Summary
Background and objectives
In children with CKD, information is limited regarding the prevalence and determinants of fibroblast growth factor 23 excess and 1,25-dihyroxyvitamin D deficiency across the spectrum of predialysis CKD. This study characterized circulating concentrations of fibroblast growth factor 23 and 1,25-dihyroxyvitamin D, and investigated their interrelationships and associations with GFR and secondary hyperparathyroidism in children with CKD who were enrolled in the Chronic Kidney Disease in Children observational cohort study.
Design, setting, participants, & measurements
Plasma fibroblast growth factor 23 concentrations and determinants of mineral metabolism were measured in 464 children ages 1–16 years with predialysis CKD. GFR was measured by plasma disappearance of iohexol in 70% of participants and estimated by the Chronic Kidney Disease in Children estimating equation using serum creatinine and cystatin C concentrations in the remainder of the participants. Participants were grouped according to CKD stage and by 10-ml/min categories of GFR.
Results
Median GFR for the cohort was 45 ml/min per 1.73 m2 (interquartile range=33–57; range=15–109). Plasma fibroblast growth factor 23 concentration was above the normal range in 67% of participants (with higher levels observed among participants with lower GFR) before higher levels of serum parathyroid hormone and phosphorus were observed. Plasma fibroblast growth factor 23 levels were 34% higher in participants with glomerular disease than in participants with nonglomerular disease, despite similar GFR. Serum phosphorus levels, adjusted for age, were significantly lower at GFR of 60–69 ml/min per 1.73 m2 than higher GFR, but thereafter they became higher in parallel with fibroblast growth factor 23 as GFR declined. Serum 1,25-dihyroxyvitamin D concentrations were lower in those participants with low GFR values, high fibroblast growth factor 23 levels, 25-hydroxyvitamin D deficiency, and proteinuria. Secondary hyperparathyroidism was present in 55% of participants with GFR<50 ml/min per 1.73 m2.
Conclusion
In children with predialysis CKD, high plasma fibroblast growth factor 23 is the earliest detectable abnormality in mineral metabolism, and levels are highest in glomerular diseases.
Introduction
Advanced CKD in children results in disordered bone and mineral metabolism and associated bone deformities, growth failure, and cardiovascular disease that severely diminish quality of life and lifespan (1). The evolution of such abnormalities during the early stages of CKD in children, however, is incompletely characterized. Thus, too little is known regarding the optimal therapies to use and their timing, which limits our ability to prevent such abnormalities or mediate their severity.
Secondary hyperparathyroidism in advanced CKD is attributed to deficiency of 1,25-dihyroxyvitamin D [1,25(OH)2D] combined with hyperphosphatemia, leading to disordered turnover and mineralization of bone. These abnormalities are now considered novel risk factors for cardiovascular disease and mortality in both children and adults with CKD. Deficiency of 1,25(OH)2D occurs early in the course of progressive CKD in adults and children (2–5), and recent data suggest that excess circulating fibroblast growth factor 23 (FGF23) is the primary mechanism and, therefore, the initiating event in the development of secondary hyperparathyroidism (6,7).
FGF23 is a bone-derived circulating hormone that acts to inhibit renal phosphate reabsorption and suppress the renal synthesis of 1,25(OH)2D (8–10). In adults with CKD, plasma FGF23 concentrations increase progressively as GFR declines before the development of hyperphosphatemia (6,7), and increased FGF23 levels are associated with CKD progression (11–13), left ventricular hypertrophy (14,15), and premature death (16,17). In children, however, information is limited regarding the prevalence and determinants of FGF23 excess and 1,25(OH)2D deficiency across the spectrum of predialysis CKD and their potential association with adverse renal and cardiovascular outcomes. Prior single-center studies of FGF23 and mineral metabolism in childhood CKD have been limited by small sample size (18,19), heterogeneous study populations that include dialysis and transplant patients (5,20), and imprecise estimates of GFR (21,22).
In the present study, we characterized circulating concentrations of FGF23 and 1,25(OH)2D and investigated their interrelationships and associations with GFR and secondary hyperparathyroidism in children with predialysis CKD who were enrolled in the Chronic Kidney Disease in Children (CKiD) observational cohort study (23).
Materials and Methods
Participants
We studied 464 children with predialysis CKD who were enrolled in CKiD from June of 2005 to June of 2011. Study details were published (23). Briefly, eligible participants ages 1–16 years with an estimated GFR between 30 and 90 ml/min per 1.73 m2 determined by the original Schwartz equation (24) were enrolled from 48 pediatric nephrology sites in the United States and Canada. Recipients of solid organ transplants were excluded. The Institutional Review Board at each site approved the study protocol, and informed consent was obtained from each patient and parent or guardian (NCT00327860; May 18, 2006).
