Summary
Background and objectives
The complexity of CKD management in children is increased by the number of comorbid conditions. This study assessed the prevalence of comorbidities in pediatric CKD and the frequency with which multiple comorbidities present together by assessing prevalent medication use by CKD stage and diagnosis and their association with clinical or sociodemographic factors. The association between number and frequency of dosing of medications prescribed and self-report of nonadherence was also assessed.
Design, setting, participants, & measurements
In this cross-sectional analysis of the Chronic Kidney Disease in Children study, medication use at study entry grouped by indication was examined by CKD stage, diagnosis, age, race, ethnicity, income, and CKD duration. Multivariate adjusted predictors of medication use and clustering were examined. Nonadherence was assessed by self-report of missed medications in the past 7 days.
Results
The 558 eligible participants had a median age of 11 years and median GFR of 44 ml/min per 1.73 m2; 62% of participants were male and 78% had nonglomerular kidney disease. The number of medications for treatment of CKD comorbidities increased with advanced CKD stage (2.5-fold for stages IV versus II; P<0.001) and glomerular disease (1.4-fold versus nonglomerular; P<0.001). Three distinct medication clusters were identified that corresponded to treatment of glomerular disease, advanced renal tubular dysfunction, and proteinuric complications, respectively. Nonadherence was associated with increased medication dosing frequency (administration >2 times/d; P<0.001) but not the number of medications.
Conclusions
Medical therapy for children with CKD is complex and is affected by glomerular diagnosis, CKD stage, and medication frequency. The need for CKD-related medication treatment cannot be easily predicted by CKD staging alone. Poorer adherence was associated with increased medication frequency, but not with the number of medical problems needing treatment. Consolidating medical treatment and reducing medication frequency may improve adherence rates in children with CKD.
Introduction
Progressive CKD in children requires a variety of medical treatments in an attempt to delay progression, prevent or treat comorbidities, and alleviate symptoms resulting from uremia. Given the number of different medical problems requiring management, care of pediatric patients with CKD is complex for physicians and a major burden for patients and their parents. Despite these issues, treatment complexity—including, but not limited to, diversity, multiplicity, and frequency of treatments— for children with CKD has not been described. Such a report is needed to better understand the spectrum of treatment requirements and identify special risk cohorts.
Successful management of complex chronic disease is influenced by our ability to understand the variable onset of associated comorbidities and how they relate to one another and to patient and disease characteristics. We therefore sought to characterize the medications prescribed to participants in the prospective Chronic Kidney Disease in Children (CKiD) study using the context of disease type, stage of progression, and other sociodemographic characteristics to help physicians and families anticipate therapy as disease progresses. Furthermore, we hypothesized that treatment of multiple comorbidities would be associated with poorer adherence.
In this analysis, we characterize prevalent medication use in children with CKD according to CKD stage and diagnosis. In addition, clinical and sociodemographic factors associated with medication nonadherence are identified. Finally, we identify classes of medication that exhibit concomitant use (coprescribed) in children with CKD.
Materials and Methods
Study Population
The CKiD study is a prospective, observational cohort study of CKD in children conducted at 48 pediatric nephrology centers across North America (Supplemental Appendix 1). Details of the CKiD study protocol and study design were previously published (1); the study protocol was approved by the institutional review board of each participating center. Briefly, eligibility criteria for enrollment in CKiD included age 1–16 years and an estimated GFR (eGFR) of 30–90 ml/min per 1.73 m2 calculated by the Schwartz formula (2,3). Clinical and demographic data are collected from CKiD participants at annual visits. This analysis draws upon data collected at the baseline study visit among children enrolled in the study before January 2010.
Data Collection
Demographic and medical history data, including age, sex, self-reported race/ethnicity, underlying CKD diagnosis (glomerular versus nonglomerular), age/date of CKD onset, and annual household income, were collected using standardized forms. GFR was determined by plasma iohexol disappearance (iGFR) (3). If iGFR was not available, eGFR based on serum creatinine, BUN, and/or cystatin C was calculated using formulae published by CKiD investigators (4). Hereafter, we refer to the combination of iGFR and eGFR measurements as simply GFR. CKD stage was defined as follows: stage II, GFR 60–89; stage IIIa, GFR 45–59; stage IIIb, GFR 30–44; and stage IV, GFR 15–29.
