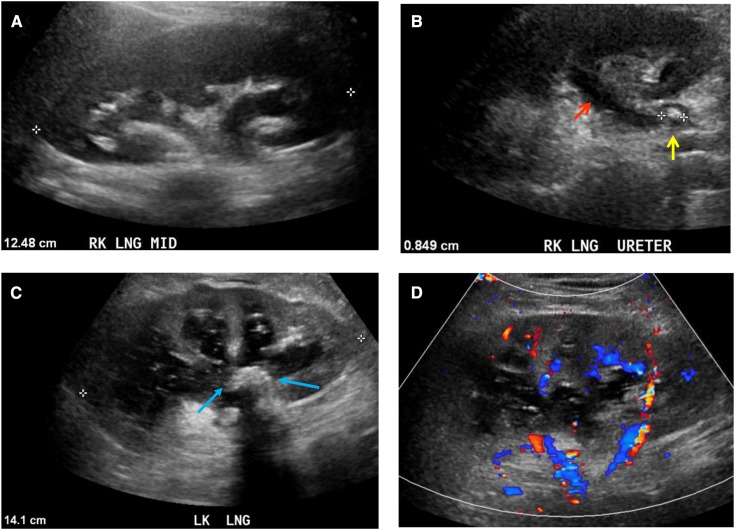
Grayscale ultrasonographic images demonstrating varying degrees of hydronephrosis. Grayscale longitudinal ultrasonographic image of the right kidney obtained at different time periods in the same patient demonstrates mild (A), moderate (B), and severe (C) hydronephrosis in a 59-year-old man with metastatic prostate cancer and creatinine level of 15 mg/dl. Grayscale longitudinal ultrasonography of both kidneys demonstrates severe right (D) and severe left (E) hydronephrosis in a 54-year-old man with AKI (creatinine increase of 1.2–1.5 mg/dl), benign prostatic hypertrophy, and acute urinary retention. MED, medial.
Figure 10.
Grayscale ultrasonographic images of hydronephrosis due to obstructing stones. Grayscale longitudinal ultrasonographic image of the right kidney demonstrates hydronephrosis (A) with a dilated ureter (B) (red arrow) due to an 8-mm hyperechoic, obstructing stone (yellow arrow) in the proximal ureter in a 26-year-old woman with right flank pain. (C) Grayscale longitudinal ultrasonographic image of the left kidney demonstrates hydronephrosis containing complex fluid due to a large, echogenic, staghorn calculus (blue arrows) in the renal pelvis causing pyonephrosis in a 60-year-old man who presented with sepsis; (D) color Doppler ultrasonogram does not demonstrate flow, confirming that it is a dilated collecting system containing urine and not blood vessels in the central renal sinus (urine and blood are both anechoic [black]; color Doppler imaging can be used to distinguish urine [no flow] from blood in vessels [flow is present]).
Figure 11.
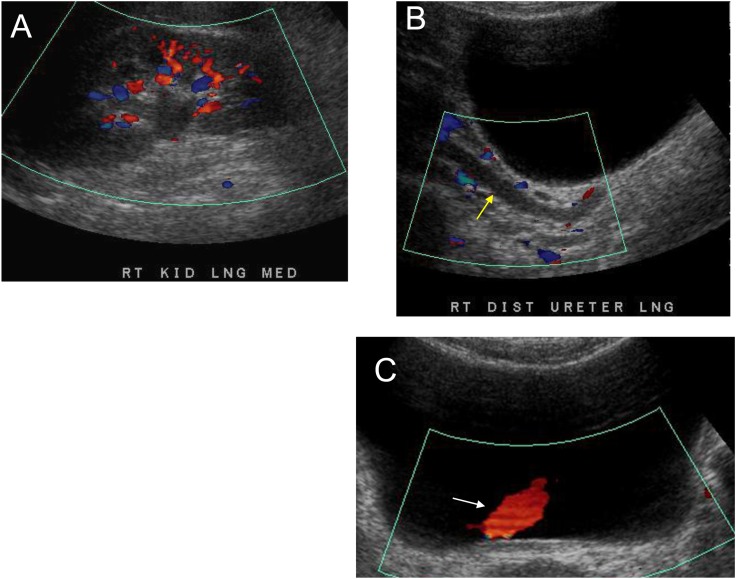
Hydronephrosis without functional obstruction. Grayscale and color Doppler ultrasonogram of a 59-year-old woman with right flank pain demonstrate mild right hydronephrosis (A) and hydroureter (yellow arrow) (B) ureteric jet (C) (white arrow) in the bladder. No calculus was seen on computed tomography of the kidneys, ureters, and bladder done at the same time. Thus, this likely represents a recently passed calculus or vesico-ureteric reflux and hydronephrosis without functional obstruction is present. (C) The utility of Doppler imaging to assess for functional obstruction by assessing the ureteric jet. When the bolus of urine being transmitted through the ureter reaches the terminal portion, it is ejected forcefully into the bladder through the vesicoureteric junction. This creates a jet of urine that can be seen within the urinary bladder on grayscale and color Doppler ultrasonography (white arrow; jet appears red). LNG, longitudinal; MED, medial.