For the present cross-sectional investigation, we obtained mineral and hormone data from a study visit that occurred 3–6 months after the enrollment visit (n=359) or at the second annual study visit (n=105). At the enrollment visit, GFR was measured by plasma disappearance of iohexol (25) (n=331) or estimated by the CKiD estimating equation using serum creatinine and cystatin C concentrations (26); at the second annual study visit, GFR was estimated. In 331 participants who underwent iohexol GFR measurement, median values for estimated and measured GFR were virtually identical (45 and 44 ml/min per 1.73 m2, respectively). Plasma C-terminal FGF23 concentrations were measured in duplicate by second generation ELISA (Immutopics Int., San Clemente, CA). We recruited a separate cohort of 42 healthy children (mean age=12±4 years) whose median FGF23 was 57 RU/ml (2.5th and 97.5th percentiles: 17 and 101 RU/ml). Thus, we defined 101 RU/ml as the upper limit of the normal range in these healthy children. This value is comparable with 105 RU/ml, which is considered the upper limit of normal in 61 healthy children (8.0±4.2 years old) (19), 100 RU/ml, which is considered the upper limit of normal in healthy adults (7), and 108 RU/ml, which is the median 90th percentile value in 172 healthy children (11.0±4.5 years old) (27). Serum concentrations of 25-hydroxyvitamin D (25OHD) were measured in duplicate by chemiluminescence immunoassay (DiaSorin LIAISON 25-OH Vitamin D TOTAL Assay) (28,29); inter- and intra-assay coefficients of variation (CVs) are 11.2% and 8.1%, respectively. 1,25(OH)2D was measured by radioimmunoassay (30); CVs are 12.6% and 9.8%, respectively. Specimens for parathyroid hormone (PTH), FGF23, and vitamin D metabolites were stored at −80°F until measurements were made. Serum concentrations of PTH, calcium, and phosphorus were determined in the CKiD Central Biochemistry Laboratory at the University of Rochester. Intact PTH was measured by chemiluminometric assay (CV<5%, n=451 participants; Advia Centaur System; Bayer Healthcare LLC; CV<5%, n=13; PTH Stat; Roche Diagnostics, Indianapolis, IN). Calcium was measured using the arsenazo dye end point method and phosphorus was measured using the phosphomolybdate reaction. Serum calcium concentrations were corrected for serum albumin concentrations: corrected calcium=measured calcium+0.8×(4.0−serum albumin). Urine phosphorus and creatinine concentrations were determined in voided morning specimens by autoanalyzer (Beckman Coulter AU400; Beckman Coulter, Inc., Brea, CA).
Statistical Analyses
We summarized continuous variables as mean±SD or median and interquartile range (IQR) for skewed data, for the overall cohort and participants grouped by CKD stage (stage 2, GFR≥60 ml/min per 1.73 m2; stage 3a, GFR=45–59 ml/min per 1.73 m2; stage 3b, GFR=30–44 ml/min per 1.73 m2; stage 4, GFR=15–29 ml/min per 1.73 m2). We expressed categorical variables as frequencies and proportions. For statistical analyses, values of FGF23 and PTH were natural log–transformed to satisfy normality assumptions. We compared mean (median) values of biochemical parameters between CKD stages and across seven finer categories of GFR (≥70, 60–69, 50–59, 40–49, 30–39, 20–29, and <20 ml/min per 1.73 m2) using one-way ANOVA or the Kruskal–Wallis test, as appropriate. We used simple linear and multivariable regression analyses to examine associations between estimated GFR and FGF23, 1,25(OH)2D, and other parameters of mineral metabolism. Because serum phosphorus concentrations vary with age in healthy children (31), we expressed the phosphorus value for each participant as a z score relative to age-matched values in 493 healthy children 1–20 years old (31). A P value<0.05 was considered statistically significant. Analyses were performed using STATA 12 (Stata Corp, College Station, TX).
Results
Study Population
Characteristics of 464 participants for the entire cohort and grouped by CKD stage are shown in Tables 1 and 2. The median age was 11.7 years (IQR=8–15), and 39% were girls. The median GFR for the cohort was 45 ml/min per 1.73 m2 (IQR=33–57 ml/min per 1.73 m2), and the range was 15–109 ml/min per 1.73 m2; 2% of participants had CKD stage 1, 20% of participants had CKD stage 2, 28% of participants had CKD stage 3a, 31% of participants had CKD stage 3b, and 19% of participants had CKD stage 4. CKD was caused by nonglomerular disease (obstruction/reflux, hypoplasia/dysplasia, cystic disease, and other) in 80% of participants and glomerular disease (FSGS, hemolytic uremic syndrome, and other) in 20% of participants; 21% of participants reported taking phosphate binding agents, 38% of participants reported taking active vitamin D sterols, and 5% of participants reported taking nutritional vitamin D supplements.
Table 1.
Characteristics of the study population by CKD stage
| Characteristic | Overall | Stage 2 (GFR≥60 ml/min per 1.73 m2) | Stage 3a (GFR=45–59 ml/min per 1.73 m2) | Stage 3b (GFR=30–44 ml/min per 1.73 m2) | Stage 4 (GFR=15–29 ml/min per 1.73 m2) |
|---|---|---|---|---|---|
| Number | 464 | 103a | 127 | 145 | 89 |
| Age, yr | 11.7 [8.4, 15.1] | 11.2 [7.0, 15.3] | 11.5 [8.1, 15.0] | 11.4 [8.6, 15.1] | 13.6 [9.6, 15.2] |
| Girls | 39 (181) | 37 (38) | 41 (52) | 36 (52) | 44 (39) |
| Race | |||||
| Caucasian | 70 (323) | 55 (57) | 71 (90) | 77 (112) | 72 (64) |
| African American | 20 (93) | 35 (36) | 19 (24) | 14 (20) | 15 (13) |
| Multiracial or other | 10 (48) | 10 (10) | 10 (13) | 9 (13) | 13 (12) |
| Hispanic ethnicity | 14 (61) | 10 (9) | 15 (18) | 14 (21) | 14 (13) |
| Primary CKD diagnosis | |||||
| Glomerular | 20 (94) | 27 (28) | 18 (23) | 17 (24) | 21 (19) |
| Obstruction/reflux | 37 (174) | 40 (41) | 43 (54) | 32 (46) | 37 (33) |
| Hypoplasia/dysplasia | 18 (82) | 13 (13) | 15 (919) | 22 (32) | 20 (18) |
| Other nonglomerular | 20 (93) | 18 (19) | 19 (24) | 26 (38) | 14 (12) |
| Cystic disease | 5 (21) | 2 (2) | 5 (7) | 3 (5) | 8 (7) |
| Medication use | |||||
| Phosphate binders | 21 (98) | 6 (6) | 11 (14) | 23 (34) | 49 (44) |
| Nutritional vitamin D | 5 (22) | 3 (3) | 4 (5) | 7 (10) | 5 (4) |
| Active vitamin D | 38 (178) | 8 (8) | 29 (37) | 49 (71) | 70 (62) |
Data are medians [25th, 75th percentiles] or percentage (number).