Medication History
As part of the study visit, children (and/or their parent or guardian) reported all medications and supplements, prescribed and over the counter, taken by the child in the previous 30 days, including the frequency of each medication dose. Nonadherence was assessed by self-report and was defined as missing ≥1 doses of the medication in the last 7 days. Parents or guardians were asked to bring the child’s medications to the study visit.
Using a standardized coding form, each medication was classified and similar medications were grouped according to indication for a specific clinical problem. For example, active vitamin D preparations were grouped together, as were phosphate binders. Groupings for CKD-related comorbidities were based on those for which specific Kidney Disease Outcomes Quality Initiative pediatric recommendations are published (5). Medications for specific indications (e.g., an angiotensin converting enzyme inhibitor [ACEI]/angiotensin receptor blocker [ARB] for CKD progression; a diuretic for edema control) that might also be classified under another broad indication (e.g., BP control) were assigned to their own grouping rather than combining potentially disparate indications. Corticosteroids were grouped separately from treatment with noncorticosteroid immunosuppressant medications. Calcium carbonate was assigned to the phosphate binder group, although it may have dual efficacy as an alkali supplement. Nutritional supplements were defined as any treatment for supplemental caloric intake.
These therapy groupings were in turn assigned to one of four broad treatment categories: therapies for the treatment of CKD-specific comorbidities (14 groups), therapies for the treatment of underlying kidney disease (4 groups), therapies for the control of symptoms (3 groups), and non-CKD–related medications (4 groups). Antibacterial agents were assigned to the non-CKD–related grouping due to their heterogeneity and the inability to determine whether unique prescriptions were for urinary tract infection prophylaxis or other indications. With the exception of medications indicated for treatment of CKD-specific comorbidities, medications under each treatment category with <5% prevalent use were conglomerated and reported as “other.”
Medication use was examined with reference to the clinical problem requiring management using the therapy groups designated by indication. The number of clinical problems being treated at the same time was determined by the number of different therapy groupings present for each patient. Each grouping could be counted only once per patient, regardless of the number of medications assigned to that group.
Statistical Analyses
Analysis was restricted to baseline data from patients with known age, race, ethnicity, GFR, CKD diagnosis, household income, and CKD duration. Demographic and clinical characteristics were reported using the median (interquartile range [IQR]) for continuous variables and the frequency (percentage) for categorical variables.
The first goal was to describe the prevalence of medication use for each of the defined therapy groups, overall and stratified by CKD stage. Prevalence trends across CKD stage were assessed by the Cochran–Armitage trend test. Differences in the prevalence of specific medication use between children with glomerular and nonglomerular CKD were assessed using Fisher exact tests.
A second goal of this analysis was to describe the cross-sectional association between the number of problems being treated together and to select clinical and sociodemographic risk factors for CKD. The primary outcome was the total number of therapy groups being reported for a child. There were 19 possible CKD-related medication groups. The primary exposures of interest were CKD stage and diagnosis. Other covariates included age, sex, race, annual household income, and CKD duration. We used multivariate generalized linear models with a negative binomial distribution to model the log (count) of unique medication groups reported. Coefficient estimates from the model (and the 95% confidence [95% CI] interval bounds) were exponentiated to yield adjusted relative differences in the number of unique medication groups associated with the exposure of interest.
The third goal was to identify clusters of indication-specific CKD-related therapies prescribed together. The analysis was restricted to the same 19 groups of medications (as above). We used a recursive algorithm whereby groups of medications were amalgamated if they were found to be associated using the following criteria: the odds ratio (OR) generated from the 2×2 table describing use (yes/no) of medication A versus use (yes/no) of medication B was >3.0 (defined as a strong association) and significant at the α=0.01 level. The two medication groups with the strongest significant association were amalgamated into one new grouping and the process was repeated. Amalgamation of medications continued in expanding clusters until no two groups were significantly associated by the above-described criteria.
A final goal was to identify clinical, pharmaceutical, and demographic factors associated with 7-day nonadherence to CKD medication. This was achieved using a multivariable generalized estimating equation logit model with an exchangeable working correlation to account for children with >1 CKD-related medication. The outcome for the model was a binary (yes/no) variable indicating nonadherence of each medication.
All analyses were performed using SAS statistical software (version 9.2; SAS Institute, Inc., Cary, NC). Statistical significance was determined at the α=0.05 level, unless otherwise indicated.
