Summary
The availability of life-saving dialysis therapy has been one of the great successes of medicine in the past four decades. Over this time period, despite treatment of hundreds of thousands of patients, the overall quality of life for patients with ESRD has not substantially improved. A narrow focus by clinicians and regulators on basic indicators of care, like dialysis adequacy and anemia, has consumed time and resources but not resulted in significantly improved survival; also, frequent hospitalizations and dissatisfaction with the care experience continue to be seen. A new quality paradigm is needed to help guide clinicians, providers, and regulators to ensure that patients’ lives are improved by the technically complex and costly therapy that they are receiving. This paradigm can be envisioned as a quality pyramid: the foundation is the basic indicators (outstanding performance on these indicators is necessary but not sufficient to drive the primary outcomes). Overall, these basics are being well managed currently, but there remains an excessive focus on them, largely because of publically reported data and regulatory requirements. With a strong foundation, it is now time to focus on the more complex intermediate clinical outcomes—fluid management, infection control, diabetes management, medication management, and end-of-life care among others. Successfully addressing these intermediate outcomes will drive improvements in the primary outcomes, better survival, fewer hospitalizations, better patient experience with the treatment, and ultimately, improved quality of life. By articulating this view of quality in the ESRD program (pushing up the quality pyramid), the discussion about quality is reframed, and also, clinicians can better target their facilities in the direction of regulatory oversight and requirements about quality. Clinicians owe it to their patients, as the ESRD program celebrates its 40th anniversary, to rekindle the aspirations of the creators of the program, whose primary goal was to improve the lives of the patients afflicted with this devastating condition.
Introduction
If you don't know where you are going, you will wind up somewhere else.
–Yogi Berra
Since the implementation of the ESRD program entitlement in 1973, the program has been under the microscope and rightfully so. Initially envisioned to provide needed coverage for a few thousand patients through Medicare, it was anticipated that the program would not only provide life-sustaining dialysis therapy but result in patients returning to full, active, and productive lives, including a return to employment. Over the past 40 years, however, the evolution of the ESRD program has been quite different, a fact that has been documented by the US Renal Data System (USRDS). This unique registry, an active repository of information since 1988, continues as a partnership between the Centers for Medicare and Medicaid Services (CMS) and the National Institutes of Health and is unique in American health care. In 2012, there were over 430,000 patients on various forms of dialysis, and although the growth rate of this population may be slowing, the complexity of the patients receiving this high cost, technically complex treatment is increasing (1). The majority of patients have three to four comorbid conditions in addition to ESRD, with diabetes and hypertension causing ESRD in nearly two thirds of patients and cardiovascular disease being highly prevalent. Patients are receiving 8–10 different medications daily, and the current most common form of dialysis (three times weekly in-center hemodialysis) replaces the equivalent of 10%–14% of small solute removal compared with natural kidneys. The ability of conventional dialysis to remove the full range of toxins necessary to optimize health, including salt and water, is inadequate.
Although continuous improvements in mortality have been seen over the past decades as the result of intense efforts by the renal community (at times partnering with federal agencies, including the Health Care Financing Administration [HCFA] and CMS) (2), ESRD patients continue to have high mortality and morbidity. Mortality remains nearly 20% annually overall and nearly 40% for patients new to dialysis, with an average of nearly two hospitalizations still occurring per patient per year. Although late-stage CKD and ESRD patients comprise just over 1% of all Medicare patients, they consume nearly 10% of the overall costs of Medicare, nearing $45 billion.
Recent publications have pointed out the need to reexamine the approach that has been taken to improving clinical outcomes and constraining costs for this vulnerable population (3–6). Largely because of the USRDS and provider consolidation, US ESRD patients are in the most data-dense chronic disease population in the world. To date, quality improvement has been largely focused on biochemical/surrogate outcomes, which has been attempted with other disease states. However, there is an urgent need to move beyond such outcomes to focus on more patient-centered care, which was emphasized by the Patient Centered Outcomes Research Institute (7). Clearly, reorganization of the care delivery system and focusing on care coordination can be effective in this population, which was shown by a recently completed CMS demonstration project (8,9). Ironically, despite the clear value shown in the demonstration project for certain interventions, such as oral nutritional supplements in selected patients, such supplements remain an uncovered benefit in the current reimbursement system and must be provided by dialysis facilities at their own expense. The Center for Medicare and Medicaid Innovation (CMMI) has recognized the potential for improving outcomes in ESRD patients through system reengineering by announcing the Comprehensive ESRD Care Initiative (10). Through a request for proposal process, applications to form an ESRD Seamless Care Organization (ESCO) will be reviewed, and up to 15 such programs will be awarded. Unfortunately, however, the small number of programs, small patient size of each program designated in the request for proposal, lack of specificity of quality metrics, and concerns over baseline rate setting make participation and success in this program challenging. In addition, results will not be available for 3–5 years, and in the meantime, hundreds of thousands of ESRD patients will continue to have suboptimal outcomes.