Includes 11 participants with CKD stage 1 (GFR=90–109 ml/min per 1.73 m2).
Table 2.
Mineral metabolism by CKD stage
| Overall | Stage 2 (GFR≥60 ml/min per 1.73 m2) | Stage 3a (GFR=45–59 ml/min per 1.73 m2) | Stage 3b (GFR=30–44 ml/min per 1.73 m2) | Stage 4 (GFR=15–29 ml/min per 1.73 m2) | |
|---|---|---|---|---|---|
| Number | 464 | 103 | 127 | 145 | 89 |
| GFR, ml/min per 1.73 m2 | 45 [33, 57] | 71 [64, 80] | 51 [48, 55] | 38 [34, 42] | 26 [24, 28] |
| Serum calcium, mg/dl | 9.4±0.4 | 9.4±0.4 | 9.4±0.4 | 9.3±0.4 | 9.3±0.4 |
| Serum phosphorus, mg/dl | 4.6±0.8 | 4.4±0.7 | 4.4±0.7 | 4.6±0.8a | 4.9±0.9b,c,d |
| Serum phosphorus z score, SD | 0.1±1.5 | −0.4±1.2 | −0.2±1.3 | 0.2±1.5b | 0.9±1.8b,c,e |
| Serum iPTH, pg/ml | 51 [30, 84] | 37 [26, 54] | 48 [26, 70] | 55 [33, 95]b | 74 [47, 181]b,c,d |
| Plasma FGF23, RU/ml | 138 [91, 210] | 93 [73, 140] | 122 [90, 171]a | 153 [102, 221]b | 223 [148, 460]b,c,f |
| Serum 25OHD, ng/mlg | 27±12 | 27±10 | 27±11 | 29±12 | 25±12 |
| Serum 1,25(OH)2D, pg/mlg | 30±11 | 33±11 | 32±10 | 30±12 | 25±10b,c,e |
| Urine FEPi, % | 16.4 [11.6, 23.2] | 10.0 [7.7, 13.6] | 14.7 [11.0, 19.2]a | 17.8 [13.5, 24.0]a,c | 25.2 [20.6, 30.6]a,c,f |
Data are means±SD or medians [25th, 75th percentiles]. Differences between CKD stages were tested using one-way ANOVA. P value represents pairwise tests of significance using the Sidak procedure. Analyses of FGF23 and PTH were performed using log-transformed values. iPTH, immunoreactive parathyroid hormone; FGF23, fibroblast growth factor 23; 25OHD, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihyroxyvitamin D; FEPi, fractional urinary excretion of phosphate calculated as (urine phosphorus×serum creatinine/urine creatinine×serum phosphorus)×100.
P<0.05 versus stage 2.
P<0.01 versus stage 2.
P<0.001 versus stage 3a.
P<0.05 versus stage 3b.
P<0.01 versus stage 3b.
P<0.001 versus stage 3b.
25OHD and 1,25(OH)2D were measured in 376 participants.
Mineral Ion and Hormone Data for the Cohort and by CKD Stage
For the overall cohort, mean serum concentrations of corrected calcium, phosphorus, 1,25(OH)2D, and median PTH were within normal ranges (Table 2). In contrast, the median plasma FGF23 level of 138 RU/ml (IQR=9–210) was 2.4 times higher than the median value of 57 RU/ml in 42 healthy children of comparable age (12±4 years); 67% of participants had FGF23 excess, defined as a value>101 RU/ml, and 58% of participants were either vitamin D–insufficient (25OHD=16 to <30 ng/ml; 38%) or –deficient (25OHD≤15 ng/ml; 20%) (32). Median urine fractional urinary excretion of phosphate (FEPi) was 16.4% (IQR=11.6–23.2).
Compared with values in CKD stage 2, median plasma FGF23 concentration and urine FEPi were significantly higher in CKD stage 3a and higher stages, whereas serum phosphorus, phosphorus z score, and serum PTH concentrations were significantly higher in CKD stage 3b and higher stages (Table 2). Mean serum concentrations of 1,25(OH)2D were significantly lower at CKD stage 4 (Table 2). Thus, when grouped by CKD stages, higher values of plasma FGF23 and urine FEPi were the earliest significant abnormalities in mineral metabolism observed.
Mineral Ion and Hormone Data by GFR Group
To more closely examine the differences in mineral metabolism with lower levels of GFR, we grouped participants into seven GFR groups spanning 10 ml/min per 1.73 m2. Participants with GFR≥70 ml/min per 1.73 m2 (n=54) served as the reference group for statistical comparison.
Calcium, Phosphorus, and PTH.