Studies suggest that clinical suspicion is a useful predictor of the presence of obstruction. In a retrospective analysis of 60 patients referred to ultrasonography for an increased creatinine level, Gottlieb et al. found that all of the patients with hydronephrosis had a clinical history suggestive of obstruction (39). Extending these observations, Licurse et al. examined whether clinical measures could indeed be used to risk-stratify patients for likelihood of obstruction (40). Although not validated in other centers, a clinical decision rule was developed and internally validated. In that study, 7 of 36 clinical variables were important in the assessment of obstruction, and a point system was devised: One point is awarded for the presence of any of the following: (1) nonblack race, (2) history of recurrent urinary tract infections (three in previous year), (3) diagnosis consistent with possible obstruction (benign prostatic hyperplasia, abdominal/pelvic cancer, neurogenic bladder, single functional kidney, or previous pelvic surgery), (4) no congestive heart failure, (5) no history of prerenal AKI, pressors, sepsis, or (6) no exposure to nephrotoxins. Four points are awarded for a history of hydronephrosis. Patients are divided into low, medium, and high risk if they had ≤2 points, 3 points, or ≥4 points, respectively. The prevalence of hydronephrosis was 3% in the low-risk group, 10.7% in the medium-risk group, and 16.1% in the high-risk group. Thus, 2 points or less helped predict a negative ultrasonography result for hydronephrosis 97% of the time.
The appropriate use of ultrasonography for the evaluation of AKI, with or without clinical suspicion of obstruction, has been vigorously debated. We and others (41) suggest that renal ultrasonography is particularly important in the evaluation of AKI if the diagnosis is unclear or the clinical course is not as expected. It is useful to note that the American College of Radiology (ACR) Appropriateness Criteria rating for the use of ultrasonography in AKI is a 9, the highest rating, indicating that its use in AKI is highly appropriate. The ACR Appropriateness Criteria are a compilation of evidence-based recommendations to aid in the selection of radiologic imaging for a variety of medical conditions. The web-based tool is available at http://www.acr.org/Quality-Safety/Appropriateness-Criteria (or simply do a web search for “appropriateness criteria”); the site is searchable for a wide variety of topics, such as renal masses or abdominal pain. The appropriateness of imaging techniques (e.g., ultrasonography and magnetic resonance imaging) are ranked on a scale from 1 to 9 with the following interpretations: 1, 2, 3: usually not appropriate; 4, 5, 6: may be appropriate; 7, 8, 9: usually appropriate. The recommendations regarding ultrasonography (and other imaging techniques) for AKI may be found using the search term “acute renal failure.” (Please note that the criteria for AKI are being updated with new recommendations anticipated soon).
Utility of Ultrasonography in Pediatric AKI
Ultrasonography is a critical imaging tool in the evaluation of AKI in children because it can help to exclude underlying chronic and/or congenital renal conditions, requires no radiation exposure, and is easily performed in children of all ages. Alternatively, computed tomography and magnetic resonance imaging often require sedation in children and are associated with concerns similar to those in adults with respect to contrast/gadolinium exposure in the setting of renal failure. Several caveats must be noted with respect to pediatric renal ultrasonography, however. Normal renal length and volume vary with body size, and individual imaging results must be compared with known norms (42–45) (Table 4). More recent data suggest that renal length is related to numerous individual variables, including age, sex, race, height, and weight (42). The appearance of the normal neonatal kidney by ultrasonography differs significantly from that of older children and adults, with relatively hyperechoic cortex and hypoechoic pyramids, little sinus fat, and frequent fetal lobulation (46). The echogenicity of the renal cortex becomes more hypoechoic than liver by approximately 4–6 months of age. In younger children, prominent renal pyramids are easily mistaken for dilated calices or renal cysts. Norms for RI are age dependent, with the highest values noted at birth and increased normal values in preterm (0.8–0.9) versus term infants. Normal RI decreases during childhood, reaching typical adult values of <0.7 by 4–5 years of age (47). This is in large part due to the normal decrease in renal vascular resistance and increase in systemic BP that occur postnatally.