Results
Between January 2005 and December 2010, 586 children were enrolled in the CKiD study. Of these, 558 participants met the criteria for inclusion in this analysis. These children’s baseline characteristics are presented in Table 1. The median age was 11 years (IQR, 7, 14), 62% were male, 23% were African American, and 14% reported being of Hispanic ethnicity. The median GFR was 44 ml/min per 1.73 m2 (IQR, 33, 57) and the majority of children (78%) had a nonglomerular CKD diagnosis. Characteristics that differed by CKD diagnosis included age, sex, race, and CKD duration.
Table 1.
Characteristics of the study population
| Characteristic | Overall (N=558) |
|---|---|
| Age (yr) | 11 (7, 14) |
| Male | 346 (62) |
| Race | |
| Caucasian | 371 (66) |
| African American | 127 (23) |
| Other | 33 (6) |
| Multiracial (non–African American) | 27 (5) |
| Hispanic ethnicity | 78 (14) |
| Glomerular CKD | 120 (22) |
| eGFR (ml/min per 1.73 m2) | 44 (33, 57) |
| Duration of CKD (yr) | 6 (3, 10) |
| Household annual income<$36,000 | 235 (42) |
| Total no. of unique medication groupsa | 3 (2, 5) |
| Total no. of CKD-specific medication groupsb | 2 (1, 4) |
Data are presented as the median (interquartile range) or n (%).
Unique medication groups includes those with <5% prevalence grouped separately (not grouped together as “other”).
CKD-specific medication groups excludes the non-CKD–related medications category and the “other” medication groupings.
Table 2 provides a summary of the prevalent use of medications grouped according to indication, the trend for use by CKD stage, and nonadherence rates for each grouping. Of the 14 medication groups for CKD-specific comorbidities, all showed higher prevalence with more advanced CKD stage except ACEI/ARB treatment, vitamin and mineral supplements, and vitamin D and nutritional supplements. In the other major medication categories, only noncorticosteroid immunosuppressants and bladder medications varied significantly according to CKD stage. Considering medications in use by >5% of participants, 7-day nonadherence ranged from 6% to 26% and was greatest for alkali treatments, phosphate binders, growth hormone, and bladder medications, all of which exceeded 20% nonadherence rates.
Table 2.
Prevalence and nonadherence rates of medications grouped by indication
| Medication Groups of Interest | Groups Using Medication Overall (N=558) | CKD Stage | Nonadherence Past 7 Days | ||||
|---|---|---|---|---|---|---|---|
| II (n=127) | IIIa (n=143) | IIIb (n=189) | IV (n=99) | P Value | |||
| Treatment of CKD-specific comorbidities | |||||||
| ACEI and/or ARB | 295 (53) | 67 (53) | 69 (48) | 98 (52) | 61 (62) | 0.20 | 52 (18) |
| Active vitamin D | 206 (37) | 8 (6) | 41 (29) | 90 (48) | 67 (68) | <0.001a | 26 (13) |
| Iron preparations | 166 (30) | 19 (15) | 38 (27) | 64 (34) | 45 (45) | <0.001a | 24 (14) |
| Alkali therapy | 163 (29) | 15 (12) | 32 (22) | 72 (38) | 44 (44) | <0.001a | 38 (23) |
| Vitamin and mineral supplements | 126 (23) | 28 (22) | 25 (17) | 40 (21) | 33 (33) | 0.06a | 18 (14) |
| Phosphate binders | 114 (20) | 11 (9) | 15 (10) | 44 (23) | 44 (44) | <0.001a | 30 (26) |
| Other BP medicationsb | 106 (19) | 19 (15) | 17 (12) | 38 (20) | 32 (32) | <0.001a | 17 (16) |
| Erythrocyte stimulating agents | 75 (13) | 3 (2) | 10 (7) | 26 (14) | 36 (36) | <0.001a | 6 (8) |
| Growth hormone | 65 (12) | 5 (4) | 5 (4) | 35 (19) | 20 (20) | <0.001a | 16 (25) |