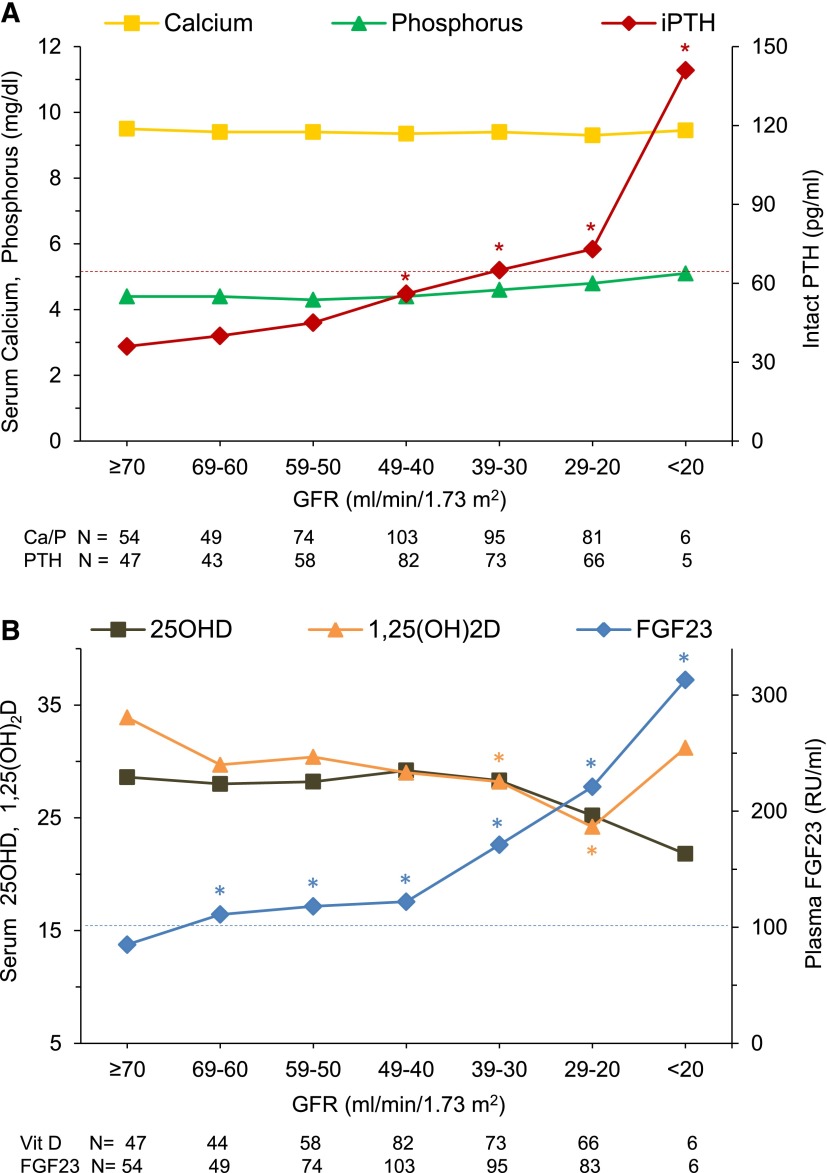
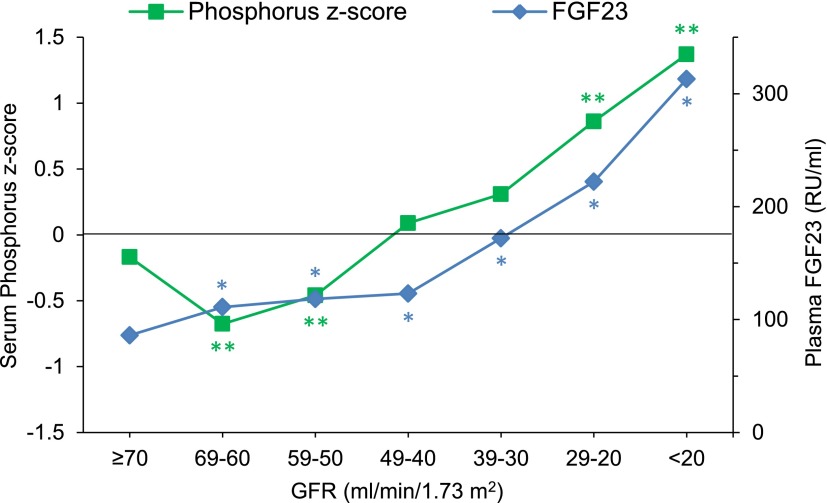
Median serum-corrected calcium and phosphorus concentrations did not differ significantly according to GFR (Figure 1A). Because serum phosphorus levels decrease with advancing age in healthy children, we examined serum phosphorus z scores scaled to age-adjusted values in healthy children (31). At GFR of 60–69 ml/min per 1.73 m2, mean phosphorus z score was 0.68 SDs below the mean of the healthy reference population (31) (P<0.001). At lower levels of GFR, phosphorus z scores were progressively higher (0.39 SD per each 10-ml/min per 1.73 m2 decrement in GFR), reaching a maximum value of 1.37 SD (P<0.001) (Figure 2). Median serum PTH concentrations were progressively higher as GFR declined, with values being significantly higher than those values in the reference group (GFR≥70 ml/min per 1.73 m2) at GFR=40–49 ml/min per 1.73 m2 and below (P<0.05) (Figure 1A).
Figure 1.
Mineral and hormone values according to estimated GFR at 10-ml/min per 1.73 m2 intervals in children with CKD. Median concentrations of (A) serum corrected–calcium, phosphorus, and immunoreactive parathyroid hormone (iPTH) and (B) serum 25-hydroxyvitamin D (25OHD) (ng/ml), 1,25-dihyroxyvitamin D [1,25(OH)2D] (pg/ml), and plasma fibroblast growth factor 23 (FGF23). Higher levels of serum iPTH precede changes in serum calcium and phosphorus. Higher levels of plasma FGF23 precede changes in 25OHD and 1,25(OH)2D. *P<0.05 versus the reference GFR group of ≥70 ml/min per 1.73 m2 using Kruskal–Wallis ANOVA. The dotted lines indicate the upper limit of normal for PTH (65 pg/ml) and FGF23 (101 RU/ml). The number of participants in each GFR group is indicated below each figure. Additional data are provided in Supplemental Table 1.