Table 4.
Normal values for renal variables by age
| Age | Mean Serum Creatinine (mg/dl) (58) | Mean Estimated GFR (ml/min per 1.73 m2) (58) | Mean Kidney Length (cm) (44) | Resistive Index (47,59,60) |
| Preterm neonate | 0.9 | 11 | Depends on gestational age (61,62) | 0.8–0.9 |
| Term neonate | 0.5 | 39 | 4.5 | 0.7–0.8 |
| 1 yr | 0.3 | 103 | 6.6 | 0.7–0.8 |
| 5 yr | 0.4 | 127 | 8.1 | <0.7 |
| 10 yr | 0.6 | 127 | 9.2 | <0.7 |
| 15 yr | 0.9 | 127 | 10.9 | <0.7 |
Numbers in parentheses are references.
As in adults, an important function of sonographic imaging in pediatric renal failure is to exclude CKD. Because most symptoms of pediatric CKD are nonspecific, detection of an increased serum creatinine concentration during acute illness does not necessarily signify AKI. Renal ultrasonography is valuable for detecting previously undiagnosed chronic renal conditions, such as congenital hypoplasia/dysplasia, cystic disease, or urinary tract dilation suggestive of underlying obstructive uropathy. For example, hypoplastic/dysplastic kidneys may be small with altered echotexture and reduced corticomedullary differentiation. Autosomal recessive polycystic kidney is associated with symmetrically enlarged hyperechoic kidneys with poor corticomedullary differentiation. Small scarred kidneys are observed in a variety of conditions causing chronic renal failure/ESRD.
Renal ultrasonography also has utility in the differentiation of prerenal, intrinsic, and postrenal causes of AKI. With prerenal azotemia, ultrasonography may demonstrate normal-appearing to edematous diffusely hyperechoic kidneys with perfusion alterations, such as decreased flow velocity with normal to increased RI, flattened systolic flow curves, or delayed systolic flow acceleration (48). In ATN, the kidneys may be symmetrically enlarged with variable echogenicity and increased RI. Gheisari and Haghighi used renal Doppler ultrasonography to differentiate prerenal azotemia from ATN in 50 oligoanuric children age 1 month–15 years (mean, 9 years) with AKI who were referred to a tertiary care center (30). Imaging was performed within 24 hours of admission and when oligoanuria had resolved. The mean RI was higher in patients with ATN than in those with prerenal azotemia (0.82±0.07 versus 0.76±0.06; P<0.01). The mean reduction in RI after treatment was also greater in patients with ATN (0.26±0.01 versus 0.04±0.0; P<0.01). The sensitivity and specificity of an RI ≥0.75 in differentiating prerenal failure and ATN were 91.3% and 85.2%, respectively. Further large-scale study is needed in this area.
Ultrasonography is less helpful in conditions of diffuse renal inflammation, such as GN or interstitial nephritis, with findings including normal to enlarged kidneys, variable corticomedullary differentiation, and normal to increased RI (48). In pediatric hemolytic-uremic syndrome, ultrasonography demonstrates enlarged kidneys with hyperechoic cortex, increased corticomedullary differentiation, increased RI, and frequent reversal of diastolic flow (48,49). It has been suggested that normalization of flow as assessed by RI or flow volume may predict renal recovery (50). More current studies with advanced imaging technology may be helpful to clarify the reliability of such measurements. Renal vein thrombosis occurs with increased frequency in neonates and in children with nephrotic syndrome and is readily diagnosed by Doppler ultrasonography (48).
Postrenal AKI rarely occurs in children (51,52) but may not necessarily be anticipated on the basis of medical history, as with obstructive urolithiasis (53,54) or intra-abdominal tumor resulting in urinary tract obstruction. As with adults, it is important to distinguish collecting system dilatation from obstructive hydronephrosis, particularly in children with underlying primary vesicoureteral reflux, chronic polyuria, or a lax collecting system, as is seen more commonly in neonates. Sonographic features observed more commonly with obstructive versus nonobstructive hydronephrosis in children include increased echogenicity, parenchymal rim measuring ≤5 mm, contralateral hypertrophy, RI ratio (ratio of the RI of the hydronephrotic kidney divided by the RI of the normal kidney) >1.1, and ureter diameter>10 mm (55). Other studies have confirmed a role for the RI ratio to delineate obstruction in the setting of chronic hydronephrosis in children (56).