| Diuretics | 38 (7) | 5 (4) | 4 (3) | 12 (6) | 17 (17) | <0.001a | 7 (18) |
| Vitamin D | 25 (4) | 3 (2) | 5 (4) | 13 (7) | 4 (4) | 0.21 | 4 (16) |
| Lipid medications | 17 (3) | 1 (<1) | 3 (2) | 6 (3) | 7 (7) | 0.01a | 4 (24) |
| Nutritional supplements | 12 (2) | 1 (<1) | 2 (1) | 5 (3) | 4 (4) | 0.07 | 6 (50) |
| Potassium binders | 5 (<1) | 0 (0) | 1 (<1) | 1 (<1) | 3 (3) | 0.04a | 0 (0) |
| Treatment of underlying kidney disease | |||||||
| Bladder medications | 65 (12) | 8 (6) | 9 (6) | 40 (21) | 8 (8) | 0.02a | 14 (22) |
| Noncorticosteroid immunosuppressants | 42 (8) | 15 (12) | 12 (8) | 11 (6) | 4 (4) | 0.02a | 8 (19) |
| Corticosteroids | 36 (6) | 12 (9) | 8 (6) | 11 (6) | 5 (5) | 0.19 | 2 (6) |
| Other treatment of kidney diseasec | 23 (4) | 4 (3) | 3 (2) | 12 (6) | 4 (4) | NA | NA |
| Symptom control | |||||||
| Antacids | 54 (10) | 13 (10) | 15 (10) | 15 (8) | 11 (11) | 0.87 | 7 (13) |
| Laxatives and stool softeners | 33 (6) | 8 (6) | 10 (7) | 9 (5) | 6 (6) | 0.68 | 4 (12) |
| Other symptom control medicationsc | 64 (11) | 12 (9) | 18 (13) | 26 (14) | 8 (8) | NA | NA |
| Non-CKD–related medications | |||||||
| Antibacterial agents | 160 (29) | 31 (24) | 34 (24) | 67 (35) | 28 (28) | 0.11 | NA |
| Asthma/allergy medications | 42 (8) | 12 (9) | 12 (8) | 9 (5) | 9 (9) | 0.48 | NA |
| Other non-CKD–related medicationsc | 78 (14) | 20 (16) | 18 (13) | 22 (12) | 18 (18) | NA | NA |
Reported P values are based on the Cochran–Armitage trend test. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; NA, not applicable.
P is significant at the α=0.05 level.
Includes BP control medications other than ACEIs, ARBs, and diuretics.
Medication groups with overall prevalence <5% were conglomerated and reported as “other” for each category. Trends across CKD stage were not calculated for “other” medication groups.
Children with glomerular disease had more prevalent use of ACEIs/ARBs, diuretics, other BP control medications, erythrocyte stimulating agents (ESAs), antacids, and lipid-lowering medications compared with children with nonglomerular disease (P<0.05, Fisher exact test; data not shown), in addition to corticosteroids and noncorticosteroid immunosuppressants to treat the primary kidney disease. Greater use of alkali therapy, growth hormone, bladder medications, laxatives, and antibacterial agents was seen in children with nonglomerular disease.
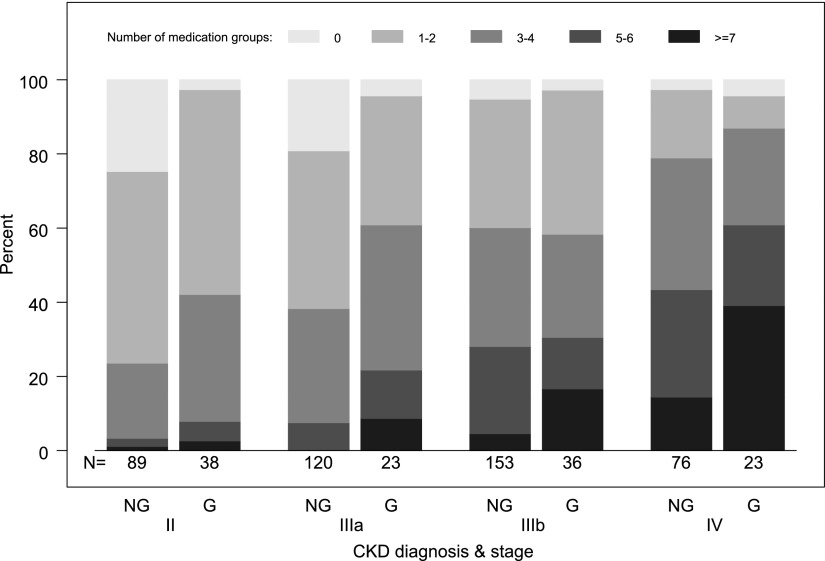
The magnitude of treatment requirements for multiple comorbidities was assessed by the number of medication groups used by each patient, restricted to medications for CKD-specific comorbidities, treatment of kidney disease, and symptom control (excluding other groups; n=19). On average, children required treatment with medications from a median of 2 (IQR, 1, 4) different groups; the number of medication groups used by patients increased significantly with worsening CKD stage (P<0.001 for trend by the Jonckheere–Terpstra test). Among children with nonglomerular kidney disease, 24%, 38%, 60%, and 79% with CKD stages II, IIIa, IIIb, and IV, respectively, required treatment with ≥3 groups of medications for CKD-related problems (see Figure 1). Such requirements were similar or greater for children with glomerular CKD at each CKD stage (42%, 61%, 58%, and 87% respectively). At the extreme, a small number of children with glomerular disease and CKD stages IIIb–IV needed medication from ≥9 different groups as part of their care (n=9; 15%).