Figure 2.
Mean phosphorus z scores and median plasma FGF23 concentrations according to estimated GFR at 10-ml/min per 1.73 m2 intervals in children with CKD. Phosphorus z score is expressed in SD units. *P<0.05 versus the reference GFR group of ≥70 ml/min per 1.73 m2 using Kruskal–Wallis ANOVA; **P<0.001 versus corresponding mean for age of a healthy control population (31).
FGF23, 25OHD, and 1,25(OH)2D.
Median plasma FGF23 concentrations were progressively higher as GFR declined, with values being significantly higher than those values in the reference group at GFR=60–69 ml/min per 1.73 m2 and below (P<0.05) (Figures 1B and 2). Median serum 25OHD levels were slightly but not significantly lower with declining GFR. Median 1,25(OH)2D concentrations were lower as GFR declined, and values were significantly lower at GFRs of 30–39 and 20–29 ml/min per 1.73 m2 (P<0.05) (Figure 1B) compared with the reference group. At GFR<20 ml/min per 1.73 m2, 1,25(OH)2D values were slightly higher; however, four of six individuals in this group were taking active vitamin D sterols.
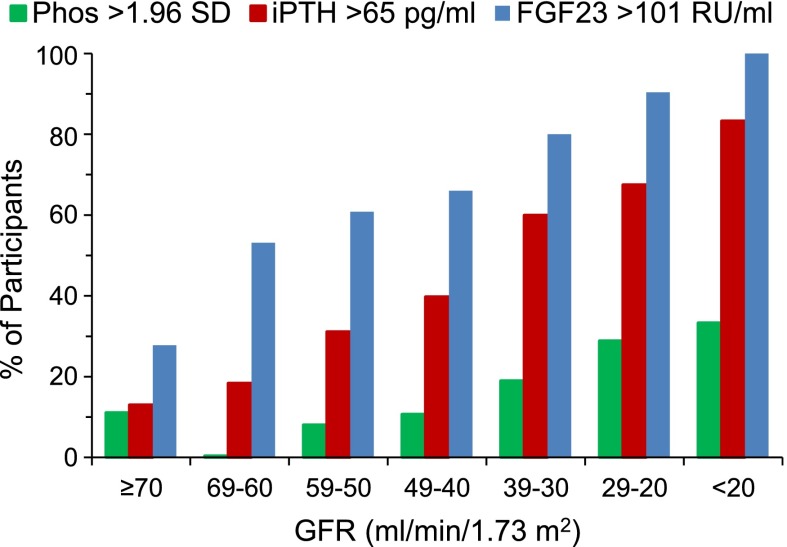
We calculated the percentage of participants with high plasma FGF23 concentrations (FGF23>101 RU/ml), hyperparathyroidism (serum PTH>65 pg/ml), and hyperphosphatemia (serum phosphorus>1.96 SD for age) within each 10-ml/min GFR group (Figure 3). Plasma FGF23 was high in >50% of participants whose GFR was 60–69 ml/min per 1.73 m2 or lower, whereas hyperparathyroidism was present in only 18% of this GFR group, being >50% only when the GFR was 30–39 ml/min per 1.73 m2 or lower. The prevalence of hyperphosphatemia remained low until the GFR declined to 20–29 ml/min per 1.73 m2 (prevalence was 29%).
Figure 3.
Prevalence of hyperphosphatemia, hyperparathyroidism, and increased plasma FGF23 according to GFR groups. Hyperphosphatemia is defined as phosphorus (Phos) z score>1.96 SD, hyperparathyroidism is defined as serum immunoreactive parathyroid hormone (iPTH) >65 pg/ml, and FGF23 excess is defined as plasma FGF23>101 RU/ml.
Glomerular Versus Nonglomerular Disease
We compared indices of mineral metabolism between participants with glomerular and nonglomerular disease. Those participants with glomerular disease were 3 years older, but GFR, serum calcium, and PTH concentrations as well as phosphorus z scores were comparable between the groups (Table 3). By contrast, plasma FGF23 concentration (P=0.01) and urine protein to creatinine ratio (P<0.001) were higher, and serum concentrations of 25OHD, 1,25(OH)2D, and albumin were lower (P<0.001) in participants with glomerular versus nonglomerular disease. In the glomerular group, proteinuria was associated with plasma FGF23 (coefficient=0.05; 95% confidence interval, 0.01 to 0.09; P=0.01) in analyses adjusted for GFR, phosphorus z score, age, and sex; no association was found in the nonglomerular group (P=0.90).
Table 3.
Mineral metabolism in glomerular and nonglomerular disease
| Characteristic | Glomerular | Nonglomerular | P Value |
|---|---|---|---|
| Number | 94 | 370 | |
| Age, yr | 15 [13, 16] | 11 [8, 14] | <0.001a |
| GFR, ml/min per 1.73 m2 | 47 [32, 63] | 44 [33, 56] | 0.31a |
| Serum calcium, mg/dl | 9.3±0.4 | 9.4±0.4 | 0.07 |
| Serum phosphorus, mg/dl | 4.4±1.0 | 4.6±0.8 | 0.02a |
| Serum phosphorus z score, SD | 0.13±1.9 | 0.08±1.4 | 0.89 |
| Serum iPTH, pg/ml | 49 [29, 114] | 52 [30, 82] | 0.98a |
| Plasma FGF23, RU/ml | 165 [95, 271] | 131 [90, 194] | 0.01a |
| Serum 25OHD,b ng/ml | 19±12 | 29±11 | <0.001 |
| Serum 1,25(OH)2D,b pg/ml | 26±12 | 31±11 | <0.001 |
| Serum albumin, g/dl | 4.0±0.7 | 4.4±0.3 | <0.001 |
| Urine FEPi, % | 15.4 [10.7, 23.0] | 16.9 [12.2, 23.3] | 0.23a |
| Urine protein to creatinine ratio, mg/mg | 0.95 [0.35, 2.61] | 0.32 [0.14, 0.85] | <0.001a |
Data are means±SD or medians [25th, 75th percentiles]. Mean (median) values were compared using t tests. FEPi calculated as (urine phosphorus×serum creatinine/urine creatinine×serum phosphorus)×100.