Conclusion
In summary, ultrasonography is a useful tool in the evaluation of adult and pediatric patients with AKI for assessing obstruction, parenchymal evaluation, and distinguishing acute from chronic kidney diseases. With better understanding of the pathogenesis of certain causes of AKI, it is anticipated that renal ultrasonography may become an even more important tool in the assessment, management, and treatment of patients with AKI.
Disclosures
S.F.: Scientific Advisory Board, Satellite Healthcare; M.L.: Salary support: Deputy Editor, Journal of Ultrasound in Medicine.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Papanicolaou N, Francis IR, Casalino DD, Arellano RS, Baumgarten DA, Curry NS, Dighe M, Israel GM, Jafri SZ, Kawashima A, Leyendecker JR, Prasad S, Ramchandani P, Remer EM, Sheth S, Fulgham P, Expert Panel on Urologic Imaging. ACR Appropriateness Criteria renal failure. Reston, VA, American College of Radiology , 2008 [Google Scholar]
- 2.Ozmen CA, Akin D, Bilek SU, Bayrak AH, Senturk S, Nazaroglu H: Ultrasound as a diagnostic tool to differentiate acute from chronic renal failure. Clin Nephrol 74: 46–52, 2010 [PubMed] [Google Scholar]
- 3.Platt JF, Rubin JM, Bowerman RA, Marn CS: The inability to detect kidney disease on the basis of echogenicity. AJR Am J Roentgenol 151: 317–319, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Manley JA, O’Neill WC: How echogenic is echogenic? Quantitative acoustics of the renal cortex. Am J Kidney Dis 37: 706–711, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Marchal G, Verbeken E, Oyen R, Moerman F, Baert AL, Lauweryns J: Ultrasound of the normal kidney: A sonographic, anatomic and histologic correlation. Ultrasound Med Biol 12: 999–1009, 1986 [DOI] [PubMed] [Google Scholar]
- 6.O’Neill WC: Sonographic evaluation of renal failure. Am J Kidney Dis 35: 1021–1038, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Page JE, Morgan SH, Eastwood JB, Smith SA, Webb DJ, Dilly SA, Chow J, Pottier A, Joseph AE: Ultrasound findings in renal parenchymal disease: Comparison with histological appearances. Clin Radiol 49: 867–870, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Nomura G, Kinoshita E, Yamagata Y, Koga N: Usefulness of renal ultrasonography for assessment of severity and course of acute tubular necrosis. J Clin Ultrasound 12: 135–139, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Toyoda K, Miyamoto Y, Ida M, Tada S, Utsunomiya M: Hyperechoic medulla of the kidneys. Radiology 173: 431–434, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Nayir A, Kadioğlu A, Sirin A, Emre S, Tonguç E, Bilge I: Causes of increased renal medullary echogenicity in Turkish children. Pediatr Nephrol 9: 729–733, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Sefczek RJ, Beckman I, Lupetin AR, Dash N: Sonography of acute renal cortical necrosis. AJR Am J Roentgenol 142: 553–554, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA, O’Neill WC: Correlation of renal histopathology with sonographic findings. Kidney Int 67: 1515–1520, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Rosenfield AT, Zeman RK, Cicchetti DV, Siegel NJ: Experimental acute tubular necrosis: US appearance. Radiology 157: 771–774, 1985 [DOI] [PubMed] [Google Scholar]
- 14.American Society of Nephrology: Nephrology Self Assessment Program: Renal Imaging. 7: 2008
- 15.Emamian SA, Nielsen MB, Pedersen JF: Intraobserver and interobserver variations in sonographic measurements of kidney size in adult volunteers. A comparison of linear measurements and volumetric estimates. Acta Radiol 36: 399–401, 1995 [PubMed] [Google Scholar]
- 16.Emamian SA, Nielsen MB, Pedersen JF, Ytte L: Kidney dimensions at sonography: Correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol 160: 83–86, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Miletić D, Fuckar Z, Sustić A, Mozetic V, Stimac D, Zauhar G: Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound 26: 185–189, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Harmse WS: Normal variance in renal size in relation to body habitus. South African Journal of Radiology 15: 2011. Available at: http://www.sajr.org.za/index.php/sajr/article/view/565/497 Accessed October 18, 2013