The nephrology community should not wait for the results of the CMMI ESCO initiative to act—these vulnerable patients deserve action now that will improve outcomes as well as provide the good stewardship of resources of this largely public program that the public expects. Recent publications show wide agreement within the nephrology community that care coordination incorporating nephrologist leadership is a promising approach to significantly improving outcomes (11–13). Care coordination as a delivery model is fundamental to improving outcomes, but by itself, it will not ensure the goal—improving the lives of patients with kidney disease—without consensus on the key clinical targets and metrics to drive to this goal.
Patient-Focused Care
Although there is widespread recognition of the areas of clinical focus that are most likely to improve survival, morbidity, patient experience, and overall quality of life, the ability of providers to deliver on these areas has been stymied by the lack of a unified conceptual framework for quality for ESRD patients and a well meaning CMS Quality Improvement Program (QIP), which unlike the approach advocated by VanLare and Conway (14) at CMS, uses primarily laboratory indicators that in and of themselves are no longer the key drivers to significantly improve the primary clinical outcomes. A recent case in point is the continued inclusion of dialysis adequacy in the CMS ESRD QIP program (15), despite the fact that over 98% of facilities meet the target for patients achieving adequate dialysis.
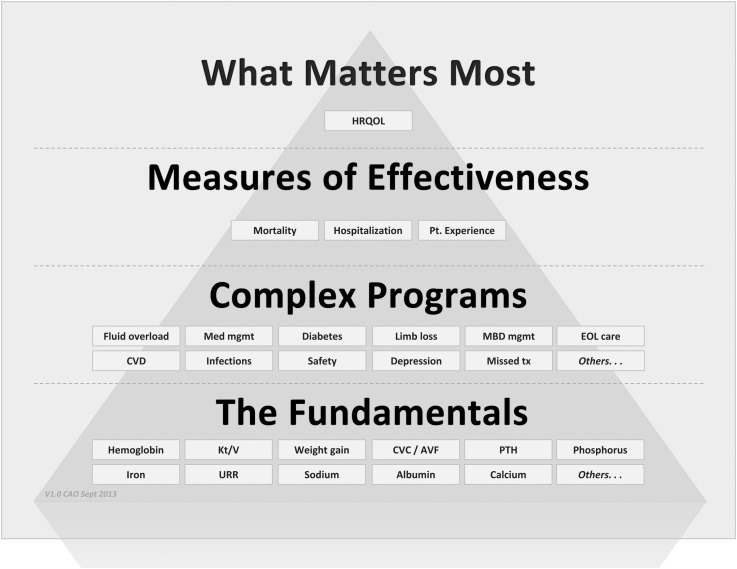
We have, therefore, developed a patient-focused needs hierarchy meant to better describe and encourage the approach to patient outcomes that is the most likely to significantly improve the lives of patients with kidney disease (Figure 1). We took an approach based on the recommendation of Stephen Covey, the business leader and author: “Begin with the end in mind” (16). We assembled a group of practicing and academic nephrologists and shared with them the results of internal nephrologist surveys as well as available literature on drivers of primary outcomes in ESRD patients. The group asked and answered a series of questions about interconnectivity. How do very basic care surrogate outcomes (sodium and albumin) impact or drive more complex health indictors (i.e., fluid status and diabetes management)? How do these complex health indicators impact widely accepted primary outcomes (survival, hospitalization, and patient experience of health)? This map was then connected to market research done directly with patients about understanding what really matters to them, research that clearly showed that, although quantity of life is important, patients focus intensely on the quality of their lives as enhanced by their dialysis care and their caregivers. After the interconnectivities were understood, they were connected in a visual form as a pyramid to provide a heuristic that could be used with physicians and nonphysician caregivers.
Figure 1.
The patient-focused quality hierarchy (the “quality pyramid”). The individual boxes are examples within the key layers that form the pyramid and are not meant to encompass all possible items within a layer. What Matters Most–outcomes that improve patients' health related quality of life; Measures of Effectiveness–primary outcomes driven by lower complex programs and fundamental clinical areas of focus; Complex Programs-comprehensive and multi-faceted clinical programs driven by fundamental clinical areas of focus and closely linked to highest-order outcomes; The Fundamentals–basic clinical information focusing largely on biochemical and surrogate data. AVF, arteriovenous fistula; CVD, cardiovascular disease; CVC, central venous catheter; EOL, end of life; HRQOL, health related quality of life; MBD, mineral and bone disorder; Med, medical; mgmt, management; Pt., patient; PTH, parathyroid hormone; tx, treatment; URR, urea reduction ratio.