Figure 1.
Distribution of number of CKD-related medication groups by CKD stage and CKD diagnosis (N=558). G, glomerular diagnosis; NG, nonglomerular diagnosis.
Table 3 reports the association of the relative number of CKD-related medication groups with demographic and clinical characteristics, by comparing a given covariate exposure level to the reference level and adjusted for the variables shown. Increased treatment requirements were independently associated with more advanced CKD stage, glomerular CKD, and a self-report of other/mixed race (non-Caucasian, non-African American).
Table 3.
Adjusted estimated relative counts of reported unique medication groups (N=558)
| Demographic and Clinical Characteristics | Relative Count of Unique Medication Groups (19 Possible) | |
|---|---|---|
| Estimate (95% CI) | P Value | |
| CKD stage | ||
| II | 1.00 (reference) | |
| IIIa | 1.25 (1.04 to 1.50) | 0.02 |
| IIIb | 1.84 (1.56 to 2.18) | <0.001 |
| IV | 2.47 (2.07 to 2.95) | <0.001 |
| Glomerular CKD | 1.30 (1.12 to 1.50) | <0.001 |
| Age (per 3-yr increase) | 1.01 (0.96 to 1.06) | 0.71 |
| Male versus female | 1.08 (0.96 to 1.21) | 0.22 |
| Race | ||
| Caucasian | 1.00 (reference) | |
| African American | 1.01 (0.87 to 1.17) | 0.87 |
| Other/mixed | 1.27 (1.07 to 1.51) | 0.01 |
| Annual household income<$36,000 | 1.02 (0.90 to 1.14) | 0.62 |
| CKD duration per 1 yr | 0.99 (0.97 to 1.00) | 0.10 |
All estimates are adjusted for the variables shown in the table. 95% CI, 95% confidence interval.
We sought to identify clusters of CKD-related medications that were prescribed together and their associated clinical characteristics. Three medication clusters were identified (Figure 2). The primary pairing in cluster 1 was between corticosteroid and noncorticosteroid immunosuppressant medications; antacid therapy and vitamin D showed secondary associations. Cluster 1 was strongly associated with glomerular CKD (OR, 6.97; 95% CI, 3.85, 12.62; P<0.001), and to a lesser degree with older age (OR, 1.08 per year increase; 95% CI, 1.00, 1.16; P=0.05) and female sex (OR, 2.04; 95% CI, 1.16, 3.45; P=0.01). Use of medications in this group was not associated with the level of GFR, severity of proteinuria, or race.
Figure 2.
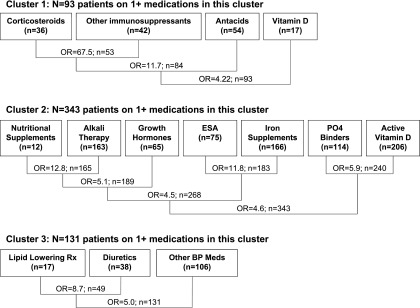
Cluster plots showing primary pair-wise associations and subsequently ranked associations between medication groups, restricted to 19 groups of medications for management of CKD-specific complications, treatment of underlying kidney disease, and symptom control. The odds ratio (OR) for strength of association for each initial and subsequent pairing is reported, along with the total number of patients in each pairing that are members of one or both groups. ESA, erythrocyte stimulating agent; PO4, phosphate; Rx, treatment.
The primary pairings in cluster 2 were nutritional supplements with alkali therapy, ESAs with iron preparations, and phosphate binders with active vitamin D. These pairings were in turn strongly associated with one another and with use of growth hormones. With the exception of nutritional supplements (n=12 reported uses), all of the individual medication groups in the cluster were independently associated with one another at the α=0.01 level. Use of a cluster 2 medication was associated with lower GFR (OR, 2.00 per 20% decrease in GFR; 95% CI, 1.71, 2.32; P<0.001), non-Caucasian/African-American race (OR, 2.44; 95% CI, 1.18, 5.05; P=0.02), and younger age (OR, 0.90 per year increase; 95% CI, 0.86, 0.95; P<0.001), but not with glomerular CKD or proteinuria.