- 19.Raj DS, Hoisala R, Somiah S, Sheeba SD, Yeung M: Quantitation of change in the medullary compartment in renal allograft by ultrasound. J Clin Ultrasound 25: 265–269, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Keogan MT, Kliewer MA, Hertzberg BS, DeLong DM, Tupler RH, Carroll BA: Renal resistive indexes: Variability in Doppler US measurement in a healthy population. Radiology 199: 165–169, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Sugiura T, Wada A: Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant 24: 2780–2785, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Sugiura T, Wada A: Resistive index predicts renal prognosis in chronic kidney disease: Results of a 4-year follow-up. Clin Exp Nephrol 15: 114–120, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Thalhammer C, Aschwanden M, Mayr M, Koller M, Steiger J, Jaeger KA: Duplex sonography after living donor kidney transplantation: New insights in the early postoperative phase. Ultraschall Med 27: 141–145, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Heine GH, Gerhart MK, Ulrich C, Köhler H, Girndt M: Renal Doppler resistance indices are associated with systemic atherosclerosis in kidney transplant recipients. Kidney Int 68: 878–885, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Tublin ME, Bude RO, Platt JF: Review. The resistive index in renal Doppler sonography: Where do we stand? AJR Am J Roentgenol 180: 885–892, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Bossard G, Bourgoin P, Corbeau JJ, Huntzinger J, Beydon L: Early detection of postoperative acute kidney injury by Doppler renal resistive index in cardiac surgery with cardiopulmonary bypass. Br J Anaesth 107: 891–898, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Lerolle N, Guérot E, Faisy C, Bornstain C, Diehl JL, Fagon JY: Renal failure in septic shock: Predictive value of Doppler-based renal arterial resistive index. Intensive Care Med 32: 1553–1559, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Schnell D, Deruddre S, Harrois A, Pottecher J, Cosson C, Adoui N, Benhamou D, Vicaut E, Azoulay E, Duranteau J: Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock 38: 592–597, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Platt JF, Rubin JM, Ellis JH: Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology 179: 419–423, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Gheisari A, Haghighi M: Diagnostic value of Doppler ultrasound in differentiating prerenal azotemia from acute tubular necrosis in children. Saudi J Kidney Dis Transpl 17: 168–170, 2006 [PubMed] [Google Scholar]
- 31.Izumi M, Sugiura T, Nakamura H, Nagatoya K, Imai E, Hori M: Differential diagnosis of prerenal azotemia from acute tubular necrosis and prediction of recovery by Doppler ultrasound. Am J Kidney Dis 35: 713–719, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM; Program to Improve Care in Acute Renal Disease: Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Liaño F, Junco E, Pascual J, Madero R, Verde E; The Madrid Acute Renal Failure Study Group: The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int Suppl 66: S16–S24, 1998 [PubMed] [Google Scholar]
- 35.Liaño F, Pascual J; Madrid Acute Renal Failure Study Group: Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Spital A, Valvo JR, Segal AJ: Nondilated obstructive uropathy. Urology 31: 478–482, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Platt JF, Rubin JM, Ellis JH: Acute renal obstruction: Evaluation with intrarenal duplex Doppler and conventional US. Radiology 186: 685–688, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Mostbeck GH, Zontsich T, Turetschek K: Ultrasound of the kidney: Obstruction and medical diseases. Eur Radiol 11: 1878–1889, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb RH, Weinberg EP, Rubens DJ, Monk RD, Grossman EB: Renal sonography: Can it be used more selectively in the setting of an elevated serum creatinine level? Am J Kidney Dis 29: 362–367, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Licurse A, Kim MC, Dziura J, Forman HP, Formica RN, Makarov DV, Parikh CR, Gross CP: Renal ultrasonography in the evaluation of acute kidney injury: Developing a risk stratification framework. Arch Intern Med 170: 1900–1907, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Liu KD, Chertow GM: Curbing the use of ultrasonography in the diagnosis of acute kidney injury: Penny wise or pound foolish? Comment on “Renal ultrasonography in the evaluation of acute kidney injury”. Arch Intern Med 170: 1907–1908, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JJ, Pugach J, Patel M, Luisiri A, Steinhardt GF: The renal length nomogram: Multivariable approach. J Urol 168: 2149–2152, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Han BK, Babcock DS: Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol 145: 611–616, 1985 [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum DM, Korngold E, Teele RL: Sonographic assessment of renal length in normal children. AJR Am J Roentgenol 142: 467–469, 1984 [DOI] [PubMed] [Google Scholar]