Mean (median) values were compared using Wilcoxon rank sum tests.
25OHD and 1,25(OH)2D were measured in 83 participants with glomerular disease and 293 patients with nonglomerular disease.
Relationships with GFR
For the entire cohort, bivariate analysis revealed that higher log plasma FGF23 was most strongly associated with decreasing GFR (r=−0.44, P<0.001). Higher log serum immunoreactive PTH (r=−0.32, P<0.001), phosphorus z score (r=−0.27, P<0.001), serum phosphorus (r=−0.25, P<0.001), and lower 1,25(OH)2D (r=0.21, P<0.001) were also significantly associated with decreasing GFR. Serum 25OHD, age, and sex were not associated with GFR. Higher levels of proteinuria were significantly associated with lower serum 25OHD concentrations (r=−0.34, P<0.001).
Determinants of FGF23
By multivariable linear regression analysis, log plasma FGF23 concentration was significantly associated with GFR, serum phosphorus z score, glomerular disease, active vitamin D use, Hispanic ethnicity, and girls (Table 4). Specifically, for each 10-ml/min per 1.73 m2 decrease in GFR, plasma FGF23 increased by 14%, and for each 1 SD increase in serum phosphorus z score, FGF23 increased by 6%. At a given level of GFR and serum phosphorus, plasma FGF23 concentrations were 34% higher in children with glomerular disease, 18% higher in children receiving active vitamin D supplements, 26% lower in Hispanics, and 15% higher in girls.
Table 4.
Multivariable regression analysis of determinants of fibroblast growth factor 23, 1,25-dihyroxyvitamin D, and parathyroid hormone
| Determinant | Regression Coefficient | 95% Confidence Interval | P Value |
|---|---|---|---|
| Change in log plasma FGF23, RU/ml | |||
| GFR, 10 ml/min per 1.73 m2 | −0.15 | −0.19 to −0.11 | <0.001 |
| Serum phosphorus z score, SD | 0.06 | 0.02 to 0.10 | 0.004 |
| Glomerular diagnosis | 0.29 | 0.13 to 0.44) | <0.001 |
| Active vitamin D use | 0.16 | 0.03 to 0.30 | 0.02 |
| Hispanic ethnicity | −0.30 | −0.48 to −0.13 | 0.001 |
| Girls | 0.14 | 0.26 to 0.02 | 0.03 |
| Age, yr | −0.004 | −0.02 to 0.01 | 0.59 |
| Change in serum 1,25(OH)2D, pg/ml | |||
| GFR, 10 ml/min per 1.73 m2 | 1.22 | 0.62 to 1.82 | <0.001 |
| Serum 25OHD, 10 ng/ml | 2.46 | 1.52 to 3.41 | <0.001 |
| Plasma FGF23, 10 pg/ml | −0.06 | −0.11 to −0.02 | 0.003 |
| Serum phosphorus z score, SD | 0.44 | −0.26 to 1.14 | 0.22 |
| Urine protein to creatinine ratio, mg/mg | −1.08 | −1.62 to −0.53 | <0.001 |
| Age, yr | −0.27 | −0.53 to −0.04 | 0.05 |
| Change in log serum PTH, pg/ml | |||
| GFR, 10 ml/min per 1.73 m2 | −0.15 | −0.21 to −0.09 | <0.001 |
| Serum 25OHD, 10 ng/ml | −0.14 | −0.23 to −0.05 | 0.003 |
| Serum calcium, mg/dl | −0.25 | −0.49 to −0.01 | 0.04 |
| Serum phosphorus z score, SD | 0.06 | −0.01 to 0.12 | 0.08 |
| Serum 1,25(OH)2D, pg/ml | 0.01 | −0.00 to 0.02 | 0.10 |
PTH, parathyroid hormone.
Determinants of 1,25(OH)2D, 25OHD, and PTH
By multivariable analysis, lower serum 1,25(OH)2D concentrations were significantly associated with lower GFR and serum 25OHD levels, higher FGF23 concentrations, proteinuria, and younger age (Table 4). Higher log serum PTH concentrations were significantly associated with lower GFR, serum 25OHD, and corrected calcium levels (Table 4).
Urine FEPi
In bivariate analysis, higher values of urine FEPi were strongly associated with lower GFR (r=−0.57, P<0.001), higher log FGF23 (r=0.27, P<0.001), and higher log PTH (r=0.26, P<0.001) concentrations.
Discussion
In the largest examination to date of mineral metabolism in children with predialysis CKD, we observed that plasma FGF23 concentrations were increased early and progressively as GFR declined, with the increase in FGF23 preceding increases in serum PTH and phosphorus and decreases in serum 1,25(OH)2D concentrations. In participants grouped by CKD stage, plasma FGF23 concentrations were significantly higher in CKD stages 3 and 4 relative to CKD stage 2, findings similar to those findings reported in children (5,18,19,21) and adults (6,7,33) with CKD. Because we studied a large number of children across a broad range of GFR, we subdivided participants more finely into seven GFR categories of 10 ml/min each. Plasma FGF23 levels were significantly higher as early as GFR=60–69 ml/min per 1.73 m2 (i.e., late stage 2 CKD). Indeed, at this level of GFR, FGF23 levels were above the reference range in 53% of participants. These data provide evidence that, in children with predialysis CKD, high plasma FGF23 is the earliest detectable abnormality in mineral metabolism, similar to findings in adults with CKD (6,7).