Cluster 3 was composed of lipid-lowering medications, diuretics, and other BP medications. It was associated with proteinuria (OR, 1.97 for protein/creatinine ratio>2 versus<2; 95% CI, 1.12, 3.48; P=0.02) and African-American race (OR, 2.40 versus Caucasian; 95% CI, 1.44, 3.97; P<0.001), and had a weaker association with lower GFR (OR, 1.29 per 20% decrease in GFR; 95% CI, 1.13, 1.47; P<0.001). Despite the association with nephrotic proteinuria, cluster 3 was not significantly associated with glomerular diagnosis or ACEI/ARB use.
A final analysis identified clinical, demographic, and medication-related factors associated with medication nonadherence. Univariate analysis showed a significant association between nonadherence and medication frequency, with the highest nonadherence rate occurring with medications dosed three times daily (25%; P=0.03 for trend with dosing frequency by the Cochran–Armitage test). In multivariate analysis (Table 4), nonadherence was associated with older age, Caucasian race, and increased dosing frequency, particularly medications dosed >2 times daily (OR, 1.70>2 times per day versus 1 time per day; P<0.001). There was no significant association between nonadherence and number of medication groups.
Table 4.
Factors associated with nonadherence of CKD-related medications (1602 medications in 477 patients)
| Risk Factor | OR (95% CI) for Nonfull Adherence in Past 7 Days | P Value |
|---|---|---|
| Medications currently taking (n) | ||
| 1–2 | 1 (reference) | |
| 3–4 | 0.82 (0.47 to 1.43) | 0.49 |
| ≥5 | 0.63 (0.38 to 1.06) | 0.08 |
| Dosing frequency (times/d) | ||
| <1 | 0.72 (0.49 to 1.05) | 0.09 |
| 1 | 1 (reference) | |
| 2 | 1.18 (0.90 to 1.55) | 0.22 |
| >2 | 1.70 (1.25 to 2.29) | <0.001 |
| Glomerular CKD | 0.75 (0.43 to 1.32) | 0.32 |
| Age (per 1-yr increase) | 1.09 (1.03 to 1.15) | 0.004 |
| Male versus female | 0.99 (0.65 to 1.50) | 0.95 |
| Race | ||
| Caucasian | 1 (reference) | |
| African American | 0.53 (0.30 to 0.92) | 0.02 |
| Other/mixed | 0.37 (0.18 to 0.77) | 0.01 |
| Annual household income<$36,000 | 1.20 (0.79 to 1.82) | 0.39 |
| CKD duration (per 1 yr) | 0.98 (0.93 to 1.03) | 0.46 |
Restricted to CKD-related medications with available adherence and dosing frequency. Analysis evaluates nonadherence of unique reported medications, not medication groups as in earlier analyses. All estimates are adjusted for the variables shown in the table. OR, odds ratio.
Discussion
This study provides the first comprehensive description of medication requirements in children with CKD. We identified 19 distinct medication groups directly or indirectly related to CKD care. ACEI/ARB treatment was most common and it was the only medication group used by >50% of patients. Predictably, medications for the treatment of CKD-related comorbidities were used more frequently in individuals with more advanced CKD stage, and medications for treatment of GN, hypertension, and medication side effects (e.g., antacids) were more prevalently used in children with primary glomerular disease.
Although medication use increased by CKD stage, we did not find that treatment for specific CKD-related comorbidities is consistently required at a given stage of CKD. Using a >50% threshold, active vitamin D supplements and ACEIs/ARBs were commonly used in stage IV CKD only. For other medications perceived to be commonly used in advanced CKD such as growth hormones, ESAs, and phosphate binders, prevalent use ranged from 20% to 44%. In mild CKD (stage II), prevalent use of BP medications and iron, alkali, vitamin, and mineral supplements exceeded 10% and the use of phosphate binders was 9%. This suggests that despite the trend, CKD staging alone may be insufficient to guide targeted screening for comorbidities.