What Matters Most
For patients with advanced kidney disease, the end described by Covey (16) is, as described above, improving the quality of their lives. It should be noted that we use quality of life in this hierarchy, because it is the term generally used as a key primary outcome in health care, although there are various definitions used from overall quality of life as defined by the World Health Organization (individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns) to Health Related Quality of Life (those aspects of overall quality of life that can be clearly shown to affect health—either physical or mental). For the proposed paradigm, we are focused on the latter definition. Although some patients may state that they cherish length of life more than anything, most patients focus on the quality rather than the quantity of life as most important. We developed this approach to the hierarchy after an independent group carried out a survey of 271 patients (DaVita data on file, 2011; some patients were on dialysis, and some patients had advanced CKD) to determine what was important to them. Although ESRD patients value living longer, they indicated that they were looking for more holistic care that improved the quality of their lives and caregivers who treated more than just their disease. In addition, specific clinical outcomes were only of average importance to patients, whereas a strong support system and compassionate treatment were equally important. Finally, when patients were asked what best described what they were seeking in ESRD care, the three most common responses were “living better,” “a better life,” and “treating the whole me.” It seems that the value of specific clinical outcomes is less important to patients, because they assume that competent dialysis facilities will provide safe and effective treatment or they would not be allowed to function by Medicare. A study from Australia focused on patient priorities for research in CKD is consistent with these data (17).
Measures of Effectiveness
Therefore, if one starts with the top of the hierarchy—the overarching goal to improve the lives of patients with kidney disease—it is necessary, if one is to accomplish this goal, to improve survival, decrease hospitalizations, and optimize the patient experience with care, which are outcomes on the highest tier of our pyramid as primary clinical outcomes. Moving farther down the hierarchy, there is a constellation of potential intermediate clinical outcomes—complex clinical areas that, if optimized, are most likely to drive the desired improvements in primary clinical outcomes. For example, recent analysis of claims data taken from USRDS shows that cardiovascular disease—caused or worsened by acute/chronic fluid overload, infection, and diabetes—accounts for the majority of hospitalizations. Additionally, we believe that appropriate medication management is clearly critically important if hospitalizations are to be avoided, but quantifying the contribution of medication errors and complications is not possible to tease out from claims data (18). Other potential intermediate clinical outcomes, like depression and missed treatments, have been shown to impact primary outcomes as well (19,20). These intermediate clinical outcomes are the areas of care that currently drive hospitalizations, rehospitalizations, and mortality and contribute to a suboptimal patient experience.
The Fundamentals
The basic indicators form the lower layer of the hierarchy and are the ones that have largely preoccupied the renal community and HCFA/CMS over the past decades. What we propose is that poor performance on these basic indicators will ensure poor intermediate clinical outcomes. However, excellent performance on these indicators, which is currently the case for a number of these indicators for most providers of care, has not resulted in significant improvements in intermediate or primary outcomes. Thus, excellent performance on the basic indicators is necessary but not sufficient to lead to excellent primary outcomes. The intermediate outcomes are more complex than the basic indicators, requiring systematic, organized clinical programs, multiple indicators, and often fundamental changes in the culture of the dialysis facility and dialysis team if they are to be successfully improved.
Two major observations have significantly affected the way that we perceive the basic, largely laboratory indicators in ESRD. First, we have a number of large prospective clinical trials that show us that what we may have observed about the achievement of certain basic biochemical markers and primary outcomes, such as mortality and morbidity, was not correct (21–24). Second, we have noted that many facilities have reached clinical optimization for many of the basic indicators. We use this term to indicate facility-level performance for a given indicator, where the mean value is well into the target range and the performance variation is minimal among patients. Hemodialysis adequacy is a good example of clinical optimization based on recent QIP data, with nearly 98% of facilities achieving optimization using CMS definitions. Although it will remain important going forward to maintain clinically optimized performance on the basic indicators, future focus and investment in clinical programs should move up the pyramid to intermediate outcomes if we are truly to drive to improve patients’ lives. What does it mean practically within a large dialysis organization? We have developed and successfully implemented programs to drive the basic indicators. Because these basic indicators have moved to a state of clinical optimization, our efforts shift from program development to active surveillance of performance across the organization to a focus on outliers and ongoing watchful monitoring to prevent deterioration in performance. Proactive program development and resource allocation now go to those areas of intermediate outcomes that are the most impactful, particularly fluid management, infection management, diabetes management, and medication management. The latter is derived from claims data analyzed and published by the USRDS. This practical application of the conceptual pyramid drives other strategic decision-making within the organization. For example, investment in new technology that specifically drives the prioritized intermediate outcomes would have a higher priority than technology that addressed other aspects of care. In addition, areas of the organization that support the clinical care delivered, such as clinical laboratories and information technology, are using the clinical hierarchy prioritization scheme to consider how best to provide the support needed to drive up the pyramid.