- 45.Schmidt IM, Main KM, Damgaard IN, Mau C, Haavisto AM, Chellakooty M, Boisen KA, Petersen JH, Scheike T, Olgaard K: Kidney growth in 717 healthy children aged 0-18 months: a longitudinal cohort study. Pediatr Nephrol 19: 992–1003, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Daneman A, Navarro OM, Somers GR, Mohanta A, Jarrín JR, Traubici J: Renal pyramids: Focused sonography of normal and pathologic processes. Radiographics 30: 1287–1307, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Murat A, Akarsu S, Ozdemir H, Yildirim H, Kalender O: Renal resistive index in healthy children. Eur J Radiol 53: 67–71, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Riccabona M: Renal failure in neonates, infants, and children: The role of ultrasound. Ultrasound Clin 1: 457–469, 2006 [Google Scholar]
- 49.Scholbach TM: Changes of renal flow volume in the hemolytic-uremic syndrome—color Doppler sonographic investigations. Pediatr Nephrol 16: 644–647, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Patriquin HB, O’Regan S, Robitaille P, Paltiel H: Hemolytic-uremic syndrome: intrarenal arterial Doppler patterns as a useful guide to therapy. Radiology 172: 625–628, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Asgari SA, Mansour Ghanaie M, Simforoosh N, Kajbafzadeh A, Zare A: Acute urinary retention in children. Urol J 2: 23–27, 2005 [PubMed] [Google Scholar]
- 52.Gatti JM, Perez-Brayfield M, Kirsch AJ, Smith EA, Massad HC, Broecker BH: Acute urinary retention in children. J Urol 165: 918–921, 2001 [PubMed] [Google Scholar]
- 53.Sun Q, Shen Y, Sun N, Zhang GJ, Chen Z, Fan JF, Jia LQ, Xiao HZ, Li XR, Puschner B: Diagnosis, treatment and follow-up of 25 patients with melamine-induced kidney stones complicated by acute obstructive renal failure in Beijing Children’s Hospital. Eur J Pediatr 169: 483–489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cara Fuentes GM, Espinosa Roman L, Melgosa Hijosa M, Navarro Torres M: Acute renal failure due to bilateral pieloureteral stone impaction in a 10-month-old boy. Clin Exp Nephrol 14: 401–403, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Peña BM, Keller MS, Schwartz DS, Korsvik HE, Weiss RM: The ultrasonographic differentiation of obstructive versus nonobstructive hydronephrosis in children: A multivariate scoring system. J Urol 158: 560–565, 1997 [PubMed] [Google Scholar]
- 56.Patti G, Menghini ML, Todini AR, Marrocco G, Calisti A: The role of the renal resistive index ratio in diagnosing obstruction and in the follow-up of children with unilateral hydronephrosis. BJU Int 85: 308–312, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Darmon M, Schortgen F, Vargas F, Liazydi A, Schlemmer B, Brun-Buisson C, Brochard L: Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med 37: 68–76, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Way AF, Bolonger AM, Gambertogli JG: Pharmacokinetics and drug dosing in children with decreased renal function. In: Pediatric Nephrology, 3rd Ed., edited by Holliday MA, Barratt TM, Avne ED, Baltimore: Williams and Wilkins, 1994: p. 1306 [Google Scholar]
- 59.Bude RO, DiPietro MA, Platt JF, Rubin JM, Miesowicz S, Lundquist C: Age dependency of the renal resistive index in healthy children. Radiology 184: 469–473, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Coley BD: Pediatric applications of abdominal vascular Doppler: Part II. Pediatr Radiol 34: 772–786, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Shin JS, Seo YS, Kim JH, Park KH: Nomogram of fetal renal growth expressed in length and parenchymal area derived from ultrasound images. J Urol 178: 2150–2154, 2007 [DOI] [PubMed] [Google Scholar]
- 62.van Venrooij NA, Junewick JJ, Gelfand SL, Davis AT, Crumb TL, Bunchman TE: Sonographic assessment of renal size and growth in premature infants. Pediatr Radiol 40: 1505–1508, 2010 [DOI] [PubMed] [Google Scholar]