We therefore sought to assess whether there was clustering of comorbidities independent of CKD stage. Because comorbidities may develop through common mechanisms of injury, their presentation and treatment may also be linked. Medication cluster 2 included strong associations (OR≥4.5) between medications for treating anemia (ESAs and iron preparations), mineral and bone disorder (active vitamin D and phosphate binders), and growth failure (growth hormones, nutrition supplements, and alkali). These processes have an association with tubulointerstitial compartment injury in common. Both erythropoietin and 1,25-hydroxy vitamin D are produced in the tubulointerstitium, and progression of kidney disease, regardless of etiology, results in gradual tubulointerstitial fibrosis and atrophy (6–8). Similarly, the mechanism of metabolic acidosis is primarily associated with tubulointerstitial injury and is independent of GFR to some extent (9–13). This clustering of medication use suggests that patients manifesting evidence of tubulointerstitial injury, regardless of CKD stage, may benefit from more active and targeted screening for related comorbidities, in addition to the usual screening that is dictated by progression of CKD stage.
Progressive CKD and glomerular disease both contribute to a greater magnitude of treatment requirements. The number of medication groups was 1.5-fold higher in children with glomerular disease and 2.5-fold comparing CKD stage II to IV. Among children with stage IIIb CKD, >25% reported use of medication for ≥5 problems and this level of medication use was reported by almost half (47%) of children with stage IV CKD. In the subset with glomerular disease, 15% of children required ≥9 different medication groups.
We hypothesized that the treatment of a larger number of comorbidities would be associated with higher levels of nonadherence, but we did not see such an association. Nonadherence was, however, independently associated with the medication dosing frequency. This suggests that what matters is not so much the number of different medications being taken together, rather the number of times each day that medication administration must be coordinated. Other studies looking at management of cardiovascular disease and HIV reach similar conclusions (14–18). Indeed, one study demonstrated better adherence after adding lipid-lowering treatment to a multidrug treatment regimen in a population with chronic hypertension (19). This effect may be due in part to the perceived importance of medications with progressive comorbidity. This has been reported in transplant recipients, in which preferential adherence is associated with immunosuppressant treatment (20). Chiu et al. (21) reported a positive association of phosphate binder pill burden and nonadherence in a chronic dialysis population. The effect on adherence of multiple daily dosing for phosphate binders would be consistent with our findings if the effect was predominantly related to typical dosing frequency. Other positive associations of medication complexity with reduced quality of life (15,17,21) and poor outcome (22) have been reported, but were not explored in this analysis.
Nonadherence was more frequently reported among Caucasians even after adjustment for socioeconomic status, sex, age, glomerular diagnosis, and CKD duration. This contrasts with other reports that suggest greater nonadherence among African-American patients (23–27).
This study has a number of limitations. First, we relied on medication self-reporting to determine the active treatment for CKD comorbidities as well as medication adherence. We were not able to include dietary interventions that may have contributed additional complexity of care and likely under-reported use of nutrition supplements for lack of specific screening. Second, we have speculated that certain disease categories may be associated with particular patterns of medication administration. Because of the large number of diseases that result in CKD, we were not able to separate these beyond the general classification as glomerular or nonglomerular. Third, some medications with multiple indications were grouped based on the predominant usage (e.g., calcium carbonate as a phosphate binder) or assigned to their own distinct group (e.g., ACEI/ARB). It is possible that some misclassification has resulted or that multiple indications determined medication selection for an individual patient.
In summary, this is the first report to provide a comprehensive summary of medication use and treatment complexity in children with CKD. The number and profile of medication requirements change consistently in association with both CKD stage and the presence of glomerular disease. These findings may permit better anticipation of CKD-related comorbidities and treatment, but also highlight the limitations of CKD staging to dependably predict onset of such comorbidities in children. The number of treatments required for some patients was onerous but was not associated specifically with nonadherence. Nonadherence was, however, linked to increased medication dosing frequency, suggesting that simplification of medication regimens may improve adherence in this population.
Disclosures
M.M.-M. is employed by the National Institute of Diabetes and Digestive and Kidney Diseases.
Supplementary Material
Acknowledgments
The authors thank the youth with kidney disease and their families for their participation in the CKiD study, as well as the study investigators and coordinators for their hard work.
The CKiD study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute. The study sponsors had a role in the study design, analysis, interpretation of data, writing this manuscript, and the decision to submit the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05750513/-/DCSupplemental.