Multivariable regression analysis revealed that the principal metabolic determinants of the increase in FGF23 levels were declining GFR, increasing age-adjusted serum phosphorus concentrations (phosphorus z score), and glomerular disease as etiology of CKD. In the overall cohort, FGF23 was positively correlated with phosphorus z score. However, as shown in Figure 2, in the GFR=60–69 ml/min per 1.73 m2 group, the earliest increase in FGF23 was associated with the lowest phosphorus z score; at lower GFR levels, both FGF23 and phosphorus z score increased progressively in parallel. These findings are similar to the findings reported in the work by Isakova et al. (7). They favor the formulation that, with a mild reduction in GFR, the early increase in plasma FGF23 is not dependent on excess phosphorus exposure, as is judged from the serum phosphorus concentration, but, rather, that FGF23 may contribute to the early decrease in serum phosphorus by its phosphaturic effect. Indeed, Wilson et al. (34) first observed mild hypophosphatemia and enhanced capacity to excrete a phosphorus load in adults with mild CKD. Recent data suggest that FGF23 is the primary mediator of this response (6,7,35).
Few studies have tested whether the underlying cause of CKD is a determinant of plasma FGF23 levels. In adults with CKD stages 1 and 2 caused by autosomal dominant polycystic kidney disease, plasma FGF23 concentrations were 4-fold higher than in adults with diabetic or nondiabetic CKD and normal controls (36). An important new finding in the present study is that plasma FGF23 concentrations were significantly higher (by 34%) and serum 25OHD and 1,25(OH)2D levels were significantly lower in children with glomerular disease than in children with nonglomerular disease, despite a lack of difference in GFR between the groups. Loss of vitamin D in the urine has been invoked as a cause of low vitamin D levels in patients with high-grade proteinuria, an association that we observed in the present cohort. However, one would expect that a primary loss of vitamin D and thereby, decrease in serum 1,25(OH)2D levels would lead to a reduction in FGF23 levels. However, we observed higher FGF23 and lower vitamin D concentrations in the glomerular disease group, consistent with the effect of FGF23 to suppress 1,25(OH)2D synthesis and increase the catabolism of both 1,25(OH)2D and 25OHD (9,10,37). Corticosteroid use has been associated with higher levels of FGF23 (5); however, the children with glomerular disease in the present study reported no corticosteroid use. Our finding that phosphorus z scores did not differ between the two groups suggests that a primary difference in phosphate metabolism was not a determinant of the higher FGF23 levels in the glomerular group. Furthermore, the findings of higher FGF23 levels in the glomerular group but no difference in phosphorus z score or FEPi between the groups suggest that participants with glomerular disease may have been relatively resistant to the phosphaturic effect of FGF23.
We observed that serum 1,25(OH)2D concentrations were significantly lower in participants with a GFR of 30–39 ml/min per 1.73 m2 (i.e., CKD stage 3b) and lower. At this level of GFR, ∼50% of participants were receiving treatment with active vitamin D analogs, which could have obscured a decrease in 1,25(OH)2D levels at earlier stages of CKD. Our finding that plasma FGF23 was a significant independent predictor of serum 1,25(OH)2D concentrations is consistent with the formulation that, as GFR declines, FGF23 contributes to deficiency of 1,25(OH)2D by suppressing its renal production (6). The independent association between lower serum 25OHD and 1,25(OH)2D concentrations suggests that vitamin D deficiency further contributes to impaired 1,25(OH)2D production.
Secondary hyperparathyroidism was present in 43% of participants in the overall cohort and 55% of those participants with GFR<50 ml/min per 1.73 m2, despite little change in serum calcium levels and a low prevalence of hyperphosphatemia. These findings suggest that serum PTH levels should be monitored in children beginning in CKD stage 3a, irrespective of serum calcium and phosphorus levels. The independent association between higher serum PTH and lower serum 25OHD concentrations suggests that vitamin D deficiency further contributes to hyperparathyroidism in CKD. We observed vitamin D insufficiency or deficiency in 58% of participants, a prevalence comparable with the prevalence in the general pediatric population (38–40) and children with predialysis CKD (41–43).
A major strength of the present study is the recruitment of a large cohort of children with predialysis CKD from multiple pediatric nephrology centers across North America, in whom the underlying cause of CKD is representative of CKD in children. Furthermore, GFR was directly measured in 70% of participants, and the estimating equation used in the remainder was shown to be highly accurate. The study has some limitations. The data are cross-sectional; thus, causal relationships among the variables cannot be defined. GFR was determined simultaneously with hormone measurements in one quarter of the children, and in the remainder of the children, GFR was determined 3–6 months before hormone measurements. Over this time period, however, GFR is predicted to decline by <1 ml/min per 1.73 m2 given that the composite rate of GFR decline in the CKiD cohort is approximately 2 ml/min per 1.73 m2 per year (44). We did not measure intact FGF23, although prior work has shown good correlation between the two assays in CKD. We did not measure soluble Klotho and thus cannot address the question of whether early CKD is associated with Klotho deficiency (45,46). At lower levels of GFR, more participants received active vitamin D sterols, possibly obscuring the true prevalence of 1,25(OH)2D deficiency.