The Patient Hierarchy in Practice
At the facility level, the hierarchy provides a powerful tool to ensure that the interdisciplinary team fully understands and embraces this new way of thinking about clinical outcomes. An example relates to a focus on the key secondary outcomes of fluid management. This complex area is, because of its many components, overwhelming to most dialysis facilities. By breaking it down using the hierarchy, however, it begins to be easier for facilities to tackle. We would use the hierarchy in this example in the following ways. (1) Explain and educate the teams in the facilities that appropriate attention to sodium, particularly avoiding sodium loading during dialysis from too high a dialysate sodium or use of sodium modeling, is a basic component of a more complex program, fluid management, that is under the control of the dialysis facility. (2) If the facility is successful in impacting fluid management, in part through controlling sodium loading during dialysis, the results will be fewer hospitalizations, better survival, and better patient experience. (3) If successful, the result will be the ultimate goal being achieved—improving the patient’s life.
We have recently published a study that is a proof of concept that moving up the pyramid and focusing on the intermediate clinical outcomes will result in lower rates of hospitalization (25). Three dialysis providers collaborated on a project to improve fluid management in in-center hemodialysis patients in Texas. In that evaluation, implementation of a technology-driven process (Crit-Line) resulted in a substantial drop in fluid overload-related hospitalizations.
From the perspective of policymakers, the hierarchy clearly shows that the renal community is interested in patient-centered care—driving to what is important to patients. It emphasizes that the basic indicators are important but that we now have a strong foundation and need to move to more impactful clinical programs to drive improvements in the primary outcomes. Policy decisions regarding quality incentives need to keep pace with this paradigm shift in clinical focus. To date, quality has been defined by ESRD policymakers largely based on the available data and not necessarily the most impactful clinical areas. This finding is ironic, because it runs counter to the stated approach articulated by Goodrich et al. (26) and Conway et al. (27). In the latter paper, Conway et al. (27) state “[the goal is] delivering care that is high quality, safe, and affordable. Reliable and meaningful quality measurement that focuses on important outcomes … is an essential prerequisite for achieving this goal” (27). The ESRD QIP has been constructed largely around those metrics that are currently measured and captured by CMS, on claims forms, or going forward, in CrownWeb, like hemoglobin and urea reduction ratio. We are increasingly aware of the disconnect between attainment of these surrogate laboratory parameters (basic indicators in the pyramid) and outcomes that matter to patients, such as mortality and morbidity and the experience of care. The pyramid can inform policymakers, providing a roadmap to which areas of focus going forward will be most likely to lead to improvements in the primary clinical outcomes. In fact, the eventual use of the National Healthcare Surveillance Network metrics for bloodstream infection in the QIP shows that this very progression is already happening.
Such a framework is helpful in communicating the importance of the new programs and initiatives to a wide audience, and it helps to get health care delivery teams and patients aligned on program rationale. It allows population-based management programs to be successfully implemented to improve outcomes. Although clinicians know what is needed to improve the lives of patients with kidney disease, up until now they did not have a conceptual framework to articulate it within their own organizations or externally, particularly to CMS. If key stakeholders can embrace such a common vision and share best practices and innovative ways to drive the secondary outcomes, it is likely that patients will see the benefits of moving the focus up the pyramid and that all will benefit as a result.
Disclosures
A.R.N. is a salaried employee of DaVita HealthCare Partners, Inc.