References
- 1.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Kidney Foundation: KDOQI Clinical Practice Guidelines. Available at: http://www.kidney.org/professionals/KDOQI/guidelines_commentaries.cfm Accessed November 11, 2009
- 6.Eddy AA: Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol 5: 1273–1287, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Eddy AA: Progression in chronic kidney disease. Adv Chronic Kidney Dis 12: 353–365, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Pichler R, Giachelli C, Young B, Alpers CE, Couser WG, Johnson RJ: The pathogenesis of tubulointerstitial disease associated with glomerulonephritis: The glomerular cytokine theory. Miner Electrolyte Metab 21: 317–327, 1995 [PubMed] [Google Scholar]
- 9.Batlle D, Kurtzman NA: Distal renal tubular acidosis: Pathogenesis and classification. Am J Kidney Dis 1: 328–344, 1982 [DOI] [PubMed] [Google Scholar]
- 10.Batlle DC, Arruda JA, Kurtzman NA: Hyperkalemic distal renal tubular acidosis associated with obstructive uropathy. N Engl J Med 304: 373–380, 1981 [DOI] [PubMed] [Google Scholar]
- 11.Miklovicova D, Cervenova O, Cernianska A, Jancovicova Z, Dedik L, Vasilenkova A: Long-term follow-up of renal function in patients after surgery for obstructive uropathy. Pediatr Nephrol 23: 937–945, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Ortega LM, Arora S: Metabolic acidosis and progression of chronic kidney disease: Incidence, pathogenesis, and therapeutic options. Nefrologia 32: 724–730, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Rothstein M, Obialo C, Hruska KA: Renal tubular acidosis. Endocrinol Metab Clin North Am 19: 869–887, 1990 [PubMed] [Google Scholar]
- 14.Buscher A, Hartman C, Kallen MA, Giordano TP: Impact of antiretroviral dosing frequency and pill burden on adherence among newly diagnosed, antiretroviral-naive HIV patients. Int J STD AIDS 23: 351–355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyle BA, Jayaweera D, Witt MD, Grimm K, Maa JF, Seekins DW: Randomization to once-daily stavudine extended release/lamivudine/efavirenz versus a more frequent regimen improves adherence while maintaining viral suppression. HIV Clin Trials 9: 164–176, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Frishman WH: Importance of medication adherence in cardiovascular disease and the value of once-daily treatment regimens. Cardiol Rev 15: 257–263, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gianotti N, Galli L, Bocchiola B, Cahua T, Panzini P, Zandonà D, Salpietro S, Maillard M, Danise A, Pazzi A, Lazzarin A, Castagna A: Number of daily pills, dosing schedule, self-reported adherence and health status in 2010: A large cross-sectional study of HIV-infected patients on antiretroviral therapy. HIV Med 14: 153–160, 2013 [DOI] [PubMed] [Google Scholar]
- 18.O’Connor JL, Gardner EM, Mannheimer SB, Lifson AR, Esser S, Telzak EE, Phillips AN, INSIGHT SMART Study Group : Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. J Infect Dis 208: 40–49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson TA, Cooke CE, Wang J, Shaya FT, Lee HY: Effect of medication burden on persistent use of lipid-lowering drugs among patients with hypertension. Am J Manag Care 14: 710–716, 2008 [PubMed] [Google Scholar]
- 20.Terebelo S, Markell M: Preferential adherence to immunosuppressive over nonimmunosuppressive medications in kidney transplant recipients. Transplant Proc 42: 3578–3585, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardinger KL, Hutcherson T, Preston D, Murillo D: Influence of pill burden and drug cost on renal function after transplantation. Pharmacotherapy 32: 427–432, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ: Patient characteristics associated with medication adherence. Clin Med Res 11: 54–65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu VJ, Tu W, Marrero DG, Rosenman MB, Overhage JM: Race and medication adherence and glycemic control: findings from an operational health information exchange. AMIA Annu Symp Proc 2011: 1649–1657, 2011 [PMC free article] [PubMed] [Google Scholar]
- 25.Gebregziabher M, Lynch CP, Mueller M, Gilbert GE, Echols C, Zhao Y, Egede LE: Using quantile regression to investigate racial disparities in medication non-adherence. BMC Med Res Methodol 11: 88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyser M, Buchacz K, Bush TJ, Conley LJ, Hammer J, Henry K, Kojic EM, Milam J, Overton ET, Wood KC, Brooks JT: Factors associated with non-adherence to antiretroviral therapy in the SUN study. AIDS Care 23: 601–611, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Oliva M, Singh TP, Gauvreau K, Vanderpluym CJ, Bastardi HJ, Almond CS: Impact of medication non-adherence on survival after pediatric heart transplantation in the U.S.A. J Heart Lung Transplant 32: 881–888, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.