In summary, the present study of children with CKD expands our knowledge of the prevalence of abnormalities of FGF23 and indices of mineral metabolism across a broad range of precisely determined GFRs. Longitudinal follow-up of this cohort will provide an opportunity to further evaluate changes in FGF23 that occur with progressive CKD and the impact of those changes on patient outcome.
Disclosures
A.A.P. has received honoraria from Sanofi. M.W. has served as a consultant or received honoraria from Abbott, Amgen, Diasorin, Sanofi, Shire, Keryx, and Vifor. H.J. is named on a patent describing the fibroblast growth factor 23 assay that was used for this study. S.L.F. has received honoraria from Johnson and Johnson. B.A.W. has received honoraria from Amgen, Sanofi, and Abbott. I.B.S. has received honoraria from Cytochroma, Abbvie, Amgen, and Sanofi.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-084978 (to A.A.P.), R01-DK-35423, R01-DK-67563, UL1-TR-000122 (to I.B.S.), and P01-DK-11794 (subproject IV; to H.J.), grants from Abbott Laboratories and Genzyme Corporation (to A.A.P.), and the Pediatric Nephrology Innovative Research Fund (to A.A.P.). The Chronic Kidney Disease in Children study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and National Heart, Lung, and Blood Institute Grants UO1-DK- 66143, UO1-DK-66174, and UO1-DK-66116.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05840513/-/DCSupplemental.
References
- 1.McDonald SP, Craig JC, Australian and New Zealand Paediatric Nephrology Association : Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Pitts TO, Piraino BH, Mitro R, Chen TC, Segre GV, Greenberg A, Puschett JB: Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 67: 876–881, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Portale AA, Booth BE, Tsai HC, Morris RC, Jr.: Reduced plasma concentration of 1,25-dihydroxyvitamin D in children with moderate renal insufficiency. Kidney Int 21: 627–632, 1982 [DOI] [PubMed] [Google Scholar]
- 5.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P: The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab 95: 1741–1748, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N: Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1α,25-dihydroxyvitamin D3 production. J Biol Chem 278: 2206–2211, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Perwad F, Zhang MY, Tenenhouse HS, Portale AA: Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293: F1577–F1583, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P, MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lundberg S, Qureshi AR, Olivecrona S, Gunnarsson I, Jacobson SH, Larsson TE: FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol 7: 727–734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Husen M, Fischer AK, Lehnhardt A, Klaassen I, Möller K, Müller-Wiefel DE, Kemper MJ: Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int 78: 200–206, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Siomou E, Challa A, Printza N, Giapros V, Petropoulou F, Mitsioni A, Papachristou F, Stefanidis CJ: Serum osteoprotegerin, RANKL and fibroblast growth factor-23 in children with chronic kidney disease. Pediatr Nephrol 26: 1105–1114, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K: Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 45: 1161–1168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha MD, Turner C, Dalton RN, Rasmussen P, Waller S, Booth CJ, Goldsmith DJ: Investigating FGF-23 concentrations and its relationship with declining renal function in paediatric patients with pre-dialysis CKD Stages 3-5. Nephrol Dial Transplant 27: 4361–4368, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Wesseling-Perry K, Pereira RC, Tseng CH, Elashoff R, Zaritsky JJ, Yadin O, Sahney S, Gales B, Jüppner H, Salusky IB: Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol 7: 146–152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz GJ, Haycock GB, Edelmann CM, Jr., Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 25.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D: Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem 49: 546–553, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Wagner D, Hanwell HE, Vieth R: An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem 42: 1549–1556, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr., Miller AB, Parikh N, Eskridge TL, Polinske A, Olson GT, MacFarlane GD: Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 37: 867–874, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL: Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem 42: 586–592, 1996 [PubMed] [Google Scholar]
- 31.Lockitch G, Halstead AC, Wadsworth L, Quigley G, Reston L, Jacobson B: Age- and sex-specific pediatric reference intervals and correlations for zinc, copper, selenium, iron, vitamins A and E, and related proteins. Clin Chem 34: 1625–1628, 1988 [PubMed] [Google Scholar]
- 32.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 33.Shigematsu T, Kazama JJ, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, Fukagawa M: Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis 44: 250–256, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Wilson L, Felsenfeld A, Drezner MK, Llach F: Altered divalent ion metabolism in early renal failure: Role of 1,25(OH)2D. Kidney Int 27: 565–573, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Pavik I, Jaeger P, Kistler AD, Poster D, Krauer F, Cavelti-Weder C, Rentsch KM, Wüthrich RP, Serra AL: Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int 79: 234–240, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, Cox JE: Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med 162: 505–512, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML: Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics 124: e362–e370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuchman S, Kalkwarf HJ, Zemel BS, Shults J, Wetzsteon RJ, Foerster D, Strife CF, Leonard MB: Vitamin D deficiency and parathyroid hormone levels following renal transplantation in children. Pediatr Nephrol 25: 2509–2516, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Menon S, Valentini RP, Hidalgo G, Peschansky L, Mattoo TK: Vitamin D insufficiency and hyperparathyroidism in children with chronic kidney disease. Pediatr Nephrol 23: 1831–1836, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Hari P, Gupta N, Hari S, Gulati A, Mahajan P, Bagga A: Vitamin D insufficiency and effect of cholecalciferol in children with chronic kidney disease. Pediatr Nephrol 25: 2483–2488, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Kalkwarf HJ, Denburg MR, Strife CF, Zemel BS, Foerster DL, Wetzsteon RJ, Leonard MB: Vitamin D deficiency is common in children and adolescents with chronic kidney disease. Kidney Int 81: 690–697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2132–2140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John GB, Cheng CY, Kuro-o M: Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis 58: 127–134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wan M, Smith C, Shah V, Gullet A, Wells D, Rees L, Shroff R: Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant 28: 153–161, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.