Acknowledgments
Special thanks for the input and creativity to Paul Asper and Michael Brouthers, whose insights made this work possible.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.US Renal Data System: Annual Data Report: Atlas of End-Stage Renal Disease in the United States, 2012. Available at: http://www.usrds.org/2012/pdf/v2_ch1_12.pdf Accessed March 25, 2013
- 2.Kidney Care Partners: Kidney Care Partners Performance Excellence and Accountability in Kidney Care, 2012. Available at: http://www.kidneycarequality.com/ Accessed March 25, 2013
- 3.Parker T, 3rd, Hakim R, Nissenson AR, Steinman T, Glassock RJ: Dialysis at a crossroads: 50 years later. Clin J Am Soc Nephrol 6: 457–461, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Parker TF, 3rd, Straube BM, Nissenson A, Hakim RM, Steinman TI, Glassock RJ: Dialysis at a crossroads—Part II: A call for action. Clin J Am Soc Nephrol 7: 1026–1032, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Wingard RL, Pupim LB, Krishnan M, Shintani A, Ikizler TA, Hakim RM: Early intervention improves mortality and hospitalization rates in incident hemodialysis patients: RightStart program. Clin J Am Soc Nephrol 2: 1170–1175, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Lacson E, Jr., Li NC, Guerra-Dean S, Lazarus M, Hakim R, Finkelstein FO: Depressive symptoms associate with high mortality risk and dialysis withdrawal in incident hemodialysis patients. Nephrol Dial Transplant 27: 2921–2928, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Patient-Centered Outcomes Research Institute: PCORI 101 Available at: http://www.pcori.org/about/governance-and-leadership/pcori101/ Accessed March 25, 2013
- 8.Nissenson AR, Deeb T, Franco E, Krishnan M, McMurray S, Mayne TJ: The ESRD Demonstration Project: What it accomplished. DaVita Inc. Nephrol News Issues 25: 39–41, 2011 [PubMed] [Google Scholar]
- 9.Sauer PF, Farrell RE, Lazarus JM: The ESRD demonstration project: What it accomplished. Fresenius Medical Care North America. Nephrol News Issues 25: 32–38, 2011 [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid Services: Comprehensive ESRD Care Initiative, 2013. Available at: http://innovation.cms.gov/initiatives/comprehensive-ESRD-care/ Accessed March 25, 2013
- 11.Nissenson AR, Maddux FW, Velez RL, Mayne TJ, Parks J: Accountable care organizations and ESRD: The time has come. Am J Kidney Dis 59: 724–733, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Williams AW, Nesse RE, Wood DL: Delivering accountable care to patients with complicated chronic illness: How does it fit into care models and do nephrologists have a role? Am J Kidney Dis 59: 601–603, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Maddux FW, McMurray S, Nissenson AR: Toward population management in an integrated care model. Clin J Am Soc Nephrol 8: 694–700, 2013 [DOI] [PubMed] [Google Scholar]
- 14.VanLare JM, Conway PH: Value-based purchasing—national programs to move from volume to value. N Engl J Med 367: 292–295, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services (CMS), HHS: Medicare program; end-stage renal disease prospective payment system, quality incentive program, and bad debt reductions for all Medicare providers. Final rule. Fed Regist 77: 67450–67531, 2012 [PubMed] [Google Scholar]
- 16.Covey S: The 7 Habits of Highly Effective People. Habit 2, New York, Simon and Schuster, 1989 [Google Scholar]
- 17.Tong A, Sainsbury P, Carter SM, Hall B, Harris DC, Walker RG, Hawley CM, Chadban S, Craig JC: Patients’ priorities for health research: Focus group study of patients with chronic kidney disease. Nephrol Dial Transplant 23: 3206–3214, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Weinhandl ED, Arneson TJ, St Peter WL: Clinical outcomes associated with receipt of integrated pharmacy services by hemodialysis patients: A quality improvement report. Am J Kidney Dis 62: 557–567, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kimmel PL, Cukor D, Cohen SD, Peterson RA: Depression in end-stage renal disease patients: A critical review. Adv Chronic Kidney Dis 14: 328–334, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Obialo C, Hunt W, Bashit K, Zager P: Relationship of missed and shortened hemodialysis treatments to hospitalization and mortality: observations from a US dialysis network. Clin Kidney J 5: 315–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS; EVOLVE Trial Investigators: Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW, and the CAST Investigators: Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324: 781–788, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Lowrie EG, Teng M, Lacson E, Lew N, Lazarus JM, Owen WF: Association between prevalent care process measures and facility-specific mortality rates. Kidney Int 60: 1917–1929, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Parker TF, 3rd, Hakim R, Nissenson AR, Krishnan M, Bond TC, Chan K, Maddux FW, Glassock R: A quality initiative. Reducing rates of hospitalizations by objectively monitoring volume removal. Nephrol News Issues 27: 30–36, 2013 [PubMed] [Google Scholar]
- 26.Goodrich K, Garcia E, Conway PH: A history of and a vision for CMS quality measurement programs. Jt Comm J Qual Patient Saf 38: 465–470, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Conway PH, Mostashari F, Clancy C: The future of quality measurement for improvement and accountability. JAMA 309: 2215–2216, 2013 [DOI] [PubMed] [Google Scholar]